Prediction of Gait Kinematics and Kinetics: A Systematic Review of EMG and EEG Signal Use and Their Contribution to Prediction Accuracy
Abstract
:1. Introduction
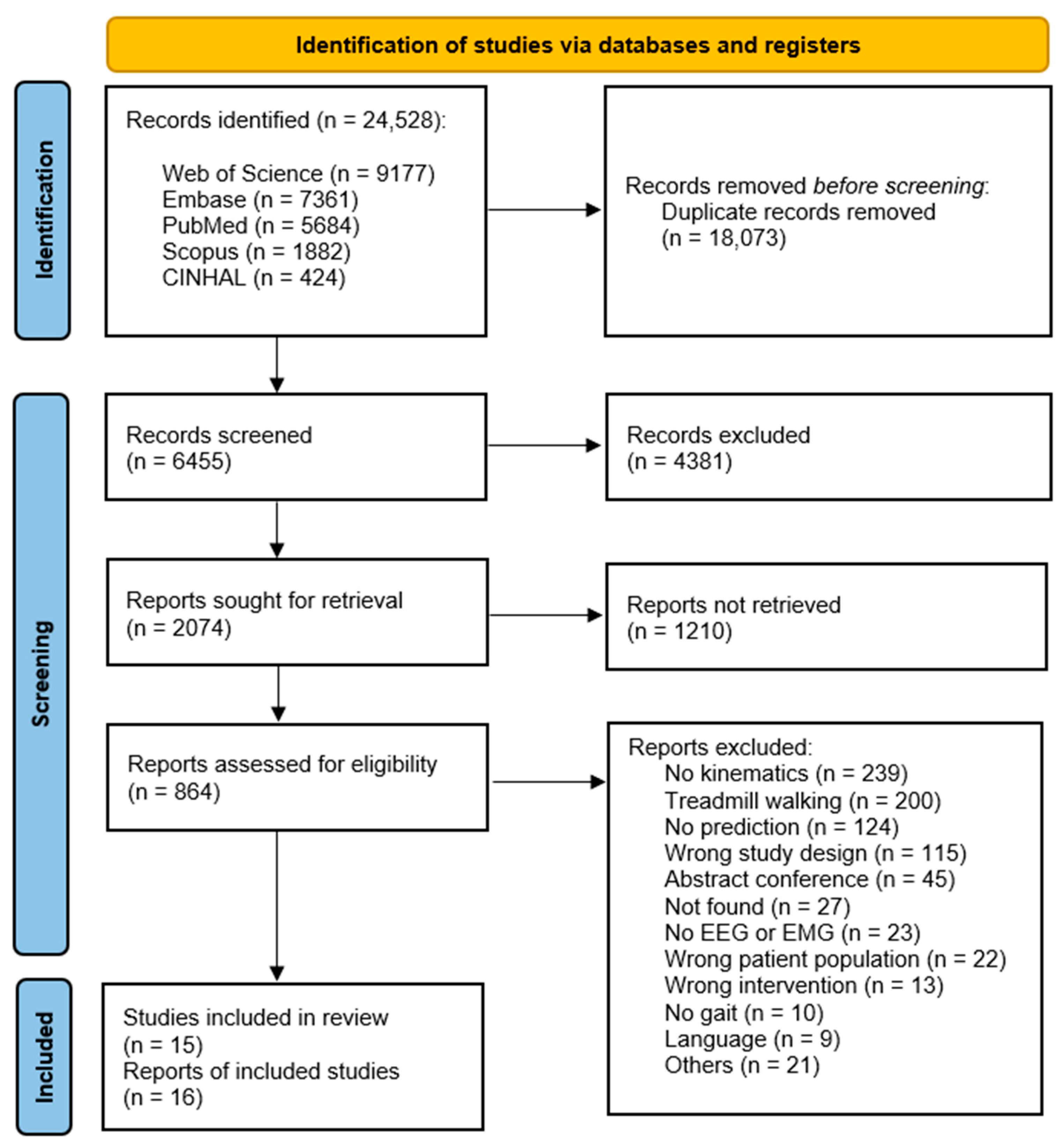
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
2.2. Study Design
2.3. Quality Assessment Tool
- Selection bias: Examining potential biases in the selection process of the study participants.
- Study design: Evaluating the robustness and appropriateness of the chosen research design.
- Confounders: Analysing potential variables that may impact the study’s conclusions.
- Blindings: Evaluating whether subjects are aware of the study design in a manner that could influence the data they contribute.
- Data collection method: Scrutinizing the accuracy and appropriateness of the instruments employed during data collection for the study.
