Controlled-Release Hydrogel Microspheres to Deliver Multipotent Stem Cells for Treatment of Knee Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Production of Hydrogel Spheres
2.1.1. Synthesis of AHA
2.1.2. Fabrication of AHA Microspheres
2.2. Assessment of Hydrogel Sphere Physical Properties
2.2.1. Assessment of Average Hydrogel Sphere Diameter
2.2.2. Assessment of Hydrogel Sphere Swelling Ratio
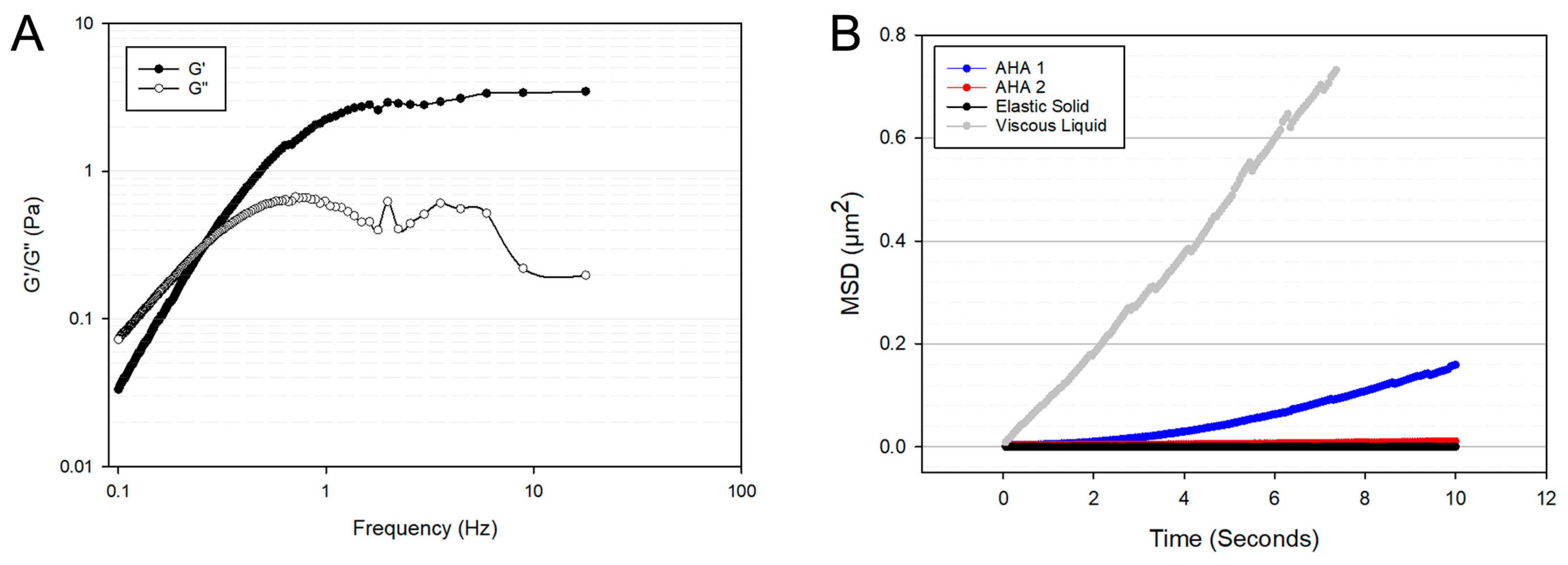
2.2.3. Microrheological Analysis of Hydrogel Microspheres
2.3. Effect of Encapsulation on Cell Viability and Identity
2.3.1. Culture of Rat Bone Marrow-Derived Multipotent Stromal Cells
2.3.2. Encapsulation of bMSCs
2.3.3. Viability of Cells in AHA Microspheres
2.3.4. Evaluation of Gene Expression Changes via qPCR on Encapsulated MSCs
2.4. In Vivo Function of Hydrogel Microspheres
2.4.1. Hydrogel Degradation and In Vivo Retention
2.4.2. Induction and Treatment of Osteoarthritis Rat model
2.4.3. Pain Assessment
2.4.4. Histological Analysis
2.5. Statistical Analysis
3. Results
3.1. Diameter, Swelling Ratio, and Microrheology of Hydrogel Microspheres
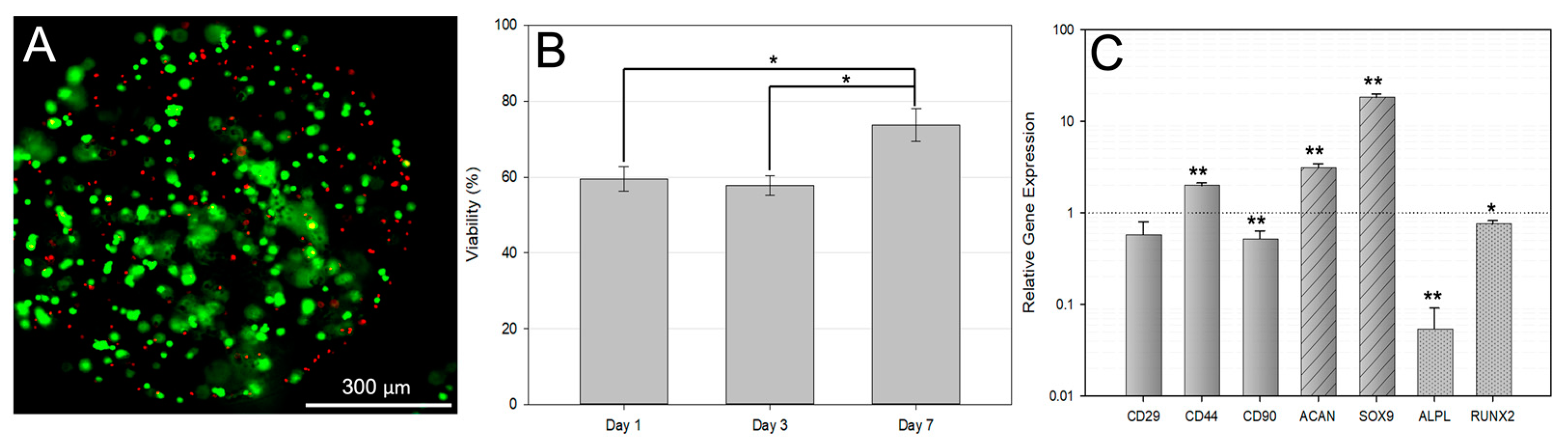
3.2. Encapsulated Cell Viability
3.3. qPCR Analysis of Encapsulated bMSCs
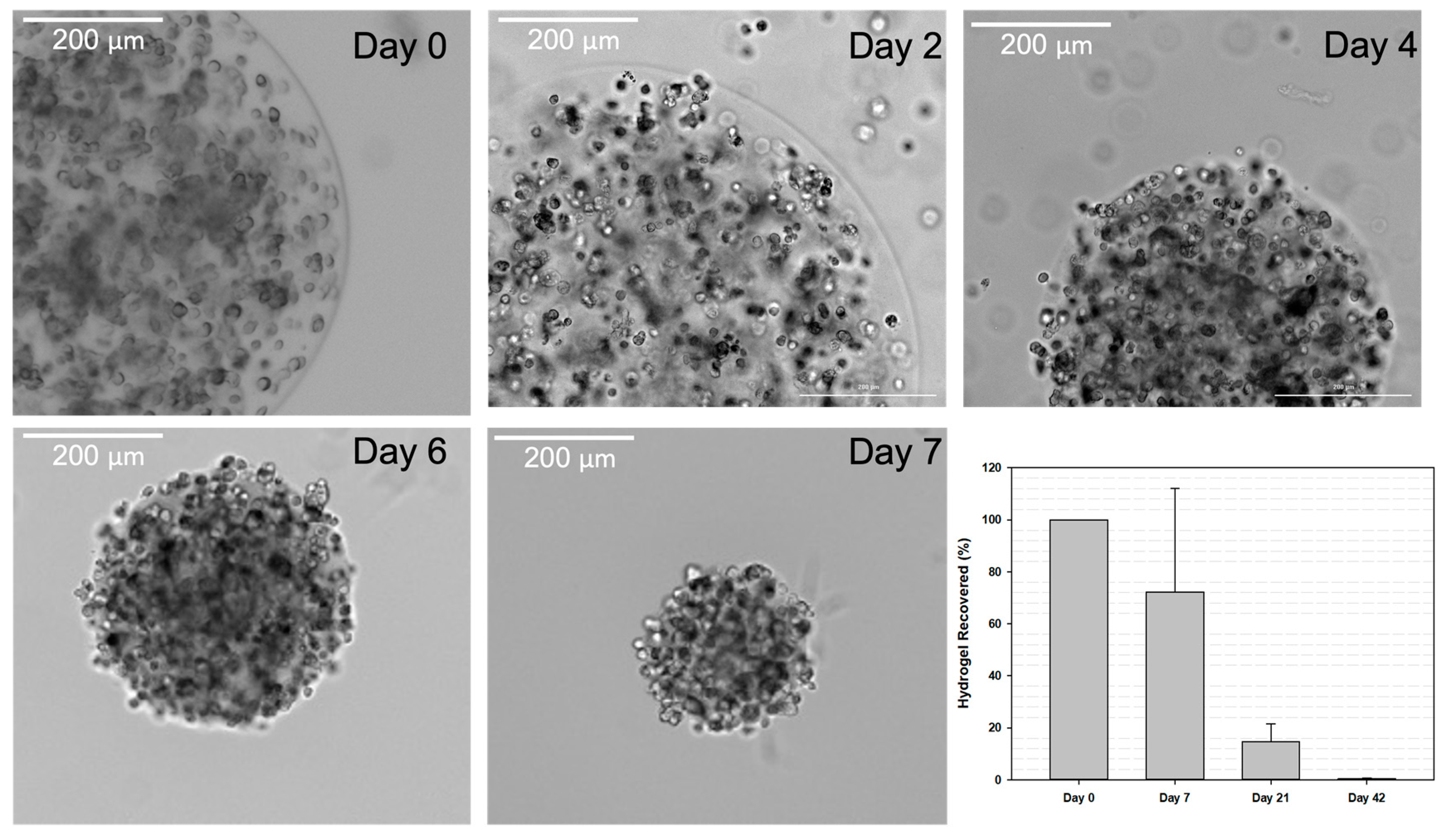
3.4. Hydrogel Retention Time
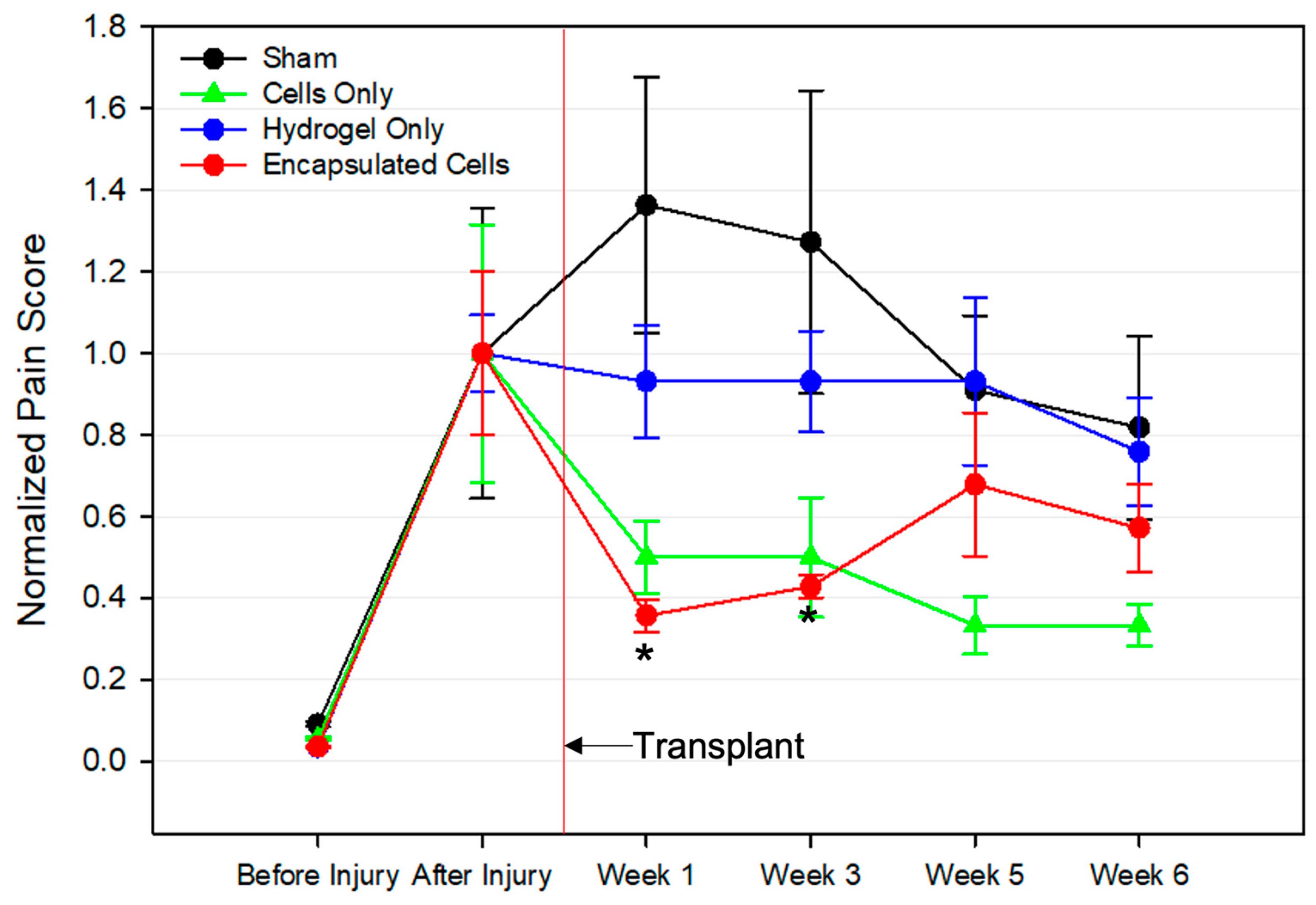
3.5. Pain Assessment following Cell Therapy Treatment
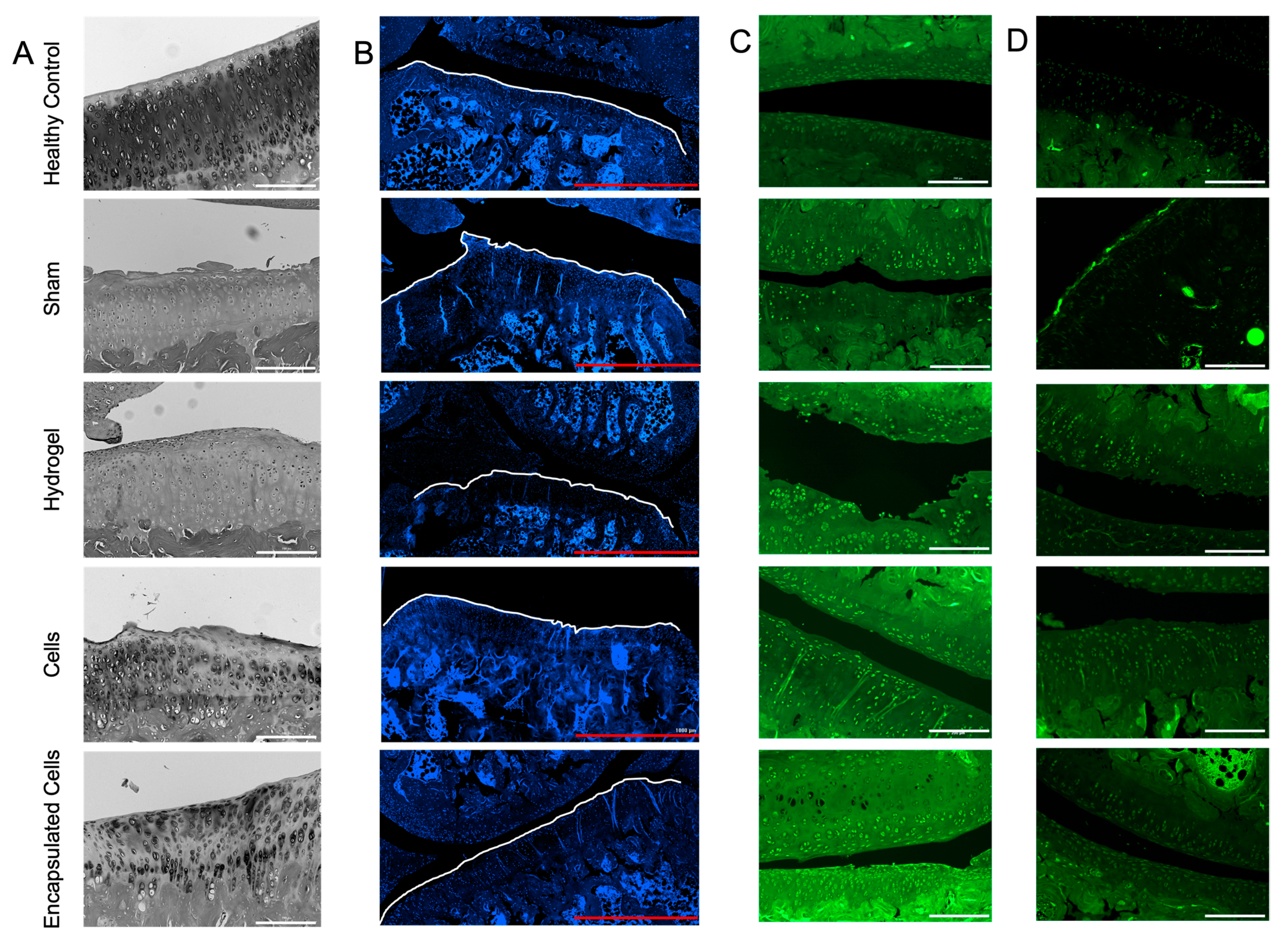
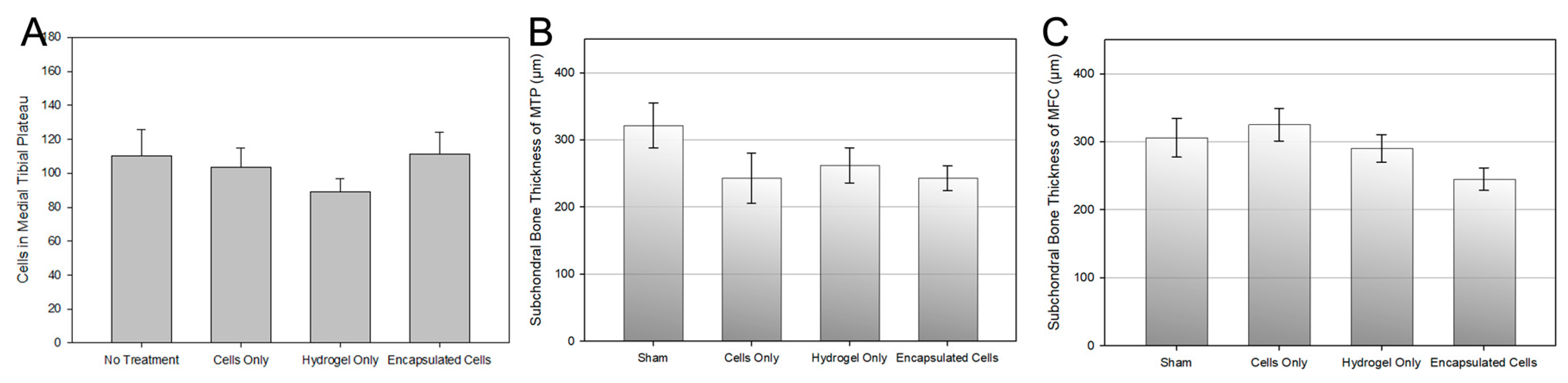
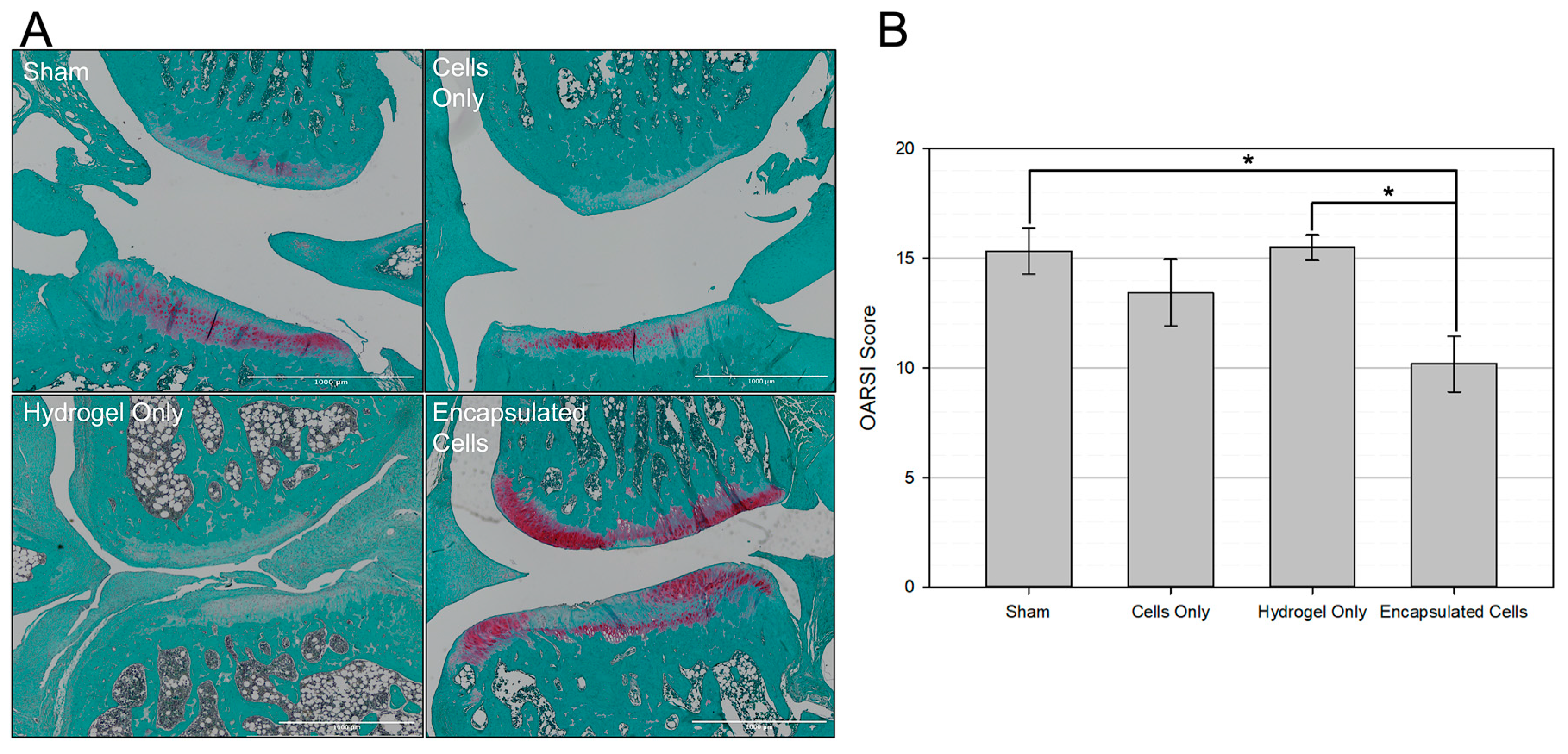
3.6. Histological Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef]
- Heidari, B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Casp. J. Intern. Med. 2011, 2, 205–212. [Google Scholar]
- Zhao, X.; Shah, D.; Gandhi, K.; Wei, W.; Dwibedi, N.; Webster, L.; Sambamoorthi, U. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthr. Cartil. 2019, 27, 1618–1626. [Google Scholar] [CrossRef]
- McAlindon, T.E.; Bannuru, R.R.; Sullivan, M.C.; Arden, N.K.; Berenbaum, F.; Bierma-Zeinstra, S.M.; Hawker, G.A.; Henrotin, Y.; Hunter, D.J.; Kawaguchi, H.; et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef] [PubMed]
- Jevsevar, D.S. Treatment of osteoarthritis of the knee: Evidence-based guideline, 2nd Edition. JAAOS—J. Am. Acad. Orthop. Surg. 2013, 21, 571–576. [Google Scholar] [CrossRef]
- Maradit Kremers, H.; Larson, D.R.; Crowson, C.S.; Kremers, W.K.; Washington, R.E.; Steiner, C.A.; Jiranek, W.A.; Berry, D.J. Prevalence of total hip and knee replacement in the United States. J. Bone Jt. Surg. Am. 2015, 97, 1386–1397. [Google Scholar] [CrossRef]
- Caldwell, K.L.; Wang, J. Cell-based articular cartilage repair: The link between development and regeneration. Osteoarthr. Cartil. 2015, 23, 351–362. [Google Scholar] [CrossRef]
