Molecular and Biological Aspects of Orthodontic Tooth Movement: Possibilities for Bioengineering Intervention: A Narrative Review
Abstract
:1. Introduction
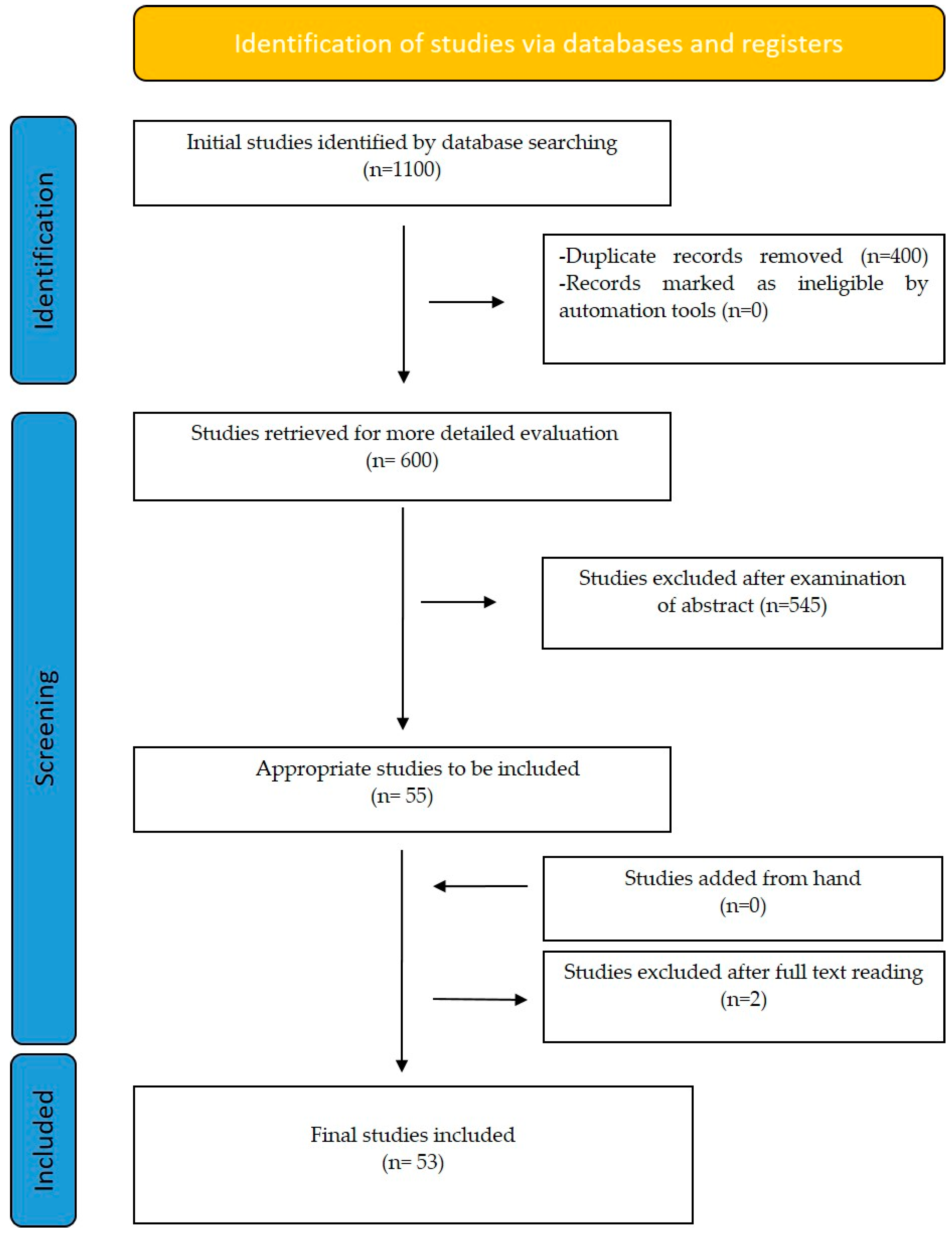
2. Materials and Methods
3. Results
3.1. Orthodontic Tooth Movement: Biological Events and Mechanisms
3.2. RANK, RANK-L, and OPG Molecular Triad
3.3. Biomarkers
3.4. Gene Therapy and Its Application in Alveolar Bone and OTM
3.5. Future of Genetic Manipulation of OTM
3.6. Invasive Methods: Procedures Affecting Orthodontic Tooth Movement
3.7. Vibration Methods
3.8. Magnetic Fields
3.9. Hormones
3.10. Clinical Applications of Accelerating OTM
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Long, H.; Pyakurel, U.; Wang, Y.; Liao, L.; Zhou, Y.; Lai, W. Interventions for accelerating orthodontic tooth movement: A systematic review. Angle Orthod. 2013, 83, 164–171. [Google Scholar] [CrossRef]
- Fleming, P.S.; Fedorowicz, Z.; Johal, A.; El-Angbawi, A.; Pandis, N. Surgical adjunctive procedures for accelerating orthodontic treatment. Cochrane Database Syst. Rev. 2015, 6, CD010572. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.; Papadopoulos, N.; Visel, D.; Visel, T.; Jost-Brinkmann, P.G.; Präger, T.M. Influence of piezotomy and osteoperforation of the alveolar process on the rate of orthodontic tooth movement: A systematic review. J. Orofac. Orthop. Fortschritte Kieferorthopadie 2017, 78, 301–311. [Google Scholar] [CrossRef]
- Yi, J.; Xiao, J.; Li, Y.; Li, X.; Zhao, Z. Efficacy of piezocision on accelerating orthodontic tooth movement: A systematic review. Angle Orthod. 2017, 87, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Williams, R.C.; Kyrkanides, S. Accelerated orthodontic tooth movement: Molecular mechanisms. Am. J. Orthod. Dentofac. Orthop. 2014, 146, 620–632. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.M.; Dalci, O.; Darendeliler, M.A.; Papadopoulou, A.K. Corticotomies and orthodontic tooth movement: A systematic review. J. Oral Maxillofac. Surg. 2016, 74, 453–473. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M. RANK/RANKL/OPG during orthodontic tooth movement. Orthod. Craniofacial Res. 2009, 12, 113–119. [Google Scholar] [CrossRef]
- Theoleyre, S.; Wittrant, Y.; Tat, S.K.; Fortun, Y.; Redini, F.; Heymann, D. The molecular triad OPG/RANK/RANKL: Involvement in the orchestration of pathophysiological bone remodeling. Cytokine Growth Factor Rev. 2014, 15, 457–475. [Google Scholar] [CrossRef]
- Davidovitch, Z. Tooth movement. Crit. Rev. Oral. Biol. Med. 1991, 24, 411–450. [Google Scholar] [CrossRef]
- Pavlin, D.; Gluhak-Heinrich, J. Effect of mechanical loading on periodontal cells. Crit. Rev. Oral. Biol. Med. 2001, 5, 414–424. [Google Scholar] [CrossRef]
- Lekic, P.; McCulloch, C.A. Periodontal ligament cell population: The central role of fibroblasts in creating a unique tissue. Anat. Rec. 1996, 2, 327–341. [Google Scholar] [CrossRef]
