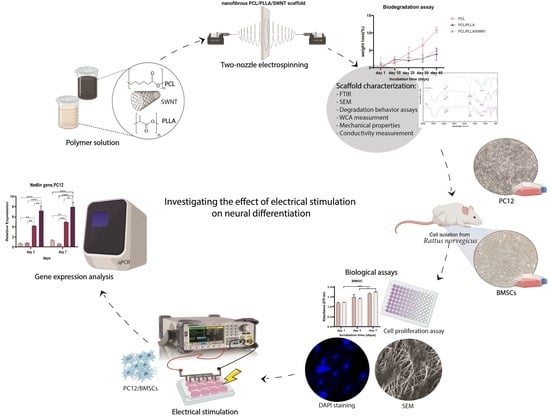
Electroconductive Nanofibrous Scaffolds Enable Neuronal Differentiation in Response to Electrical Stimulation without Exogenous Inducing Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanofibrous Scaffold Fabrication
2.2. Nanofibrous Scaffolds Characterization
2.2.1. Fourier-Transform Infrared Spectroscopy (FTIR)
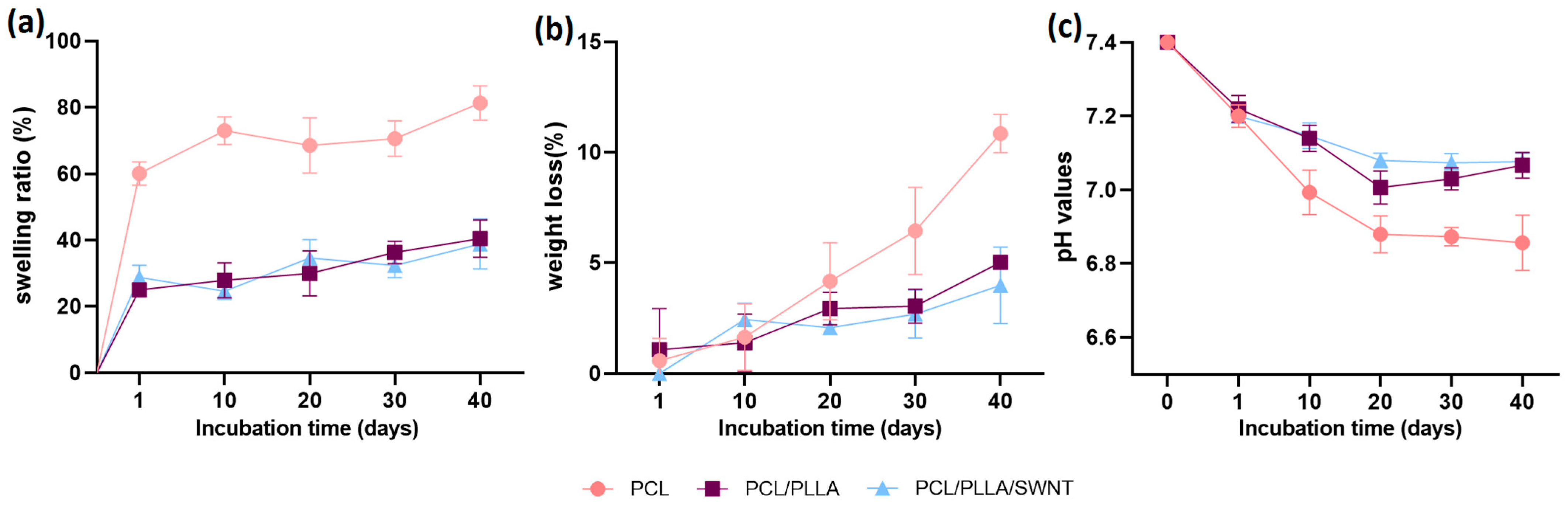
2.2.2. Degradation Behavior
2.2.3. Water Contact Angle (WCA) Assay
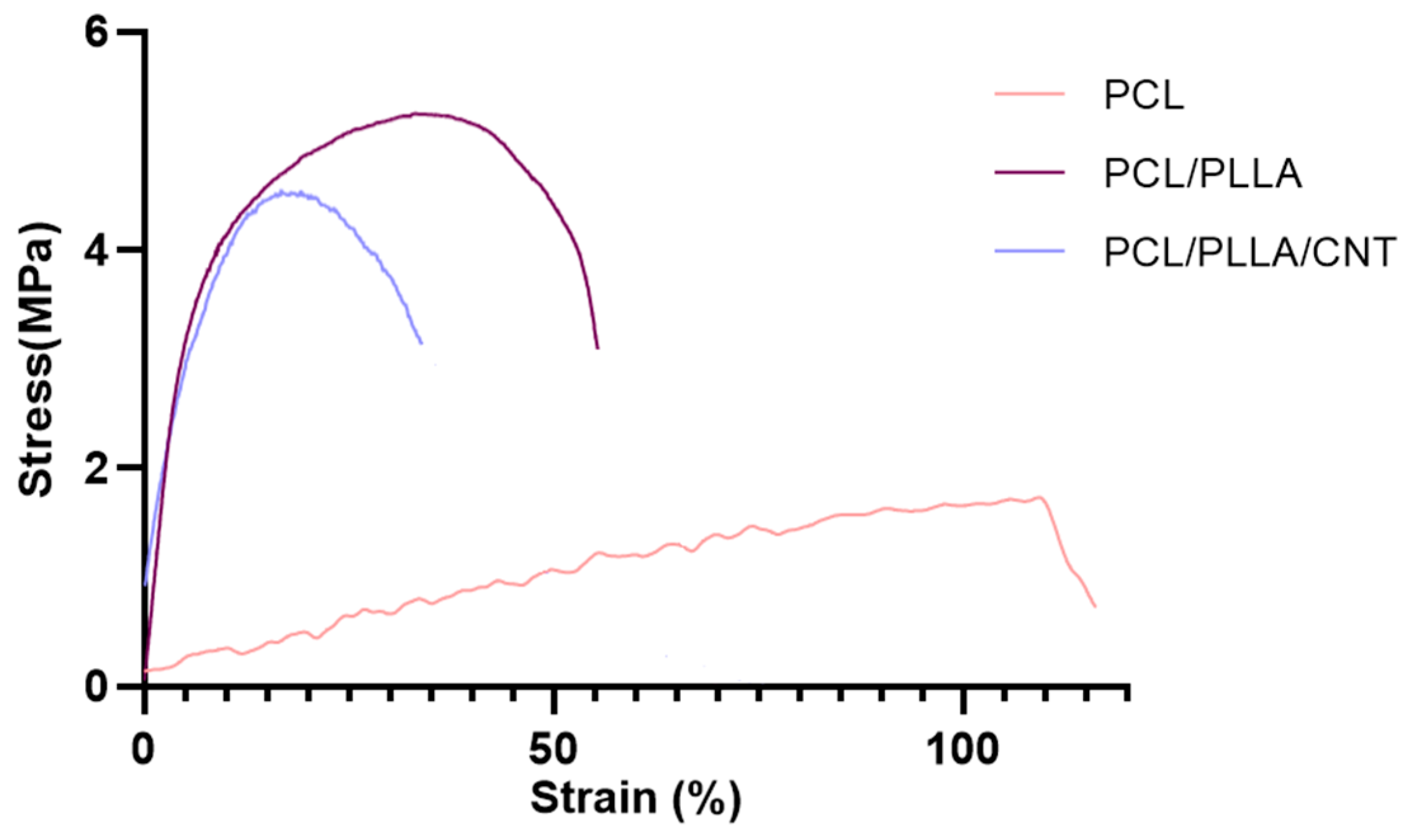
2.2.4. Mechanical Properties
2.2.5. Conductivity Measurement
2.3. Cell Culture and Visualization
2.4. Cell Proliferation Assay
2.5. Electrical Induction
2.6. Transcriptional Analyses of Neural Marker Genes
2.7. Statistics
3. Results and Discussion
3.1. Nanofibrous Scaffolds Characterization
3.1.1. Electrospun Nanofibers Morphology
3.1.2. WCA of Electrospun PCL/PLLA/SWNTs Nanofibers
3.1.3. FTIR of Electrospun PCL/PLLA/SWNTs Nanofibers
3.1.4. Degradation Behavior of Electrospun PCL/PLLA/SWNTs Nanofibers
3.1.5. Mechanical Properties of Electrospun PCL/PLLA/SWNTs Nanofibers
3.1.6. Electrical Conductivity of Electrospun PCL/PLLA/SWNTs Nanofibers
3.2. Biocompatibility of the Electrospun PCL/PLLA/SWNTs Nanofibers
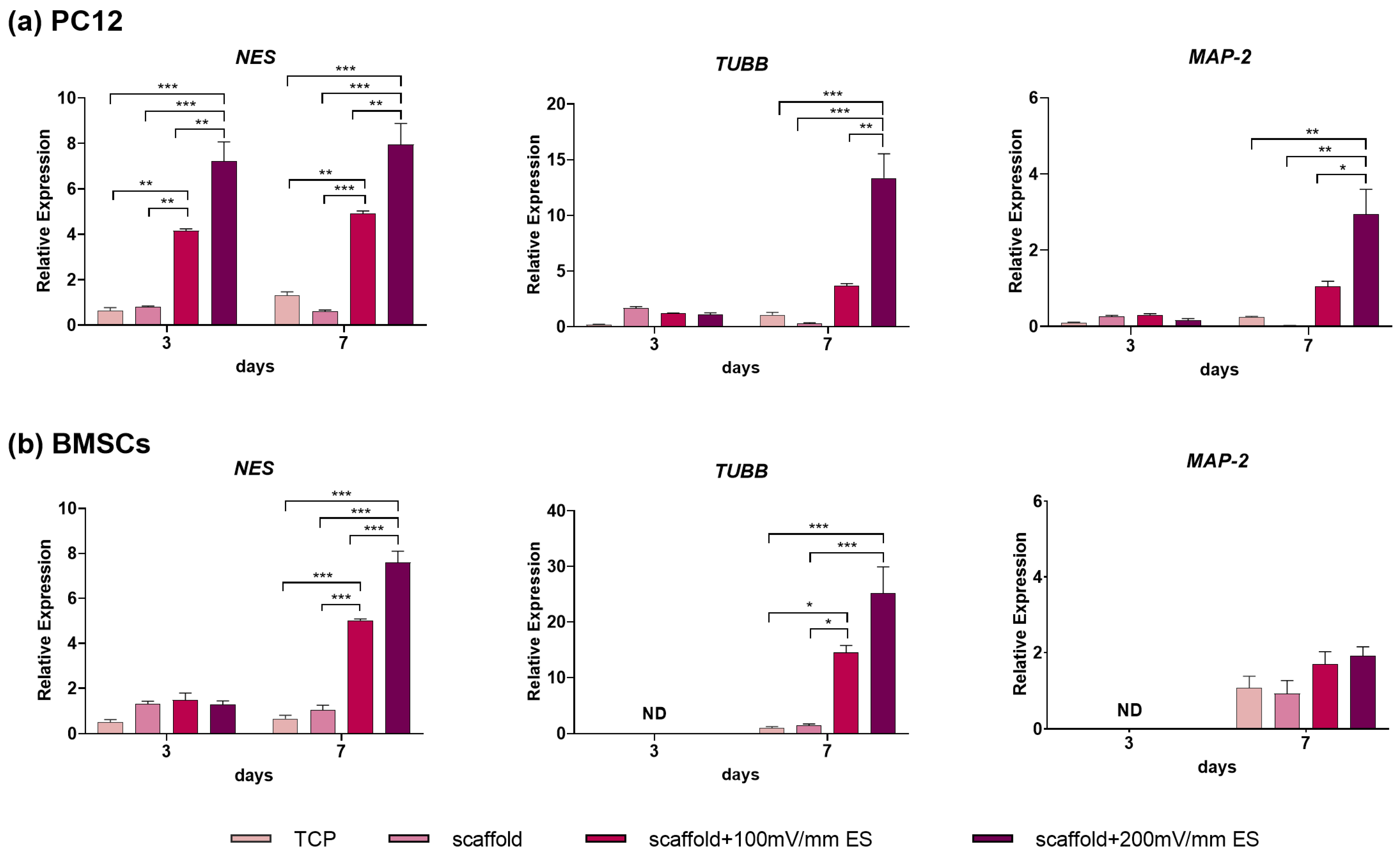
3.3. The Effect of Electrical Stimulation on Neural Differentiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, X.; Ding, F.; Yang, Y.; Liu, J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog. Neurobiol. 2011, 93, 204–230. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xie, L.; Lin, W.Z.Y.; Chen, Q. Biomimetic nanofibrous scaffolds for neural tissue engineering and drug development. Drug Discov. Today 2017, 22, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Zarrintaj, P.; Bakhshandeh, B.; Rezaeian, I.; Heshmatian, B.; Ganjali, M.R. A Novel Electroactive Agarose-Aniline Pentamer Platform as a Potential Candidate for Neural Tissue Engineering. Sci. Rep. 2017, 7, 17187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhou, H.; Jin, G.; Jin, B.; Geng, S.; Luo, Z.; Ge, Z.; Xu, F. Rational Design of Electrically Conductive Biomaterials toward Excitable Tissues Regeneration. Prog. Polym. Sci. 2022, 131, 101573. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Ranjbar, N.; Abbasi, A.; Amiri, E.; Abedi, A.; Mehrabi, M.R.; Dehghani, Z.; Pennisi, C.P. Recent Progress in the Manipulation of Biochemical and Biophysical Cues for Engineering Functional Tissues. Bioeng. Transl. Med. 2022, 8, e10383. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, F.; Arab, F.L.; Nikkhah, K.; Amiri, H.; Mahmoudi, M. Novel approaches using mesenchymal stem cells for curing peripheral nerve injuries. Life Sci. 2019, 221, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, C.A.; Grochmal, J.; Kung, T.A.; Cederna, P.S.; Midha, R.; Kemp, S.W. Stem-cell-based therapies to enhance peripheral nerve regeneration. Muscle Nerve 2020, 61, 449–459. [Google Scholar] [CrossRef]
- Prabhakaran, M.P.; Venugopal, J.R.; Ramakrishna, S. Mesenchymal stem cell differentiation to neuronal cells on electrospun nanofibrous substrates for nerve tissue engineering. Biomaterials 2009, 30, 4996–5003. [Google Scholar] [CrossRef]
- Jiang, Y.; Jahagirdar, B.N.; Reinhardt, R.L.; Schwartz, R.E.; Keene, C.D.; Ortiz-Gonzalez, X.R.; Reyes, M.; Lenvik, T.; Lund, T.; Blackstad, M. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 2002, 418, 41–49. [Google Scholar] [CrossRef]
