Abstract
Stormwater runoff containing road deicing salts has led to the increasing salinization of surface waters in northern climates, and urban municipalities are increasingly being mandated to manage stormwater runoff to improve water quality. We assessed chloride concentrations in runoff from late-winter snowmelt and rainfall events flowing into an urban Minnesota, USA, lake during two different years, predicting that specific stormwater drainages with greater concentrations of roadways and parking lots would produce higher chloride loads during runoff than other drainages with fewer impervious surfaces. Chloride levels were measured in runoff draining into Lake Winona via 11 stormwater outfalls, a single channelized creek inlet, and two in-lake locations during each snowmelt or rainfall event from mid-February through early April in 2021 and 2023. In total, 33% of outfall runoff samples entering the lake collected over two years had chloride concentrations exceeding the 230 ppm chronic standard for aquatic life in USA surface waters, but no sample exceeded the 860 ppm acute standard. Chloride concentrations in outfall runoff (mean ± SD; 190 ± 191 ppm, n = 143) were significantly higher than in-lake concentrations (43 ± 14 ppm, n = 25), but chloride levels did not differ significantly between snowmelt and rainfall runoff events. Runoff from highway locations had higher chloride concentrations than runoff from residential areas. Site-specific chloride levels were highly variable both within and between years, with only a single monitored outfall displaying high chloride levels in both years. There are several possible avenues available within the city to reduce deicer use, capture and treat salt-laden runoff, and prevent or reduce the delivery of chlorides to the lake.
1. Introduction
Freshwater lakes, streams, and rivers historically have been impacted by a myriad of human activities within their watersheds [1,2,3,4]. Urbanization, agriculture, mining, and forestry practices have changed the ways in which precipitation runoff reaches surface waters [2,4,5,6,7] and how its chemical composition is modified along the way [8,9,10,11]. These changes collectively have had negative effects on the physical and biological condition of lakes and streams throughout the world [2,4,12,13] and have led to efforts to reduce or eliminate the impacts, mitigate the damages, and restore the aquatic habitats and communities back to something more closely resembling their natural state [2,4,14,15,16,17,18,19,20,21].
Despite laws to protect surface waters from pollution and efforts to improve water quality, many aquatic systems continue to experience what has become known as freshwater salinization syndrome [8,9,10,11,22,23], the increasing concentrations of salt ions produced by altered land uses that can lead to watershed-wide releases of trace elements and other chemicals (i.e., chemical cocktails) and their delivery to surface and groundwaters [11]. Such water quality degradation in surface waters can lead to reduced productivity of aquatic organisms, decreased biotic richness, and/or the loss of critical ecosystem services [8,24,25,26,27,28]. Within northern climates, a major driver of freshwater salinization syndrome is the use of deicing salts to clear ice and snow from roads, parking lots, and pedestrian walkways [11,24,27,29,30].
Deicing salts (primarily sodium chloride but also including calcium and magnesium chlorides) are used during winter to reduce/prevent accumulations or remove snow and ice from paved roads and walkways [31,32,33,34]. In the United States, >18 million metric tons of deicing salts are used each winter to improve driver and pedestrian safety, mostly in northern and northeastern states [35,36]. Salt use on highways in northern USA states ranges from 1.5 to 25.9 metric tons/lane km/year [36,37], with even higher use rates per area on some parking lots and residential sidewalks and driveways [34]. This rate of use of salt on impervious surfaces during winter, along with other salts released into watersheds by human activities (e.g., water softener discharges to septic systems or municipal wastewater treatment plants, agricultural fertilizers, dust suppressants) [29], has led to steadily increasing chloride concentrations in many USA lakes and rivers [10,22,33,38]. Consequently, the US Environmental Protection Agency has established national numerical water quality criteria for chloride (230 ppm for chronic toxicity and 860 ppm for acute toxicity) designed to protect aquatic organisms [39].
Since the 1940s, the use of salt for road deicing to maintain safe winter driving conditions in northern latitudes of the USA, Canada, and Europe has expanded dramatically (15 to 140-fold) [40,41,42,43]. Increased use of NaCl as a deicer has been driven by its low cost, widespread availability, effectiveness at temperatures down to −18 °C, and development of improved application equipment and practices [42,44]. Early on, a dramatic reduction (>90%) in winter traffic accidents due to salt use led some jurisdictions to develop “bare pavement” policies during winter, effectively mandating deicer use to ensure highway safety [40,42,44]. However, the environmental effects of increasing salt use soon became apparent [40,41,42], leading to efforts to reduce and/or manage salt use to reduce environmental impacts while maintaining road safety [44,45]. Deicing salts are applied to impervious surfaces by a variety of applicators, including city, county, state, province, and national roadway maintenance personnel (streets and highways), school and business staff (parking lots and pedestrian walkways), commercial operators (parking lots, driveways, and pedestrian walkways), and area residents (driveways and pedestrian walkways). Applicator training and improved application equipment and methods are being encouraged or mandated to reduce salt use [42,44,45]. Applications generally are in two forms: solid rock salt (NaCl) applied to melt snow and ice remaining on surfaces after snow plowing and liquid salt brine sprayed onto surfaces prior to expected snowfall or freezing rain events (i.e., pre-treatment to lessen roadway freezing and reduce the need for post-event salt applications) [24]. Solid rock salt often is mixed with sand to improve traction until the snow and ice melt.
