Functional Near-Infrared Spectroscopy-Based Evidence of the Cerebral Oxygenation and Network Characteristics of Upper Limb Fatigue
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
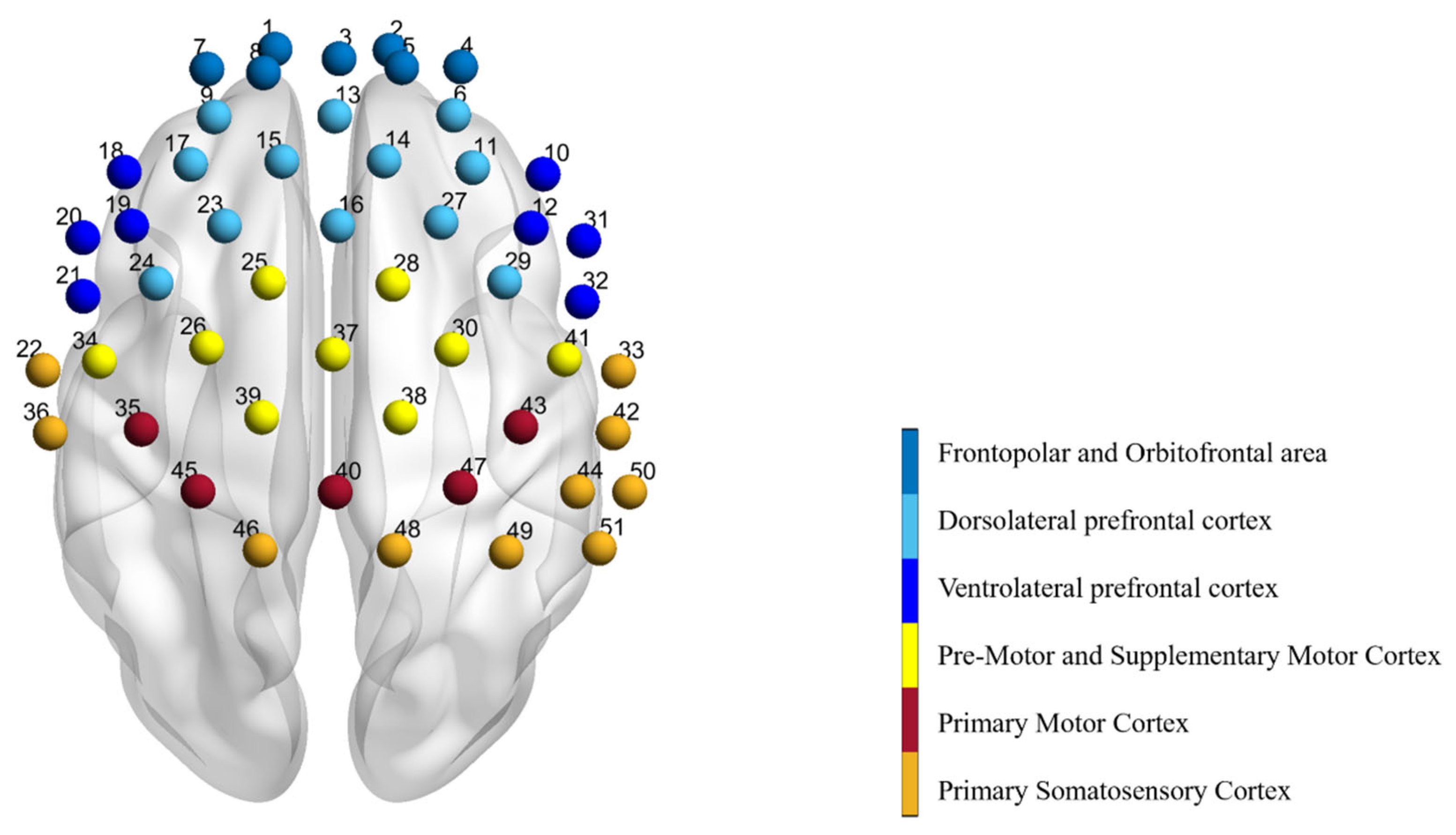
2.3. Functional Near-Infrared Spectroscopy System Acquisition
2.4. Data Pre-Processing
2.5. Functional Connectivity Analysis
2.6. Statistical Analysis
3. Results
3.1. Cerebral Oxygenation Changes
3.2. Functional Connectivity Changes
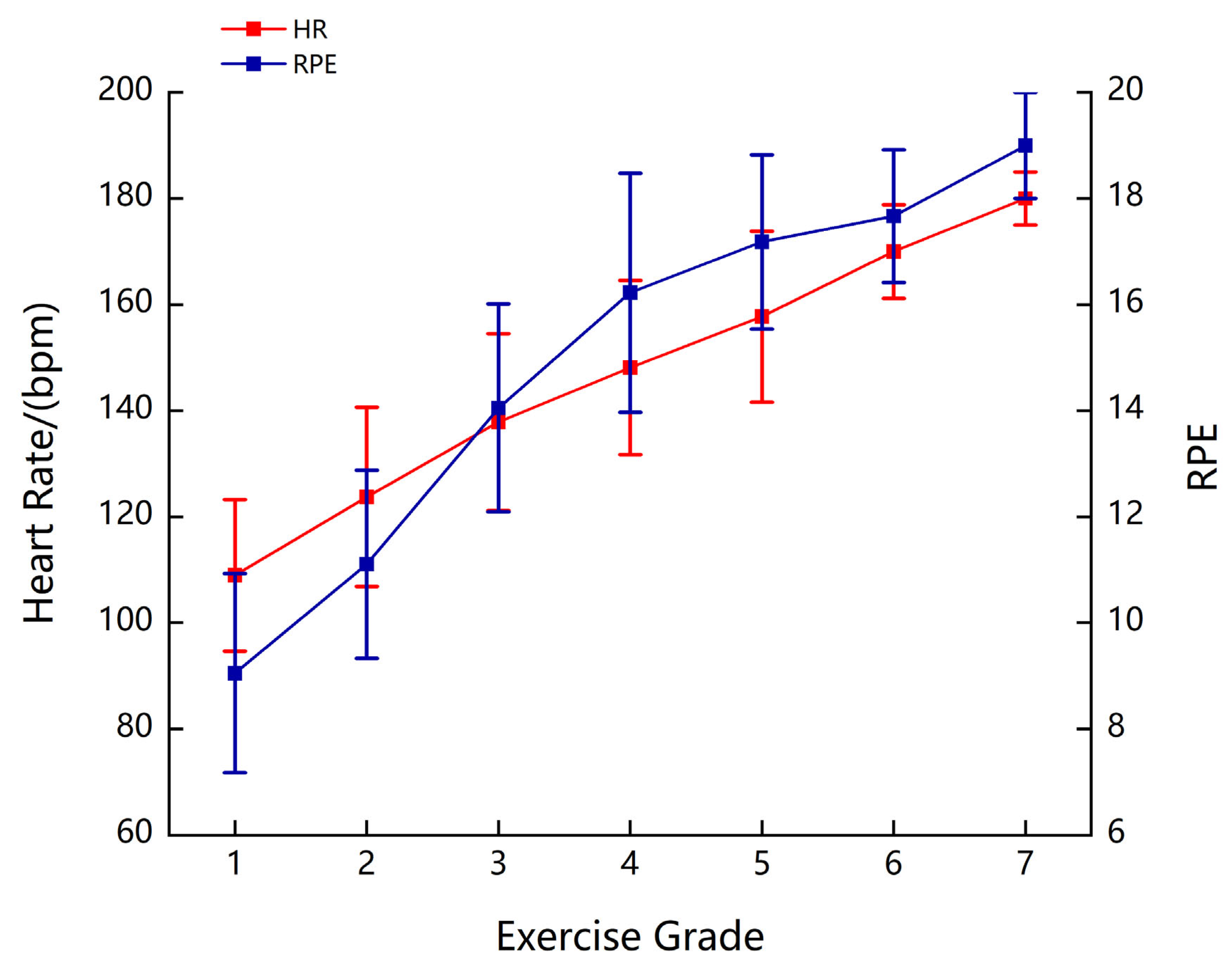
3.3. Physiological Response to Fatigue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abd-Elfattah, H.M.; Abdelazeim, F.H.; Elshennawy, S. Physical and cognitive consequences of fatigue: A review. J. Adv. Res. 2015, 6, 351–358. [Google Scholar] [CrossRef] [PubMed]
- MacIntosh, B.R.; Gardiner, P.F.; McComas, A.J. (Eds.) Skeletal Muscle: Form and Function; Human Kinetics: Leeds, UK; Champaign, IL, USA, 2006. [Google Scholar]
- McKenna, M.J.; Hargreaves, M. Resolving fatigue mechanisms determining exercise performance: Integrative physiology at its finest! J. Appl. Physiol. 2008, 104, 286–287. [Google Scholar] [CrossRef] [PubMed]
- Ament, W.; Verkerke, G.J. Exercise and fatigue. Sports Med. 2009, 39, 389–422. [Google Scholar] [CrossRef]
- Korzeniewski, B. Pi-induced muscle fatigue leads to near-hyperbolic power-duration dependence. Eur. J. Appl. Physiol. 2019, 119, 2201–2213. [Google Scholar] [CrossRef] [PubMed]
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Batson, G. Exercise-induced central fatigue: A review of the literature with implications for dance science research. J. Danc. Med. Sci. Off. Publ. Int. Assoc. Danc. Med. Sci. 2013, 17, 53–62. [Google Scholar] [CrossRef]
- Twomey, R.; Aboodarda, S.J.; Kruger, R.; Culos-Reed, S.N.; Temesi, J.; Millet, G.Y. Neuromuscular fatigue during exercise: Methodological considerations, etiology and potential role in chronic fatigue. Neurophysiol. Clin. Clin. Neurophysiol. 2017, 47, 95–110. [Google Scholar] [CrossRef]
- Douris, P.C.; Handrakis, J.P.; Gendy, J.; Salama, M.; Kwon, D.; Brooks, R.; Salama, N.; Southard, V. Fatiguing upper body aerobic exercise impairs balance. J. Strength Cond. Res. 2011, 25, 3299–3305. [Google Scholar] [CrossRef]