- Rahmati, M.; Pennisi, C.P.; Mobasheri, A.; Mozafari, M. Bioengineered scaffolds for stem cell applications in tissue engineering and regenerative medicine. In Advances in Experimental Medicine and Biology; Turksen, K., Ed.; Springer: Cham, Switzeland, 2018; Volume 1107, pp. 73–89. [Google Scholar] [CrossRef]
- Roth, J.G.; Huang, M.S.; Li, T.L.; Feig, V.R.; Jiang, Y.; Cui, B.; Greely, H.T.; Bao, Z.; Paşca, S.P.; Heilshorn, S.C. Advancing models of neural development with biomaterials. Nat. Rev. Neurosci. 2021, 22, 593–615. [Google Scholar] [CrossRef]
- Hafizi, M.; Bakhshandeh, B.; Soleimani, M.; Atashi, A. Exploring the enkephalinergic differentiation potential in adult stem cells for cell therapy and drug screening implications. In Vitro Cell. Dev. Biol. Anim. 2012, 48, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Hafizi, M.; Atashi, A.; Bakhshandeh, B.; Kabiri, M.; Nadri, S.; Hosseini, R.H.; Soleimani, M. MicroRNAs as markers for neurally committed CD133+/CD34+ stem cells derived from human umbilical cord blood. Biochem. Genet. 2013, 51, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.G.; Kwon, Y.W.; Lee, T.W.; Park, G.T.; Kim, J.H. Recent advances in stem cell therapeutics and tissue engineering strategies. Biomater. Res. 2018, 22, 36. [Google Scholar] [CrossRef]
- Zarrintaj, P.; Bakhshandeh, B.; Saeb, M.R.; Sefat, F.; Rezaeian, I.; Ganjali, M.R.; Ramakrishna, S.; Mozafari, M. Oligoaniline-based conductive biomaterials for tissue engineering. Acta Biomater. 2018, 72, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Aadil, K.R.; Ranjan, S.; Kumar, V.B. Advances in nanotechnology and nanomaterials based strategies for neural tissue engineering. J. Drug Deliv. Sci. Technol. 2020, 57, 101617. [Google Scholar] [CrossRef]
- Rahmati, M.; Mills, D.K.; Urbanska, A.M.; Saeb, M.R.; Venugopal, J.R.; Ramakrishna, S.; Mozafari, M. Electrospinning for tissue engineering applications. Prog. Mater. Sci. 2021, 117, 100721. [Google Scholar] [CrossRef]
- Jing, W.; Zhang, Y.; Cai, Q.; Chen, G.; Wang, L.; Yang, X.; Zhong, W. Study of electrical stimulation with different electric-field intensities in the regulation of the differentiation of PC12 cells. ACS Chem. Neurosci. 2018, 10, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Foldberg, S.; Petersen, M.; Fojan, P.; Gurevich, L.; Fink, T.; Pennisi, C.P.; Zachar, V. Patterned poly(lactic acid) films support growth and spontaneous multilineage gene expression of adipose-derived stem cells. Colloids Surf. B Biointerfaces 2012, 93, 92–99. [Google Scholar] [CrossRef]
- Karami, Z.; Rezaeian, I.; Zahedi, P.; Abdollahi, M. Preparation and performance evaluations of electrospun poly(ε-caprolactone), poly(lactic acid), and their hybrid (50/50) nanofibrous mats containing thymol as an herbal drug for effective wound healing. J. Appl. Polym. Sci. 2013, 129, 756–766. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, T.; Shao, M.; Lyu, F. Recent advances in PLLA-based biomaterial scaffolds for neural tissue engineering: Fabrication, modification, and applications. Front. Bioeng. Biotechnol. 2022, 10, 1011783. [Google Scholar] [CrossRef]
- Nune, M.; Bhat, M.; Nagarajan, A. Design of ECM Functionalized Polycaprolactone Aligned Nanofibers for Peripheral Nerve Tissue Engineering. J. Med. Biol. Eng. 2022, 42, 147–156. [Google Scholar] [CrossRef]
