Whole-Body Vibration Training on Oxidative Stress Markers, Irisin Levels, and Body Composition in Women with Fibromyalgia: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
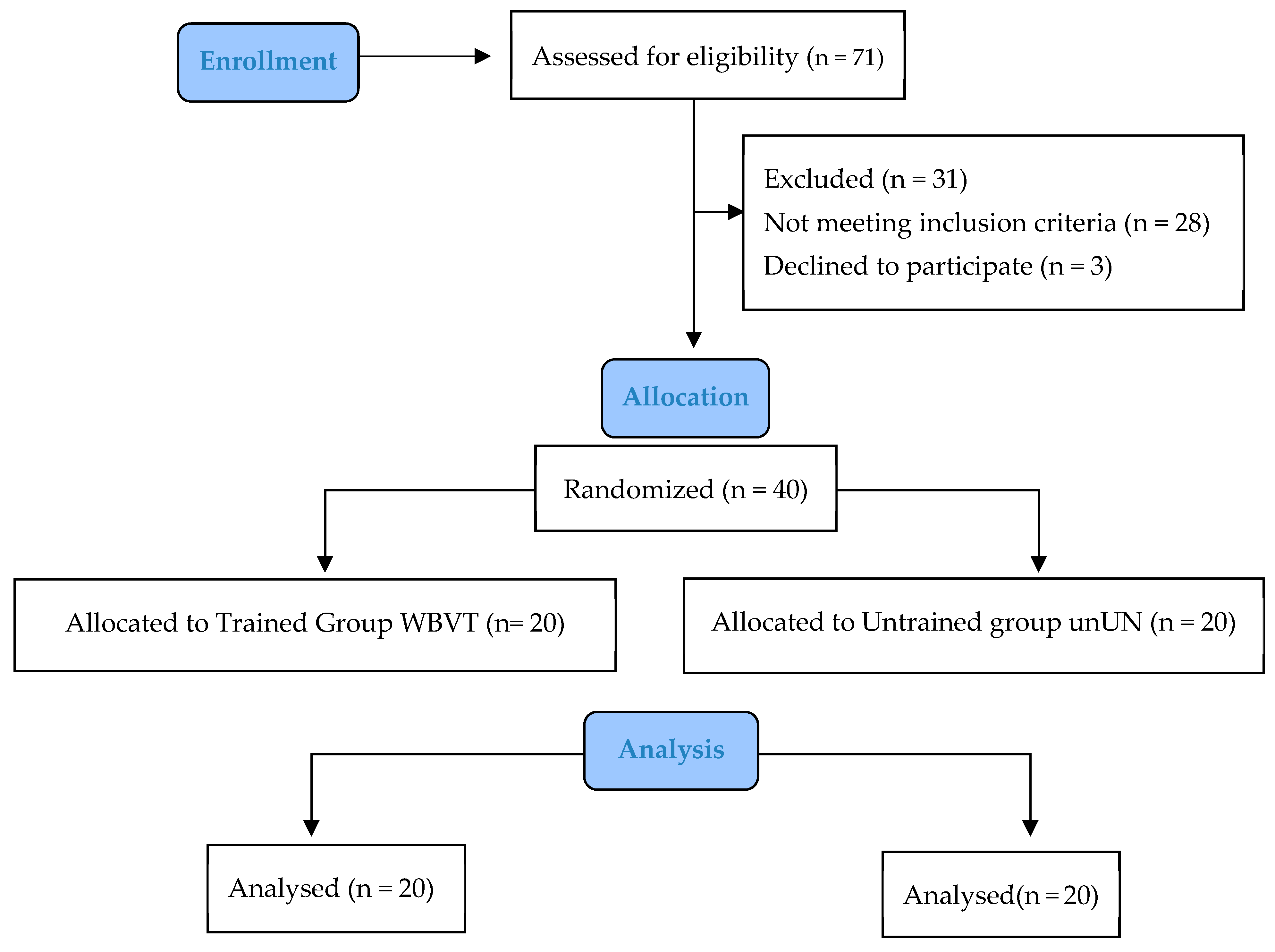
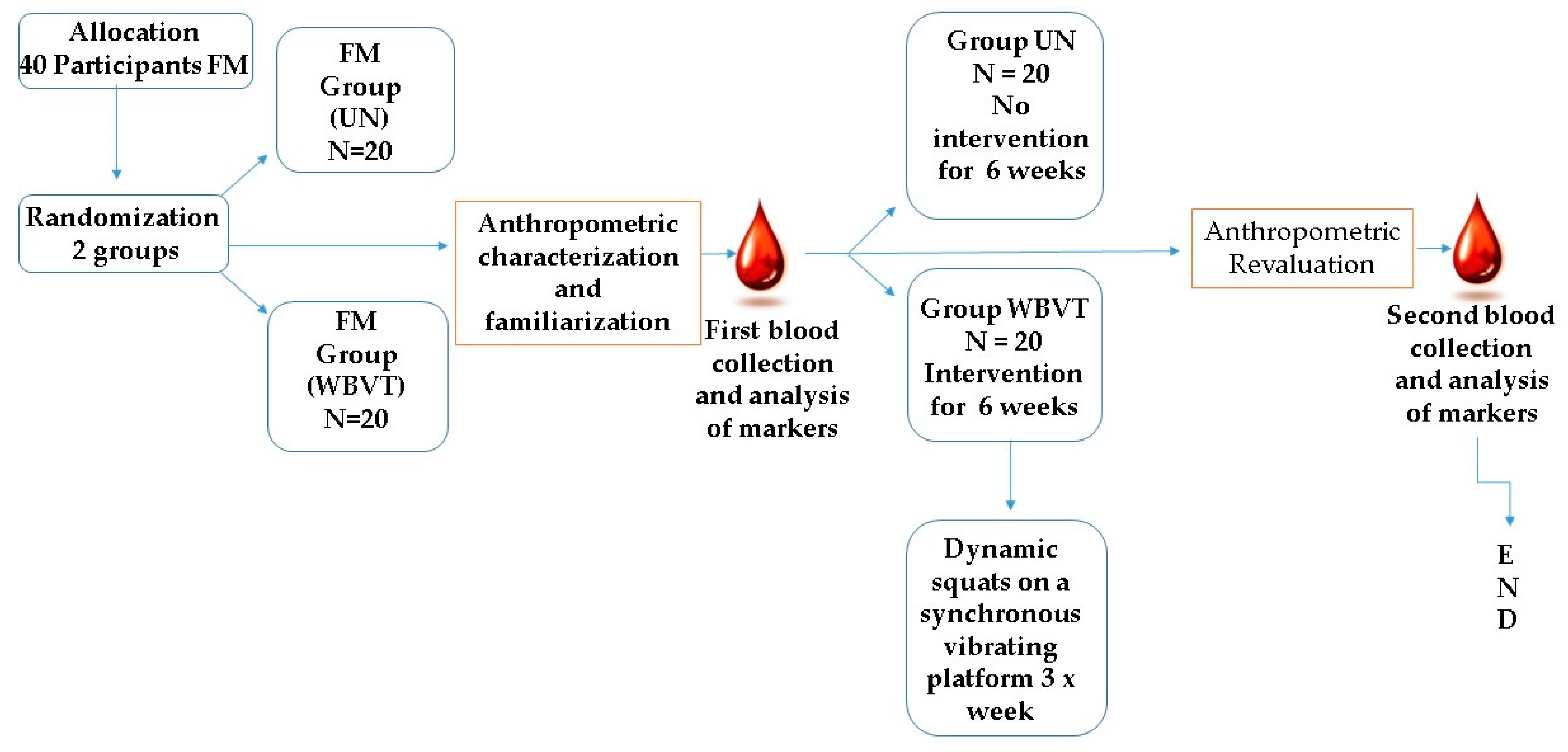
2.2. Study Design
2.3. Inclusion Criteria
2.4. Exclusion Criteria
2.5. Participants
2.6. Evaluations
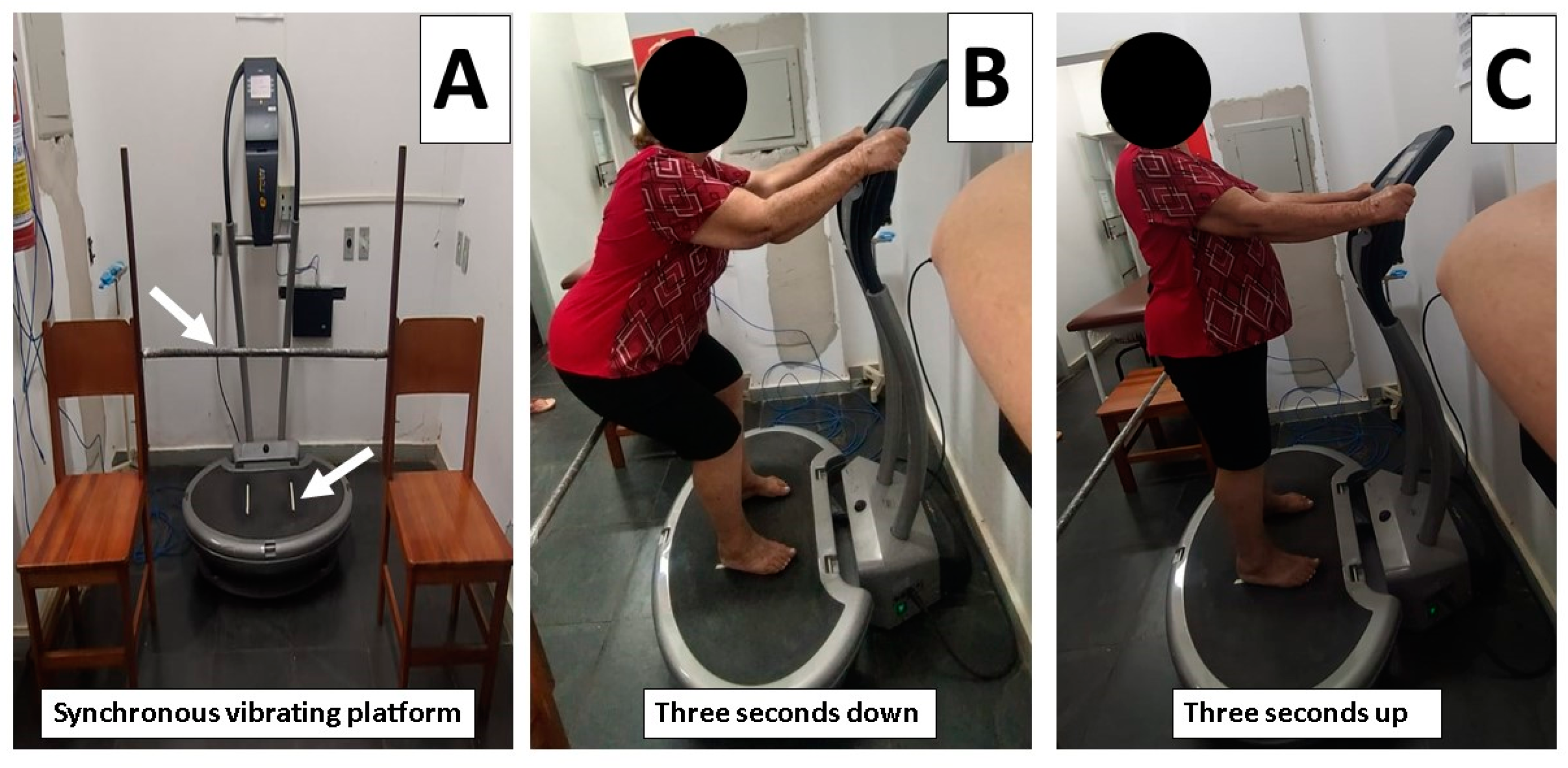
2.7. Intervention
2.8. Oxidative Stress Biomarkers
2.9. Irisin Plasma Levels
2.10. Secondary Outcomes: Body Composition—Determination of Body Composition
2.11. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
6. Practical Application
7. Strengths and limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellato, E.; Marini, E.; Castoldi, F.; Barbasetti, N.; Mattei, L.; Bonasia, D.E.; Blonna, D. Fibromyalgia syndrome: Etiology, pathogenesis, diagnosis, and treatment. Pain Res. Treat. 2012, 42, 30–61. [Google Scholar] [CrossRef] [PubMed]
- Clauw, D.J.; D’Arcy, Y.; Gebke, K.; Semel, D.; Pauer, L.; Jones, K.D. Normalizing Fibromyalgia as a chronic illness. Postgrad. Med. 2018, 130, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; Culic, O.; Navarro-Pando, J.M.; Sánchez-Alcázar, J.A.; Bullón, P. Effect of coenzyme Q(10) on psychopathological symptoms in fibromyalgia patients. CNS Neurosci. Ther. 2017, 23, 188–189. [Google Scholar] [CrossRef] [PubMed]
- Shukla, V.; Das, S.K.; Mahdi, A.A.; Agarwal, S.; Alok, R.; Ansari, J.A.; Khandpur, S. Metal-induced oxidative stress level in patients with fibromyalgia syndrome and its contribution to the severity of the disease: A correlational study. J. Back Musculoskelet Rehabil. 2021, 34, 319–326. [Google Scholar] [CrossRef]
- Bagis, S.; Tamer, L.; Sahin, G.; Bilgin, R.; Guler, H.; Ercan, B.; Erdogan, C. Freeradicals and antioxidants in primary Fibromyalgia: An oxidative stress disorder? Rheumatol. Int. 2005, 25, 188–190. [Google Scholar] [CrossRef]
- Sánchez-Domínguez, B.; Bullón, P.; Román-Malo, L.; Marín-Aguilar, F.; Alcocer-Gómez, E.; Carrión, A.M.; Sánchez-Alcazar, J.A.; Cordero, M.D. Oxidative stress, mitochondrial dysfunction and, inflammation common events in skin of patients with Fibromyalgia. Mitochondrion 2015, 21, 69–75. [Google Scholar] [CrossRef]
- Fatima, G.; Das, S.K.; Mahdi, A.A. Oxidative stress and antioxidative parameters and metal ion content in patients with fibromyalgia syndrome: Implications in the pathogenesis of the disease. Clin. Exp. Rheumatol. 2013, 31, S128–S133. [Google Scholar]