- Takano-Yamamoto, T.; Takemura, T.; Kitamura, Y. Site-specific expression of mRNAs for osteonectin, osteocalcin, and osteopontin revealed by in situ hybridization in rat periodontal ligament during physiological tooth movement. J. Histochem. Cytochem. 1994, 7, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Terai, K.; Takano-Yamamoto, T.; Ohba, Y. Role of osteopontin in bone remodeling caused by mechanical stress. J. Bone Miner. Res. 1999, 6, 839–849. [Google Scholar] [CrossRef]
- Wise, G.E.; King, G.J. Mechanisms of tooth eruption and orthodontic tooth movement. J. Dent. Res. 2008, 5, 414–434. [Google Scholar] [CrossRef]
- Yan, Y.; Sun, H.; Gong, Y. Mechanical strain promotes osteoblastic differentiation through integrin-β1-mediated β-catenin signaling. Int. J. Mol. Med. 2016, 2, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Davidovitch, Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 469.e1–469.e32. [Google Scholar] [CrossRef]
- Davidovitch, Z.; Nicolay, O.F.; Ngan, P.W.; Shanfeld, J.L. Neurotrans- mitters, cytokines, and the control of alveolar bone remodeling in orthodontics. Dent. Clin. N. Am. 1988, 32, 411–435. [Google Scholar] [CrossRef] [PubMed]
- Alhashimi, N.; Frithiof, L.; Brudvik, P.; Bakhiet, M. Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am. J. Orthod. Dentofac. Orthop. 2001, 119, 307–312. [Google Scholar] [CrossRef]
- Lo Giudice, R.; Militi, A.; Nicita, F.; Bruno, G.; Tamà, C.; Lo Giudice, F.; Puleio, F.; Calapai, F.; Mannucci, C. Correlation between Oral Hygiene and IL-6 in Children. Dent. J. 2020, 8, 91. [Google Scholar] [CrossRef]
- Aonuma, T.; Tamamura, N.; Fukunaga, T.; Sakai, Y.; Takeshita, N.; Shigemi, S.; Yamashiro, T.; Thesleff, I.; Takano-Yamamoto, T. Delayed tooth movement in Runx2+/− mice associated with mTORC2 in stretch-induced bone formation. Bone. Rep. 2020, 27, 100285. [Google Scholar] [CrossRef]
- Liu, L.; Shao, L.; Li, B.; Zong, C. Extracellular signal regulated kinase1/2 activated by fluid shear stress promotes osteogeneic differentiation of human bone marrow-derived mesenchymal stem cells through novel signaling pathways. Int. J. Biochem. Cell. Biol. 2011, 11, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Takahashi, T.; Tsujisawa, T. Mechanical stress-mediated Runx2 activation is dependent on Ras/ERK1/2 MAPK signaling in osteoblasts. J. Cell. Biochem. 2007, 5, 1266–1277. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ge, C.; Long, J.P. Biomechanical stimulation of osteoblast gene expression requires phosphorylation of the Runx2 transcription factor. J. Bone Miner. Res. 2012, 6, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Salingcarnboriboon, R.; Tsuji, K.; Komori, T. Runx2 is a target of mechanical unloading to alter osteoblastic activity and bone formation in vivo. Endocrinology 2006, 5, 2296–2305. [Google Scholar] [CrossRef]
- Enomoto, H.; Shiojiri, S.; Hoshi, K. Induction of osteoclast differentiation by Runx2 through receptor activator of nuclear factor-kappa B ligand (RANKL) and osteoprotegerin regulation and partial rescue of osteoclastogenesis in Runx2−/− mice by RANKL transgene. J. Biol. Chem. 2003, 26, 23971–23977. [Google Scholar] [CrossRef]
- Frost, H.M. The regional acceleratory phenomenon: A review. Henry Ford Hosp. Med. J. 1982, 31, 3–9. [Google Scholar]