- Tian, L.; Prabhakaran, M.P.; Hu, J.; Chen, M.; Besenbacher, F.; Ramakrishna, S. Synergistic effect of topography, surface chemistry and conductivity of the electrospun nanofibrous scaffold on cellular response of PC12 cells. Colloids Surf. B Biointerfaces 2016, 145, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Hopley, E.L.; Salmasi, S.; Kalaskar, D.M.; Seifalian, A.M. Carbon nanotubes leading the way forward in new generation 3D tissue engineering. Biotechnol. Adv. 2014, 32, 1000–1014. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.M.; Do, A.T.; Wang, J.K.; Yeoh, C.L.; Yeo, K.S.; Choong, C. Development of a miniaturized stimulation device for electrical stimulation of cells. J. Biol. Eng. 2015, 9, 14. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-R.; Ryu, S.; Kim, S.; Kim, B.-S. Behaviors of stem cells on carbon nanotube. Biomater. Res. 2015, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Salvetat, J.-P.; Briggs, G.A.D.; Bonard, J.-M.; Bacsa, R.R.; Kulik, A.J.; Stöckli, T.; Burnham, N.A.; Forró, L. Elastic and shear moduli of single-walled carbon nanotube ropes. Phys. Rev. Lett. 1999, 82, 944. [Google Scholar] [CrossRef]
- Aliev, A.E. Bolometric detector on the basis of single-wall carbon nanotube/polymer composite. Infrared Phys. Technol. 2008, 51, 541–545. [Google Scholar] [CrossRef]
- Javeed, S.; Faraji, A.H.; Dy, C.; Ray, W.Z.; MacEwan, M.R. Application of electrical stimulation for peripheral nerve regeneration: Stimulation parameters and future horizons. Interdiscip. Neurosurg. 2021, 24, 101117. [Google Scholar] [CrossRef]
- Bakhshandeh, B.; Sorboni, S.G.; Ranjbar, N.; Deyhimfar, R.; Abtahi, M.S.; Izady, M.; Kazemi, N.; Noori, A.; Pennisi, C.P. Mechanotransduction in tissue engineering: Insights into the interaction of stem cells with biomechanical cues. Exp. Cell Res. 2023, 431, 113766. [Google Scholar] [CrossRef]
- Zuo, K.J.; Gordon, T.; Chan, K.M.; Borschel, G.H. Electrical stimulation to enhance peripheral nerve regeneration: Update in molecular investigations and clinical translation. Exp. Neurol. 2020, 332, 113397. [Google Scholar] [CrossRef]
- Roh, J.; Schellhardt, L.; Keane, G.C.; Hunter, D.A.; Moore, A.M.; Snyder-Warwick, A.K.; Mackinnon, S.E.; Wood, M.D. Short-Duration, Pulsatile, Electrical Stimulation Therapy Accelerates Axon Regeneration and Recovery following Tibial Nerve Injury and Repair in Rats. Plast. Reconstr. Surg. 2022, 149, 681e–690e. [Google Scholar] [CrossRef] [PubMed]
- Babaie, A.; Bakhshandeh, B.; Abedi, A.; Mohammadnejad, J.; Shabani, I.; Ardeshirylajimi, A.; Moosavi, S.R.; Amini, J.; Tayebi, L. Synergistic effects of conductive PVA/PEDOT electrospun scaffolds and electrical stimulation for more effective neural tissue engineering. Eur. Polym. J. 2020, 140, 110051. [Google Scholar] [CrossRef]
- Liu, Q.; Song, B. Electric field regulated signaling pathways. Int. J. Biochem. Cell Biol. 2014, 55, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, S.J.; Lagacé, M.; St-Amour, I.; Arsenault, D.; Cisbani, G.; Chabrat, A.; Fecteau, S.; Lévesque, M.; Cicchetti, F. The morphological and molecular changes of brain cells exposed to direct current electric field stimulation. Int. J. Neuropsychopharmacol. 2015, 18, pyu090. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.C.; Briquez, P.S.; Hubbell, J.A.; Cochran, J.R. Engineering growth factors for regenerative medicine applications. Acta Biomater. 2016, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Z.; Lu, W.W.; Zhen, W.; Yang, D.; Peng, S. Novel biomaterial strategies for controlled growth factor delivery for biomedical applications. NPG Asia Mater. 2017, 9, e435. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 cell line: Cell types, coating of culture vessels, differentiation and other culture conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Madras, G.; Basu, B. Intermittent electrical stimuli for guidance of human mesenchymal stem cell lineage commitment towards neural-like cells on electroconductive substrates. Biomaterials 2014, 35, 6219–6235. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Madras, G.; Basu, B. Electrically driven intracellular and extracellular nanomanipulators evoke neurogenic/cardiomyogenic differentiation in human mesenchymal stem cells. Biomaterials 2016, 77, 26–43. [Google Scholar] [CrossRef]