- Miyamae, T.; Seki, M.; Naga, T.; Uchino, S.; Asazuma, H.; Yoshida, T.; Iizuka, Y.; Kikuchi, M.; Imagawa, T.; Natsumeda, Y.; et al. Increased oxidative stress and coenzyme Q10 399 deficiency in juvenile Fibromyalgia: Amelioration of hypercholesterolemia and fatigue 400 by ubiquinol-10 supplementation. Redox Rep. 2013, 18, 12–19. [Google Scholar] [CrossRef]
- Cordero, M.D.; De-Miguel, M.; Carmona-López, I.; Bonal, P.; Campa, F.; Moreno-Fernández, A.M. Oxidative Stress Mitochondrial Dysfunction in Fibromyalgia. Neuro Endocrinol. Lett. 2010, 31, 169–173. Available online: https://pubmed.ncbi.nlm.nih.gov/20424583 (accessed on 2 February 2022).
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Curr. Osteoporos. Rep. 2020, 8, 388–400. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Duan, Y.; Hu CA, A.; Tang, Y.; Yin, Y. Myokines and adipokines: Involvement in the crosstalk between skeletal muscle and adipose tissue. Cytokine Growth Factor Rev. 2017, 33, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Paris, M.T.; Bell, K.E.; Mourtzakis, M. Myokines and adipokines in sarcopenia: Understanding cross-talk between skeletal muscle and adipose tissue and the role of exercise. Curr. Opin. Pharmacol. 2020, 52, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, M.C.; Vella, J.P.; Cafeo, F.R.; Affonso Fonseca, F.L.; Bacci, M.R. Association between irisin and major chronic diseases: A review. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 4072–4077. [Google Scholar] [PubMed]
- Zhao, J.; Qiao, L.; Dong, J.; Wu, R. Antioxidant Effects of Irisin in Liver Diseases: Mechanistic Insights. Oxid Med. Cell Longev. 2022, 35, 65–89. [Google Scholar] [CrossRef] [PubMed]
- Jahan, F.; Nanji, K.; Qidwai, W.; Qasim, R. Fibromyalgia syndrome: An overview of pathophysiology, diagnosis and management. Oman Med. J. 2012, 27, 192–195. [Google Scholar] [CrossRef] [PubMed]
- García, D.A.; Martínez, I.; Saturno, P.J. Clinical approach to Fibromyalgia: Synthesis of evidence-based recommendations, a systematic review. Reumatol. Clin. 2016, 12, 65–69. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Skraparlis, A.; Kabasakalis, A.; Mantzoros, C.S. Irisin in response to acute and chronic whole-body vibration exercise in humans. Metabolism 2014, 63, 918–921. [Google Scholar] [CrossRef]