- Hwang, J.J.; Rim, Y.A.; Nam, Y.; Ju, J.H. Recent developments in clinical applications of mesenchymal stem cells in the treatment of rheumatoid arthritis and osteoarthritis. Front. Immunol. 2021, 12, 631291. [Google Scholar] [CrossRef]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J.M. Mesenchymal stem cell therapy for osteoarthritis: The critical role of the cell secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef]
- Murphy, J.M.; Fink, D.J.; Hunziker, E.B.; Barry, F.P. Stem cell therapy in a caprine model of osteoarthritis. Arthritis Rheum. 2003, 48, 3464–3474. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The pros and cons of mesenchymal stem cell-based therapies. Cell Transpl. 2019, 28, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.; Murphy, M. Mesenchymal stem cells in joint disease and repair. Nat. Rev. Rheumatol. 2013, 9, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.Y.; Kim, D.H.; Ha, J.; Jin, H.J.; Kwon, S.-J.; Chang, J.W.; Choi, S.J.; Oh, W.; Yang, Y.S.; Kim, G.; et al. Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells 2013, 31, 2136–2148. [Google Scholar] [CrossRef]
- Xing, D.; Liu, W.; Wang, B.; Li, J.J.; Zhao, Y.; Li, H.; Liu, A.; Du, Y.; Lin, J. Intra-articular Injection of cell-laden 3D microcryogels empower low-dose cell therapy for osteoarthritis in a rat model. Cell Transpl. 2020, 29, 963689720932142. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Zeng, Y.; Zhang, X.; Wang, J.; Xie, L.; Li, X.; Du, Y. Microcryogels as injectable 3-D cellular microniches for site-directed and augmented cell delivery. Acta Biomater. 2014, 10, 1864–1875. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wei, Z.; Zhu, W.; Weng, X. Recent developments and current applications of hydrogels in osteoarthritis. Bioengineering 2022, 9, 132. [Google Scholar] [CrossRef]
- Horie, M.; Choi, H.; Lee, R.H.; Reger, R.L.; Ylostalo, J.; Muneta, T.; Sekiya, I.; Prockop, D.J. Intra-articular injection of human mesenchymal stem cells (MSCs) promote rat meniscal regeneration by being activated to express Indian hedgehog that enhances expression of type II collagen. Osteoarthr. Cartil. 2012, 20, 1197–1207. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and strategies for improving the regenerative effects of mesenchymal stromal cell-based therapies. Int. J. Mol. Sci. 2017, 18, 87. [Google Scholar] [CrossRef]
- Soto-Gutierrez, A.; Yagi, H.; Uygun, B.E.; Navarro-Alvarez, N.; Uygun, K.; Kobayashi, N.; Yang, Y.-G.; Yarmush, M.L. Cell delivery: From cell transplantation to organ engineering. Cell Transpl. 2010, 19, 655–665. [Google Scholar] [CrossRef]