- Baum, B.J.; O’Connell, B.C. The impact of gene therapy on dentistry. J. Am. Dent. Assoc. 1995, 126, 179–189. [Google Scholar] [CrossRef]
- Kneser, U.; Schaefer, D.J.; Polykandriotis, E.; Horch, R.E. Tissue engineering of bone: The reconstructive surgeon0s point of view. J. Cell. Mol. Med. 2006, 10, 7–19. [Google Scholar] [CrossRef]
- Kirker-Head, C.A. Potential applications and delivery strategies for bone morphogenetic proteins. Adv. Drug Deliv. Rev. 2000, 43, 65–92. [Google Scholar] [CrossRef]
- Baum, B.J.; Kok, M.; Tran, S.D.; Yamano, S. The impact of gene therapy on dentistry: A revisiting after six years. J. Am. Dent. Assoc. 2002, 133, 35–44. [Google Scholar] [CrossRef]
- Cho, T.; Gerstenfeld, L.C.; Einhorn, T.A. Differential temporal expression of members of the transforming growth factor _ superfamily during murine fracture healing. J. Bone Miner. Res. 2002, 17, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Chiba, M.; Arai, K.; Takahashi, I.; Haruyama, N.; Nishimura, M.; Mitani, H. Local RANKL gene transfer to the periodontal tissue accelerates orthodontic tooth movement. Gene Ther. 2006, 13, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Chiba, M.; Takahashi, I.; Haruyama, N.; Nishimura, M.; Mitani, H. Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J. Dent. Res. 2004, 83, 920–925. [Google Scholar] [CrossRef]
- Long, H.; Wang, Y.; Jian, F.; Liao, L.; Yang, X.; Lai, W. Current advances in orthodontic pain. Int. J. Oral. Sci. 2016, 8, 67–75. [Google Scholar] [CrossRef]
- Verna, C. Regional Acceleratory Phenomenon. Front. Oral. Biol. 2016, 18, 28–35. [Google Scholar] [PubMed]
- Dhungel, B.; Ramlogan-Steel, C.A.; Steel, J.C. MicroRNA-regulated gene delivery systems for research and therapeutic purposes. Molecules 2018, 23, 1500. [Google Scholar] [CrossRef]
- Irwandi, R.A.; Vacharaksa, A. The role of microRNA in periodontal tissue: A review of the literature. Arch. Oral Biol. 2016, 72, 66–74. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, L.; Tong, X.; Zhang, M.; Zhao, Y.; Guo, J.; Lei, L.; Chen, X.; Tickner, J.; Xu, J.; et al. Mechanical stress regulates bone metabolism through microRNAs. J. Cell. Physiol. 2017, 232, 1239–1245. [Google Scholar] [CrossRef]
- Wei, F.; Wang, J.; Ding, G.; Yang, S.; Li, Y.; Hu, Y.; Wang, S. Mechanical force-induced specific MicroRNA expression in human periodontal ligament stem cells. Cells Tissues Organs 2014, 199, 353–363. [Google Scholar] [CrossRef]
- Chen, Y.; Mohammed, A.; Oubaidin, M.; Evans, C.A.; Zhou, X.; Luan, X.; Diekwisch, T.G.H.; Atsawasuwan, P. Cyclic stretch and compression forces alter microRNA-29 expression of human periodontal ligament cells. Gene 2015, 566, 13–17. [Google Scholar] [CrossRef]
- Qamruddin, I.; Alam, M.K.; Mahroof, V.; Karim, M.; Fida, M.; Khamis, M.F.; Husein, A. Biostimulatory Effects of Low-Intensity Pulsed Ultrasound on Rate of Orthodontic Tooth Movement and Associated Pain, Applied at 3-Week Intervals: A Split-Mouth Study. Pain Res. Manag. 2021, 2021, 6624723. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lee, W.; Lei, D.L.; Liu, Y.P.; Yamashita, D.D.; Yen, S.L. Tisssue responses in corticotomy- and osteotomy-assisted tooth movements in rats: Histology and immunostaining. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 770. [Google Scholar] [CrossRef]