- Greulich, T.; Nell, C.; Koepke, J.; Fechtel, J.; Franke, M.; Schmeck, B.; Koczulla, A.R. Benefits of whole body vibration training in patients hospitalised for COPD exacerbations-a randomized clinical trial. BMC Pulm. Med. 2014, 14, 1–9. [Google Scholar] [CrossRef]
- Santos, J.; Mendonça, V.; Ribeiro, V.; Tossige-Gomes, R.; Fonseca, S.; Prates, A.; Flor, J.; Oliveira, A.; Martins, J.; Garcia, B.; et al. Does whole body vibration exercise improve oxidative stress markers in women with fibromyalgia? Braz. J. Med. Biol. Res. 2019, 16, 82–98. [Google Scholar] [CrossRef]
- Ribeiro, V.; Mendonça, V.; Souza, A.; Fonseca, S.; Camargos, A.; Lage, V.; Neves, C.; Santos, J.; Teixeira, A.L.; Vieira, E.; et al. Inflammatory biomarkers responses after acute whole body vibration in Journal of Healthcare Engineering 7 fibromyalgia. Braz. J. Med. Biol. Res. 2018, 24, 74–82. [Google Scholar] [CrossRef]
- Ribeiro, V.G.C.; Lacerda, A.C.R.; Santos, J.M.; Coelho-Oliveira, A.C.; Fonseca, S.F.; Prates, A.C.N.; Flor, J.; Garcia, B.C.C.; Tossige-Gomes, R.; Leite, H.R.; et al. Efficacy of Whole-Body Vibration Training on Brain-Derived Neurotrophic Factor, Clinical and Functional Outcomes, and Quality of Life in Women with Fibromyalgia Syndrome: A Randomized controlled Trial. J. Healthc. Eng. 2021, 30, 75–93. [Google Scholar] [CrossRef]
- Alentorn-Geli, E.; Padilla, J.; Moras, G.; Haro, C.L.; Fernández-Solà, J. Six weeks of whole-body vibration exercise improves pain and fatigue in women with fibromyalgia. J. Altern. Complement. Med. 2008, 14, 975–981. [Google Scholar] [CrossRef]
- Sañudo, B.; De Hoyo, M.; Carrasco, L.; McVeigh, J.G.; Corral, J.; Cabeza, R.; Rodríguez, C.; Oliva, A. The effect of 6-week exercise programme and whole body vibration on strength and quality of life in women with fibromyalgia: A randomised study. Clin. Exp. Rheumatol. 2010, 28 (Suppl. 63), S40–S45. [Google Scholar]
- Alev, A.; Mihriban, A.; Bilge, E.; Ayça, E.; Merve, K.; Şeyma, C.; Uğur, E.; Adnan, B.; Zeynel, K.; Mahmut, G.S. Effects of whole body vibration therapy in pain, function and depression of the patients with fibromyalgia. Complement. Ther. Clin. Pract. 2017, 28, 200–203. [Google Scholar] [CrossRef]
- Morey, S.S. ACSM/AHA Release Recommendations for fitness facilities. American College of Sports Medicine / American Heart Association. Am. Fam. Physician 1999, 59, 693–694. [Google Scholar] [PubMed]