- Moroder, P.; Runge, M.B.; Wang, H.; Ruesink, T.; Lu, L.; Spinner, R.J.; Windebank, A.J.; Yaszemski, M.J. Material properties and electrical stimulation regimens of polycaprolactone fumarate–polypyrrole scaffolds as potential conductive nerve conduits. Acta Biomater. 2011, 7, 944–953. [Google Scholar] [CrossRef]
- Sadeghi, M.; Bakhshandeh, B.; Dehghan, M.M.; Mehrnia, M.R.; Khojasteh, A. Functional synergy of anti-mir221 and nanohydroxyapatite scaffold in bone tissue engineering of rat skull. J. Mater. Sci. Mater. Med. 2016, 27, 132. [Google Scholar] [CrossRef] [PubMed]
- Porgham Daryasari, M.; Dusti Telgerd, M.; Hossein Karami, M.; Zandi-Karimi, A.; Akbarijavar, H.; Khoobi, M.; Seyedjafari, E.; Birhanu, G.; Khosravian, P.; SadatMahdavi, F. Poly-l-lactic acid scaffold incorporated chitosan-coated mesoporous silica nanoparticles as pH-sensitive composite for enhanced osteogenic differentiation of human adipose tissue stem cells by dexamethasone delivery. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4020–4029. [Google Scholar] [CrossRef] [PubMed]
- Birhanu, G.; Tanha, S.; Akbari Javar, H.; Seyedjafari, E.; Zandi-Karimi, A.; Kiani Dehkordi, B. Dexamethasone loaded multi-layer poly-l-lactic acid/pluronic P123 composite electrospun nanofiber scaffolds for bone tissue engineering and drug delivery. Pharm. Dev. Technol. 2019, 24, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Leena, R.; Vairamani, M.; Selvamurugan, N. Alginate/Gelatin scaffolds incorporated with Silibinin-loaded Chitosan nanoparticles for bone formation in vitro. Colloids Surf. B Biointerfaces 2017, 158, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Nukavarapu, S.P.; Deng, M.; Jabbarzadeh, E.; Kofron, M.D.; Doty, S.B.; Abdel-Fattah, W.I.; Laurencin, C.T. Chitosan–poly (lactide-co-glycolide) microsphere-based scaffolds for bone tissue engineering: In vitro degradation and in vivo bone regeneration studies. Acta Biomater. 2010, 6, 3457–3470. [Google Scholar] [CrossRef] [PubMed]
- Nandagiri, V.K.; Gentile, P.; Chiono, V.; Tonda-Turo, C.; Matsiko, A.; Ramtoola, Z.; Montevecchi, F.M.; Ciardelli, G. Incorporation of PLGA nanoparticles into porous chitosan–gelatin scaffolds: Influence on the physical properties and cell behavior. J. Mech. Behav. Biomed. Mater. 2011, 4, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Hiremath, R.; Rabinal, M.; Mulimani, B. Simple setup to measure electrical properties of polymeric films. Rev. Sci. Instrum. 2006, 77, 126106. [Google Scholar] [CrossRef]
- Xu, T.; Yao, Q.; Miszuk, J.M.; Sanyour, H.J.; Hong, Z.; Sun, H.; Fong, H. Tailoring weight ratio of PCL/PLA in electrospun three-dimensional nanofibrous scaffolds and the effect on osteogenic differentiation of stem cells. Colloids Surf. B Biointerfaces 2018, 171, 31–39. [Google Scholar] [CrossRef]
- Curcio, E.; Macchiarini, P.; De Bartolo, L. Oxygen mass transfer in a human tissue-engineered trachea. Biomaterials 2010, 31, 5131–5136. [Google Scholar] [CrossRef]
