Double Filtration Plasmapheresis with Polyvinyl Alcohol-Based Membrane Lowers Serum Inflammation and Toxins in Patients with Hyperlipidemia
Abstract
1. Introduction
1.1. Persistent Organic Pollutants: Perfluorochemicals (PFCs)
1.2. Double Filtration (DF)
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
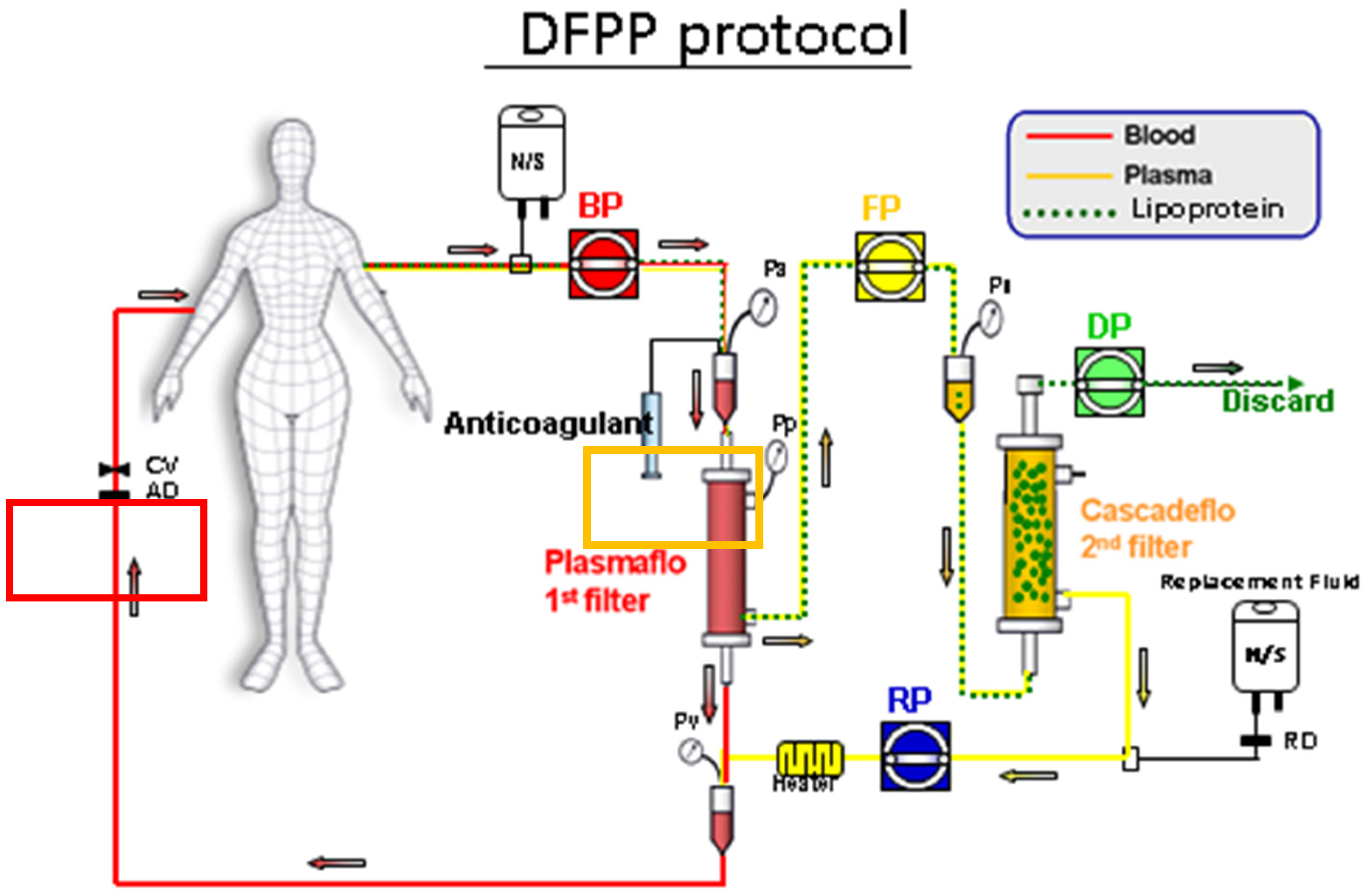
2.2. Double Filtration (DF) Protocol
2.3. Statistical Analysis
3. Results
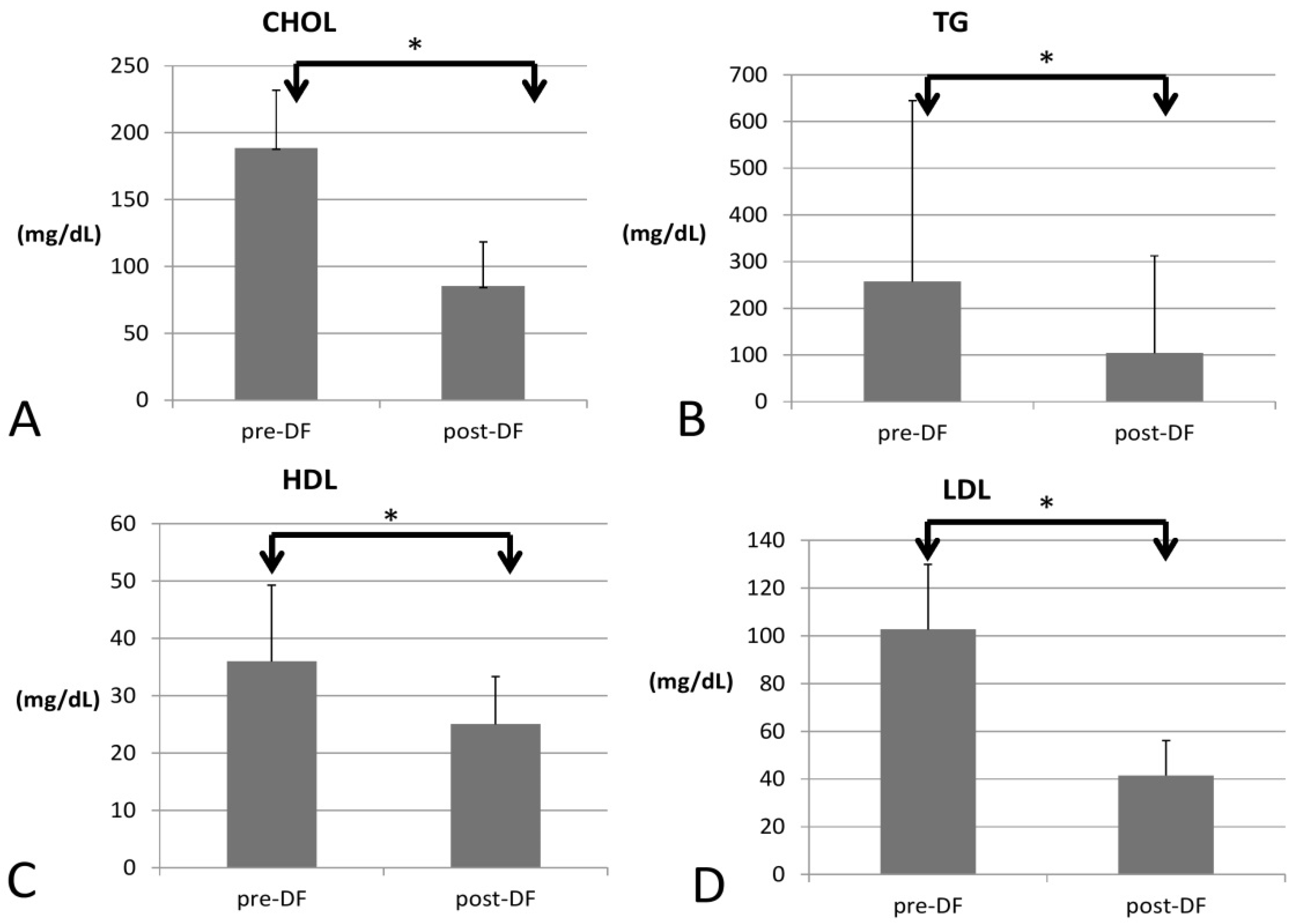
3.1. DF Effect on Lipid Profile
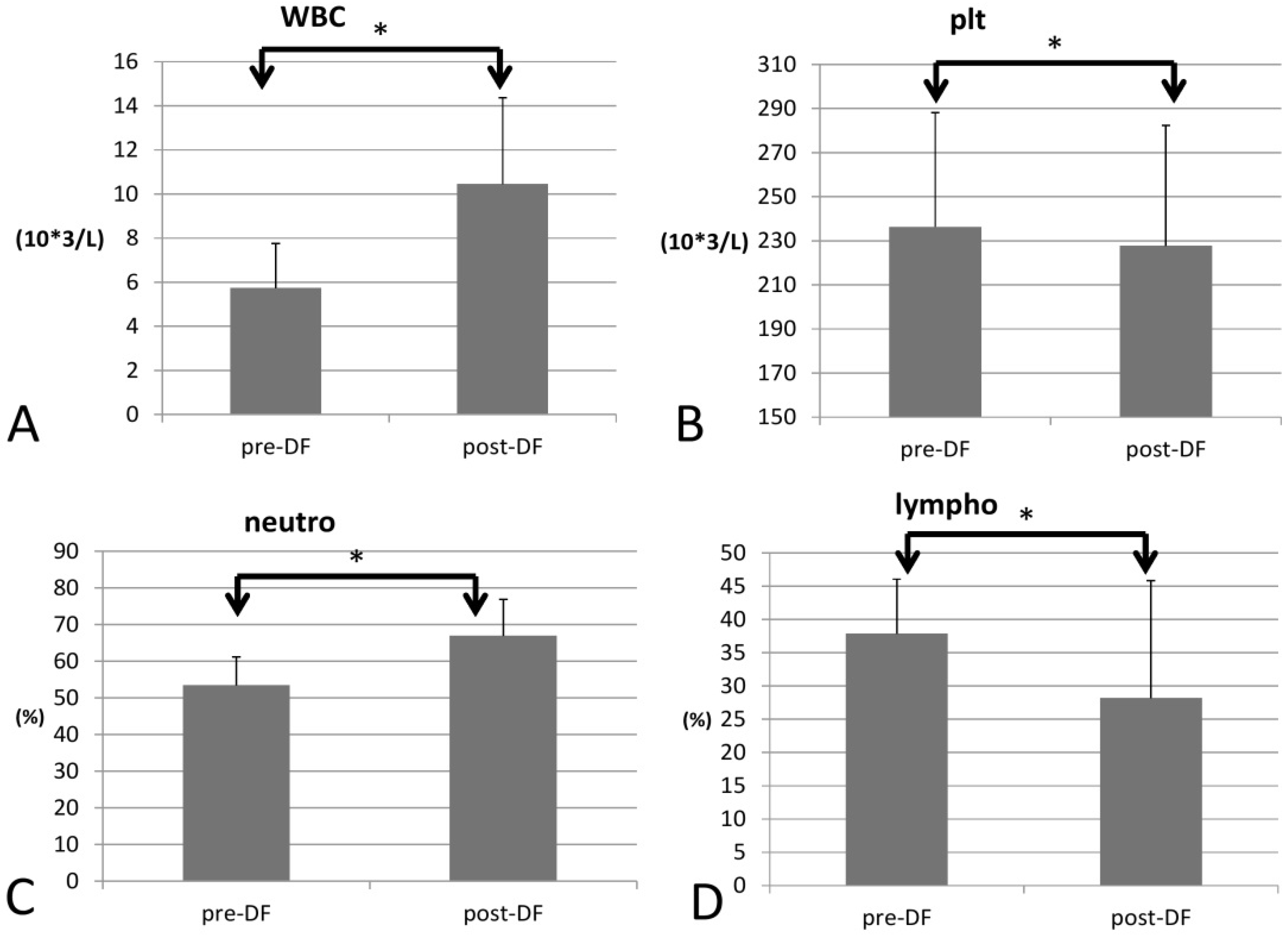
3.2. DF Effect on Complete Blood Count (CBC)
3.3. DF Effect on Liver Function, Uric Acid, Inflammation, and PFOS
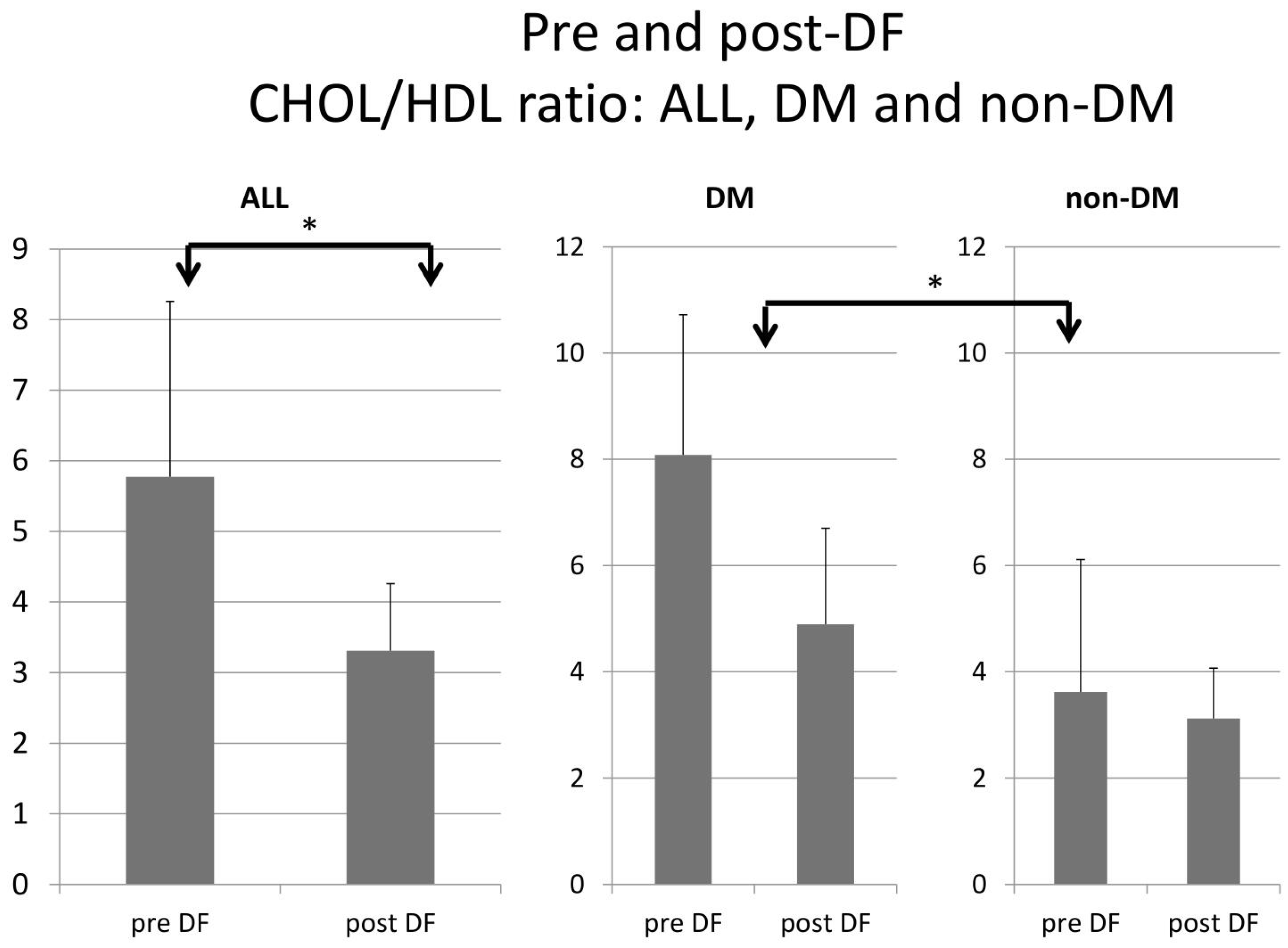
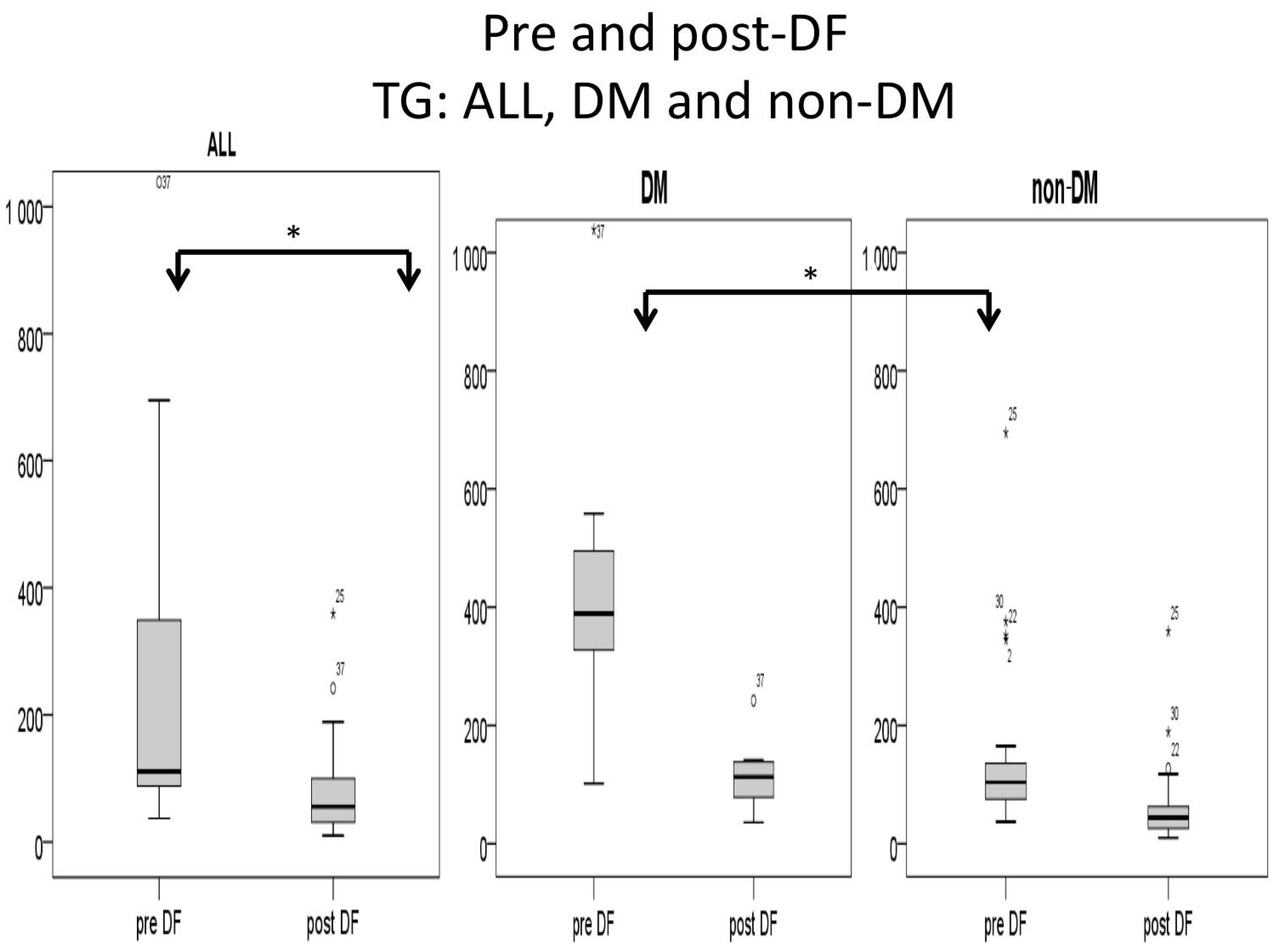
3.4. Comparison of DF Effect on DM and Non-DM Patients
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix A.1. Measurement of PFOA and PFOS by Isotope Dilution High Performance Liquid Chromatography Coupled with Mass Spectrometry (IDLCMS)
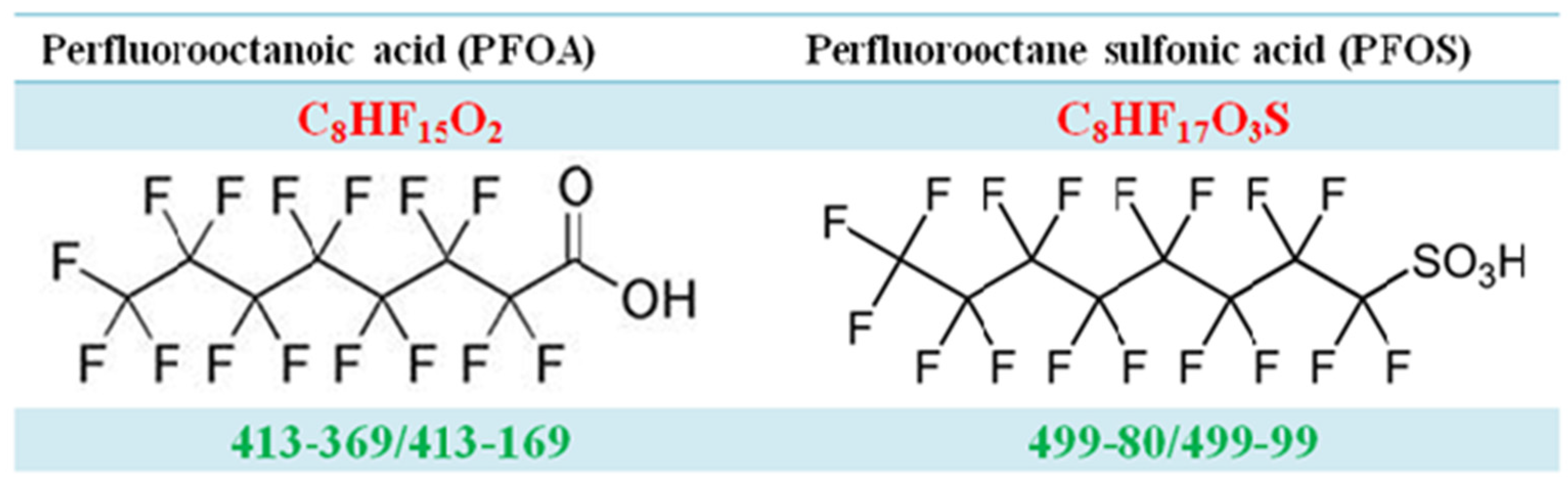
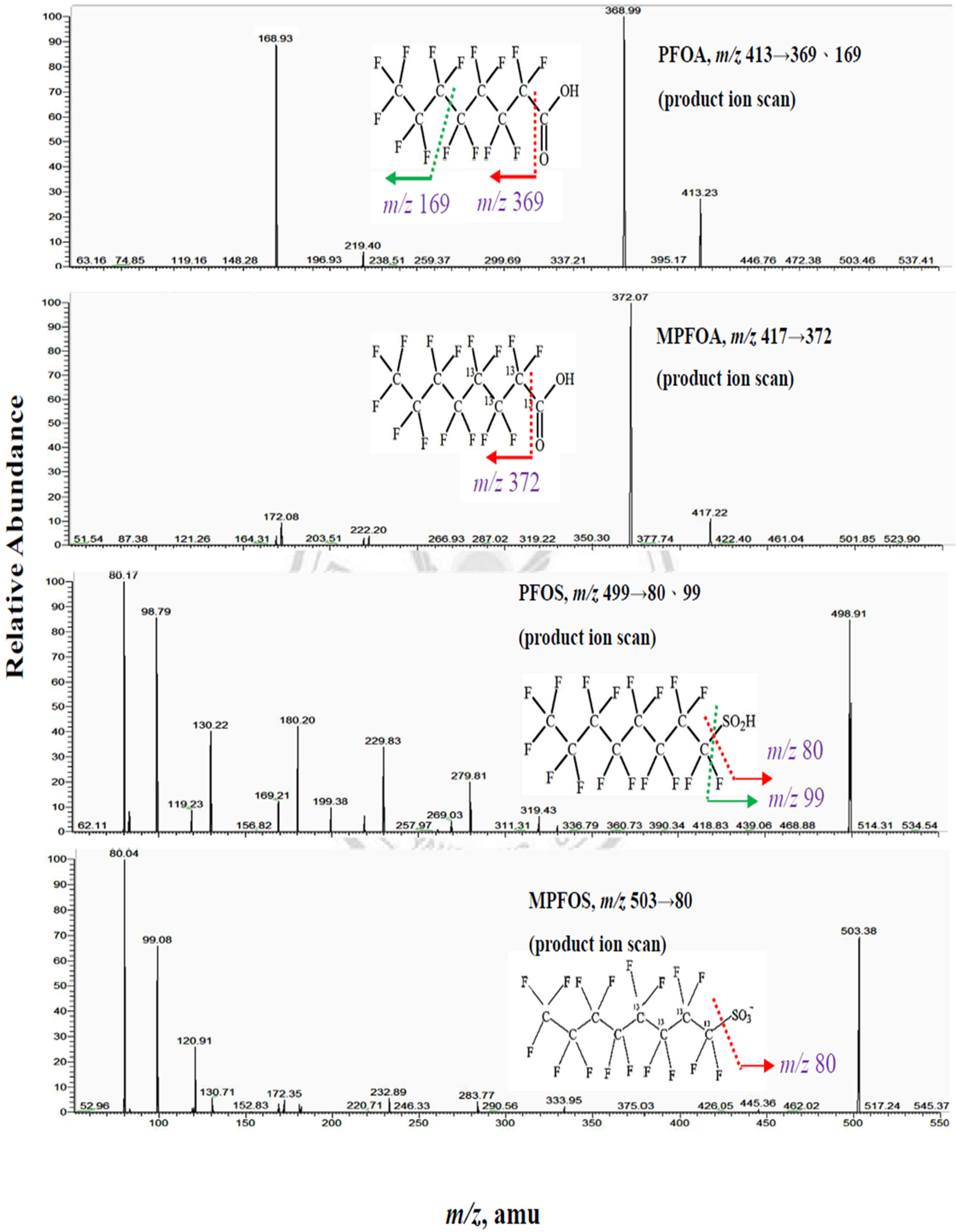
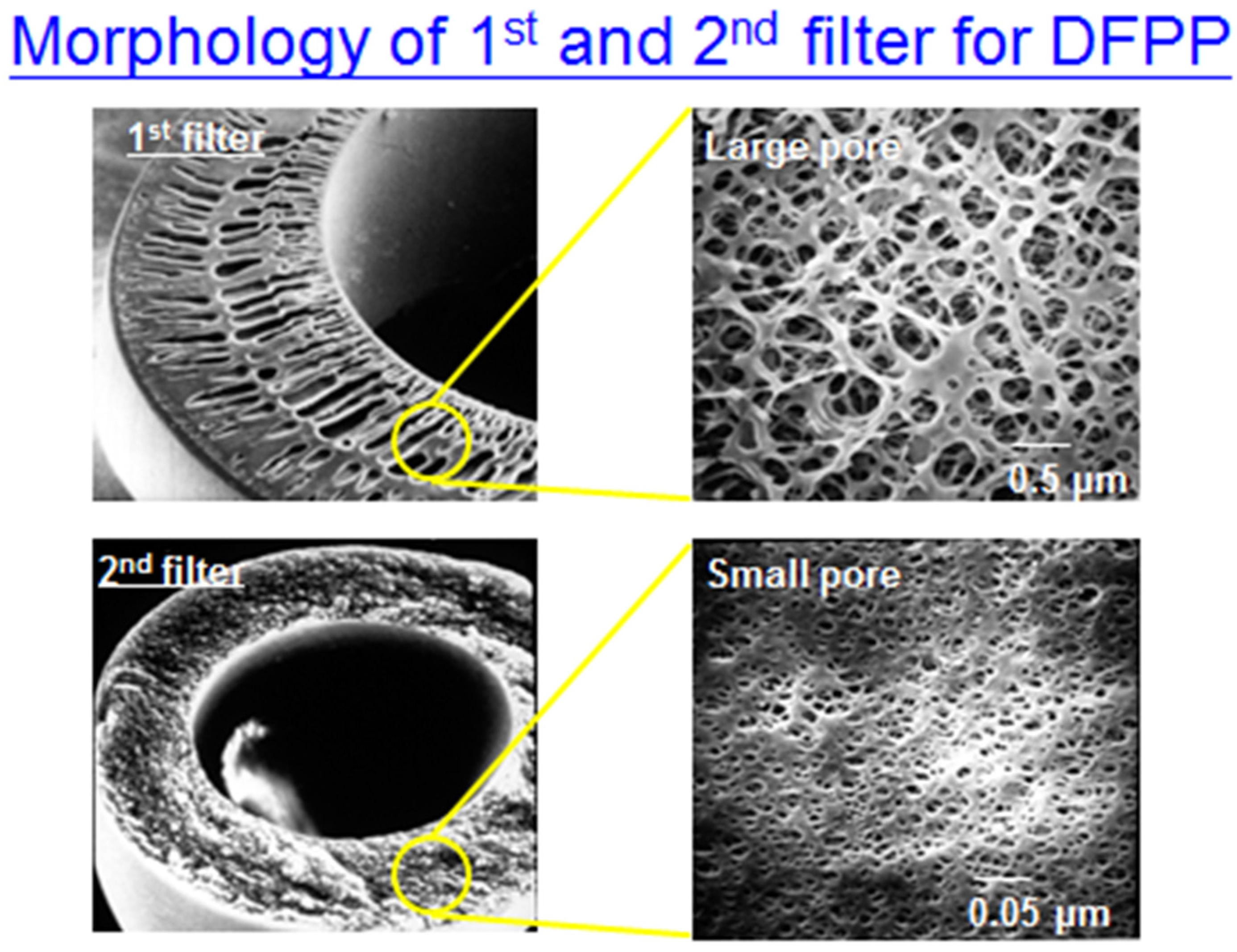
Appendix A.2. Process for Double Filtration Plasmaphresis (DFPP), Which Means the Same as Double Filtration (DF) in Our Manuscript (Copyright: Asahi Kasei Medical, Tokyo, Japan)
References
- Gotto, A.M., Jr. Treatment of hyperlipidemia. Am. J. Cardiol. 1986, 57, G11–G16. [Google Scholar] [CrossRef]
- Fernandez-Fuertes, L.F.; Tapia Martin, M.; Nieves Pla, I.; Novoa Mogollon, F.J.; Diaz Cremades, J. Low-density lipoprotein apheresis using double filtration plasmapheresis: 27-month use in a child with homozygous familial hypercholesterolemia. Ther. Apher. Dial. 2010, 14, 484–485. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.L.; Wu, M.J.; Shu, K.H.; Tsai, S.F. Long-Term Follow-Up of a Homozygous Familial Hypercholesterolemic Patient Receiving Regular Double Filtration Plasmapheresis-Case Report and Literature Review. Blood Purif. 