- Mooney, D.J.; Vandenburgh, H. Cell delivery mechanisms for tissue repair. Cell Stem Cell 2008, 2, 205–213. [Google Scholar] [CrossRef]
- Lamo-Espinosa, J.M.; Mora, G.; Blanco, J.F.; Granero-Moltó, F.; Nuñez-Córdoba, J.M.; Sánchez-Echenique, C.; Bondía, J.M.; Aquerreta, J.D.; Andreu, E.J.; Ornilla, E.; et al. Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: Multicenter randomized controlled clinical trial (phase I/II). J. Transl. Med. 2016, 14, 246. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.H.; Chai, J.W.; Jeong, E.C.; Oh, S.; Shin, J.S.; Shim, H.; Yoon, K.S. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: A 2-Year follow-up study. Am. J. Sports Med. 2017, 45, 2774–2783. [Google Scholar] [CrossRef]
- Matas, J.; Orrego, M.; Amenabar, D.; Infante, C.; Tapia-Limonchi, R.; Cadiz, M.I.; Alcayaga-Miranda, F.; González, P.L.; Muse, E.; Khoury, M.; et al. Umbilical cord-derived mesenchymal stromal cells (MSCs) for knee osteoarthritis: Repeated MSC dosing Is superior to a single MSC dose and to hyaluronic acid in a controlled randomized phase I/II trial. Stem Cells Transl. Med. 2019, 8, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, N.; Muneta, T.; Koga, H.; Nakagawa, Y.; Mizuno, M.; Tsuji, K.; Mabuchi, Y.; Akazawa, C.; Kobayashi, E.; Matsumoto, K.; et al. Not single but periodic injections of synovial mesenchymal stem cells maintain viable cells in knees and inhibit osteoarthritis progression in rats. Osteoarthr. Cartil. 2016, 24, 1061–1070. [Google Scholar] [CrossRef]
- Lukomska, B.; Stanaszek, L.; Zuba-Surma, E.; Legosz, P.; Sarzynska, S.; Drela, K. Challenges and controversies in human mesenchymal stem cell therapy. Stem Cells Int. 2019, 2019, 9628536. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Rao, V.V.; Borelli, A.N.; Anseth, K.S. Engineering the MSC secretome: A hydrogel focused approach. Adv. Heal. Mater. 2021, 10, e2001948. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1880. [Google Scholar] [CrossRef]
- Han, W.M.; Mohiuddin, M.; Anderson, S.E.; García, A.J.; Jang, Y.C. Co-delivery of Wnt7a and muscle stem cells using synthetic bioadhesive hydrogel enhances murine muscle regeneration and cell migration during engraftment. Acta Biomater. 2019, 94, 243–252. [Google Scholar] [CrossRef]
- Cardoso, L.M.d.F.; Barreto, T.; Gama, J.F.G.; Alves, L.A. Natural biopolymers as additional tools for cell microencapsulation applied to cellular therapy. Polymers 2022, 14, 2641. [Google Scholar] [CrossRef]
- Leslie, S.K.; Cohen, D.J.; Sedlaczek, J.; Pinsker, E.J.; Boyan, B.D.; Schwartz, Z. Controlled release of rat adipose-derived stem cells from alginate microbeads. Biomaterials 2013, 34, 8172–8184. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, M.D.; Van Hove, A.H.; Benoit, D.S.W. Degradable hydrogels for spatiotemporal control of mesenchymal stem cells localized at decellularized bone allografts. Acta Biomater. 2014, 10, 3431–3441. [Google Scholar] [CrossRef]