- Wassinger, C.A.; McKinney, H.; Roane, S.; Davenport, M.J.; Owens, B.; Breese, U.; Sokell, G.A. The Influence of Upper Body Fatigue on Dynamic Standing Balance. Int. J. Sports Phys. Ther. 2014, 9, 40–46. [Google Scholar]
- Cogswell, F.D.; Huang, F.; Dietze, B. The Effects of Upper-Body and Lower-Body Fatigue on Standing Balance. West. Undergrad. Res. J. Health Nat. Sci. 2016, 7, 1. [Google Scholar] [CrossRef][Green Version]
- Noakes, T.D.; Gibson, A.S.C. Logical limitations to the “catastrophe” models of fatigue during exercise in humans. Br. J. Sports Med. 2004, 38, 648–649. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Leitkam, S.; Côté, J.N. Effects of different fatigue locations on upper body kinematics and inter-joint coordination in a repetitive pointing task. PLoS ONE 2019, 14, e0227247. [Google Scholar] [CrossRef]
- Vegter, R.J.K.; van den Brink, S.; Mouton, L.J.; Sibeijn-Kuiper, A.; van der Woude, L.H.V.; Jeneson, J.A.L. Magnetic Resonance-Compatible Arm-Crank Ergometry: A New Platform Linking Whole-Body Calorimetry to Upper-Extremity Biomechanics and Arm Muscle Metabolism. Front. Physiol. 2021, 12, 599514. [Google Scholar] [CrossRef] [PubMed]
- Orr, J.L.; Williamson, P.; Anderson, W.; Ross, R.; McCafferty, S.; Fettes, P. Cardiopulmonary exercise testing: Arm crank vs cycle ergometry. Anaesthesia 2013, 68, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Zinner, C.; Matzka, M.; Krumscheid, S.; Holmberg, H.-C.; Sperlich, B. Cardiorespiratory, Metabolic and Perceived Responses to Electrical Stimulation of Upper-Body Muscles While Performing Arm Cycling. J. Hum. Kinet. 2021, 77, 117–123. [Google Scholar] [CrossRef]
- Robinson, N.J.; Montgomery, C.; Swettenham, L.; Whitehead, A. A pilot study investigating cortical haemodynamic and physiological correlates of exercise cognition in trained and untrained cyclists over an incremental self-paced performance test, while thinking aloud. Psychol. Sport Exerc. 2021, 54, 101912. [Google Scholar] [CrossRef]
- Pinti, P.; Tachtsidis, I.; Hamilton, A.; Hirsch, J.; Aichelburg, C.; Gilbert, S.; Burgess, P.W. The present and future use of functional near-infrared spectroscopy (fNIRS) for cognitive neuroscience. Ann. N. Y. Acad. Sci. 2020, 1464, 5–29. [Google Scholar] [CrossRef]
