The Influence of Different Inter-Trial Intervals on the Quantification of Intracortical Facilitation in the Primary Motor Cortex
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Design
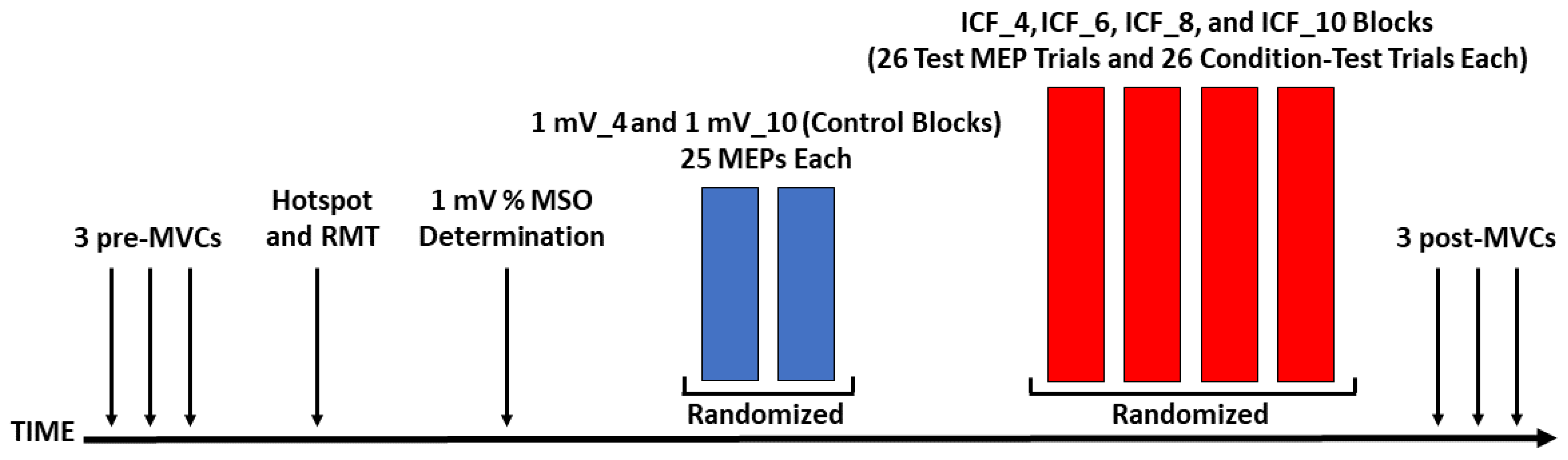
2.3. Experimental Arrangement
2.4. Experimental Procedures
2.4.1. MVCs
2.4.2. Motor Hotspot Localization
2.4.3. RMT
2.4.4. mV Stimulation Intensity (% MSO) Determination
2.4.5. Control Blocks
2.4.6. ICF Blocks
2.5. Data Analysis
2.5.1. MVC Force, MVC EMG, RMT, and 1 mV (% MSO) Stimulation Intensity Analyses
2.5.2. Control Block Analyses
2.5.3. ICF Block Analyses
2.6. Statistical Analysis
2.6.1. MVCs
2.6.2. Control Blocks
2.6.3. ICF Blocks
3. Results
3.1. MVCs
3.2. Control Blocks
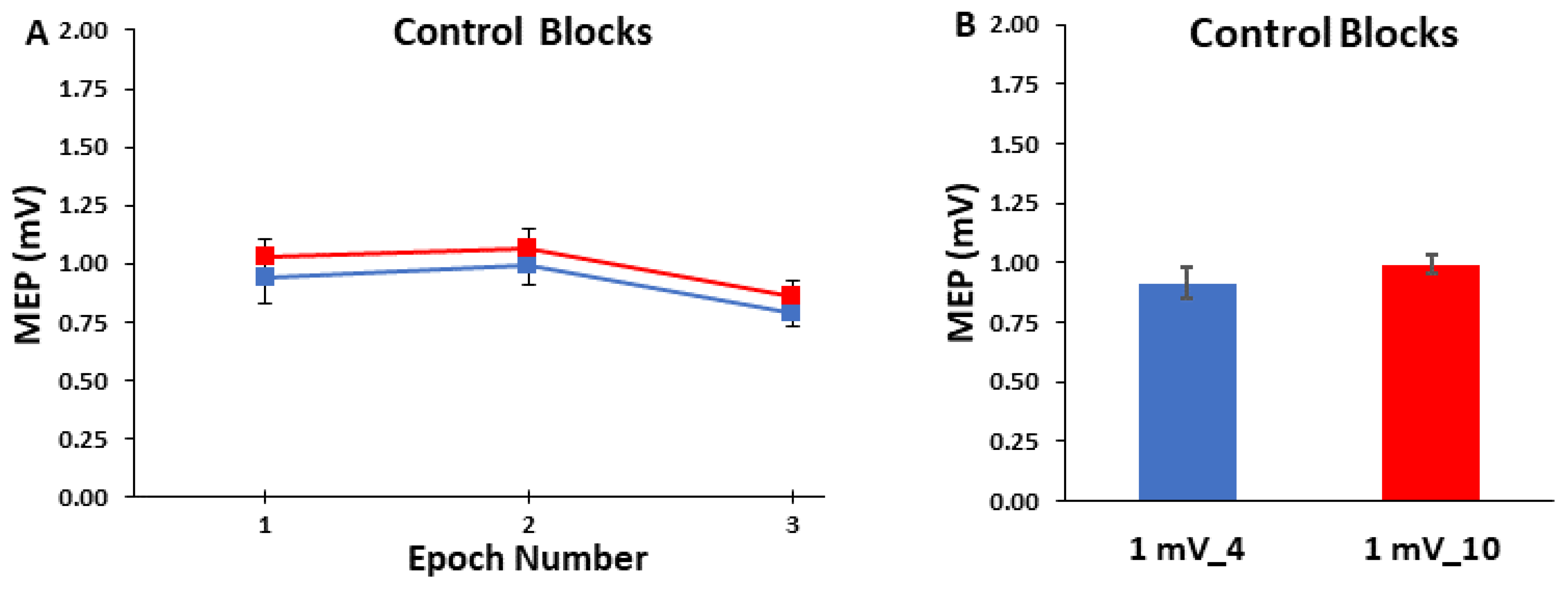
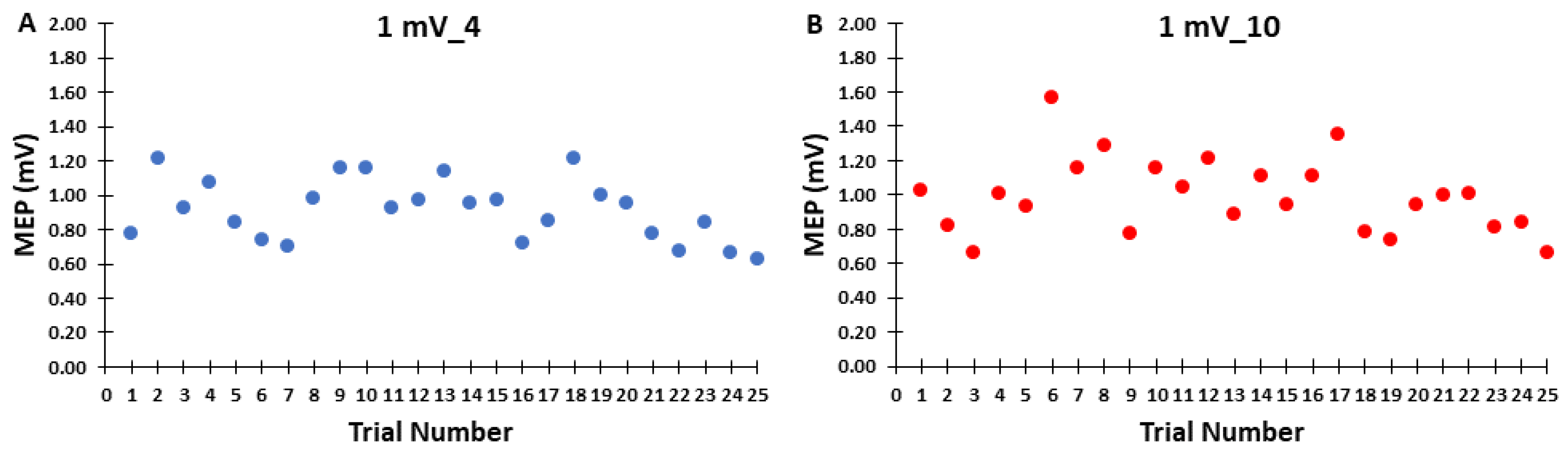
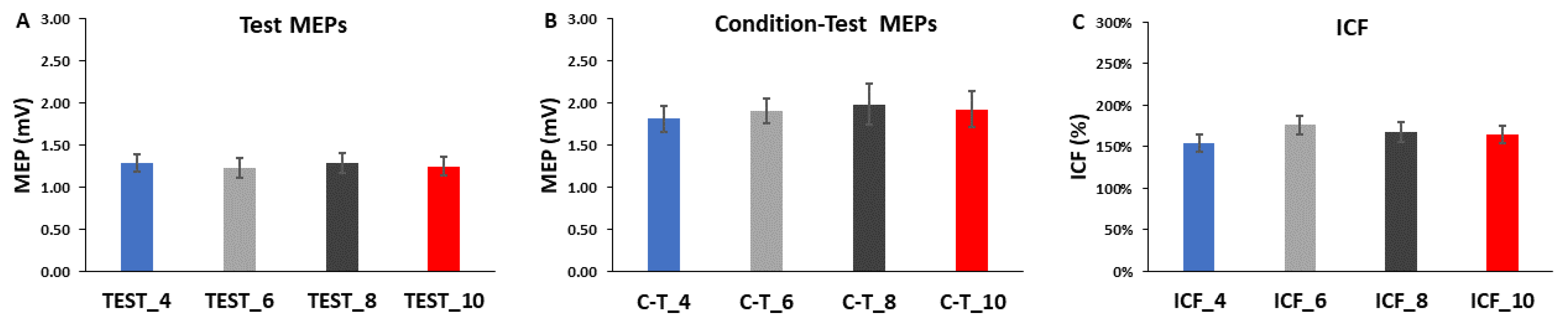
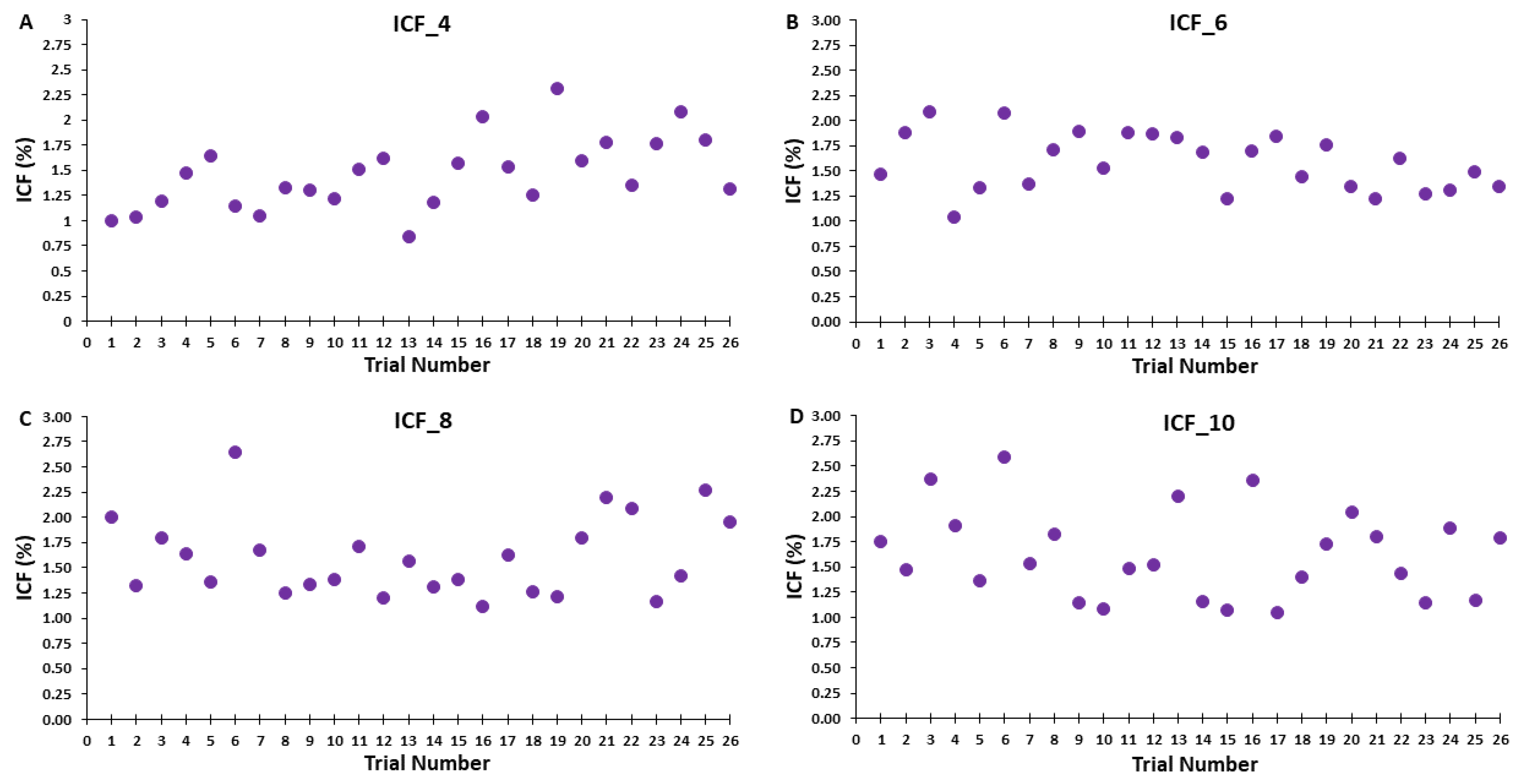
3.3. ICF Blocks
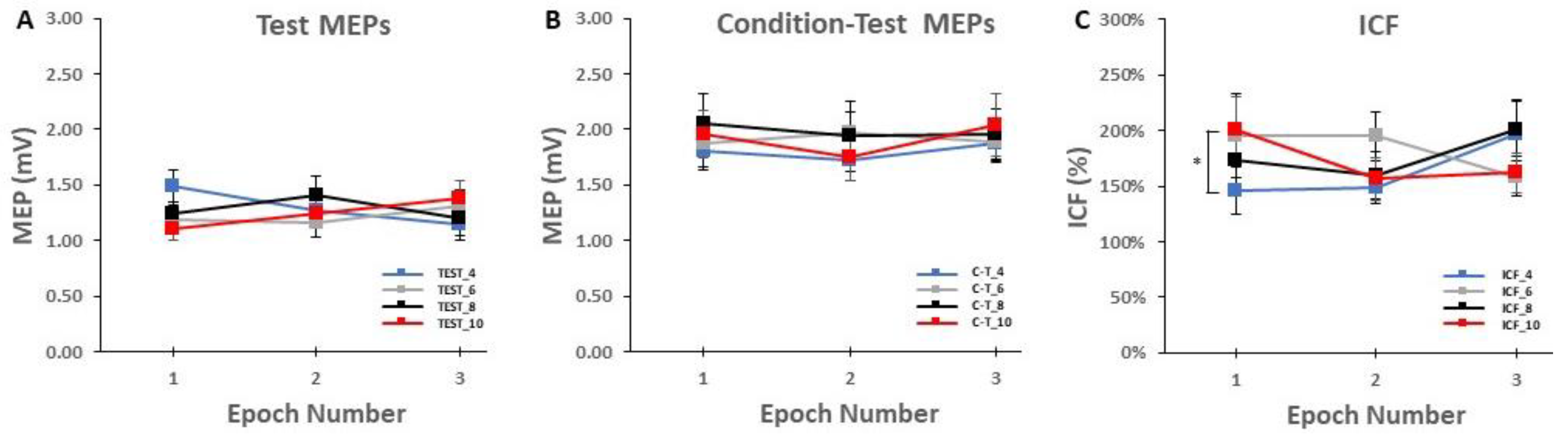

4. Discussion
4.1. Effects of ITI on MEP Amplitude in the Control Blocks
4.2. Effects of ITI on Test MEPs. Condition-Test MEPs, and ICF
4.2.1. Effects of ITI on Test MEP Trials Alone
4.2.2. Effects of ITI on Condition-Test MEP Trials Alone
4.2.3. Effects of ITI on ICF
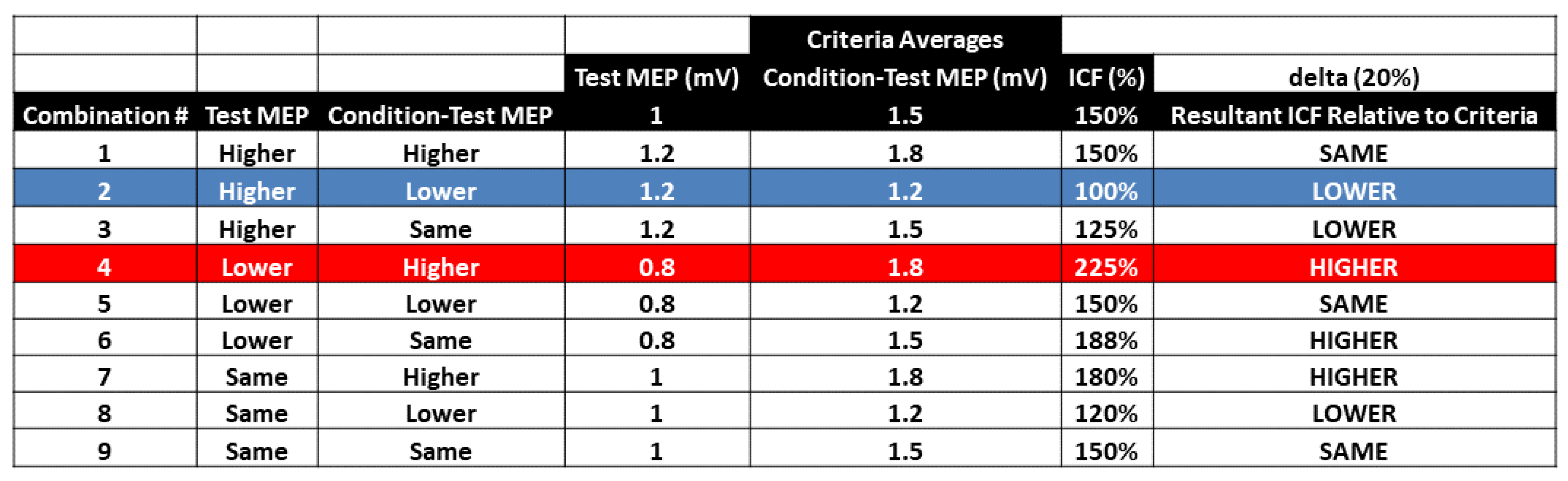
4.3. Overall Interpretation of the Combined Control Blocks and ICF Blocks Results
4.4. Possible Impact of Methodological Issues on the Results
4.5. Implications and Practical Application of the Findings for ICF Studies
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rothwell, J.C. Techniques and mechanisms of action of transcranial stimulation of the human motor cortex. J. Neurosci. Methods 1997, 74, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M. Transcranial magnetic stimulation and the human brain. Nature 2000, 406, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Bestmann, S.; Krakauer, J.W. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp. Brain Res. 2015, 233, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.B.; Pyndt, H.S.; Petersen, N.T. Investigating human motor control by transcranial magnetic stimulation. Exp. Brain Res. 2003, 152, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, D.A.; Ibanez, J.; Rocchi, L.; Rothwell, J. Motor potentials evoked by transcranial magnetic stimulation: Interpreting a simple measure of a complex system. J. Physiol. 2023, 601, 2827–2851. [Google Scholar] [CrossRef] [PubMed]
- Chen, R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp. Brain Res. 2004, 154, 1–10. [Google Scholar] [CrossRef]
- Vucic, S.; Chen, K.-H.S.; Kiernan, M.C.; Hallett, M.; Benninger, D.H.; Di Lazzaro, V.; Rossini, P.M.; Benussi, A.; Berardelli, A.; Currà, A.; et al. Clinical Diagnostic Utility of Transcranial Magnetic Stimulation in Neurological Disorders. Updated Report of an IFCN Committee. Clin. Neurophysiol. 2023, 150, 131–175. [Google Scholar] [CrossRef]
- Siebner, H.R.; Funke, K.; Aberra, A.S.; Antal, A.; Bestmann, S.; Chen, R.; Classen, J.; Davare, M.; Di Lazzaro, V.; Fox, P.T.; et al. Transcranial magnetic stimulation of the brain: What is stimulated?—A consensus and critical position paper. Clin. Neurophysiol. 2022, 140, 59–97. [Google Scholar] [CrossRef]
- Kujirai, T.; Caramia, M.D.; Rothwell, J.C.; Day, B.L.; Thompson, P.D.; Ferbert, A.; Wroe, S.; Asselman, P.; Marsden, C.D. Corticocortical inhibition in human motor cortex. J. Physiol. 1993, 471, 501–519. [Google Scholar] [CrossRef]
- Berardelli, A.; Abbruzzese, G.; Chen, R.; Orth, M.; Ridding, M.C.; Stinear, C.; Suppa, A.; Trompetto, C.; Thompson, P.D. Consensus paper on short-interval intracortical inhibition and other transcranial magnetic stimulation intracortical paradigms in movement disorders. Brain Stimul. 2008, 1, 183–191. [Google Scholar] [CrossRef]