- Curcio, E.; Piscioneri, A.; Morelli, S.; Salerno, S.; Macchiarini, P.; De Bartolo, L. Kinetics of oxygen uptake by cells potentially used in a tissue engineered trachea. Biomaterials 2014, 35, 6829–6837. [Google Scholar] [CrossRef]
- Scaffaro, R.; Lopresti, F.; Botta, L. Preparation, characterization and hydrolytic degradation of PLA/PCL co-mingled nanofibrous mats prepared via dual-jet electrospinning. Eur. Polym. J. 2017, 96, 266–277. [Google Scholar] [CrossRef]
- Srikanth, M.; Asmatulu, R.; Cluff, K.; Yao, L. Material characterization and bioanalysis of hybrid scaffolds of carbon nanomaterial and polymer nanofibers. ACS Omega 2019, 4, 5044–5051. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Zhou, S.; Li, L.; Li, J.; Luo, C.; Wang, J.; Li, X.; Weng, J. Osteoblast function on electrically conductive electrospun PLA/MWCNTs nanofibers. Biomaterials 2011, 32, 2821–2833. [Google Scholar] [CrossRef] [PubMed]
- Magiera, A.; Markowski, J.; Pilch, J.; Blazewicz, S. Degradation behavior of electrospun PLA and PLA/CNT nanofibres in aqueous environment. J. Nanomater. 2018, 2018, 8796583. [Google Scholar] [CrossRef]
- Oztemur, J.; Yalcin-Enis, I. Development of biodegradable webs of PLA/PCL blends prepared via electrospinning: Morphological, chemical, and thermal characterization. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1844–1856. [Google Scholar] [CrossRef]
- Jacobs, T.; Morent, R.; De Geyter, N.; Dubruel, P.; Leys, C. Plasma surface modification of biomedical polymers: Influence on cell-material interaction. Plasma Chem. Plasma Process. 2012, 32, 1039–1073. [Google Scholar] [CrossRef]
- Cheng, Q.; Lee, B.L.-P.; Komvopoulos, K.; Yan, Z.; Li, S. Plasma surface chemical treatment of electrospun poly(L-lactide) microfibrous scaffolds for enhanced cell adhesion, growth, and infiltration. Tissue Eng. Part A 2013, 19, 1188–1198. [Google Scholar] [CrossRef]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef]
- Shalumon, K.; Sreerekha, P.; Sathish, D.; Tamura, H.; Nair, S.; Chennazhi, K.; Jayakumar, R. Hierarchically designed electrospun tubular scaffolds for cardiovascular applications. J. Biomed. Nanotechnol. 2011, 7, 609–620. [Google Scholar] [CrossRef]
- Raja, M.; Ryu, S.H.; Shanmugharaj, A. Thermal, mechanical and electroactive shape memory properties of polyurethane (PU)/poly (lactic acid)(PLA)/CNT nanocomposites. Eur. Polym. J. 2013, 49, 3492–3500. [Google Scholar] [CrossRef]
- Lebedev, S.M. PCL-CNT nanocomposites prepared by melt compounding and evaluation of their basic properties. Polym. Compos. 2020, 41, 1830–1840. [Google Scholar] [CrossRef]
- Zahid, S.; Khalid, H.; Ikram, F.; Iqbal, H.; Samie, M.; Shahzadi, L.; Shah, A.T.; Yar, M.; Chaudhry, A.A.; Awan, S.J. Bi-layered α-tocopherol acetate loaded membranes for potential wound healing and skin regeneration. Mater. Sci. Eng. C 2019, 101, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Łysik, D.; Mystkowska, J.; Markiewicz, G.; Deptuła, P.; Bucki, R. The influence of mucin-based artificial saliva on properties of polycaprolactone and polylactide. Polymers 2019, 11, 1880. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Ye, Z.; Ren, H.; Chen, N.; Zeng, Q.; Wu, X.; Lu, T. Bioactivity assessment of PLLA/PCL/HAP electrospun nanofibrous scaffolds for bone tissue engineering. Life Sci. 2016, 148, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zheng, W.; Li, L.; Zheng, Y. Fabrication and characterization of three-dimensional nanofiber membrance of PCL–MWCNTs by electrospinning. Mater. Sci. Eng. C 2010, 30, 1014–1021. [Google Scholar] [CrossRef]