- Frijia, E.M.; Billing, A.; Lloyd-Fox, S.; Rosas, E.V.; Collins-Jones, L.; Crespo-Llado, M.M.; Amadó, M.P.; Austin, T.; Edwards, A.; Dunne, L.; et al. Functional imaging of the developing brain with wearable high-density diffuse optical tomography: A new benchmark for infant neuroimaging outside the scanner environment. NeuroImage 2021, 225, 117490. [Google Scholar] [CrossRef]
- Perrey, S. Non-invasive NIR spectroscopy of human brain function during exercise. Methods 2008, 45, 289–299. [Google Scholar] [CrossRef]
- Perrey, S.; Besson, P. Studying brain activity in sports performance: Contributions and issues. Prog. Brain Res. 2018, 240, 247–267. [Google Scholar]
- Song, Q.; Cheng, X.; Zheng, R.; Yang, J.; Wu, H. Effects of different exercise intensities of race-walking on brain functional connectivity as assessed by functional near-infrared spectroscopy. Front. Hum. Neurosci. 2022, 16, 1002793. [Google Scholar] [CrossRef]
- Takahashi, R.; Fujita, K.; Kobayashi, Y.; Ogawa, T.; Teranishi, M.; Kawamura, M. Effect of muscle fatigue on brain activity in healthy individuals. Brain Res. 2021, 1764, 147469. [Google Scholar] [CrossRef] [PubMed]
- Pires, F.O.; Hammond, J.; Lima-Silva, A.E.; Bertuzzi, R.C.M.; Kiss, M.A.P.D.M. Ventilation behavior during upper-body incremental exercise. J. Strength Cond. Res. 2011, 25, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Borg, G. Perceived exertion as an indicator of somatic stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Bertuzzi, R.; Silva-Cavalcante, M.D.; Couto, P.G.; Azevedo, R.D.A.; Coelho, D.B.; Zagatto, A.; Lima-Silva, A.E.; Millet, G.Y. Prior Upper Body Exercise Impairs 4-km Cycling Time-Trial Performance Without Altering Neuromuscular Function. Res. Q. Exerc. Sport 2021, 92, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Dan, H.; Sakamoto, K.; Takeo, K.; Shimizu, K.; Kohno, S.; Oda, I.; Isobe, S.; Suzuki, T.; Kohyama, K.; et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. NeuroImage 2004, 21, 99–111. [Google Scholar] [CrossRef]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. HomER: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef]
- Gollnick, P.D.; Armstrong, R.B.; Saubert, C.W.; Piehl, K.; Saltin, B. Enzyme activity and fiber composition in skeletal muscle of untrained and trained men. J. Appl. Physiol. 1972, 33, 312–319. [Google Scholar] [CrossRef]
- Johnson, M.A.; Polgar, J.; Weightman, D.; Appleton, D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J. Neurol. Sci. 1973, 18, 111–129. [Google Scholar] [CrossRef]