- Menzel, E.J.; Farr, C. Hyaluronidase and its substrate hyaluronan: Biochemistry, biological activities and therapeutic uses. Cancer Lett. 1998, 131, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Harrington, S.; Ott, L.; Karanu, F.; Ramachandran, K.; Stehno-Bittel, L. A versatile microencapsulation platform for hyaluronic acid and polyethylene glycol. Tissue Eng. Part A 2021, 27, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Choe, G.; Park, J.; Park, H.; Lee, J.Y. Hydrogel biomaterials for stem cell microencapsulation. Polymers 2018, 10, 997. [Google Scholar] [CrossRef]
- Hamilton, M.; Harrington, S.; Dhar, P.; Stehno-Bittel, L. Hyaluronic acid hydrogel microspheres for slow release stem cell delivery. ACS Biomater. Sci. Eng. 2021, 7, 3754–3763. [Google Scholar] [CrossRef]
- Sahu, N.; Agarwal, P.; Grandi, F.; Bruschi, M.; Goodman, S.; Amanatullah, D.; Bhutani, N. Encapsulated mesenchymal stromal cell microbeads promote endogenous regeneration of osteoarthritic cartilage ex vivo. Adv. Health Mater. 2021, 10, e2002118. [Google Scholar] [CrossRef]
- Harrington, S.; Karanu, F.; Ramachandran, K.; Williams, S.J.; Stehno-Bittel, L. PEGDA microencapsulated allogeneic islets reverse canine diabetes without immunosuppression. PLoS ONE 2022, 17, e0267814. [Google Scholar] [CrossRef]
- Molnar, V.; Pavelić, E.; Vrdoljak, K.; Čemerin, M.; Klarić, E.; Matišić, V.; Bjelica, R.; Brlek, P.; Kovačić, I.; Tremolada, C.; et al. Mesenchymal stem cell mechanisms of action and clinical effects in osteoarthritis: A narrative review. Genes 2022, 13, 949. [Google Scholar] [CrossRef]
- Sigen, A.; Xu, Q.; McMichael, P.; Gao, Y.S.; Li, X.L.; Wang, X.; Greiser, U.; Zhou, D.Z.; Wang, W.X. A facile one-pot synthesis of acrylated hyaluronic acid. Chem. Commun. 2018, 54, 1081–1084. [Google Scholar] [CrossRef]
- Nicholls, M.; Manjoo, A.; Shaw, P.; Niazi, F.; Rosen, J. A comparison between rheological properties of intra-articular hyaluronic acid preparations and reported human aynovial fluid. Adv. Ther. 2018, 35, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Aromaa, M.K.; Vallittu, P.K. Delayed post-curing stage and oxygen inhibition of free-radical polymerization of dimethacrylate resin. Dent. Mater. 2018, 34, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Nielsen, P.; Ali, J. Passive and active microrheology for biomedical systems. Front. Bioeng. Biotechnol. 2022, 10, 916354. [Google Scholar] [CrossRef] [PubMed]