- Duek, E.; Zavaglia, C.; Belangero, W. In vitro study of poly (lactic acid) pin degradation. Polymer 1999, 40, 6465–6473. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y.; Hu, J.; Wu, Z.; Chen, H. Fabrication and characterization of polylactic acid/polycaprolactone composite macroporous micro-nanofiber scaffolds by phase separation. New J. Chem. 2020, 44, 17382–17390. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, J.; Fang, C.; Xiong, J. Investigation of polylactide/poly (ε-caprolactone)/multi-walled carbon nanotubes electrospun nanofibers with surface texture. RSC Adv. 2015, 5, 99179–99187. [Google Scholar] [CrossRef]
- Shamsah, A.H.; Cartmell, S.H.; Richardson, S.M.; Bosworth, L.A. Material characterization of PCL: PLLA electrospun fibers following six months degradation in vitro. Polymers 2020, 12, 700. [Google Scholar] [CrossRef]
- Sharifi, M.; Bahrami, S.H.; Nejad, N.H.; Milan, P.B. Electrospun PCL and PLA hybrid nanofibrous scaffolds containing Nigella sativa herbal extract for effective wound healing. J. Appl. Polym. Sci. 2020, 137, 49528. [Google Scholar] [CrossRef]
- Amin, A.; Sarkar, R.; Moorefield, C.N.; Newkome, G.R. Preparation of different dendritic-layered silicate nanocomposites. Polym. Eng. Sci. 2013, 53, 2166–2174. [Google Scholar] [CrossRef]
- Lebedev, S.; Gefle, O.; Amitov, E.; Zhuravlev, D.; Berchuk, D.; Mikutskiy, E. Mechanical properties of PLA-based composites for fused deposition modeling technology. Int. J. Adv. Manuf. Technol. 2018, 97, 511–518. [Google Scholar] [CrossRef]
- Urquijo, J.; Dagréou, S.; Guerrica-Echevarría, G.; Eguiazábal, J. Morphology and properties of electrically and rheologically percolated PLA/PCL/CNT nanocomposites. J. Appl. Polym. Sci. 2017, 134, 45265. [Google Scholar] [CrossRef]
- Sanchez-Garcia, M.; Lagaron, J.; Hoa, S. Effect of addition of carbon nanofibers and carbon nanotubes on properties of thermoplastic biopolymers. Compos. Sci. Technol. 2010, 70, 1095–1105. [Google Scholar] [CrossRef]
- Kim, H.; Na, H.Y.; Lee, J.H.; Lee, S.J. Preparation, morphology and electrical conductivity of polystyrene/polydopamine-carbon nanotube microcellular foams via high internal phase emulsion polymerization. Polymer 2015, 39, 293–299. [Google Scholar] [CrossRef]
- Huang, J.; Mao, C.; Zhu, Y.; Jiang, W.; Yang, X. Control of carbon nanotubes at the interface of a co-continuous immiscible polymer blend to fabricate conductive composites with ultralow percolation thresholds. Carbon 2014, 73, 267–274. [Google Scholar] [CrossRef]
- He, L.; Zhang, Y.; Zeng, X.; Quan, D.; Liao, S.; Zeng, Y.; Lu, J.; Ramakrishna, S. Fabrication and characterization of poly(l-lactic acid) 3D nanofibrous scaffolds with controlled architecture by liquid–liquid phase separation from a ternary polymer–solvent system. Polymer 2009, 50, 4128–4138. [Google Scholar] [CrossRef]
- Mombini, S.; Mohammadnejad, J.; Bakhshandeh, B.; Narmani, A.; Nourmohammadi, J.; Vahdat, S.; Zirak, S. Chitosan-PVA-CNT nanofibers as electrically conductive scaffolds for cardiovascular tissue engineering. Int. J. Biol. Macromol. 2019, 140, 278–287. [Google Scholar] [CrossRef]
- Serrano, M.C.; Gutiérrez, M.C.; del Monte, F. Role of polymers in the design of 3D carbon nanotube-based scaffolds for biomedical applications. Prog. Polym. Sci. 2014, 39, 1448–1471. [Google Scholar] [CrossRef]
- Zimmerman, L.; Lendahl, U.; Cunningham, M.; McKay, R.; Parr, B.; Gavin, B.; Mann, J.; Vassileva, G.; McMahon, A. Independent regulatory elements in the nestin gene direct transgene expression to neural stem cells or muscle precursors. Neuron 1994, 12, 11–24. [Google Scholar] [CrossRef]