2.4. Data Extraction Strategy
- Participants characteristics (sex, age, healthy subject, or with a central neurological disorder, weight, height)
- Test protocol
- Data collected (input and output)
- Pre-processing pipeline applied to input and output signals
- Prediction tool utilised
- Accuracy of prediction
3. Results
3.1. Lower-Limb, Kinematic, and Kinetic Prediction with EEGs
3.2. Lower-Limb, Kinematic, and Kinetic Prediction with EMGs
3.3. Lower-Limb, Kinematic, and Kinetic Prediction with EMG and Additional Data
4. Discussion
4.1. Scientific Gaps
4.2. EEG
4.3. EMG
4.4. Prediction Tools
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tesio, L.; Rota, V. The Motion of Body Center of Mass During Walking: A Review Oriented to Clinical Applications. Front. Neurol. 2019, 10, 1664–2295. [Google Scholar] [CrossRef] [PubMed]
- Rossignol, S.; Dubuc, R.; Gossard, P. Dynamic Sensorimotor Interactions in Locomotion. Physiol. Rev. 2006, 86, 89–154. [Google Scholar] [CrossRef] [PubMed]
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef]
- Belda-Lois, J.M.; Mena-del Horno, S.; Bermejo-Bosch, I.; Moreno, J.C.; Pons, J.L.; Farina, D.; Iosa, M.; Molinari, M.; Tamburella, F.; Ramos, A.; et al. Rehabilitation of gait after stroke: A review towards a top-down approach. J. Neuroeng. Rehabil. 2011, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; McDonough, D.J.; Gao, Z. The Effectiveness of Virtual Reality Exercise on Individual’s Physiological, Psychological and Rehabilitative Outcomes: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4133. [Google Scholar] [CrossRef]
- Taylor, M.J.; Griffin, M. The use of gaming technology for rehabilitation in people with multiple sclerosis. Mult. Scler. 2015, 21, 355–371. [Google Scholar] [CrossRef]
- Vidal, J.J. Toward direct brain-computer communication. Annu. Rev. Biophys. Bioeng. 1973, 2, 157–180. [Google Scholar] [CrossRef]
- Konger, C.; Principe, J.C. Neural network classification of event related potentials for the development of a new computer interface. In Proceedings of the 1990 IJCNN International Joint Conference on Neural Networks, San Diego, CA, USA, 17–21 June 1990; Volume 1, pp. 367–372. [Google Scholar]
- Moore, M.M. Real-world applications for brain-computer interface technology. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 162–165. [Google Scholar] [CrossRef]
- Wolpaw, J.R.; Birbaumer, N.; Heetderks, W.J.; McFarland, D.J.; Peckham, P.H.; Schalk, G.; Donchin, E.; Quatrano, L.A.; Robinson, C.J.; Vaughan, T.M. Brain-computer interface technology: A review of the first international meeting. IEEE Trans. Rehabil. Eng. J. 2000, 8, 164–173. [Google Scholar] [CrossRef]
- Oweiss, K.G.; Badreldin, I.S. Neuroplasticity subserving the operation of brain-machine interfaces. Neurobiol. Dis. 2015, 83, 161–171. [Google Scholar] [CrossRef]
- Bai, Z.; Fong, K.N.; Zhang, J.J.; Chan, J.; Ting, K.H. Immediate and long-term effects of BCI-based rehabilitation of the upper extremity after stroke: A systematic review and meta-analysis. J. Neuroeng. Rehabil. 2020, 17, 57. [Google Scholar] [CrossRef] [PubMed]
- Cervera, M.A.; Soekadar, S.R.; Ushiba, J.; Millán, J.D.R.; Liu, M.; Birbaumer, N.; Garipelli, G. Brain-computer interfaces for post-stroke motor rehabilitation: A meta-analysis. Ann. Clin. Transl. Neurol. 2018, 5, 651–663. [Google Scholar] [CrossRef]
- Mak, J.N.; Wolpaw, J.R. Clinical Applications of Brain-Computer Interfaces: Current State and Future Prospects. IEEE Rev. Biomed. Eng. 2009, 2, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.; Ang, K.K.; Nair, K.P.S.; Phua, K.S.; Arvaneh, M. Efficacy of Brain-Computer Interface and the Impact of Its Design Characteristics on Poststroke Upper-limb Rehabilitation: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. EEG Neurosci. 2022, 53, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B. ACSM’s Guidelines for Exercise Testing and Prescription, 9th ed.; Canadian Chiropractic Association (CCA): Toronto, ON, Canada, 2014; Volume 58, p. 328. [Google Scholar]