- First, E.M.; Squires, T.M. Microrheology; Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Hasler, J.; Hatt, L.P.; Stoddart, M.J.; Armiento, A.R. Stable reference genes for qPCR analysis in BM-MSCs undergoing osteogenic differentiation within 3D hyaluronan-based hydrogels. Int. J. Mol. Sci. 2020, 21, 9195. [Google Scholar] [CrossRef] [PubMed]
- Glasson, S.S.; Blanchet, T.J.; Morris, E.A. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr. Cartil. 2007, 15, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Iijima, H.; Aoyama, T.; Ito, A.; Tajino, J.; Nagai, M.; Zhang, X.; Yamaguchi, S.; Akiyama, H.; Kuroki, H. Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthr. Cartil. 2014, 22, 1036–1043. [Google Scholar] [CrossRef]
- Piel, M.J.; Kroin, J.S.; van Wijnen, A.J.; Kc, R.; Im, H.-J. Pain assessment in animal models of osteoarthritis. Gene 2014, 537, 184–188. [Google Scholar] [CrossRef]
- Aho, O.M.; Finnilä, M.; Thevenot, J.; Saarakkala, S.; Lehenkari, P. Subchondral bone histology and grading in osteoarthritis. PLoS ONE 2017, 12, e0173726. [Google Scholar] [CrossRef]
- Glasson, S.S.; Chambers, M.G.; Van Den Berg, W.B.; Little, C.B. The OARSI histopathology initiative—Recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr. Cartil. 2010, 18, S17–S23. [Google Scholar] [CrossRef]
- Wei, J.-l.; Fu, W.; Ding, Y.-j.; Hettinghouse, A.; Lendhey, M.; Schwarzkopf, R.; Kennedy, O.D.; Liu, C.-j. Progranulin derivative Atsttrin protects against early osteoarthritis in mouse and rat models. Arthritis Res. Ther. 2017, 19, 280. [Google Scholar] [CrossRef]
- Schneider, T.; Welker, P.; Licha, K.; Haag, R.; Schulze-Tanzil, G. Influence of dendritic polyglycerol sulfates on knee osteoarthritis: An experimental study in the rat osteoarthritis model. BMC Musculoskelet. Disord. 2015, 16, 387. [Google Scholar] [CrossRef] [PubMed]
- Corciulo, C.; Lendhey, M.; Wilder, T.; Schoen, H.; Cornelissen, A.S.; Chang, G.; Kennedy, O.D.; Cronstein, B.N. Endogenous adenosine maintains cartilage homeostasis and exogenous adenosine inhibits osteoarthritis progression. Nat. Commun. 2017, 8, 15019. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.; Hu, H.; Barroga, C.; Bossard, C.; Kc, S.; Dellamary, L.; Stewart, J.; Chiu, K.; Ibanez, M.; Pedraza, M.; et al. A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthr. Cartil. 2018, 26, 18–27. [Google Scholar] [CrossRef]
- Charrier, E.E.; Pogoda, K.; Wells, R.G.; Janmey, P.A. Control of cell morphology and differentiation by substrates with independently tunable elasticity and viscous dissipation. Nat. Commun. 2018, 9, 449. [Google Scholar] [CrossRef]
- Lee, S.; Choi, E.; Cha, M.J.; Hwang, K.C. Cell adhesion and long-term survival of transplanted mesenchymal stem cells: A prerequisite for cell therapy. Oxid. Med. Cell Longev. 2015, 2015, 632902. [Google Scholar] [CrossRef]