- Katsetos, C.D.; Herman, M.M.; Mörk, S.J. Class III β-tubulin in human development and cancer. Cell Motil. Cytoskelet. 2003, 55, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Soltani, M.H.; Pichardo, R.; Song, Z.; Sangha, N.; Camacho, F.; Satyamoorthy, K.; Sangueza, O.P.; Setaluri, V. Microtubule-associated protein 2, a marker of neuronal differentiation, induces mitotic defects, inhibits growth of melanoma cells, and predicts metastatic potential of cutaneous melanoma. Am. J. Pathol. 2005, 166, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Gupta, S.D.; Basu, B. Optimization of electrical stimulation parameters for enhanced cell proliferation on biomaterial surfaces. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 98, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Khatib, L.; Golan, D.E.; Cho, M. Physiologic electrical stimulation provokes intracellular calcium increase mediated by phospholipase C activation in human osteoblasts. FASEB J. 2004, 18, 1903–1905. [Google Scholar] [CrossRef] [PubMed]
- Grossemy, S.; Chan, P.P.; Doran, P.M. Electrical stimulation of cell growth and neurogenesis using conductive and nonconductive microfibrous scaffolds. Integr. Biol. 2019, 11, 264–279. [Google Scholar] [CrossRef] [PubMed]
- Borah, R.; Ingavle, G.C.; Sandeman, S.R.; Kumar, A.; Mikhalovsky, S. Electrically conductive MEH-PPV: PCL electrospun nanofibres for electrical stimulation of rat PC12 pheochromocytoma cells. Biomater. Sci. 2018, 6, 2342–2359. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, L.; Chen, N.; Ramakrishna, S.; Mo, X. The cellular response of nerve cells on poly-l-lysine coated PLGA-MWCNTs aligned nanofibers under electrical stimulation. Mater. Sci. Eng. C 2018, 91, 715–726. [Google Scholar] [CrossRef]
- Tsai, N.C.; She, J.W.; Wu, J.G.; Chen, P.; Hsiao, Y.S.; Yu, J. Poly (3,4-ethylenedioxythiophene) polymer composite bioelectrodes with designed chemical and topographical cues to manipulate the behavior of pc12 neuronal cells. Adv. Mater. Interfaces 2019, 6, 1801576. [Google Scholar] [CrossRef]
- Matsumoto, M.; Imura, T.; Fukazawa, T.; Sun, Y.; Takeda, M.; Kajiume, T.; Kawahara, Y.; Yuge, L. Electrical stimulation enhances neurogenin2 expression through β-catenin signaling pathway of mouse bone marrow stromal cells and intensifies the effect of cell transplantation on brain injury. Neurosci. Lett. 2013, 533, 71–76. [Google Scholar] [CrossRef]
- Pires, F.; Ferreira, Q.; Rodrigues, C.A.; Morgado, J.; Ferreira, F.C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 1158–1168. [Google Scholar] [CrossRef]
- Qi, F.; Wang, Y.; Ma, T.; Zhu, S.; Zeng, W.; Hu, X.; Liu, Z.; Huang, J.; Luo, Z. Electrical regulation of olfactory ensheathing cells using conductive polypyrrole/chitosan polymers. Biomaterials 2013, 34, 1799–1809. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.J.; Bosch, C.; Venance, L.; Pouget, P. Microscale inhomogeneity of brain tissue distorts electrical signal propagation. J. Neurosci. 2013, 33, 2821–2827. [Google Scholar] [CrossRef] [PubMed]
- Sykova, E. Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience 2004, 129, 861–876. [Google Scholar] [CrossRef] [PubMed]