2016, 41, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Navarese, E.P.; Szczesniak, A.; Kolodziejczak, M.; Gorny, B.; Kubica, J.; Suryapranata, H. Statins and risk of new-onset diabetes mellitus: Is there a rationale for individualized statin therapy? Am. J. Cardiovasc. Drugs 2014, 14, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Brinton, E.A. Does the addition of fibrates to statin therapy have a favorable risk to benefit ratio? Curr. Atheroscler. Rep. 2008, 10, 25–32. [Google Scholar] [CrossRef]
- Wanner, C.; Schmidt, K.R.; Krane, V. Results of the 4D study: Ten years of follow-up? Clin. Exp. Nephrol. 2014, 18, 274–277. [Google Scholar] [CrossRef]
- Fellström, B.C.; Jardine, A.G.; Schmieder, R.E.; Holdaas, H.; Bannister, K.; Beutler, J.; Chae, D.W.; Chevaile, A.; Cobbe, S.M.; Grönhagen-Riska, C.; et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009, 360, 1395–1407. [Google Scholar] [CrossRef]
- Palmer, S.C.; Navaneethan, S.D.; Craig, J.C.; Johnson, D.W.; Perkovic, V.; Nigwekar, S.U.; Hegbrant, J.; Strippoli, G.F. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane. Database Syst. Rev. 2013, 12, CD004289. [Google Scholar] [CrossRef]
- Buemi, M.; Floccari, F.; Nostro, L.; Campo, S.; Caccamo, C.; Sturiale, A.; Aloisi, C.; Giacobbe, M.S.; Frisina, N. Statins in the prevention of cardiovascular events in patients with renal failure. Cardiovasc. Hematol. Disord. Drug Targets 2007, 7, 7–13. [Google Scholar]
- Demetriou, K.; H’Maltezou, E.; Pierides, A.M. Familial homozygous hypercholesterolemia: Effective long-term treatment with cascade double filtration plasmapheresis. Blood Purif. 2001, 19, 308–313. [Google Scholar] [CrossRef]