- Furst, E.M.; Squires, T.M. Passive Microrheology. In Microrheology; Oxford University Press: New York, NY, USA, 2017. [Google Scholar]
- Lieleg, O.; Vladescu, I.; Ribbeck, K. Characterization of particle translocation through mucin hydrogels. Biophys. J. 2010, 98, 1782–1789. [Google Scholar] [CrossRef]
- Chung, C.; Burdick, J.A. Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis. Tissue Eng. Part A 2009, 15, 243–254. [Google Scholar] [CrossRef]
- Loo, S.J.Q.; Wong, N.K. Advantages and challenges of stem cell therapy for osteoarthritis (Review). Biomed. Rep. 2021, 15, 67. [Google Scholar] [CrossRef]
- Luo, Y.; Liang, F.; Wan, X.; Liu, S.; Fu, L.; Mo, J.; Meng, X.; Mo, Z. Hyaluronic acid facilitates angiogenesis of endothelial colony forming cell combining with mesenchymal stem cell via CD44/ microRNA-139-5p pathway. Front. Bioeng. Biotechnol. 2022, 10, 794037. [Google Scholar] [CrossRef]
- Moraes, D.A.; Sibov, T.T.; Pavon, L.F.; Alvim, P.Q.; Bonadio, R.S.; Da Silva, J.R.; Pic-Taylor, A.; Toledo, O.A.; Marti, L.C.; Azevedo, R.B.; et al. A reduction in CD90 (THY-1) expression results in increased differentiation of mesenchymal stromal cells. Stem Cell Res. Ther. 2016, 7, 97. [Google Scholar] [CrossRef]
- Burr, D.B.; Gallant, M.A. Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Bobacz, K.; Erlacher, L.; Smolen, J.; Soleiman, A.; Graninger, W.B. Chondrocyte number and proteoglycan synthesis in the aging and osteoarthritic human articular cartilage. Ann. Rheum. Dis. 2004, 63, 1618–1622. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Kim, H.A. Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 2015, 16, 26035–26054. [Google Scholar] [CrossRef] [PubMed]
- Chawla, S.; Mainardi, A.; Majumder, N.; Dönges, L.; Kumar, B.; Occhetta, P.; Martin, I.; Egloff, C.; Ghosh, S.; Bandyopadhyay, A.; et al. Chondrocyte hypertrophy in osteoarthritis: Mechanistic studies and models for the identification of new therapeutic strategies. Cells 2022, 11, 34. [Google Scholar] [CrossRef]
- Jiang, L.; Lin, J.; Zhao, S.; Wu, J.; Jin, Y.; Yu, L.; Wu, N.; Wu, Z.; Wang, Y.; Lin, M. ADAMTS5 in osteoarthritis: Biological functions, regulatory network, and potential targeting therapies. Front. Mol. Biosci. 2021, 8, 703110. [Google Scholar] [CrossRef]
- Funck-Brentano, T.; Cohen-Solal, M. Subchondral bone and osteoarthritis. Curr. Opin. Rheumatol. 2015, 27, 420–426. [Google Scholar] [CrossRef]
- Li, G.; Yin, J.; Gao, J.; Cheng, T.S.; Pavlos, N.J.; Zhang, C.; Zheng, M.H. Subchondral bone in osteoarthritis: Insight into risk factors and microstructural changes. Arthritis Res. Ther. 2013, 15, 223. [Google Scholar] [CrossRef]