- Morawski, M.; Reinert, T.; Meyer-Klaucke, W.; Wagner, F.E.; Tröger, W.; Reinert, A.; Jäger, C.; Brückner, G.; Arendt, T. Ion exchanger in the brain: Quantitative analysis of perineuronally fixed anionic binding sites suggests diffusion barriers with ion sorting properties. Sci. Rep. 2015, 5, 16471. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Wei, C.; Chow, J.K.; Nguy, L.; Nguyen, H.K.; Schmidt, C.E. Electric field stimulation through a substrate influences Schwann cell and extracellular matrix structure. J. Neural Eng. 2013, 10, 046011. [Google Scholar] [CrossRef]
- Koppes, A.N.; Seggio, A.M.; Thompson, D.M. Neurite outgrowth is significantly increased by the simultaneous presentation of Schwann cells and moderate exogenous electric fields. J. Neural Eng. 2011, 8, 046023. [Google Scholar] [CrossRef]
- Ma, K.; Fox, L.; Shi, G.; Shen, J.; Liu, Q.; Pappas, J.; Cheng, J.; Qu, T. Generation of neural stem cell-like cells from bone marrow-derived human mesenchymal stem cells. Neurol. Res. 2011, 33, 1083–1093. [Google Scholar] [CrossRef]
- Jin, K.; Mao, X.O.; Batteur, S.; Sun, Y.; Greenberg, D.A. Induction of neuronal markers in bone marrow cells: Differential effects of growth factors and patterns of intracellular expression. Exp. Neurol. 2003, 184, 78–89. [Google Scholar] [CrossRef]
- Feng, J.-F.; Liu, J.; Zhang, X.-Z.; Zhang, L.; Jiang, J.-Y.; Nolta, J.; Zhao, M. Guided migration of neural stem cells derived from human embryonic stem cells by an electric field. Stem Cells 2012, 30, 349–355. [Google Scholar] [CrossRef]
- Zhu, R.; Sun, Z.; Li, C.; Ramakrishna, S.; Chiu, K.; He, L. Electrical stimulation affects neural stem cell fate and function in vitro. Exp. Neurol. 2019, 319, 112963. [Google Scholar] [CrossRef]



| Gene Symbol | Gene Description | Primer Base Sequences (5′-3′) | Tm (°C) | |
|---|---|---|---|---|
| Forward | Reverse | |||
| NES | Nestin | TGG AAC AGA GAT TGG AAG GC | CAG CAG AGT CCT GTA TGT AGC | 58 |
| MAP-2 | Microtubule-associated protein 2 | ACC AAC TCA TCT CTC CTG TG | GGT TAT TCC ATC AGT GAC TTT GT | 57 |
| TUBB | β Tubulin-3 | TTT ATC TTC GGT CAG AGT GGT G | GGC AGT CAC AAT TCT CAC ATT C | 58 |
| HPRT1 | Hypoxanthine phosphoribosyl transferase 1 | CCA GCG TCG TGA TTA GTG | CGA GCA AGT CTT TCA GTC C | 56 |
| Scaffold | Young’s Modulus (MPa) | Tensile Strength (MPa) | Volume Resistivity (μohm·cm) | Volume Conductivity (μS/cm) |
|---|---|---|---|---|
| PCL | 1.510 ± 0.8 | 1.73 ± 0.3 | 29.6 | 0.0311 |
| PCL/PLLA | 73.50 ± 1.7 | 5.24 ± 0.4 | 30.1 | 0.0332 |
| PCL/PLLA/SWNT | 39.49 ± 2.3 | 4.53 ± 0.5 | 15.1 | 0.0663 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ranjbar, N.; Bakhshandeh, B.; Pennisi, C.P. Electroconductive Nanofibrous Scaffolds Enable Neuronal Differentiation in Response to Electrical Stimulation without Exogenous Inducing Factors. Bioengineering 2023, 10, 1438. https://doi.org/10.3390/bioengineering10121438
Ranjbar N, Bakhshandeh B, Pennisi CP. Electroconductive Nanofibrous Scaffolds Enable Neuronal Differentiation in Response to Electrical Stimulation without Exogenous Inducing Factors. Bioengineering. 2023; 10(12):1438. https://doi.org/10.3390/bioengineering10121438
Chicago/Turabian StyleRanjbar, Nika, Behnaz Bakhshandeh, and Cristian Pablo Pennisi. 2023. "Electroconductive Nanofibrous Scaffolds Enable Neuronal Differentiation in Response to Electrical Stimulation without Exogenous Inducing Factors" Bioengineering 10, no. 12: 1438. https://doi.org/10.3390/bioengineering10121438
APA StyleRanjbar, N., Bakhshandeh, B., & Pennisi, C. P. (2023). Electroconductive Nanofibrous Scaffolds Enable Neuronal Differentiation in Response to Electrical Stimulation without Exogenous Inducing Factors. Bioengineering, 10(12), 1438. https://doi.org/10.3390/bioengineering10121438