- Lee, W.; Karapetyan, G.; Moats, R.; Yamashita, D.-D.; Moon, H.-B.; Ferguson, D.; Yen, S. Corticotomy-/osteotomy-assisted tooth movement microCTs differ. J. Dent. Res. 2008, 87, 861–867. [Google Scholar] [CrossRef]
- Bowman, S.J. The effect of vibration on the rate of leveling and alignment. J. Clin. Orthod. 2014, 48, 678–688. [Google Scholar] [PubMed]
- Pavlin, D.; Anthony, R.; Raj, V.; Gakunga, P.T. Cyclic loading (vibration) accelerates tooth movement in orthodontic patients: A double-blind, randomized controlled trial. Semin. Orthod. 2015, 21, 187–194. [Google Scholar] [CrossRef]
- Mayama, A.; Seiryu, M.; Takano-Yamamoto, T. Effect of vibration on orthodontic tooth movement in a double blind prospective randomized controlled trial. Sci. Rep. 2022, 1, 1288. [Google Scholar] [CrossRef]
- Darendeliler, M.A.; Sinclair, P.M.; Kusy, R.P. The effects of samarium- cobalt magnets and pulsed electromagnetic fields on tooth movement. Am. J. Orthod. Dentofac. Orthop. 1995, 107, 578–588. [Google Scholar] [CrossRef]
- Sakata, M.; Yamamoto, Y.; Imamura, N.; Nakata, S.; Nakasima, A. The effects of a static magnetic field on orthodontic tooth movement. J. Orthod. 2008, 35, 249–254. [Google Scholar] [CrossRef]
- Stark, T.M.; Sinclair, P.M. Effect of pulsed electromagnetic fields on orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 1987, 91, 91–104. [Google Scholar] [CrossRef]
- Chen, Q. Effect of pulsed electromagnetic field on orthodontic tooth movement through transmission electromicroscopy. Zhonghua Kou Qiang Yi Xue Za Zhi 1991, 10, 61. [Google Scholar]
- Showkatbakhsh, R.; Jamilian, A.; Showkatbakhsh, M. The effect of pulsed electromagnetic fields on the acceleration of tooth movement. World J. Orthod. 2010, 11, e52–e56. [Google Scholar] [PubMed]
- Tengku, B.S.; Joseph, B.K.; Harbrow, D.; Taverne, A.A.; Symons, A.L. Effect of a static magnetic field on orthodontic tooth movement in the rat. Eur. J. Orthod. 2000, 22, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Soma, S.; Matsumoto, S.; Higuchi, Y.; Takano-Yamamoto, T.; Yamashita, K.; Kurisu, K.; Iwamoto, M. Local and chronic application of PTH accelerates tooth movement in rats. J. Dent. Res. 2000, 79, 1717–1724. [Google Scholar] [CrossRef] [PubMed]
- Suda, T.; Ueno, Y.; Fujii, K.; Shinki, T. Vitamin D and bone. J. Cell. Biochem. 2003, 88, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.K.; Sinclair, P.M. The local use of vitamin D to increase the rate of orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 1988, 94, 278–284. [Google Scholar] [CrossRef]
- Takano-Yamamoto, T.; Kawakami, M.; Yamashiro, T. Effect of age on the rate of tooth movement in combination with local use of 1,25(OH)2D3 and mechanical force in the rat. J. Dent. Res. 1992, 71, 1487–1492. [Google Scholar] [CrossRef]