- Ortu, E.; Deriu, F.; Suppa, A.; Tolu, E.; Rothwell, J.C. Effects of volitional contraction on intracortical inhibition and facilitation in the human motor cortex. J. Physiol. 2008, 586, 5147–5159. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Swayne, O.B.; Vandermeeren, Y.; Camus, M.; Dimyan, M.A.; Harris-Love, M.; Perez, M.A.; Ragert, P.; Rothwell, J.C.; Cohen, L.G. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J. Physiol. 2008, 586, 325–351. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Chen, R. Transcranial magnetic stimulation to understand pathophysiology and as potential treatment for neurodegenerative diseases. Transl. Neurodegener. 2015, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Groppa, S.; Oliviero, A.; Eisen, A.; Quartarone, A.; Cohen, L.; Mall, V.; Kaelin-Lang, A.; Mima, T.; Rossi, S.; Thickbroom, G.; et al. A practical guide to diagnostic transcranial magnetic stimulation: Report of an IFCN committee. Clin. Neurophysiol. 2012, 123, 858–882. [Google Scholar] [CrossRef] [PubMed]
- Chipchase, L.; Schabrun, S.; Cohen, L.; Hodges, P.; Ridding, M.; Rothwell, J.; Taylor, J.; Ziemann, U. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: An international consensus study. Clin. Neurophysiol. 2012, 123, 1698–1704. [Google Scholar] [CrossRef]
- Zehr, E.P. Considerations for use of the Hoffmann reflex in exercise studies. Eur. J. Appl. Physiol. 2002, 86, 455–468. [Google Scholar] [CrossRef]
- Stein, R.B.; Estabrooks, K.L.; McGie, S.; Roth, M.J.; Jones, K.E. Quantifying the effects of voluntary contraction and inter-stimulus interval on the human soleus H-reflex. Exp. Brain Res. 2007, 182, 309–319. [Google Scholar] [CrossRef]
- Rosburg, T.; Zimmerer, K.; Huonker, R. Short-term habituation of auditory evoked potential and neuromagnetic field components in dependence of the interstimulus interval. Exp. Brain Res. 2010, 205, 559–570. [Google Scholar] [CrossRef]
- Doeltgen, S.H.; McAllister, S.M.; Ridding, M.C. Simultaneous application of slow-oscillation transcranial direct current stimulation and theta burst stimulation prolongs continuous theta burst stimulation-induced suppression of corticomotor excitability in humans. Eur. J. Neurosci. 2012, 36, 2661–2668. [Google Scholar] [CrossRef]