- Song, S.; Zhang, Y.; Qiao, X.; Duo, Y.; Xu, J.; Peng, Z.; Zhang, J.; Chen, Y.; Nie, X.; Sun, Q.; et al. ALT/AST as an Independent Risk Factor of Gestational Diabetes Mellitus Compared with TG/HDL-C. Int. J. Gen. Med. 2022, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.S.; Lai, Y.T.; Chan, H.L.; Li, S.Y.; Lin, C.C.; Liu, C.K.; Tsou, H.H.; Liu, T.Y. Associations between perfluorinated chemicals and serum biochemical markers and performance status in uremic patients under hemodialysis. PLoS ONE 2018, 13, e0200271. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.A.; Arbuckle, T.E.; Lye, E.; Legrand, M.; Fisher, M.; Langlois, R.; Fraser, W. Reporting results of human biomonitoring of environmental chemicals to study participants: A comparison of approaches followed in two Canadian studies. J. Epidemiol. Community Health 2011, 65, 191–198. [Google Scholar] [CrossRef]
- Fromme, H.; Tittlemier, S.A.; Völkel, W.; Wilhelm, M.; Twardella, D. Perfluorinated compounds--exposure assessment for the general population in Western countries. Int. J. Hyg. Environ. Health 2009, 212, 239–270. [Google Scholar] [CrossRef] [PubMed]
- Powley, C.R.; Michalczyk, M.J.; Kaiser, M.A.; Buxton, L.W. Determination of perfluorooctanoic acid (PFOA) extractable from the surface of commercial cookware under simulated cooking conditions by LC/MS/MS. Analyst 2005, 130, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.W.; Mabury, S.A.; Solomon, K.R.; Muir, D.C. Dietary accumulation of perfluorinated acids in juvenile rainbow trout (Oncorhynchus mykiss). Environ. Toxicol. Chem. 2003, 22, 189–195. [Google Scholar] [CrossRef]
- Renner, R. Growing concern over perfluorinated chemicals. Environ. Sci. Technol. 2001, 35, 154A–160A. [Google Scholar] [CrossRef]
- Frisbee, S.J.; Shankar, A.; Knox, S.S.; Steenland, K.; Savitz, D.A.; Fletcher, T.; Ducatman, A.M. Perfluorooctanoic acid, perfluorooctanesulfonate, and serum lipids in children and adolescents: Results from the C8 Health Project. Arch. Pediatr. Adolesc. Med. 2010, 164, 860–869. [Google Scholar] [CrossRef]
- Olsen, G.W.; Burris, J.M.; Ehresman, D.J.; Froehlich, J.W.; Seacat, A.M.; Butenhoff, J.L.; Zobel, L.R. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ. Health Perspect. 2007, 115, 1298–1305. [Google Scholar] [CrossRef]