- Rittweger, J. Vibration as an exercise modality: How it may work, and what its potential might be. Eur. J. Appl. Physiol. 2010, 2010, 877–904. [Google Scholar] [CrossRef] [PubMed]
- Avelar, N.C.P.; Simão, A.P.; Tossige-Gomes, R.; Neves, C.D.C.; Rocha-Vieira, E.; Coimbra, C.C.; Lacerda, A.C.R. Effect of adding whole-body vibration to squat training on the functional performance and self-report of disease status in elderly patients with knee osteoarthritis: A randomized, controlled clinical study. J. Altern. Complement. Med. 2011, 17, 1155. [Google Scholar] [CrossRef] [PubMed]
- Bidonde, J.; Busch, A.J.; van der Spuy, I.; Tupper, S.; Kim, S.Y.; Boden, C. Whole body vibration exercise training for Fibromyalgia. Cochrane Database Syst. Rev. 2017, 26, 17–55. [Google Scholar] [CrossRef]
- Marín, P.J.; Bunker, D.; Rhea, M.R.; Ayllon, F.N. Neuromuscular activity during whole-body vibration of different amplitudes and footwear conditions: Implications for prescription of vibratory stimulation. J. Strength Cond. Res. 2009, 23, 2311–2316. [Google Scholar] [CrossRef] [PubMed]
- van Heuvelen, M.J.G.; Rittweger, J.; Judex, S.; Sañudo, B.; Seixas, A.; Fuermaier, A.B.M.; Tucha, O.; Nyakas, C.; Marín, P.J.; Taiar, R.; et al. Reporting Guidelines for Whole-Body Vibration Studies in Humans, Animals and Cell Cultures: A Consensus Statement from an International Group of Experts. Biology 2021, 10, 965. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the Superoxide Anion Radical in the Autoxidation of Pyrogallol and a Convenient Assay for Superoxide Dismutase. Eur. J. Biochem. 1974, 24, 469–474. [Google Scholar] [CrossRef]
- Nelson, D.; Kiesow, L. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 degrees C (with molar extinction coefficients of H 2 O 2 solutions in the U.V.). Anal. Biochem. 1972, 49, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of Antioxidant Power: The FRAP Assay. Anal. Biochem. 1996, 236, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in mass measurement muscle: The need for a reference standard. J. Sarcopenia Muscle Cachexia 2018, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.M.; Jang, H.C.; Lim, S. Differences between skeletal muscle mass indices derived from height, weight and body mass index-adjusted models in the assessment of sarcopenia. Korean J. Intern. Med. 2016, 31, 643–650. [Google Scholar] [CrossRef]