- Tinazzi, M.; Farina, S.; Tamburin, S.; Facchini, S.; Fiaschi, A.; Restivo, D.; Berardelli, A. Task-dependent modulation of excitatory and inhibitory functions within the human primary motor cortex. Exp. Brain Res. 2003, 150, 222–229. [Google Scholar] [CrossRef]
- Flament, D.; Goldsmith, P.; Buckley, C.J.; Lemon, R.N. Task dependence of responses in first dorsal interosseous muscle to magnetic brain stimulation in man. J. Physiol. 1993, 464, 361–378. [Google Scholar] [CrossRef]
- Kimiskidis, V.K.; Papagiannopoulos, S.; Sotirakoglou, K.; Kazis, D.A.; Kazis, A.; Mills, K.R. Silent period to transcranial magnetic stimulation: Construction and properties of stimulus–response curves in healthy volunteers. Exp. Brain Res. 2005, 163, 21–31. [Google Scholar] [CrossRef]
- Devanne, H.; Lavoie, B.A.; Capaday, C. Input-output properties and gain changes in the human corticospinal pathway. Exp. Brain Res. 1997, 114, 329–338. [Google Scholar] [CrossRef]
- Hoogendam, J.M.; Ramakers, G.M.J.; Di Lazzaro, V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 2010, 3, 95–118. [Google Scholar] [CrossRef]
- Ridding, M.C.; Rothwell, J.C. Is There a Future for Therapeutic Use of Transcranial Magnetic Stimulation? Nat. Rev. Neurosci. 2007, 8, 559–567. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Valls-Solé, J.; Wassermann, E.M.; Hallett, M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 1994, 117 Pt 4, 847–858. [Google Scholar] [CrossRef]
- Möller, C.; Arai, N.; Lücke, J.; Ziemann, U. Hysteresis effects on the input–output curve of motor evoked potentials. Clin. Neurophysiol. 2009, 120, 1003–1008. [Google Scholar] [CrossRef]
- Hansen, N.L.; Nielsen, J.B. The effect of transcranial magnetic stimulation and peripheral nerve stimulation on corticomuscular coherence in humans. J. Physiol. 2004, 561, 295–306. [Google Scholar] [CrossRef]
- Julkunen, P.; Saisanen, L.; Hukkanen, T.; Danner, N.; Kononen, M. Does Second-Scale Intertrial Interval Affect Motor Evoked Potentials Induced by Single-Pulse Transcranial Magnetic Stimulation? Brain Stimul. 2012, 5, 526–532. [Google Scholar] [CrossRef]