- Liu, W.S.; Chan, H.L.; Lai, Y.T.; Lin, C.C.; Li, S.Y.; Liu, C.K.; Tsou, H.H.; Liu, T.Y. Dialysis Membranes Influence Perfluorochemical Concentrations and Liver Function in Patients on Hemodialysis. Int. J. Environ. Res. Public Health 2018, 15, 2574. [Google Scholar] [CrossRef]
- Naciri Bennani, H.; Marlu, R.; Terrec, F.; Motte, L.; Seyve, L.; Chevallier, E.; Malvezzi, P.; Jouve, T.; Rostaing, L.; Noble, J. How to improve clotting factors depletion in double-filtration plasmapheresis. J. Clin. Apher. 2021, 36, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Min, Y.; Qi, B.; Zhu, C.M.; Chen, J.H.; Deng, G.X.; Wang, Y.; Li, J.F.; Li, R.S. Causal effect between total cholesterol and HDL cholesterol as risk factors for chronic kidney disease: A mendelian randomization study. BMC Nephrol. 2021, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Teng, H.W.; Lai, Y.T.; Li, S.Y.; Lin, C.C.; Yang, A.C.; Chan, H.L.; Hsieh, Y.H.; Lin, C.F.; Hsu, F.Y.; et al. Statins Reduces the Risk of Dementia in Patients with Late-Onset Depression: A Retrospective Cohort Study. PLoS ONE 2015, 10, e0137914. [Google Scholar] [CrossRef]
- Steenland, K.; Tinker, S.; Shankar, A.; Ducatman, A. Association of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) with uric acid among adults with elevated community exposure to PFOA. Environ. Health Perspect. 2010, 118, 229–233. [Google Scholar] [CrossRef]
- Zarkovic, M.; Kwaan, H.C. Correction of hyperviscosity by apheresis. Semin. Thromb. Hemost. 2003, 29, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.L.; Hollingsworth, K.G.; Aribisala, B.S.; Chen, M.J.; Mathers, J.C.; Taylor, R. Reversal of type 2 diabetes: Normalisation of beta cell function in association with decreased pancreas and liver triacylglycerol. Diabetologia 2011, 54, 2506–2514. [Google Scholar] [CrossRef]