- Lee, I.M.; Buchner, D.M. The importance of walking to public health. Med. Sci. Sports Exerc. 2008, 40, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Müller-Putz, G.R.; Schlögl, A.; Graimann, B.; Scherer, R.; Leeb, R.; Brunner, C.; Keinrath, C.; Lee, F.; Townsend, G.; et al. 15 years of BCI research at Graz University of Technology: Current projects. IEEE Trans. Neural Syst. Rehabil Eng. 2006, 14, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Powell, D.; DeVita, P.; Hortobagyi, T. Inertial Loading during Gait Evokes Unique Neuromuscular Adaptations in Old Adults. Percept. Mot. Ski. 2008, 107, 881–892. [Google Scholar] [CrossRef]
- DeVita, P.; Hortobagyi, T. Age causes a redistribution of joint torques and powers during gait. J. Appl. Physiol. 2000, 88, 1804–1811. [Google Scholar] [CrossRef]
- Bailey, C.A.; Corona, F.; Pilloni, G.; Porta, M.; Chiara Fastame, M.; Hitchcott, P.K.; Pietronilla Penna, M.; Pau, M.; Côté, J.N. Sex-dependent and sex-independent muscle activation patterns in adult gait as a function of age. Exp. Gerontol. 2018, 110, 1–8. [Google Scholar] [CrossRef]
- Kolaghassi, R.; Al-Hares, M.K.; Sirlantzis, K. Systematic Review of Intelligent Algorithms in Gait Analysis and Prediction for Lower Limb Robotic Systems. IEEE Access 2021, 9, 113788–113812. [Google Scholar] [CrossRef]
- Armijo-Olivo, S.; Stiles, C.R.; Hagen, N.A.; Biondo, P.D.; Cummings, G.G. Assessment of study quality for systematic reviews: A comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological research. J. Eval. Clin. Pract 2012, 18, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Caldas, R.; Fadel, T.; Buarque, F.; Markert, B. Adaptive predictive systems applied to gait analysis: A systematic review. Gait Posture 2020, 77, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Duan, X.; Li, F.; Liu, Y.; Wang, G.; Shi, T.; Liu, K. RBF Neural Network-Sliding Model Control Approach for Lower Limb Rehabilitation Robot Based on Gait Trajectories of SEMG Estimation. In Proceedings of the Tenth International Conference on Intelligent Control and Information Processing (ICICIP), Marrakesh, Morocco, 14–19 December 2019; pp. 14–19. [Google Scholar]
- Contreras-Vidal, J.L.; Bortole, M.; Zhu, F.; Nathan, K.; Venkatakrishnan, A.; Francisco, G.E.; Soto, R.; Pons, J.L. Neural Decoding of Robot-Assisted Gait During Rehabilitation After Stroke. Am. J. Phys. Med. Rehabil. 2018, 97, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Mercado, L.; Alvarado, L.; Quiroz-Compean, G.; Romo-Vázquez, R.; Velez Perez, H.; Platas, M.; González-Garrido, A.; Gómez-Correa, J.; Morales, J.A.; Rodriguez-Liñan, A.; et al. Decoding the Torque of Lower Limb Joints from EEG Recordings of Pre-gait Movements Using a Machine Learning Scheme. Neurocomputing 2021, 446, 118–129. [Google Scholar] [CrossRef]
- He, Y.; Nathan, K.; Venkatakrishnan, A.; Rovekamp, R.; Beck, C.; Ozdemir, R.; Francisco, G.E.; Contreras-Vidal, J.L. An integrated neuro-robotic interface for stroke rehabilitation using the NASA X1 powered lower limb exoskeleton. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 3985–3988. [Google Scholar]
- Goncharova, I.I.; McFarland, D.J.; Vaughan, T.M.; Wolpaw, J.R. EMG contamination of EEG: Spectral and topographical characteristics. Clin. Neurophysiol. 2003, 114, 1580–1593. [Google Scholar] [CrossRef]
- Brantley, J.A.; Luu, T.P.; Nakagome, S.; Contreras-Vidal, J.L. Prediction of lower-limb joint kinematics from surface EMG during overground locomotion. In Proceedings of the 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Banff, AB, Canada, 5–8 October 2017; pp. 1705–1709. [Google Scholar]
- Chen, J.; Zhang, X.; Cheng, Y.; Xi, N. Surface EMG based continuous estimation of human lower limb joint angles by using deep belief networks. Biomed. Signal Process. Control 2018, 40, 335–342. [Google Scholar] [CrossRef]
- Cheron, G.; Leurs, F.; Bengoetxea, A.; Draye, J.P.; Destrée, M.; Dan, B. A dynamic recurrent neural network for multiple muscles electromyographic mapping to elevation angles of the lower limb in human locomotion. J. Neurosci. Methods 2003, 129, 95–104. [Google Scholar] [CrossRef]