- Hassanzahraee, M.; Zoghi, M.; Jaberzadeh, S. Longer Transcranial Magnetic Stimulation Intertrial Interval Increases Size, Reduces Variability, and Improves the Reliability of Motor Evoked Potentials. Brain Connect. 2019, 9, 770–776. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Matilainen, N.; Soldati, M.; Laakso, I. The Effect of Inter-pulse Interval on TMS Motor Evoked Potentials in Active Muscles. Front. Hum. Neurosci. 2022, 16, 845476. [Google Scholar] [CrossRef]
- Schmidt, S.; Cichy, R.; Kraft, A.; Brocke, J.; Irlbacher, K.; Brandt, S. An initial transient-state and reliable measures of corticospinal excitability in TMS studies. Clin. Neurophysiol. 2009, 120, 987–993. [Google Scholar] [CrossRef]
- Vaseghi, B.; Zoghi, M.; Jaberzadeh, S. Inter-pulse Interval Affects the Size of Single-pulse TMS-induced Motor Evoked Potentials: A Reliability Study. Basic Clin. Neurosci. 2015, 6, 44–51. [Google Scholar]
- Poston, B.; Kukke, S.N.; Paine, R.W.; Francis, S.; Hallett, M. Cortical silent period duration and its implications for surround inhibition of a hand muscle. Eur. J. Neurosci. 2012, 36, 2964–2971. [Google Scholar] [CrossRef]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. A Single Application of Cerebellar Transcranial Direct Current Stimulation Fails to Enhance Motor Skill Acquisition in Parkinson’s Disease: A Pilot Study. Biomedicines 2023, 11, 2219. [Google Scholar] [CrossRef]
- Dominici, F.; Popa, T.; Ginanneschi, F.; Mazzocchio, R.; Rossi, A. Cortico-motoneuronal output to intrinsic hand muscles is differentially influenced by static changes in shoulder positions. Exp. Brain Res. 2005, 164, 500–504. [Google Scholar] [CrossRef]
- Ginanneschi, F.; Del Santo, F.; Dominici, F.; Gelli, F.; Mazzocchio, R.; Rossi, A. Changes in corticomotor excitability of hand muscles in relation to static shoulder positions. Exp. Brain Res. 2005, 161, 374–382. [Google Scholar] [CrossRef]