- Sidhu, D.; Snyder, E.L.; Tormey, C.A. Two approaches to the clinical dilemma of treating TTP with therapeutic plasma exchange in patients with a history of anaphylactic reactions to plasma. J. Clin. Apher. 2017, 32, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Kuragano, T.; Inoue, T.; Yoh, K.; Shin, J.; Fujita, Y.; Yoshiya, K.; Kim, J.I.; Sakai, R.; Sekita, K.; Goto, T.; et al. Effectiveness of beta(2)-microglobulin adsorption column in treating dialysis-related amyloidosis: A multicenter study. Blood Purif. 2011, 32, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Uchita, K.; Orita, H.; Kamimura, M.; Oda, M.; Hasegawa, H.; Kobata, H.; Fukunishi, M.; Shimazaki, M.; Akizawa, T.; et al. Effect of beta(2)-microglobulin adsorption column on dialysis-related amyloidosis. Kidney Int. 2003, 64, 1522–1528. [Google Scholar] [CrossRef]


| Characteristic | All (n = 45) | DM = 14 | Non-DM = 31 | p-Value |
|---|---|---|---|---|
| Male:Female | 23:22 | 6:8 | 17:14 | 0.53 |
| Age (years) | 54.48 ± 10.57 | 57.64 ± 10.66 | 53.06 ± 10.38 | 0.182 |
| Height (cm) | 165.24 ± 7.85 | 167.50 ± 6.81 | 164.22 ± 8.18 | 0.199 |
| Body weight (kg) | 72.80 ± 15.94 | 79.64 ± 14.67 | 69.71 ± 15.75 | 0.05 |
| Body mass index (BMI) | 26.44 ± 4.29 | 28.20 ± 3.14 | 25.65 ± 4.54 | 0.037 * |
| n = 45 Male = 23 | before DF (mean ± SD) | after DF (Mean ± SD) | p-Value | Linear Regression of Δ Value to Δ PFOS (R/p) |
|---|---|---|---|---|
| WBC 103/L | 5.73 ± 2.03 | 10.46 ± 3.91 | <0.001 * | −0.289 (0.033) * |
| RBC 106/L | 4.58 ± 0.67 | 5.18 ± 0.77 | <0.001 * | −1.286 (0.606) |
| Hb (g/dL) | 13.61 ± 1.96 | 15.39 ± 2.26 | <0.001 * | −0.690 (0.476) |
| HCT (%) | 41.76 ± 5.82 | 46.02 ± 6.07 | <0.001 * | 0.064 (0.858) |
| MCV (fl) | 91.93 ± 6.03 | 90.31 ± 5.13 | 0.005 * | 0.363 (0.331) |
| MCH (pg) | 29.81 ± 1.66 | 29.95 ± 1.59 | 0.083 | −2.680 (0.208) |
| Platelets 103/L | 236.30 ± 51.79 | 227.78 ± 54.55 | 0.022 * | −0.025 (0.104) |