- Leiker, B.J.; Nanda, R.S.; Currier, G.F.; Howes, R.I.; Sinha, P.K. The effects of exogenous prostaglandins on orthodontic tooth movement in rats. Am. J. Orthod. Dentofac. Orthop. 1995, 108, 380–388. [Google Scholar] [CrossRef]
- Seifi, M.; Eslami, B.; Saffar, A.S. The effect of prostaglandin E2 and calcium gluconate on orthodontic tooth movement and root resorption in rats. Eur. J. Orthod. 2003, 25, 199–204. [Google Scholar] [CrossRef]
- Fitzpatrick, B.N. Corticotomy. Aust. Dent. J. 1980, 25, 255–258. [Google Scholar] [CrossRef]
- Kole, H. Surgical operations on the alveolar ridge to correct occlusal abnormalities. Oral. Surg. Oral. Med. Oral. Pathol. 1959, 12, 277–288. [Google Scholar] [CrossRef]
- Merrill, R.G.; Pedersen, G.W. Interdental osteotomy for immediate repositioning of dental-osseous elements. J. Oral. Surg. 1976, 34, 118–125. [Google Scholar] [PubMed]
- Wilcko, M.T.; Wilcko, W.M.; Pulver, J.J.; Bissada, N.F.; Bouquot, J.E. Accelerated osteogenic orthodontics technique: A 1-stage surgically facilitated rapid orthodontic technique with alveolar augmentation. J. Oral. Maxillofac. Surg. 2009, 67, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Sanjideh, P.A.; Rossouw, P.E.; Campbell, P.M.; Opperman, L.A.; Buschang, P.H. Tooth movements in foxhounds after one or two alveolar corticotomies. Eur. J. Orthod. 2010, 32, 106–113. [Google Scholar] [CrossRef]
- Sekhavat, A.R.; Mousavizadeh, K.; Pakshir, H.R.; Aslani, F.S. Effect of mi- soprostol, a prostaglandin E1 analog, on orthodontic tooth move- ment in rats. Am. J. Orthod. Dentofac. Orthop. 2002, 122, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Shibata, Y.; Imai, S.; Tani, Y.; Shibasaki, Y.; Fukuhara, T. Clinical application of prostaglandin E1 (PGE1) upon orthodontic tooth movement. Am. J. Orthod. Dentofac. Orthop. 1984, 85, 508–518. [Google Scholar] [CrossRef]
- Walker, J.B.; Buring, S.M. NSAID impairment of orthodontic tooth movement. Ann. Pharmacother. 2001, 35, 113–115. [Google Scholar] [CrossRef]
- Iglesias-Linares, A.; Moreno-Fernandez, A.M.; Yañez-Vico, R.; Mendoza- Mendoza, A.; Gonzalez-Moles, M.; Solano-Reina, E. The use of gene therapy vs. corticotomy surgery in accelerating orthodontic tooth movement. Orthod. Craniofacial Res. 2011, 14, 138–148. [Google Scholar] [CrossRef]
- Tsolakis, A.I.; Khaldi, L.; Bitsanis, I.; Alexandridis, C.; Triantafyllou, A.; Spyropoulos, M.N.; Dontas, I.A. The effect of osteopenia on tooth movement in ovariectomized rats. An experimental study. J. Musculoskelet. Neuronal Interact. 2018, 3, 366–374. [Google Scholar]
- Frost, H.M. Bone ‘’mass’’ and the ‘’mechanostat’’. A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef]
- Spyropoulos, M.N.; Tsolakis, A.I. Mechanobiological perspectives of orthodontic tooth movement related to bone physiology. In Essentials of Facial Growth, 2nd ed.; Enlow, D.H., Hans, M.G., Eds.; Needham Press: Ann Arbor, MI, USA, 2008; pp. 398–410. [Google Scholar]
- Tsolakis, A.I.; Khaldi, L.; Rontogianni, A.; Georgaki, M.; Christopoulou, I.; Dontas, I.A. Alveolar bone response distal to applied orthodontic forces in ovariectomized rats. J. Musculoskelet. Neuronal Interact. 2022, 2, 235–241. [Google Scholar]