- Xing, D.; Kwong, J.; Yang, Z.; Hou, Y.; Zhang, W.; Ma, B.; Lin, J. Intra-articular injection of mesenchymal stem cells in treating knee osteoarthritis: A systematic review of animal studies. Osteoarthr. Cartil. 2018, 26, 445–461. [Google Scholar] [CrossRef]
- Jung, H. Hyaluronidase: An overview of its properties, applications, and side effects. Arch. Plast. Surg. 2020, 47, 297–300. [Google Scholar] [CrossRef]
- Nativel, F.; Smith, A.; Boulestreau, J.; Lépine, C.; Baron, J.; Marquis, M.; Vignes, C.; Le Guennec, Y.; Veziers, J.; Lesoeur, J.; et al. Micromolding-based encapsulation of mesenchymal stromal cells in alginate for intraarticular injection in osteoarthritis. Mater. Today Bio 2023, 19, 100581. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Ogle, M.; Daron, G.; Levy, M.; Temenoff, J.S. Hydrogel culture surface stiffness modulates mesenchymal stromal cell secretome and alters senescence. Tissue Eng. Part A 2020, 26, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, A.; Arha, M.; Choudhary, S.; Ashton, R.S.; Bhatia, S.R.; Schaffer, D.V.; Kane, R.S. The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials 2009, 30, 4695–4699. [Google Scholar] [CrossRef]
- Ahearne, M. Introduction to cell-hydrogel mechanosensing. Interface Focus. 2014, 4, 20130038. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, B.N.; Horrocks, M.S.; Malmström, J. Hydrogels with tunable physical cues and their emerging roles in studies of cellular mechanotransduction. Adv. NanoBiomed Res. 2021, 1, 2100059. [Google Scholar] [CrossRef]
- Lee, K.; Chen, Y.; Li, X.; Kawazoe, N.; Yang, Y.; Chen, G. Influence of viscosity on chondrogenic differentiation of mesenchymal stem cells during 3D culture in viscous gelatin solution-embedded hydrogels. J. Mater. Sci. Technol. 2021, 63, 1–8. [Google Scholar] [CrossRef]
- Monaco, G.; El Haj, A.J.; Alini, M.; Stoddart, M.J. Sodium hyaluronate supplemented culture media as a new hMSC chondrogenic differentiation media-model for in vitro/ex vivo screening of potential cartilage repair therapies. Front. Bioeng. Biotechnol. 2020, 8, 243. [Google Scholar] [CrossRef]
- Heldens, G.T.; Blaney Davidson, E.N.; Vitters, E.L.; Schreurs, B.W.; Piek, E.; van den Berg, W.B.; van der Kraan, P.M. Catabolic factors and osteoarthritis-conditioned medium inhibit chondrogenesis of human mesenchymal stem cells. Tissue Eng. Part A 2012, 18, 45–54. [Google Scholar] [CrossRef]
- Qu, H.; Sun, S. Efficacy of mesenchymal stromal cells for the treatment of knee osteoarthritis: A meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2021, 16, 11. [Google Scholar] [CrossRef]