| Neutrophils (%) | 53.40 ± 7.77 | 66.93 ± 9.92 | <0.001 * | −0.015 (0.823) |
| Lymphocytes (%) | 37.87 ± 8.19 | 28.20 ± 17.63 | 0.001 * | 0.005 (0.939) |
| Monocytes (%) | 5.56 ± 1.79 | 4.60 ± 1.28 | 0.006 * | 0.048 (0.874) |
| Eosinophils (%) | 2.51 ± 1.45 | 2.12 ± 1.28 | <0.001 * | 0.057 (0.879) |
| Basophils (%) | 0.65 ± 0.35 | 0.52 ± 0.27 | 0.004 * | 0.251 (0.869) |
| Cr (mg/dL) | 0.87 ± 0.70 | 0.81 ± 0.71 | 0.005 * | −7.152 (0.067) |
| eGFR (mL/min) | 102.45 ± 30.86 | 115.12 ± 45.05 | 0.033 * | 0.036 (0.415) |
| Uric acid (mg/dL) | 6.19 ± 1.30 | 5.97 ± 1.35 | 0.032 * | 2.277 (0.482) |
| Glucose (mg/dL) | 118.63 ± 53.83 | 137.67 ± 101.70 | 0.190 | 0.003 (0.973) |
| HbA1c (%) | 6.25 ± 1.52 | 6.25 ± 1.55 | 1 | 9.047 (0.302) |
| Insulin (mU/L) | 13.63 ± 9.24 | 13.50 ± 11.73 | 0.975 | 0.087 (0.193) |
| AST (U/L) | 26.00 ± 15.56 | 25.70 ± 15.23 | 0.755 | −0.041 (0.772) |
| ALT (U/L) | 30.14 ± 20.81 | 26.70 ± 17.91 | <0.001 * | −0.126 (0.280) |
| CHOL (mg/dL) | 188.60 ± 43.12 | 85.23 ± 33.01 | <0.001 * | 0.025 (0.098) |
| TG (mg/dL) median, IQR for TG | 257.5 ± 387.47 111 (87.25–349.75) + | 104.25 ± 208.06 56 (31–100) + | <0.001 * | 0.006 (0.307) |
| HDL (mg/dL) | 35.96 ± 13.29 | 25.03 ± 8.29 | <0.001 * | −0.064 (0.224) |
| LDL (mg/dL) | 102.67 ± 27.28 | 41.48 ± 14.62 | <0.001 * | 0.036 (0.175) |
| CHOL/HDL ratio | 5.77 ± 2.49 | 3.31 ± 0.95 | <0.001 * | 0.479 (0.121) |
| hsCRP (mg/dL) | 0.28 ± 0.38 | 0.12 ± 0.18 | <0.001 * | 4.301 (0.419) |
| PFOA (ng/mL) | 3.70 ± 2.54 | 3.87 ± 2.78 | 0.847 | 18.951 (0.008) * |
| PFOS (ng/mL) | 2.77 ± 2.09 | 1.58 ± 1.22 | 0.017 * | - |
| n = 45 (Mean ± SD) | before DF: DM pt | before DF: Non-DM pt | p-Value | after DF: DM pt | after DF: Non-DM pt | p-Value |
|---|---|---|---|---|---|---|
| WBC 103/L | 5.82 ± 1.39 | 5.71 ± 2.23 | 0.888 | 10.86 ± 1.43 | 10.34 ± 4.56 | 0.713 |
| RBC 106/L | 4.92 ± 0.17 | 4.41 ± 0.71 | 0.001 * | 5.63 ± 0.19 | 4.94 ± 0.83 | 0.010 |