- Saltin, B.; Henriksson, J.; Nygaard, E.; Andersen, P.; Jansson, E. Fiber types and metabolic potentials of skeletal muscles in sedentary man and endurance runners. Ann. N. Y. Acad. Sci. 1977, 301, 3–29. [Google Scholar] [CrossRef]
- Zinner, C.; Morales-Alamo, D.; Ørtenblad, N.; Larsen, F.J.; Schiffer, T.A.; Willis, S.J.; Gelabert-Rebato, M.; Perez-Valera, M.; Boushel, R.; Calbet, J.A.L.; et al. The Physiological Mechanisms of Performance Enhancement with Sprint Interval Training Differ between the Upper and Lower Extremities in Humans. Front. Physiol. 2016, 7, 426. [Google Scholar] [CrossRef]
- Cochrane-Snyman, K.C.; Housh, T.J.; Smith, C.M.; Hill, E.C.; Jenkins, N.D.M. Treadmill running using an RPE-clamp model: Mediators of perception and implications for exercise prescription. Eur. J. Appl. Physiol. 2019, 119, 2083–2094. [Google Scholar] [CrossRef] [PubMed]
- Jurasz, M.; Boraczyński, M.; Wójcik, Z.; Gronek, P. Neuromuscular Fatigue Responses of Endurance- and Strength-Trained Athletes during Incremental Cycling Exercise. Int. J. Environ. Res. Public Health 2022, 19, 8839. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.B.; Louie, D.R.; Peters, S.; Liu-Ambrose, T.; Boyd, L.A.; Eng, J.J. Brain activity during real-time walking and with walking interventions after stroke: A systematic review. J. Neuroeng. Rehabil. 2021, 18, 8. [Google Scholar] [CrossRef]
- Secher, N.H.; Seifert, T.; van Lieshout, J.J. Cerebral blood flow and metabolism during exercise: Implications for fatigue. J. Appl. Physiol. 2008, 104, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhong, G.; Wu, Y.; Vangel, M.G.; Jiang, B.; Kong, J. Using Granger-Geweke causality model to evaluate the effective connectivity of primary motor cortex (M1), supplementary motor area (SMA) and cerebellum. J. Biomed. Sci. Eng. 2010, 3, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Hao, D.-M.; Li, M.-A. Investigating connectional characteristics of Motor Cortex network. J. Biomed. Sci. Eng. 2009, 2, 30–35. [Google Scholar] [CrossRef][Green Version]
- Zafar, A.; Hong, K.-S. Neuronal Activation Detection Using Vector Phase Analysis with Dual Threshold Circles: A Functional Near-Infrared Spectroscopy Study. Int. J. Neural Syst. 2018, 28, 1850031. [Google Scholar] [CrossRef]