- Do, M.; Clark, G.M.; Fuelscher, I.; Kirkovski, M.; Cerins, A.; Corp, D.T.; Bereznicki, H.G.; Albein-Urios, N.; Enticott, P.G. Magstim 2002 and Bistim Mode maximum stimulus output values are not equivalent: Configuration selection is critical. Brain Stimul. 2020, 13, 444–446. [Google Scholar] [CrossRef]
- de Albuquerque, L.L.; Fischer, K.M.; Pauls, A.L.; Pantovic, M.; Guadagnoli, M.A.; Riley, Z.A.; Poston, B. An acute application of transcranial random noise stimulation does not enhance motor skill acquisition or retention in a golf putting task. Hum. Mov. Sci. 2019, 66, 241–248. [Google Scholar] [CrossRef]
- Lidstone, D.E.; Miah, F.Z.; Poston, B.; Beasley, J.F.; Mostofsky, S.H.; Dufek, J.S. Children with Autism Spectrum Disorder Show Impairments During Dynamic Versus Static Grip-force Tracking. Autism Res. 2020, 13, 2177–2189. [Google Scholar] [CrossRef]
- De Albuquerque, L.L.; Pantovic, M.; Clingo, M.; Fischer, K.; Jalene, S.; Landers, M.; Mari, Z.; Poston, B. An Acute Application of Cerebellar Transcranial Direct Current Stimulation Does Not Improve Motor Performance in Parkinson’s Disease. Brain Sci. 2020, 10, 735. [Google Scholar] [CrossRef]
- Gandevia, S.C. Spinal and Supraspinal Factors in Human Muscle Fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef]
- Pantovic, M.; de Albuquerque, L.L.; Mastrantonio, S.; Pomerantz, A.S.; Wilkins, E.W.; Riley, Z.A.; Guadagnoli, M.A.; Poston, B. Transcranial Direct Current Stimulation of Primary Motor Cortex over Multiple Days Improves Motor Learning of a Complex Overhand Throwing Task. Brain Sci. 2023, 13, 1441. [Google Scholar] [CrossRef]
- Ammann, C.; Guida, P.; Caballero-Insaurriaga, J.; Pineda-Pardo, J.A.; Oliviero, A.; Foffani, G. A framework to assess the impact of number of trials on the amplitude of motor evoked potentials. Sci. Rep. 2020, 10, 21422. [Google Scholar] [CrossRef]
- de Albuquerque, L.L.; Pantovic, M.; Clingo, M.G.; Fischer, K.M.; Jalene, S.; Landers, M.R.; Mari, Z.; Poston, B. Long-Term Application of Cerebellar Transcranial Direct Current Stimulation Does Not Improve Motor Learning in Parkinson’s Disease. Cerebellum 2022, 21, 333–349. [Google Scholar] [CrossRef]