| Hb (g/dL) | 15.04 ± 0.71 | 12.97 ± 1.99 | <0.001 * | 17.25 ± 0.85 | 14.66 ± 2.26 | 0.001 |
| HCT (%) | 46.53 ± 2.94 | 39.88 ± 5.67 | <0.001 * | 50.76 ± 2.29 | 44.49 ± 6.27 | <0.001 * |
| MCV (fl) | 94.48 ± 5.40 | 90.93 ± 6.09 | 0.096 | 90.12 ± 2.72 | 90.39 ± 5.81 | 0.887 |
| MCH (pg) | 30.58 ± 1.17 | 29.50 ± 1.74 | 0.065 | 30.61 ± 0.96 | 29.71 ± 1.74 | 0.111 |
| Platelets 103/L | 217.36 ± 44.46 | 245.06 ± 54.17 | 0.136 | 211.54 ± 56.55 | 233.73 ± 54.47 | 0.260 |
| Neutrophils (%) | 55.93 ± 5.64 | 52.62 ± 8.33 | 0.231 | 67.15 ± 3.98 | 66.85 ± 11.55 | 0.901 |
| Lymphocytes (%) | 35.61 ± 6.06 | 28.50 ± 8.79 | 0.311 | 26.39 ± 3.27 | 25.63 ± 10.20 | 0.722 |
| Monocytes (%) | 5.98 ± 1. 93 | 5.37 ± 1.76 | 0.341 | 4.15 ± 6. 83 | 4.76 ± 1.42 | 0.187 |
| Eosinophils (%) | 1.90 ± 1.12 | 2.72 ± 1.50 | 0.105 | 0.50 ± 0.20 | 0.52 ± 0.29 | 0.238 |
| Basophils (%) | 0.56 ± 0.39 | 0.70 ± 0.34 | 0.279 | 0.56 ± 0.39 | 0.70 ± 0.34 | 0.782 |
| Cr (mg/dL) | 0.85 ± 0.20 | 0.86 ± 0.82 | 0.952 | 0.83 ± 0.17 | 0.79 ± 0.84 | 0.894 |
| eGFR (mL/min) | 100.33 ± 20.71 | 103.39 ± 35.72 | 0.824 | 104.00 ± 27.41 | 119.10 ± 52.14 | 0.426 |
| Uric acid (mg/dL) | 6.11 ± 0.86 | 6.23 ± 1.58 | 0.821 | 6.19 ± 0.85 | 5.92 ± 1.62 | 0.821 |
| Glucose (mg/dL) | 191.00 ± 43.28 | 90.61 ± 23.65 | <0.001 * | 180.58 ± 40.85 | 121.06 ± 114.85 | 0.049 * |
| HbA1c (%) | 8.67 ± 1.02 | 5.43 ± 0.39 | <0.001 * | 8.62 ± 1.05 | 5.43 ± 0.44 | <0.001 * |
| Insulin (mU/L) | 19.50 ± 10.35 | 11.47 ± 8.16 | 0.013 * | 15.59 ± 7.24 | 12.29 ± 12.24 | 0.48 * |
| AST (U/L) | 22.00 ± 5.65 | 26.88 ± 17.14 | 0.710 | 17.00 ± 5.65 | 26.66 ± 16.71 | 0.568 |
| ALT (U/L) | 44.83 ± 16.35 | 24.45 ± 20.06 | 0.003 | 39.91 ± 14.00 | 21.58 ± 17.05 | 0.002 |
| CHOL (mg/dL) | 206.63 ± 57.73 | 183.64 ± 35.51 | 0.128 | 85.75 ± 48.72 | 85.44 ± 25.09 | 0.979 |
| TG (mg/dL) + | 389 (306–558) | 104 (74–141) | 0.048 | 106.5 (76.75–140.25) | 44 (22–64) | 0.186 |
| HDL (mg/dL) | 24.63 ± 5.57 | 40.45 ± 12.91 | <0.001 * | 20.16 ± 5.82 | 27.24± 8.42 | 0.012 |