- Chen, W.-L.; Wagner, J.; Heugel, N.; Sugar, J.; Lee, Y.-W.; Conant, L.; Malloy, M.; Heffernan, J.; Quirk, B.; Zinos, A.; et al. Functional Near-Infrared Spectroscopy and Its Clinical Application in the Field of Neuroscience: Advances and Future Directions. Front. Neurosci. 2020, 14, 724. [Google Scholar] [CrossRef]
- Hong, K.-S.; Yaqub, M.A. Application of functional near-infrared spectroscopy in the healthcare industry: A review. J. Innov. Opt. Health Sci. 2019, 12, 1930012. [Google Scholar] [CrossRef]
- Sadler, C.M.; Cressman, E.K. Central fatigue mechanisms are responsible for decreases in hand proprioceptive acuity following shoulder muscle fatigue. Hum. Mov. Sci. 2019, 66, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Peltier, S.J.; LaConte, S.M.; Niyazov, D.M.; Liu, J.Z.; Sahgal, V.; Yue, G.H.; Hu, X.P. Reductions in interhemispheric motor cortex functional connectivity after muscle fatigue. Brain Res. 2005, 1057, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Z.; Shan, Z.Y.; Zhang, L.D.; Sahgal, V.; Brown, R.W.; Yue, G.H. Human brain activation during sustained and intermittent submaximal fatigue muscle contractions: An FMRI study. J. Neurophysiol. 2003, 90, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Chi, P.; Song, J.; Pang, Y.; Wu, Q.; Liu, Y.; Chi, A. Effects of Sleep Deprivation on Functional Connectivity of Brain Regions after High-Intensity Exercise in Adolescents. Sustainability 2022, 14, 16175. [Google Scholar] [CrossRef]
- Hill, M.; Oxford, S.; Duncan, M.; Price, M. Arm-crank training improves postural stability and physical functioning in older people. Exp. Gerontol. 2018, 113, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Kaupp, C.; Pearcey, G.E.P.; Klarner, T.; Sun, Y.; Cullen, H.; Barss, T.S.; Zehr, E.P. Rhythmic arm cycling training improves walking and neurophysiological integrity in chronic stroke: The arms can give legs a helping hand in rehabilitation. J. Neurophysiol. 2018, 119, 1095–1112. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef]
- Laczko, J.; Mravcsik, M.; Katona, P. Control of Cycling Limb Movements: Aspects for Rehabilitation. Adv. Exp. Med. Biol. 2016, 957, 273–289. [Google Scholar]
- Renner, S.W.; Cauley, J.A.; Brown, P.J.; Boudreau, R.M.; Bear, T.M.; Blackwell, T.; Lane, N.E.; Glynn, N.W. Higher Fatigue Prospectively Increases the Risk of Falls in Older Men. Innov. Aging 2021, 5, igaa061. [Google Scholar] [CrossRef]