- Cohen, J. Eta-squared and partial eta-squared in fixed factor ANOVA designs. Educ. Psychol. Meas. 1973, 107–112. [Google Scholar] [CrossRef]
- Gaudreault, N.; Boulay, P. Cardiorespiratory fitness among adults with fibromyalgia. Breathe 2018, 33, e25–e33. [Google Scholar] [CrossRef]
- Ursini, F.; Naty, S.; Grembiale, R.D. Fibromyalgia and obesity: The hidden link. Rheumatol. Int. 2011, 31, 1403–1408. [Google Scholar] [CrossRef]
- Gota, C.E.; Kaouk, S.; Wilke, W.S. Fibromyalgia and Obesity. J. Clin. Rheumatol. 2015, 21, 289–295. [Google Scholar] [CrossRef]
- Rossi, A.; Di Lollo, A.C.; Guzzo, M.P.; Giacomelli, C.; Atzeni, F.; Bazzichi, L.; Di Franco, M. Fibromyalgia and nutrition: What news? Clin. Exp. Rheumatol. 2015, 33 (Suppl. 88), S117–S125. [Google Scholar] [PubMed]
- Kadayifci, F.Z.; Bradley, M.J.; Onat, A.M.; Shi, H.N.; Zheng, S. Review of nutritional approaches to fibromyalgia. Nutr. Rev. 2022, 80, 2260–2274. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, V.A.; Ortega, F.B.; Carbonell-Baeza, A.; Gatto-Cardia, C.; Sjöström, M.; Ruiz, J.R.; Delgado-Fernández, M. Fibromyalgia's Key Symptoms in Normal-Weight, Overweight, and Obese Female Patients. Pain Manag. Nurs. 2013, 14, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Okifuji, A.; Donaldson, G.W.; Barck, L.; Fine, P.G. Relationship between Fibromyalgia and obesity in pain, function, mood, and sleep. J. Pain Off. J. Am. Pain Soc. 2010, 11, 1329–1337. [Google Scholar] [CrossRef]
- Neumann, L.; Lerner, E.; Glazer, Y.; Bolotin, A.; Shefer, A.; Buskila, D. A cross-sectional study of the relationship between body mass index and clinical characteristics, tenderness measures, quality of life, and physical functioning in fibromyalgia patients. Clin. Rheumatol. 2008, 27, 1543–1547. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Luedtke, C.A.; Vincent, A.; Thompson, J.M.; Oh, T.H. Association of body mass index with symptom severity and quality of life in patients with Fibromyalgia. Arthritis Care Res. 2012, 64, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Koçyigit, B.F.; Okyay, R.A. The relationship between body mass index and pain, disease activity, depression and anxiety in women with Fibromyalgia. PeerJ 2018, 6, e4917. [Google Scholar] [CrossRef] [PubMed]
- Sañudo, B.; de Hoyo, M.; Carrasco, L.; Rodríguez-Blanco, C.; Oliva, A.; McVeigh, J.G. Effect of whole-body vibration exercise on balance in women with fibromyalgia syndrome: A randomized controlled trial. J. Altern. Complement. Med. 2012, 18, 158–164. [Google Scholar] [CrossRef]