| LDL (mg/dL) | 88.18 ± 20.11 | 108.64 ± 27.79 | <0.001 * | 33.50 ± 9.47 | 45.13 ± 15.26 | 0.019 |
| CHOL/HDL ratio | 8.08 ± 2.64 | 4.89 ± 1.81 | 0.002 | 3.62 ± 0.61 | 3.12 ± 1.05 | 0.242 |
| hsCRP (mg/dL) | 0.34 ± 0.11 | 0.25 ± 0.44 | 0.491 | 0.12 ± 0.068 | 0.11 ± 0.21 | 0.767 |
| PFOA (ng/mL) | 2.20 ± 1.93 | 2.96 ± 2.75 | 0.623 | 2.18 ± 0.66 | 4.22 ± 2.96 | 0.276 |
| PFOS (ng/mL) | 1.93 ± 2.46 | 2.07 ± 2.03 | 0.917 | 1.32 ± 0.93 | 1.69 ± 1.27 | 0.654 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-S.; Lin, C.-H.; Tsai, C.-Y.; Wang, H.-T.; Li, S.-Y.; Liu, T.-Y.; Tan, A.C.; Tsou, H.-H.; Tseng, K.-H.; Lin, C.-C. Double Filtration Plasmapheresis with Polyvinyl Alcohol-Based Membrane Lowers Serum Inflammation and Toxins in Patients with Hyperlipidemia. Bioengineering 2023, 10, 89. https://doi.org/10.3390/bioengineering10010089
Liu W-S, Lin C-H, Tsai C-Y, Wang H-T, Li S-Y, Liu T-Y, Tan AC, Tsou H-H, Tseng K-H, Lin C-C. Double Filtration Plasmapheresis with Polyvinyl Alcohol-Based Membrane Lowers Serum Inflammation and Toxins in Patients with Hyperlipidemia. Bioengineering. 2023; 10(1):89. https://doi.org/10.3390/bioengineering10010089
Chicago/Turabian StyleLiu, Wen-Sheng, Chien-Hung Lin, Ching-Yao Tsai, Hsiang-Tsui Wang, Szu-Yuan Li, Tsung-Yun Liu, Ann Charis Tan, Han-Hsing Tsou, Kuo-Hsien Tseng, and Chih-Ching Lin. 2023. "Double Filtration Plasmapheresis with Polyvinyl Alcohol-Based Membrane Lowers Serum Inflammation and Toxins in Patients with Hyperlipidemia" Bioengineering 10, no. 1: 89. https://doi.org/10.3390/bioengineering10010089
APA StyleLiu, W.-S., Lin, C.-H., Tsai, C.-Y., Wang, H.-T., Li, S.-Y., Liu, T.-Y., Tan, A. C., Tsou, H.-H., Tseng, K.-H., & Lin, C.-C. (2023). Double Filtration Plasmapheresis with Polyvinyl Alcohol-Based Membrane Lowers Serum Inflammation and Toxins in Patients with Hyperlipidemia. Bioengineering, 10(1), 89. https://doi.org/10.3390/bioengineering10010089