In the USA, urban areas are mandated to manage their stormwater runoff to protect aquatic resources [46]. During typical urbanization projects, engineered systems including catchment basins and a combination of surface ditches and underground pipelines were designed and installed to collect water falling on impervious streets, roadways, and parking lots and convey it for discharge into surface waters [7,47,48]. Unfortunately, chemicals, sand, or other materials on these impervious surfaces entered this same flowpath and led to the contamination of lakes, streams, rivers, and wetlands [9,10,11,22]. To better protect surface waters from contaminated stormwater runoff, municipalities developed a variety of technologies to intercept and/or redirect runoff into rain gardens, bioswales, natural and artificial wetlands, and retention ponds or basins where the water could better infiltrate into soils or more slowly flow into surface waters [7,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. These technologies were intended to reduce the volume and slow the delivery of stormwater runoff to surface waters, as well as filter out sediments and allow for biological uptake and treatment of various chemicals (especially nutrients and some metals) present in runoff [7,47,48,50,51,52,53,54,55,56,57,58,59,60,61,62]. Unfortunately, the majority of stormwater runoff in most urban areas still follows the traditional engineered flowpath directly from roadway to stream/river/lake via a pipeline or ditch [58,61,62].
The present study was initiated to examine potential patterns of late-winter runoff of deicing salts from a small urban area into a lake via a traditional system of stormwater catch basins, underground pipes, and surface ditches. We monitored the chloride concentration of runoff entering the lake via multiple stormwater outfalls during periods of both snowmelt and rainfall during two different years. We hypothesized that the chloride levels of runoff from outfalls draining primarily highway catchments would be higher than those of runoff from primarily residential drainages and that rainfall runoff would contain higher chloride levels than snowmelt runoff.
2. Study Area
The City of Winona (current population size = 26,083), Minnesota, USA, was established in 1851, located on a large sand bar in the Mississippi River and surrounded by channels and backwaters of the river. The city has a long history of directing its stormwater runoff into the Mississippi River and other surrounding lakes, wetlands, and streams [12]. Today, approximately half of the city’s land surface is connected to an engineered stormwater system that drains to the Mississippi River, with most of the remainder draining through a similar system to Lake Winona (42 km of storm sewer lines draining 650 ha) [12]. The city has undertaken several projects to intercept stormwater runoff and/or encourage infiltration via rain gardens, bioswales, and retention ponds and basins, funded by stormwater utility fees paid by local property owners based on property size and zoned usage [63].
Lake Winona (44°02′19.54″ N, 91°38′37.24″ W; 197 m above sea level) is a 129 ha eutrophic (total phosphorus levels > 80 ppb) urban lake, located entirely within the jurisdictional boundaries of Winona [12]. Its waters are alkaline (pH 8.0–8.5) with a strong carbonate–bicarbonate buffer system. It exhibits strong thermal stratification during summer, with deeper (>7 m) waters frequently becoming anoxic [12]. This floodplain lake (3.2 km long × 0.5 km wide) occupies a cut-off side channel of the Mississippi River and comprises two basins (a 36 ha western basin and 93 ha eastern basin) separated by a dike and automobile causeway (Figure 1). The basins are connected by a single large (~3 m in diameter) concrete culvert that allows for water movement between basins. The downstream portion of a nearby creek, Gilmore Creek, has been channelized and re-routed into the lake (providing continuous, perennial flows), entering the western basin and exiting from the eastern basin. Numerous stormwater outfalls draining portions of the city enter the channelized section of Gilmore Creek prior to it reaching Lake Winona.
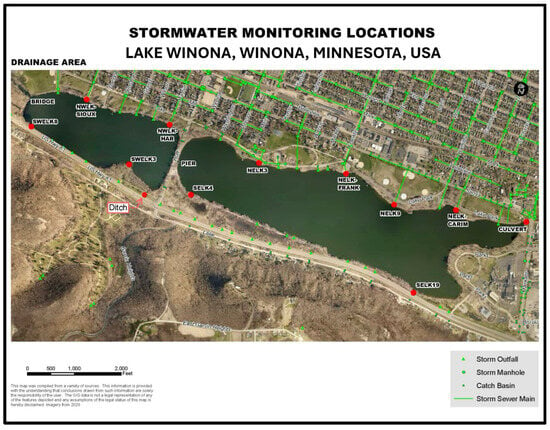
Figure 1.
Aerial view of Lake Winona, Winona, Minnesota, USA, displaying 14 stormwater monitoring locations where chloride concentrations of runoff were measured during snowmelt and rainfall events, late winter, 2021 and 2023.