- Sensini, F.; Inta, D.; Palme, R.; Brandwein, C.; Pfeiffer, N.; Riva, M.A.; Gass, P.; Mallien, A.S. The impact of handling technique and handling frequency on laboratory mouse welfare is sex-specific. Sci. Rep. 2020, 10, 17281. [Google Scholar] [CrossRef]
- Yang, Y.; Li, P.; Zhu, S.; Bi, R. Comparison of early-stage changes of osteoarthritis in cartilage and subchondral bone between two different rat models. PeerJ 2020, 8, e8934. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, X.; Wang, S.; Jing, Y.; Su, J. Subchondral bone microenvironment in osteoarthritis and pain. Bone Res. 2021, 9, 20. [Google Scholar] [CrossRef] [PubMed]

| Assay ID | |
|---|---|
| GUSB | Rn00566655_m1 |
| CD34 | Rn03416140_m1 |
| CD45 | Rn00709901_m1 |
| CD90 | Rn00562048_ml |
| CD29 | Rn00562048_m1 |
| CD44 | Rn00681157_m1 |
| ACAN | Rn00573424_m1 |
| SOX9 | Rn01751069_mH |
| Col2a1 | Rn01637087_m1 |
| ALPL | Rn01516028_m1 |
| RUNX2 | Rn01512298_m1 |
| AHA Microspheres | |
|---|---|
| Polymer mass fraction in gel precursor | 5% AHA 200 kDa 2% 1 kDa PEGDA |
| Precursor viscosity | 116 cST |
| Sphere size range | 627–1347 µm |
| Polymer mass fraction in final spheres | 0.9% |
| Mass swelling ratio “Q” | 108 ± 3 |
| Average final diameter | 1036 ± 180 µm |
| Unencapsulated MSCs | Encapsulated MSCs | |
|---|---|---|
| GUSB | 27.75 ± 0.64 | 28.12 ± 0.64 |
| CD34 | Not detected | Not detected |
| CD45 | Not detected | Not detected |
| CD90 | 27.13 ± 0.56 | 28.46 ± 0.50 |
| CD29 | 26.19 ± 2.16 | 27.41 ± 2.31 |
| CD44 | 26.45 ± 0.54 | 25.82 ± 0.61 |
| ACAN | 23.42 ± 0.78 | 22.14 ± 0.90 |
| SOX9 | 30.51 ± 1.11 | 26.67 ± 1.24 |
| Col2a1 | Not detected | Not detected |
| ALPL | 28.97 ± 0.75 | 33.99 ± 1.61 |
| RUNX2 | 29.68 ± 1.47 | 30.45 ± 1.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamilton, M.; Wang, J.; Dhar, P.; Stehno-Bittel, L. Controlled-Release Hydrogel Microspheres to Deliver Multipotent Stem Cells for Treatment of Knee Osteoarthritis. Bioengineering 2023, 10, 1315. https://doi.org/10.3390/bioengineering10111315
Hamilton M, Wang J, Dhar P, Stehno-Bittel L. Controlled-Release Hydrogel Microspheres to Deliver Multipotent Stem Cells for Treatment of Knee Osteoarthritis. Bioengineering. 2023; 10(11):1315. https://doi.org/10.3390/bioengineering10111315
Chicago/Turabian StyleHamilton, Megan, Jinxi Wang, Prajnaparamita Dhar, and Lisa Stehno-Bittel. 2023. "Controlled-Release Hydrogel Microspheres to Deliver Multipotent Stem Cells for Treatment of Knee Osteoarthritis" Bioengineering 10, no. 11: 1315. https://doi.org/10.3390/bioengineering10111315
APA StyleHamilton, M., Wang, J., Dhar, P., & Stehno-Bittel, L. (2023). Controlled-Release Hydrogel Microspheres to Deliver Multipotent Stem Cells for Treatment of Knee Osteoarthritis. Bioengineering, 10(11), 1315. https://doi.org/10.3390/bioengineering10111315