- Brasil-Neto, J.P.; Cohen, L.G.; Hallett, M. Central fatigue as revealed by postexercise decrement of motor evoked potentials. Muscle Nerve 1994, 17, 713–719. [Google Scholar] [CrossRef]
- Brasil-Neto, J.P.; Pascual-Leone, A.; Cammarota, A.; Cohen, L.G.; Hallett, M.; Valls-Solé, J. Postexercise depression of motor evoked potentials: A measure of central nervous system fatigue. Exp. Brain Res. 1993, 93, 181–184. [Google Scholar] [CrossRef]
- Cavaleri, R.; Schabrun, S.M.; Chipchase, L.S. The number of stimuli required to reliably assess corticomotor excitability and primary motor cortical representations using transcranial magnetic stimulation (TMS): A systematic review and meta-analysis. Syst. Rev. 2017, 6, 48. [Google Scholar] [CrossRef]
- Corp, D.T.; Bereznicki, H.G.; Clark, G.M.; Youssef, G.J.; Fried, P.J.; Jannati, A.; Davies, C.B.; Gomes-Osman, J.; Kirkovski, M.; Albein-Urios, N.; et al. Large-scale analysis of interindividual variability in single and paired-pulse TMS data. Clin. Neurophysiol. 2021, 132, 2639–2653. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Oliviero, A.; Saturno, E.; Pilato, F.; Insola, A.; Mazzone, P.; Profice, P.; Tonali, P.; Rothwell, J. The effect on corticospinal volleys of reversing the direction of current induced in the motor cortex by transcranial magnetic stimulation. Exp. Brain Res. 2001, 138, 268–273. [Google Scholar] [CrossRef]
- Di Lazzaro, V.; Restuccia, D.; Oliviero, A.; Profice, P.; Ferrara, L.; Insola, A.; Mazzone, P.; Tonali, P.; Rothwell, J.C. Effects of voluntary contraction on descending volleys evoked by transcranial stimulation in conscious humans. J. Physiol. 1998, 508 Pt 2, 625–633. [Google Scholar] [CrossRef]
- Corp, D.T.; He, J.; Cooke, D.; Perellón-Alfonso, R.; Joutsa, J.; Pascual-Leone, A.; Fox, M.D.; Hyde, C. ‘Expedited Interhemispheric Inhibition’: A Simple Method to Collect Additional IHI Data in the Same Amount of Time. Brain Topogr. 2021, 34, 1–5. [Google Scholar] [CrossRef]