- Cifrek, M.; Medved, V.; Tonković, S.; Ostojić, S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin. Biomech. 2009, 24, 327–340. [Google Scholar] [CrossRef]
- Emery, K.; Côté, J.N. Repetitive arm motion-induced fatigue affects shoulder but not endpoint position sense. Exp. Brain Res. 2012, 216, 553–564. [Google Scholar] [CrossRef] [PubMed]


| Area | Channel | Pre-Resting (×10−4 mmol/L) | Post-Resting (×10−4 mmol/L) | T | p |
|---|---|---|---|---|---|
| Orbitofrontal area | Ch1 | 0.53 ± 4.57 | 14.24 ± 12.41 | −5.1650 | <0.001 |
| Ch2 | −0.46 ± 3.75 | −14.22 ± 12.97 | −4.9246 | <0.001 | |
| Ch4 | 0.17 ± 3.10 | −5.87 ± 7.40 | −3.1858 | 0.005 | |
| Ch7 | 0.01 ± 3.40 | −8.71 ± 10.83 | −3.1562 | 0.006 | |
| Frontopolar area | Ch3 | −0.08 ± 3.17 | −6.46 ± 9.86 | −2.5924 | 0.019 |
| Ch5 | 0.60 ± 3.21 | −3.74 ± 3.48 | −3.9272 | 0.001 | |
| Ch8 | 0.10 ± 2.97 | −6.62 ± 6.39 | −3.9427 | 0.001 | |
| Dorsolateral prefrontal cortex | Ch6 | 0.16 ± 3.24 | −3.81 ± 5.50 | −2.6980 | 0.015 |
| Ch9 | 0.40 ± 1.92 | −6.89 ± 7.04 | −4.0708 | 0.001 | |
| Ch13 | 0.56 ± 4.31 | −6.05 ± 11.03 | −2.4281 | 0.027 | |
| Ch14 | 0.53 ± 3.35 | −3.30 ± 5.65 | −2.7408 | 0.014 | |
| Ch17 | 3.16 ± 9.84 | −7.24 ± 9.30 | −2.4574 | 0.025 | |
| Ventrolateral prefrontal cortex | Ch18 | 2.13 ± 6.80 | −3.43 ± 7.69 | −2.9275 | 0.009 |
| Ch32 | 11.44 ± 37.49 | −8.60 ± 29.87 | −2.1332 | 0.048 | |
| Includes Frontal eye fields | Ch16 | −0.41 ± 5.05 | −7.16 ± 13.41 | −2.1850 | 0.043 |
| Ch23 | 0.73 ± 3.91 | −5.50 ± 9.19 | −3.6556 | 0.002 | |
| Pre-Motor and Supplementary Motor Cortex | Ch26 | 2.08 ± 4.22 | −4.52 ± 6.43 | −3.7644 | 0.002 |
| Ch28 | 0.29 ± 8.68 | −4.59 ± 7.64 | −2.6413 | 0.017 | |
| Ch39 | 1.28 ± 4.93 | −3.42 ± 7.13 | −2.1521 | 0.046 | |
| Ch41 | 0.76 ± 5.94 | −3.33 ± 6.66 | −3.1625 | 0.006 | |
| Primary Motor Cortex | Ch40 | 0.56 ± 3.34 | −2.07 ± 6.22 | −2.3963 | 0.028 |
| Primary Somatosensory Cortex | Ch46 | 1.01 ± 3.65 | −1.98 ± 4.19 | −2.2490 | 0.038 |
| Ch50 | 0.17 ± 4.11 | −3.32 ± 4.03 | −2.4860 | 0.024 |
| Region-Region | T-Value | p-Value | |
|---|---|---|---|
| PM&SMA_L | VLPFC_L | −4.221 | <0.001 |
| PM&SMA_L | DLPFC_L | −4.27 | <0.001 |
| PM&SMA_L | DLPFC_R | −4.3057 | <0.001 |
| PM&SMA_L | VLPFC_R | −4.3882 | <0.001 |