- Tsolakis, A.I.; Khaldi, L.; Bitsanis, I.; Dontas, I.A. Regional Acceleratory Phenomenon after Orthodontic Force Exertion in Ovariectomized Rats. J. Hell. Vet. Med. Soc. 2018, 2, 925–930. [Google Scholar] [CrossRef]
- Tsolakis, A.I.; Khaldi, L.; Bitsanis, I.; Makou, M.; Dontas, I.A. Distraction-like phenomena in maxillary bone due to application of orthodontic forces in ovariectomized rats. Indian J. Dent. Res. 2012, 4, 501–505. [Google Scholar] [CrossRef] [PubMed]
| “Orthodontics” [Major] and biomarkers | 395 results |
| Orthodontic tooth movement [MeSH Major Topic] AND acceleration | 375 results |
| Orthodontic tooth movement [MeSH Major Topic] AND gene therapy | 139 results |
| Orthodontic tooth movement [MeSH Major Topic] AND stem cells | 78 results |
| Orthodontic tooth movement [MeSH Major Topic] AND biomarkers | 113 results |
| Methods Affecting OTM | Accelerate OTM | Conflicting Results |
|---|---|---|
| RANKL | + | |
| OPG | + | |
| Inflammatory mediators (IL-1b, IL-6, TNF-a, epidermal growth factor) | + | |
| Runx2 gene | + | |
| Corticotomy | + | |
| Osteotomy | + | |
| Piezocision | + | |
| AcceleDentTM | + | |
| Low-laser stimulation | + | |
| PTH | + | |
| Prostaglandins (PGs) | + | |
| 1,25-dihydroxyvitamin D3 | + | |
| Magnetic fields | + |
| Methods Affecting OTM | Animal Studies | Human Studies | Both Kinds of Studies |
|---|---|---|---|
| RANKL | + | ||
| OPG | + | ||
| Inflammatory mediators (IL-1b, IL-6, TNF-a, epidermal growth factor) | + | ||
| Runx2 gene | + | ||
| Corticotomy | + | ||
| Osteotomy | + | ||
| Piezocision | + | ||
| AcceleDentTM | + | ||
| Low-laser stimulation | + | ||
| PTH | + | ||
| Prostaglandins (PGs) | + | ||
| 1,25-dihydroxyvitamin D3 | + | ||
| Magnetic fields | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsolakis, I.A.; Christopoulou, I.; Sitaras, S.; Lyros, I.; Rontogianni, A.; Dalampira, M.; Tsolakis, A.I. Molecular and Biological Aspects of Orthodontic Tooth Movement: Possibilities for Bioengineering Intervention: A Narrative Review. Bioengineering 2023, 10, 1275. https://doi.org/10.3390/bioengineering10111275
Tsolakis IA, Christopoulou I, Sitaras S, Lyros I, Rontogianni A, Dalampira M, Tsolakis AI. Molecular and Biological Aspects of Orthodontic Tooth Movement: Possibilities for Bioengineering Intervention: A Narrative Review. Bioengineering. 2023; 10(11):1275. https://doi.org/10.3390/bioengineering10111275
Chicago/Turabian StyleTsolakis, Ioannis A., Isidora Christopoulou, Symeon Sitaras, Ioannis Lyros, Aliki Rontogianni, Maria Dalampira, and Apostolos I. Tsolakis. 2023. "Molecular and Biological Aspects of Orthodontic Tooth Movement: Possibilities for Bioengineering Intervention: A Narrative Review" Bioengineering 10, no. 11: 1275. https://doi.org/10.3390/bioengineering10111275
APA StyleTsolakis, I. A., Christopoulou, I., Sitaras, S., Lyros, I., Rontogianni, A., Dalampira, M., & Tsolakis, A. I. (2023). Molecular and Biological Aspects of Orthodontic Tooth Movement: Possibilities for Bioengineering Intervention: A Narrative Review. Bioengineering, 10(11), 1275. https://doi.org/10.3390/bioengineering10111275