- Gautam, A.; Panwar, M.; Biswas, D.; Acharyya, A. MyoNet: A Transfer-Learning-Based LRCN for Lower Limb Movement Recognition and Knee Joint Angle Prediction for Remote Monitoring of Rehabilitation Progress From sEMG. IEEE J. Transl. Eng. Health Med. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Jia, L.; Ai, Q.; Meng, W.; Liu, Q.; Xie, S.Q. Individualized Gait Trajectory Prediction Based on Fusion LSTM Networks for Robotic Rehabilitation Training. In Proceedings of the 2021 IEEE/ASME International Conference on Advanced Intelligent Mechatronics (AIM), Delft, The Netherlands, 12–16 July 2021; pp. 988–993. [Google Scholar]
- Li, Z.; Guan, X.; Zou, K.; Xu, C. Estimation of Knee Movement from Surface EMG Using Random Forest with Principal Component Analysis. Electronics 2020, 9, 43. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, L.; Han, B.; Zhang, T.; Wang, Z.; Wei, P. sEMG-Based Continuous Estimation of Knee Joint Angle Using Deep Learning with Convolutional Neural Network. In Proceedings of the 2019 IEEE 15th International Conference on Automation Science and Engineering (CASE), Vancouver, BC, Canada, 22–26 August 2019; pp. 140–145. [Google Scholar]
- Wang, F.; Yin, T.; Lei, C.; Zhang, Y.; Wang, Y.; Liu, J. Prediction of lower limb joint angle using sEMG based on GA-GRNN. In Proceedings of the 2015 IEEE International Conference on Cyber Technology in Automation, Control, and Intelligent Systems (CYBER), Shenyang, China, 8–12 June 2015; pp. 1894–1899. [Google Scholar]
- Moreira, L.; Figueiredo, J.; Vilas-Boas, J.P.; Santos, C.P. Kinematics, Speed, and Anthropometry-Based Ankle Joint Torque Estimation: A Deep Learning Regression Approach. Machines 2021, 9, 154. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Z.; Hu, Y.; Smith, C.; Farewik, E.M.G.; Wang, R. Ankle Joint Torque Estimation Using an EMG-Driven Neuromusculoskeletal Model and an Artificial Neural Network Model. IEEE Trans. Autom. Sci. Eng. 2021, 18, 564–573. [Google Scholar] [CrossRef]
- Chong, E.; Choi, T.; Kim, H.; Kim, S.J.; Hwang, Y.; Lee, J.M. Informative sensor selection and learning for prediction of lower limb kinematics using generative stochastic neural networks. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Republic of Korea, 11–15 July 2017; pp. 2043–2046. [Google Scholar]
- Hahn, M.; O’Keefe, K. A neural network model for estimation of net joint moments during normal gait. J. Musculoskelet. Res. 2008, 11, 117–126. [Google Scholar] [CrossRef]
- Zhu, A.; Shen, H.; Shen, Z.; Li, Y.; Mao, H.; Zhang, X.; Cao, G. Prediction of Human Dynamic Ankle Moment Based on Surface Electromyography Signals. In Proceedings of the 2019 16th International Conference on Ubiquitous Robots (UR), Jeju, Republic of Korea, 24–27 June 2019; pp. 755–759. [Google Scholar]
- Seeber, M.; Scherer, R.; Wagner, J.; Solis-Escalante, T.; Müller-Putz, G.R. High and low gamma EEG oscillations in central sensorimotor areas are conversely modulated during the human gait cycle. NeuroImage 2015, 112, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Whittle, M.W. Clinical gait analysis: A review, Human Movement Science. Hum. Mov. Sci. 1996, 15, 369–387. [Google Scholar] [CrossRef]
- Ricamato, A.L.; Hidler, J.M. Quantification of the dynamic properties of EMG patterns during gait. J. Electromyogr. Kinesiol. 2005, 15, 384–392. [Google Scholar] [CrossRef]
- Alton, F.; Baldey, L.; Caplan, S.; Morrissey, M.C. A kinematic comparison of overground and treadmill walking. Clin. Biomech. 1998, 13, 434–440. [Google Scholar] [CrossRef]
- Riley, P.O.; Paolini, G.; Della Croce, U.; Paylo, K.W.; Kerrigan, D.C. A kinematic and kinetic comparison of overground and treadmill walking in healthy subjects. Gait Posture 2007, 26, 17–24. [Google Scholar] [CrossRef]
- Wentink, E.C.; Beijen, S.I.; Hermens, H.J.; Rietman, J.S.; Veltink, P.H. Intention detection of gait initiation using EMG and kinematic data. Gait Posture 2013, 37, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Hooda, N.; Das, R.; Kumar, N. Fusion of EEG and EMG signals for classification of unilateral foot movements. Biomed. Signal Process. Control 2020, 60, 101990. [Google Scholar] [CrossRef]
- Moreira, L.; Figueiredo, J.; Cerqueira, J.; Santos, C.P. A Review on Locomotion Mode Recognition and Prediction When Using Active Orthoses and Exoskeletons. Sensors 2022, 22, 7109. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Park, S.J. Prediction of Myoelectric Biomarkers in Post-Stroke Gait. Sensors 2021, 21, 5334. [Google Scholar] [CrossRef] [PubMed]