- Gusi, N.; Parraca, J.A.; Olivares, P.R.; Leal, A.; Adsuar, J.C. Tilt vibratory exercise and the dynamic balance in Fibromyalgia: A randomized controlled trial. Arthrit. Care Res. 2010, 62, 1072–1078. [Google Scholar] [CrossRef]
- Adsuar, J.C.; Del Pozo-Cruz, B.; Parraca, J.A.; Olivares, P.R.; Gusi, N. Whole Body Vibration Improves the Single-Leg Stance static Balance in Women with Fibromyalgia: A Randomized Controlled Trial. J. Sports Med. Phys. Fit. 2012, 52, 85–91. [Google Scholar]
- Bekkelund, S.I.; Jorde, R. Lean body mass and creatine kinase are associated with reduced inflammation in obesity. Eur. J. Clin. Investig. 2017, 47, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017, 35, 200–221. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Arias, J.Á.; Martínez-Aranda, L.M.; Andreu-Caravaca, L.; Sanz, G.; Benito, P.J.; Ramos-Campo, D.J. Effects of Whole-Body Vibration Training on Body Composition, Cardiometabolic Risk, and Strength in the Population Who are Overweight and Obese: A Systematic Review with Meta-analysis. Arch. Phys. Med. Rehabil. 2021, 102, 2442–2453. [Google Scholar] [CrossRef]
- Park, S.Y.; Son, W.M.; Kwon, O.S. Effects of whole body vibration training on body composition, skeletal muscle strength, and cardiovascular health. J. Exerc. Rehabil. 2015, 11, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Kaeding, T.S. Sarkopenie und Vibrationstraining: Eine Ubersicht [Sarcopenia and whole body vibration training: An overview]. Z Gerontol Geriatr. 2009, 42, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Vissers, D.; Verrijken, A.; Mertens, I.; Van Gils, C.; Van de Sompel, A.; Truijen, S.; Van Gaal, L. Effect of long-term whole body vibration training on visceral adipose tissue: A preliminary report. Obes. Facts. 2010, 3, 93–100. [Google Scholar] [CrossRef]
- Correa-Rodríguez, M.; Mansouri-Yachou, J.E.; Casas-Barragán, A.; Molina, F.; Rueda-Medina, B.; Aguilar-Ferrandiz, M.E. The Association of Body Mass Index and Body Composition with Pain, Disease Activity, Fatigue, Sleep and Anxiety in Women with Fibromyalgia. Nutrients 2019, 11, 1193. [Google Scholar] [CrossRef]
- Samanci, R.; Ataoglu, S.; Ozsahin, M.; Ankarali, H.; Admis, O. An investigation of serum irisin levels and inflammatory markers in fibromyalgia syndrome. N. Clin. Istanb. 2019, 24, 341–347. [Google Scholar] [CrossRef]