- Hashemirad, F.; Zoghi, M.; Fitzgerald, P.B.; Jaberzadeh, S. Reliability of Motor Evoked Potentials Induced by Transcranial Magnetic Stimulation: The Effects of Initial Motor Evoked Potentials Removal. Basic Clin. Neurosci. J. 2017, 8, 43–50. [Google Scholar] [CrossRef]
- Szucs, D.; Ioannidis, J.P. Sample size evolution in neuroimaging research: An evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. NeuroImage 2020, 221, 117164. [Google Scholar] [CrossRef]
- Consideration of Sample Size in Neuroscience Studies. J. Neurosci. 2020, 40, 4076–4077. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantovic, M.; Boss, R.; Noorda, K.J.; Premyanov, M.I.; Aynlender, D.G.; Wilkins, E.W.; Boss, S.; Riley, Z.A.; Poston, B. The Influence of Different Inter-Trial Intervals on the Quantification of Intracortical Facilitation in the Primary Motor Cortex. Bioengineering 2023, 10, 1278. https://doi.org/10.3390/bioengineering10111278
Pantovic M, Boss R, Noorda KJ, Premyanov MI, Aynlender DG, Wilkins EW, Boss S, Riley ZA, Poston B. The Influence of Different Inter-Trial Intervals on the Quantification of Intracortical Facilitation in the Primary Motor Cortex. Bioengineering. 2023; 10(11):1278. https://doi.org/10.3390/bioengineering10111278
Chicago/Turabian StylePantovic, Milan, Rhett Boss, Kevin J. Noorda, Mario I. Premyanov, Daniel G. Aynlender, Erik W. Wilkins, Sage Boss, Zachary A. Riley, and Brach Poston. 2023. "The Influence of Different Inter-Trial Intervals on the Quantification of Intracortical Facilitation in the Primary Motor Cortex" Bioengineering 10, no. 11: 1278. https://doi.org/10.3390/bioengineering10111278
APA StylePantovic, M., Boss, R., Noorda, K. J., Premyanov, M. I., Aynlender, D. G., Wilkins, E. W., Boss, S., Riley, Z. A., & Poston, B. (2023). The Influence of Different Inter-Trial Intervals on the Quantification of Intracortical Facilitation in the Primary Motor Cortex. Bioengineering, 10(11), 1278. https://doi.org/10.3390/bioengineering10111278