- Malone, A.; Meldrum, D.; Bolger, C. Gait impairment in cervical spondylotic myelopathy: Comparison with age- and gender matched healthy controls. Eur. Spine J. 2012, 21, 2456–2466. [Google Scholar] [CrossRef]
- Sburlea, A.I.; Montesano, L.; Minguez, J. Advantages of EEG phase patterns for the detection of gait intention in healthy and stroke subjects. J. Neural Eng. 2017, 14, 036004. [Google Scholar] [CrossRef]
- Baker, R. Gait analysis methods in rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 4. [Google Scholar] [CrossRef]
- Papagiannis, G.I.; Triantafyllou, A.I.; Roumpelakis, I.M.; Zampeli, F.; Eleni, P.G.; Koulouvaris, P.; Papadopoulos, E.C.; Papagelopoulos, P.J.; Babis, G.C. Methodology of surface electromyography in gait analysis: Review of the literature. J. Med. Eng. Technol. 2019, 43, 59–65. [Google Scholar] [CrossRef]
- Morbidoni, C.; Cucchiarelli, A.; Fioretti, S.; Di Nardo, F. A deep learning approach to EMG-based classification of gait phases during level ground walking. Electronics 2019, 8, 894. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, J.; Tian, F.; Hong, J. A comparison of neural networks algorithms for EEG and sEMG features based gait phases recognition. Biomed. Signal Process. Control 2021, 68, 102587. [Google Scholar] [CrossRef]
- Mane, R.; Chouhan, T.; Guan, C. BCI for stroke rehabilitation: Motor and beyond. J. Neural Eng. 2020, 17, 041001. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.T.; Bastian, A.J. Adaptation reveals independent control networks for human walking. Nat. Neurosci. 2007, 10, 1055–1062. [Google Scholar] [CrossRef]
- Presacco, A.; Goodman, R.; Forrester, L.; Contreras-Vidal, J.L. Neural decoding of treadmill walking from non-invasive electroencephalographic signals. J. Neurophysiol. 2011, 106, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Presacco, A.; Forrester, L.W.; Contreras-Vidal, J.L. Decoding intra-limb and inter-limb kinematics during treadmill walking from scalp electroencephalographic (EEG) signals. IEEE Trans. Neural Syst. Rehabil. Eng. A Publ. IEEE Eng. Med. Biol. Soc. 2012, 20, 212–219. [Google Scholar] [CrossRef]
- Xiong, D.; Zhang, D.; Zhao, X.; Zhao, Y. Deep learning for EMG-based human-machine interaction: A review. IEEE/CAA J. Autom. Sin. 2021, 8, 512–533. [Google Scholar] [CrossRef]
- Enders, H.; Nigg, B.M. Measuring human locomotor control using EMG and EEG: Current knowledge, limitations and future considerations. Eur. J. Sport Sci. 2016, 16, 416–426. [Google Scholar] [CrossRef]
- Kljajić, M.; Krajnik, J. The use of ground reaction measuring shoes in gait evaluation. Clin. Phys. Physiol. Meas. 1987, 8, 133–142. [Google Scholar] [CrossRef]
- Tortora, S.; Tonin, L.; Chisari, C.; Micera, S.; Menegatti, E.; Artoni, F. Hybrid Human-Machine Interface for Gait Decoding Through Bayesian Fusion of EEG and EMG Classifiers. Front. Neurorobotics 2020, 14, 582728. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.; Figueiredo, J.; Fonseca, P.; Vilas-Boas, J.P.; Santos, C.P. Lower limb kinematic, kinetic, and EMG data from young healthy humans during walking at controlled speeds. Sci. Data 2021, 8, 103. [Google Scholar] [CrossRef]
- Labarrière, F.; Thomas, E.; Calistri, L.; Optasanu, V.; Gueugnon, M.; Ornetti, P.; Laroche, D. Machine Learning Approaches for Activity Recognition and/or Activity Prediction in Locomotion Assistive Devices—A Systematic Review. Sensors 2020, 20, 6345. [Google Scholar] [CrossRef]
- Koopman, B.; van Asseldonk, E.H.; van der Kooij, H. Speed-dependent reference joint trajectory generation for robotic gait support. J. Biomech. 2014, 47, 1447–1458. [Google Scholar] [CrossRef]
- Hanlon, M.; Anderson, R. Prediction methods to account for the effect of gait speed on lower limb angular kinematics. Gait Posture 2006, 24, 280–287. [Google Scholar] [CrossRef]
- Kline, J.E.; Huang, H.J.; Snyder, K.L.; Ferris, D.P. Isolating gait-related movement artifacts in electroencephalography during human walking. J. Neural Eng. 2015, 12, 46022. [Google Scholar] [CrossRef]
- Chowdhury, R.H.; Reaz, M.B.; Ali, M.A.; Bakar, A.A.; Chellappan, K.; Chang, T.G. Surface electromyography signal processing and classification techniques. Sensors 2013, 13, 12431–12466. [Google Scholar] [CrossRef] [PubMed]