- Moreno, M.; Moreno-Navarrete, J.M.; Fernández-Real, J.M. Irisina: ¿transmisor de mensajes del Olimpo? [Irisin: A messenger from the gods? Clin. Investig. Arterioscler 2014, 26, 140–146. [Google Scholar] [CrossRef]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future. J. Physiol. 2016, 15, 5081–5092. [Google Scholar] [CrossRef]
- Blobaum, P. Physiotherapy evidence database (PEDro). J. Med. Libr. Assoc. 2006, 47, 77–86. [Google Scholar]

| Vibration Parameters (Intervention) | |||||||
|---|---|---|---|---|---|---|---|
| Weeks | Frequency (Hz) | Amplitude (mm) | Acceleration (g) | Total Time per Set | Number of Repetitions per Set | Total Number of Sets | Rest Time between Sets |
| 1 | 35 | 4 | 2.78 | 16 s | 5 | 6 | 30 s |
| 2 | 35 | 4 | 2.78 | 24 s | 8 | 7 | 30 s |
| 3 | 35 | 4 | 2.78 | 32 s | 10 | 8 | 30 s |
| 4 | 40 | 4 | 3.26 | 35 s | 11 | 8 | 30 s |
| 5 | 40 | 4 | 3.26 | 40 s | 13 | 8 | 30 s |
| 6 | 40 | 4 | 3.26 | 48 s | 16 | 8 | 30 s |
| Characteristics | UN | WBVT | p Value |
|---|---|---|---|
| Age (years) | 54.31 ± 7.62 | 55.12 ± 6.44 | 0.71 |
| Time from diagnosis (years) | 8.15 ± 2.37 | 8.40 ± 2.68 | 0.75 |
| BMI (kg/m2) | 30.86 ± 3.98 | 30.26 ± 4.92 | 0.67 |
| Lean mass index (kg) | 8.31 ± 1.04 | 8.18 ± 0.76 | 0.67 |
| Fat mass index (kg) | 15.99 ± 1.24 | 15.93 ± 2.08 | 0.92 |
| VAT (kg) | 1.37 ± 0.48 | 1.34 ± 0.48 | 0.37 |
| TBARS (nmol MDA/mg protein) | 0.39 ± 0.20 | 0.34 ± 0.13 | 0.40 |
| FRAP (FeSO4.1−1mgprotein−1) | 8.34 ± 2.37 | 6.87 ± 2.40 | 0.98 |
| CAT (U/mg protein) | 2.64 ± 0.71 | 2.72 ± 2.02 | 0.86 |
| SOD (U/mg protein) | 0.30 ± 0.16 | 0.33 ± 0.24 | 0.59 |
| Irisin (ng/mL) | 333.88 ± 90.01 | 316.98 ± 109.24 | 0.59 |
| Outcomes | Between Groups | Within Groups | Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | Groups | Follow-Up | p1 | F | η2 | p2 | F | η2 | p3 | F | η2 |
| TBARS (nmol MDA/mg protein) | UN WBVT | 0.29 ± 0.09 0.24 ± 0.06 * | 0.10 | 2.69 | 0.73 | 0.008 | 12.17 | 0.92 | 0.92 | 0.01 | 0.10 |
| FRAP (FeSO4.1−.mg protein−1) | UN WBVT | 7.24 ± 1.53 8.33 ± 2.99 | 0.74 | 0.11 | 0.10 | 0.72 | 0.13 | 0.12 | 0.01 | 5.78 | 0.85 |
| CAT (U/mg protein) | UN. WBVT | 4.08 ± 1.52 *# 2.83 + 1.21 | 0.002 | 9.96 | 0.50 | 0.006 | 7.85 | 0.44 | 0.01 | 6.46 | 0.40 |
| SOD (U/mg protein) | UN. WBVT | 0.31 ± 0.14 0.36 ± 0.20 | 0.36 | 0.95 | 0.48 | 0.68 | 0.19 | 0.15 | 0.87 | 0.03 | 0.10 |