| PM&SMA_L | VLPFC_R | −4.2824 | <0.001 |
| PM&SMA_L | SAC_R | −4.3782 | <0.001 |
| PM&SMA_L | DLPFC_M | −3.9482 | <0.001 |
| PM&SMA_R | OFA_L | −5.8336 | <0.001 |
| PM&SMA_R | FPA_L | −3.9229 | <0.001 |
| PM&SMA_R | FPA_L | −4.9266 | <0.001 |
| PM&SMA_R | DLPFC_L | −4.549 | <0.001 |
| PM&SMA_R | DLPFC_L | −4.3457 | <0.001 |
| PM&SMA_R | S1_L | −4.0253 | <0.001 |
| PM&SMA_R | DLPFC_R | −4.1395 | <0.001 |
| PM&SMA_R | VLPFC_R | −4.2891 | <0.001 |
| PM&SMA_R | VLPFC_R | −4.5104 | <0.001 |
| PM&SMA_R | S1_R | −3.9672 | <0.001 |
| M1_L | DLPFC_M | −3.9406 | <0.001 |
| M1_R | OFA_L | −5.3158 | <0.001 |
| M1_R | FPA_L | −4.3207 | <0.001 |
| M1_R | DLPFC_L | −5.3984 | <0.001 |
| M1_R | OFA_R | −3.9362 | <0.001 |
| M1_R | DLPFC_R | −4.1346 | <0.001 |
| M1_R | VLPFC_R | −5.4862 | <0.001 |
| M1_R | VLPFC_R | −4.7553 | <0.001 |
| M1_R | DLPFC_M | −4.524 | <0.001 |
| M1_M | OFA_L | −4.5653 | <0.001 |
| M1_M | DLPFC_L | −3.9048 | <0.001 |
| M1_M | DLPFC_R | −3.9669 | <0.001 |
| M1_M | VLPFC_R | −5.1262 | <0.001 |
| M1_M | VLPFC_R | −5.0606 | <0.001 |
| DLPFC_L | DLPFC_L | −4.267 | <0.001 |
| DLPFC_L | VLPFC_R | −4.8204 | <0.001 |
| DLPFC_M | FPA_R | −4.2324 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Bi, J.; Liang, Z.; Li, L.; Liu, Y.; Huang, L. Functional Near-Infrared Spectroscopy-Based Evidence of the Cerebral Oxygenation and Network Characteristics of Upper Limb Fatigue. Bioengineering 2023, 10, 1112. https://doi.org/10.3390/bioengineering10101112
Li F, Bi J, Liang Z, Li L, Liu Y, Huang L. Functional Near-Infrared Spectroscopy-Based Evidence of the Cerebral Oxygenation and Network Characteristics of Upper Limb Fatigue. Bioengineering. 2023; 10(10):1112. https://doi.org/10.3390/bioengineering10101112
Chicago/Turabian StyleLi, Feng, Jiawei Bi, Zhiqiang Liang, Lu Li, Yu Liu, and Lingyan Huang. 2023. "Functional Near-Infrared Spectroscopy-Based Evidence of the Cerebral Oxygenation and Network Characteristics of Upper Limb Fatigue" Bioengineering 10, no. 10: 1112. https://doi.org/10.3390/bioengineering10101112
APA StyleLi, F., Bi, J., Liang, Z., Li, L., Liu, Y., & Huang, L. (2023). Functional Near-Infrared Spectroscopy-Based Evidence of the Cerebral Oxygenation and Network Characteristics of Upper Limb Fatigue. Bioengineering, 10(10), 1112. https://doi.org/10.3390/bioengineering10101112