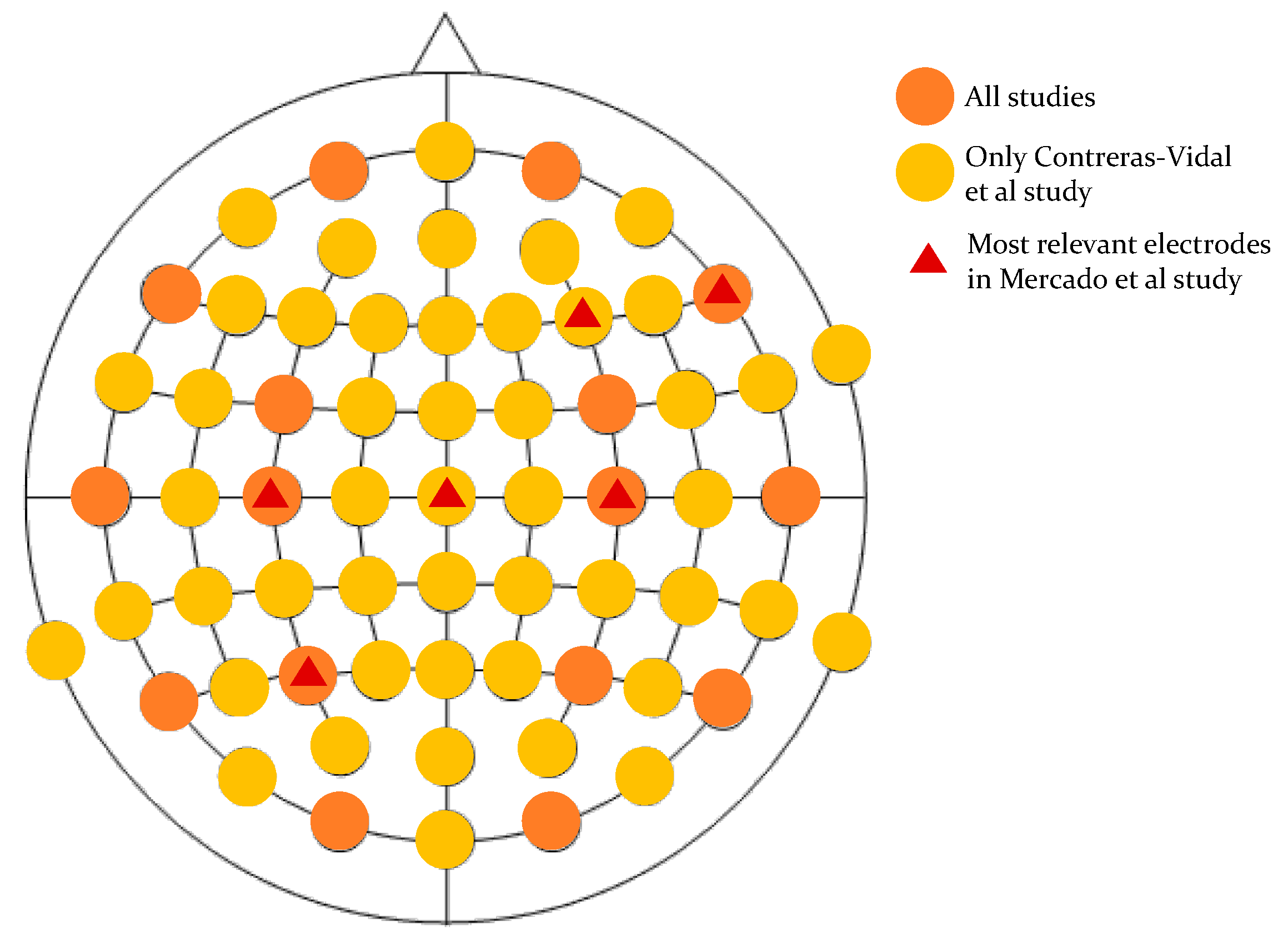
- Mercado, L.; Quiroz-Compean, G.; Azorín, J.M. Analyzing the performance of segmented trajectory reconstruction of lower limb movements from EEG signals with combinations of electrodes, gaps, and delays. Biomed. Signal Process. Control. 2021, 68, 102783. [Google Scholar] [CrossRef]
- Schmitz, A.; Silder, A.; Heiderscheit, B.; Mahoney, J.; Thelen, D.G. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J. Electromyogr. Kinesiology. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2009, 19, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Hori, H.; Kobayashi, Y. Contribution of muscle activity at different gait phases for improving walking performance in chronic stroke patients with hemiparesis. J. Phys. Ther. Sci. 2018, 30, 1381–1385. [Google Scholar] [CrossRef]
- Kumar, U.A. Comparison of neural networks and regression analysis: A new insight. Expert Syst. Appl. 2005, 29, 424–430. [Google Scholar] [CrossRef]
- Paris, G.; Robilliard, D.; Fonlupt, C. Exploring Overfitting in Genetic Programming. In Artificial Evolution. EA 2003. Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 2936. [Google Scholar]
- Lennon, O.; Tonellato, M.; Del Felice, A.; Di Marco, R.; Fingleton, C.; Korik, A.; Guanziroli, E.; Molteni, F.; Guger, C.; Otner, R.; et al. A systematic review establishing the current state-of-the-art, the limitations, and the DESIRED checklist in studies of direct neural interfacing with robotic gait devices in stroke rehabilitation. Front. Neurosci. 2020, 14, 578. [Google Scholar] [CrossRef]
- Bia, L.; Feleke, G.; Guan, C. A review on EMG-based motor intention prediction of continuos human upper limb motion for human-robot collaboration. Biomed. Signal Process. Control 2019, 51, 113–127. [Google Scholar] [CrossRef]

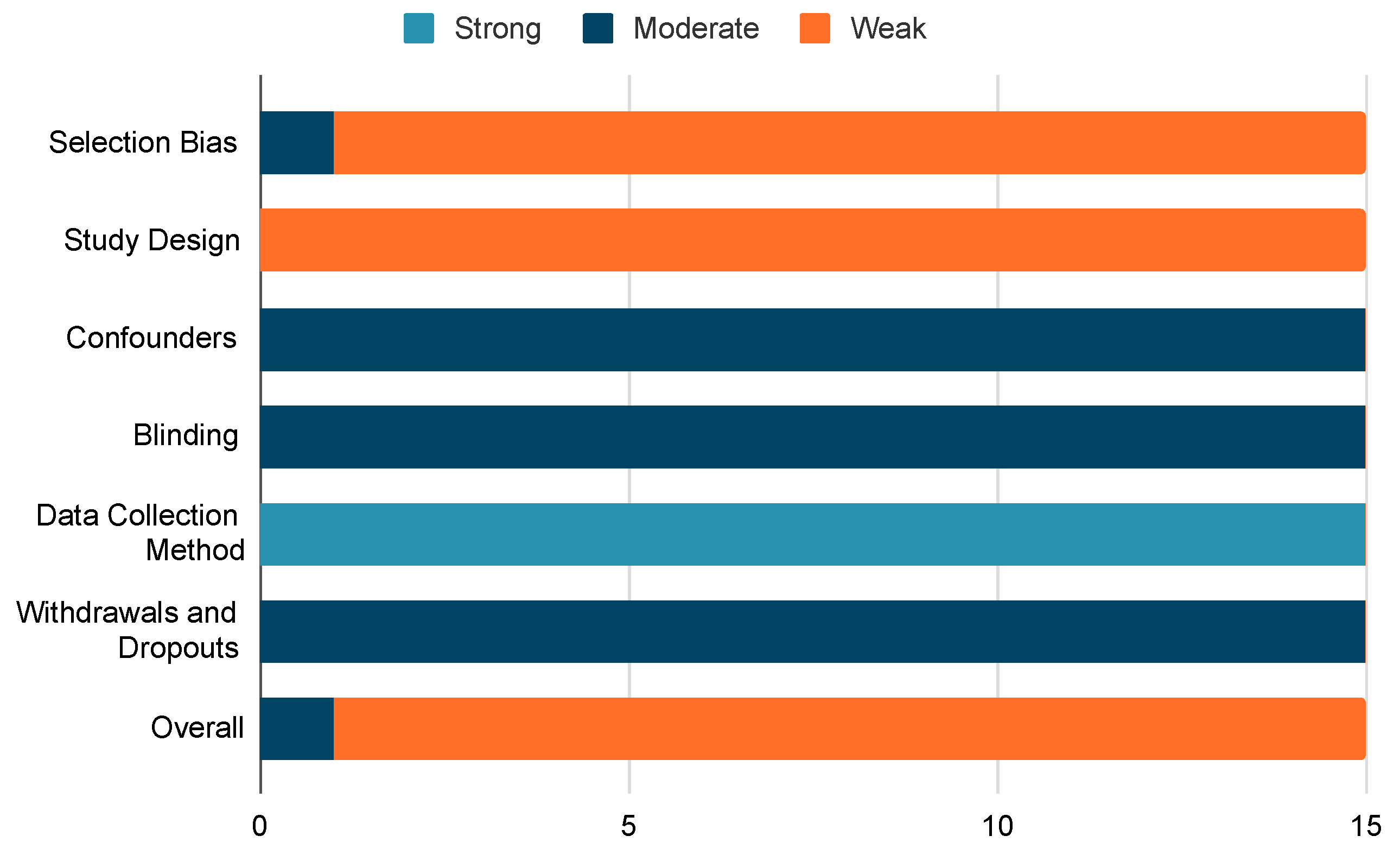


| Keyword | Synonyms | |
|---|---|---|
| #1 | EEG | (electroencephalog* OR EEG OR “brain activity” OR “brain electric? Activity” OR “brain wave*” OR “brainwave*” OR “e.e.g” OR “electr* encephalogram*” OR cEEG OR “EEG-based BCI” OR “EEG-BCI” OR “EEG-based brain-computer interface” OR “EEG-brain computer interface”) |
| #2 | EMG | (electromyogra* OR EMG OR sEMG OR “e.m.g” OR “electr* myogram”) |
| #3 | BCI | (“brain-machine interfac*” OR “brain machine interfac*” OR “brain-computer interfac*” OR “brain computer interface” OR “brain computing interface*” OR “mind-machine interface” OR “mind machine interface” OR “cerebral-computer interfac*” OR “cerebellum-machine interfac*” OR “direct neural interface*” OR BCI OR BCIs OR “neural interface system*” OR “neural-interface system” OR “BCI-controlled neuroprosthetic” OR “human machine interface” OR “human-machine interface” OR HMI OR HMIs OT HCI OR HCIs OR “robotic walking device*” OR “restorative robotic device*”) |
| #4 | ERS/ERD/ERSP | (“ERD/S” OR “ERD/ERS” OR ERD OR “event-related desynchroni$ation” OR “event-related synchroni$ation” OR ERS OR ERSP OR “event-related spectral perturbation” OR “event-related spectral power” OR “event-related slow potential” OR “event related spectral perturbation” OR “event related spectral power” OR “event related slow potential” OR “corticomuscular coherence” OR CMC OR “evoked action potential” OR “evoked discharge” OR “evoked nerve action potential” OR “evoked nerve response” OR “evoked potential” OR “evoked potentials” OR “cortical synchroni$ation*” OR “cortical phase synchroni$ation” OR “cortical desynchroni$ation*” OR “cortical phase desynchroni$ation” OR “cortical phase desynchroni$ation*” OR “phase desynchronization*” OR “event related potential*” OR “event-related potential”) |
| #5 | Stepping | (step* OR walk* OR gait* OR stride OR ambulat* OR stance OR swing OR “toe-off” OR “heel-strike” OR mobili$at*) |
| #6 | Kinematics | (kinematic* OR biomechanic* OR dynamic* OR “motion analys*” OR “movement analys*” OR “ kinesiology” OR “motion tracking system” OR “motion-tracking biomechanical function analysis system” OR “biomechanical phenomena”) |
| #7 | MTP | (“motion trajectory prediction” OR “motion analysis system” OR “motion analysis device” OR “motion capture system” OR “monitor capture device”) |