| Irisin (ng/mL) | UN. WBVT | 368.65 ± 125.70 477.62 ± 267.93 # | 0.009 | 7.10 | 0.88 | 0.21 | 1.57 | 0.61 | 0.09 | 2.95 | 0.74 |
| VAT (kg) | UN. WBVT | 1.04 ± 0.62 # 0.69 ± 0.12 | 0.001 | 16.53 | 0.94 | 0.12 | 2.45 | 0.71 | 0.19 | 1.75 | 0.63 |
| Lean mass index | UN. WBVT | 8.20 ± 1.00 8.60 ± 0.67 | 0.41 | 0.65 | 0.39 | 0.48 | 0.48 | 0.32 | 0.18 | 1.74 | 0.63 |
| Fat mass index | UN. WBVT | 16.24 ± 1.22 15.78 ± 1.98 | 0.90 | 0.18 | 0.13 | 0.50 | 0.46 | 0.31 | 0.60 | 0.28 | 0.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, J.M.; Taiar, R.; Ribeiro, V.G.C.; da Silva Lage, V.K.; Scheidt Figueiredo, P.H.; Costa, H.S.; Pereira Lima, V.; Sañudo, B.; Bernardo-Filho, M.; Sá-Caputo, D.d.C.d.; et al. Whole-Body Vibration Training on Oxidative Stress Markers, Irisin Levels, and Body Composition in Women with Fibromyalgia: A Randomized Controlled Trial. Bioengineering 2023, 10, 260. https://doi.org/10.3390/bioengineering10020260
dos Santos JM, Taiar R, Ribeiro VGC, da Silva Lage VK, Scheidt Figueiredo PH, Costa HS, Pereira Lima V, Sañudo B, Bernardo-Filho M, Sá-Caputo DdCd, et al. Whole-Body Vibration Training on Oxidative Stress Markers, Irisin Levels, and Body Composition in Women with Fibromyalgia: A Randomized Controlled Trial. Bioengineering. 2023; 10(2):260. https://doi.org/10.3390/bioengineering10020260
Chicago/Turabian Styledos Santos, Jousielle Márcia, Redha Taiar, Vanessa Gonçalves César Ribeiro, Vanessa Kelly da Silva Lage, Pedro Henrique Scheidt Figueiredo, Henrique Silveira Costa, Vanessa Pereira Lima, Borja Sañudo, Mário Bernardo-Filho, Danúbia da Cunha de Sá-Caputo, and et al. 2023. "Whole-Body Vibration Training on Oxidative Stress Markers, Irisin Levels, and Body Composition in Women with Fibromyalgia: A Randomized Controlled Trial" Bioengineering 10, no. 2: 260. https://doi.org/10.3390/bioengineering10020260
APA Styledos Santos, J. M., Taiar, R., Ribeiro, V. G. C., da Silva Lage, V. K., Scheidt Figueiredo, P. H., Costa, H. S., Pereira Lima, V., Sañudo, B., Bernardo-Filho, M., Sá-Caputo, D. d. C. d., Dias Peixoto, M. F., Mendonça, V. A., Rapin, A., & Lacerda, A. C. R. (2023). Whole-Body Vibration Training on Oxidative Stress Markers, Irisin Levels, and Body Composition in Women with Fibromyalgia: A Randomized Controlled Trial. Bioengineering, 10(2), 260. https://doi.org/10.3390/bioengineering10020260