| #8 | Locomotion | (locomot* OR “motor behavior” OR “motor behaviour”) |
| EEGs | EMGs | EMGs + Data | |
|---|---|---|---|
| COMMENTS |
|
|
|
| Authors | Participants | Protocol | Data | Pre-Processing | Prediction | Accuracy | |||
|---|---|---|---|---|---|---|---|---|---|
| Input | Output | Input | Output | ||||||
| Contreras-Vidal et al., 2018 [27] | 1F/5M Chronic poststroke hemiparesis (H: 160–192 cm, A: 40–68 yrs, W: 62–99 kg) | Walking with exoskeleton Natural speed | EEGs (64 channels, 10–20 system) | Hip, knee, and ankle angles | Artificial subspace reconstruction Peripheral channel removal Detrendation Common average referencing Down-sampling to 100 Hz Butterworth band-pass filter (0.1–3 Hz, 4th) Standardization | Low-pass filter (3 Hz) | 10th order unscented Kalman filter | RMSE 1 | |
| Mercado et al., 2021 [28] | 8F/12M Healthy subjects (A: 21–23) | Step forward, up, and back Natural speed | EEG (19 channels, 10–20 system) | Hip and knee torques | Notch filter at 60 Hz SOBI-RO K-nearest neighbours | Conversion of RGB video to BW | Multi-layer perceptron | RMSE (°): Right hip Left hip Right knee Left knee | 0.0023 0.0018 0.0095 0.0051 |
| Authors | Participants | Protocol | Data | Pre-Processing | Prediction | Accuracy | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Input | Output | Input | Output | |||||||
| Brantley et al., 2017 [31] | 1F/5M Healthy subjects | Walking, stair descent/ascent, ramp descent/ascent Natural speed | EMG of VL, RF, BF, SEM | Ankle and knee angles | Normalization Butterworth band-pass filter (30–350 Hz, 4th) Rectification Butterworth low-pass filter (6 Hz, 4th) | Butterworth low-pass filter (6 Hz, 4th) | Unscented Kalman filter | Pearson’s correlation coefficient: 0.643 | ||
| Chen et al., 2017 [32] | 0F/6M Healthy subjects (H: 170.6 ± 3.6, A: 26 ± 2.2 yrs, W: 62.6 ± 3.7 kg) | Walking Controlled speed | EMG right limb of BF, SEM, VM, VL, RF, SR, MG, LG, TA, SOL | Hip, knee and ankle angles | Notch filter 50 Hz Zero-lag fourth-order recursive Butterworth filter with 20 Hz Full-wave rectification Sub-sampling at 100 Hz Butterworth low-pass filter (4 Hz) | BP Neural network with: DBN and PCA. | RMSE(°) 1: | |||
| DBN | PCA | |||||||||
| Hip | 3.58 ± 0.67 | 6.22 ± 1.67 | ||||||||
| Knee | 3.96 ± 0.69 | 8.11 ± 2.02 | ||||||||
| Ankle | 2.45 ± 0.57 | 4.65 ± 1.32 | ||||||||
| Cheron et al., 2003 [33] | 5F/4M Healthy subjects (A: 35 + 6 yrs) | Walking Natural speed | EMG left limb of RF, VL, BF, TA, GL, SOL | Hip, knee and ankle angles, angular velocity and angular acceleration | Band-pass filter (5–2000 Hz) Full-wave rectification Smoothing with a third-order averaging filter | DRNN | Consult reference | |||
| Gautam et al., 2020 [34] | 0F/11M Healthy subjects | Walking, sitting and standing Natural speed | EMG left limb of VM, SEM, BF, RF | Knee angle | Band-pass filter (20–460 Hz) | Empirical Mode Iterative Algorithm (EIA) | CNN + LSTM | MAE ± SDMAE (%): 8.1 ± 1.2 | ||
| Jia et al., 2021 [35] | 0F/4M Healthy subjects (H: 172.1 ± 5.8 cm, A: 23.6 ± 1.4 yrs, W: 65.2 ± 7.5 kg) | Walking Natural speed | EMG left limb of RF, VL, GM | Knee angle | Full-wave rectification Butterworth low-pass filter (30 Hz, 6th) | Traditional LSTM Traditional RNN Adopted LSTM | RMSE ± RMSE (°) employing: Traditional LSTM 1.5 ± 0.098 Traditional RNN 2.523 ± 0.373 Adopted LSTM 0.464 ± 0.096 Correlation Coefficient 1: Traditional LSTM 0.984 ± 0.00219 Traditional RNN 0.963 ± 0.01223 Adopted LSTM 0.999 ± 0.00001 | |||
| Li et al., 2019 [36] | 0F/6M Healthy subjects (H: 181 ± 3.8 cm, A: 24.2 ± 1.6 yrs, W: 72.5 ± 6.9 kg) | Walking Natural speed | Unilateral EMG of VL, RF, VM, GM, GL | Knee angle | Butterworth band-pass filter (10–500 Hz, 4th) Rectification Low-pass filter (6 Hz, 2nd) Resample 100 Hz | Principal Component with: Backpropagation Random Forest | RMSE (°): Backpropagation 13 Random Forest 5 | |||
| Liu et al., 2019 [37] | 0F/3M Healthy subjects (H: 177.6 ± 2.5 cm, A: 22 ± 1 yrs, W: 70.6 ± 1.9 kg, BMI: 22.5 ± 0.4 kg/cm) | Walking Natural speed | EMG left limb of VL, RF, VM, BF, SEM, MG | Knee angle | Butterworth band-pass filter (20–460 Hz) Butterworth notch filter at 50 Hz Full-wave rectification Normalization | Butterworth low-pass filter (6 Hz, 4th) | BPNN Original data-based CNN Feature-based CNN | RMSE (°): BPNN 9.15 Original data-based CNN 10.57 Feature data-based CNN 5.88 Coefficient of correlation: BPNN 0.96 Original data-based CNN 0.93 Feature data-based CNN 0.98 | ||
| Wang et al., 2015 [38] | 5 Subjects (H: 173 cm, A: 21 yrs, W: 67 kg) | Walking Natural speed | EMG left limb of VL, TA, GM | Knee angle | GA-GRNN | RMSE ± MPE 2(°): 0.6406 ± 0.9331 Coefficient of correlation: 0.9983 | ||||
| Authors | Participants | Protocol | Data | Pre-Processing | Prediction | Accuracy | ||
|---|---|---|---|---|---|---|---|---|
| Input | Output | Input | Output | |||||
| Zhang et al., 2021 [40] | 4F/6M Healthy subjects (H: 175.06 ± 8.45 cm, A: 26 ± 2.86 yrs, W: 70.36 ± 11.49 kg) | Walking Natural and controlled speed | EMG right limb of SOL, TA, GM Ankle joint | Ankle torque | EMG: Band-pass filter (20–500 Hz) Rectification Low-pass filter (6 Hz) Normalisation | Butterworth low-pass filter (6 Hz, 4th order) GRF: Low-pass filter | EMG-driven NMS model ANN model | RMSE (Nm/Kg): Fast walking speed 0.06 ± 0.03 Slow walking speed 0.01 ± 0.01 Self-selected walking speed 0.08 ± 0.06 |
| Chong et al., 2017 [41] | 0F/4M Healthy subjects (H: 177.9 ± 3.18 cm, A: 26.75 ± 4.32 yrs, W: 81.5 ± 8.44 kg) | Walking Natural and controlled speed | EMG of RF, VM, TA, GM, BF, GT, SOL ACC FSR | Knee and hip angles | Boltzmann machine (RBM) | MSE ± STD(MSE) (°): Right knee 0.1768 ± 0.3736 Right hip 0.1444 ± 0.3628 Left knee 0.1680 ± 0.3592 Left hip 0.1756 ± 0.4040 | ||
| Hahn et al., 2008 [42] | 12F/7M Healthy subjects (H: 173 ± 0.08 cm, A: 22.3 ± 1.6 yrs, W: 72 ± 13.3 kg) | Walking Natural speed | EMG of GMAX, GMED, BF, RF, VL, TA, MG Demographics Anthropometrics Joints angles, acceleration, and angular velocity | Hip, knee and ankle moments | EMG: Bandwidth filter (10–1000 Hz) Full-wave rectification Envelopment with a low-pass filter (5 Hz, 4th) Magnitude-normalisation to the maximum value of the trial Joints coordinates: Woltring filter | Three-layer feedforward ANN structure | Coefficient of determination: Hip 0.95 Knee 0.94 Ankle 0.99 | |
| Moreira et al., 2021 [39] | 7F/6M Healthy subjects (H: 168 + 1.2, A: 24.2 + 1.85 yrs, W: 65.2 + 10.3 kg) | Walking Controlled speed | EMG of TA, GAL Ankle angle Angular velocities Angular accelerations Walking speed Body mass, and height Foot and shank length Gender Age | Ankle torque | EMG: Butterworth band-pass filter (20–450 Hz) Enveloping with Root Mean Square Kinematics: Low-pass filter (6 Hz) | LSTM CNN | NRMSE: LSTM 0.48 CNN 0.72 Coefficient of correlation: LSTM 0.73 CNN 0.92 | |
| Zhu et al., 2019 [43] | 0F/5M Healthy subjects (H: 173 ± 0.05, A: 24 ± 1.5 yrs, W: 60.5 ± 4.6 kg) | Walking Natural speed | EMG of BF, VL, GA, SE Thigh angle and shank angle | Knee joint moment | Butterworth band-pass filter (8–500 Hz, 4th) Notch filter 50 Hz Wave rectifier | Elman neural network | NRMSE: 0.116 Coefficient of correlation: 0.979 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amrani El Yaakoubi, N.; McDonald, C.; Lennon, O. Prediction of Gait Kinematics and Kinetics: A Systematic Review of EMG and EEG Signal Use and Their Contribution to Prediction Accuracy. Bioengineering 2023, 10, 1162. https://doi.org/10.3390/bioengineering10101162
Amrani El Yaakoubi N, McDonald C, Lennon O. Prediction of Gait Kinematics and Kinetics: A Systematic Review of EMG and EEG Signal Use and Their Contribution to Prediction Accuracy. Bioengineering. 2023; 10(10):1162. https://doi.org/10.3390/bioengineering10101162
Chicago/Turabian StyleAmrani El Yaakoubi, Nissrin, Caitlin McDonald, and Olive Lennon. 2023. "Prediction of Gait Kinematics and Kinetics: A Systematic Review of EMG and EEG Signal Use and Their Contribution to Prediction Accuracy" Bioengineering 10, no. 10: 1162. https://doi.org/10.3390/bioengineering10101162
APA StyleAmrani El Yaakoubi, N., McDonald, C., & Lennon, O. (2023). Prediction of Gait Kinematics and Kinetics: A Systematic Review of EMG and EEG Signal Use and Their Contribution to Prediction Accuracy. Bioengineering, 10(10), 1162. https://doi.org/10.3390/bioengineering10101162