In addition to receiving inflows from Gilmore Creek, waters flow into the lake periodically via numerous stormwater outfalls (Figure 1 and Figure 2). Runoff from 18 separate stormwater drainages (primarily encompassing urban residential areas and their associated paved streets) enters the north side of the lake via culverts (western basin = 6 and eastern basin = 12). An additional 17 culverts or shallow drainage ditches carry runoff from US Highway 14/61 into the south side of the lake (western basin = 7 and eastern basin = 10). Flows enter the lake via all of these culverts and ditches only during or shortly after rainfall and snowmelt events.

Figure 2.
Photographs of two chloride monitoring locations in Lake Winona: stormwater outfall culvert (nELK3) entering the northern shore of the eastern basin (A) and stormwater outfall ditch (sWLK8) entering the southern shore of the western basin (B).
3. Methods
3.1. Runoff Collection and Chloride Measurement
To assess the concentrations of chloride entering Lake Winona in late winter (February–April) of 2021 and 2023, samples of runoff were collected during periods of runoff (snowmelt or rainfall) at 14 locations in and around Lake Winona (Figure 1). Sampling locations included the Gilmore Creek inlet and outlet, the culvert connection between the two basins, and eleven stormwater outfalls (six along the north shore and five along the south shore). No attempts were made to measure inflow rates at any of the sites. Snowmelt samples were collected after air temperatures remained above freezing (>0 °C) for a time period (>36 h) sufficient to produce meltwater runoff from streets and parking lots. Similarly, rain event samples were collected after enough rain (>0.25 cm) had fallen to produce street and parking lot runoff. We attempted to collect one sample during each runoff event from each of the 14 sites, but ice remaining at some sites during some events prevented sample collection at those sites. No collections were made from one specific outfall site during 2021, as that site was added to the monitoring list only in 2023.
Runoff samples were collected from sites and tested using a simple standardized procedure. A clean and sanitized plastic bottle was rinsed three times with runoff water at the collection site before collecting a sample for measurement. The chloride concentration of that sample was measured using factory-calibrated Quantab chloride test strip titrators (Hach Company, Loveland, CO, USA). The lower end of the titrator was placed in the runoff sample and remained (up to 10 min) until the sample had completely saturated the titrator wick and an indicator band changed color to indicate that the reaction was complete. The chloride concentration (ppm Cl−) of the sample was then read from the titrator scale and recorded. These specific chloride titrators are recommended for testing chloride levels in surface waters in nationwide programs in the USA [64] and Canada [65]. Titrators accurately (simple linear regression: r2 = 0.947) assessed chloride concentrations across a range of standard chloride solutions (50, 100, 250, 500 ppm) and displayed low coefficients of variation (CV) when multiple titrators (n = 3–4) were used to measure chloride levels in the same outfall sample (e.g., 68 ppm, CV = 3.41%; 123 ppm, CV = 2.82%; 429 ppm, CV = 3.61%).
3.2. Historical Chloride Data for Lake Winona
Although the Minnesota Pollution Control Agency and the Minnesota Department of Natural Resources conduct periodic assessments of Lake Winona’s water quality, these assessments do not include chloride. The City of Winona also does not monitor chloride in the lake. Consequently, only limited historical data were available for chloride concentrations in Lake Winona [12], collected both during spring/summer (open water) periods in 1968 and 1971 and during periods of winter ice cover (1971, 1974, 1975). In some instances, chloride concentrations were measured at various depths during winter.
3.3. Data Analyses
Several different approaches were used to examine patterns in chloride concentrations in runoff from the various sites at Lake Winona. First, chloride concentrations were compared among eastern and western basin outfalls and the lake water sites (the culvert connection between basins and the outlet on the eastern basin) with a Kruskal–Wallis test. If a significant difference was detected among groups, Tukey’s honest significant difference (HSD) test was performed to determine which groups differed. Next, the distributions of chloride concentrations in runoff across the range of values observed (grouped into 100 ppm categories) were compared between eastern and western basins with a Chi-square contingency table test to assess whether basins had similar chloride inputs from stormwater outfalls. Next, annual mean and maximum chloride concentrations measured at each site were examined graphically to determine whether similar patterns were evident between years, with some sites potentially having either consistently higher or lower values than other sites. Each year, we also compared the chloride concentrations of runoff entering the north side of the lake (outfalls draining mostly residential areas) to those entering the south side of the lake (outfalls draining the highway) with Mann–Whitney tests. Chloride concentrations also were compared between snowmelt and rainfall events for each basin and year separately with a series of Mann–Whitney tests to assess whether chloride concentrations in runoff differed based on the type of event producing the runoff. Finally, historical chloride data collected during winter were compared to more contemporary (2021/2023) winter data for Lake Winona with a Mann–Whitney test. Historical winter versus historical summer chloride data also were compared with a Mann–Whitney test, and historical winter chloride concentrations were assessed for possible correlations with water depths using simple linear regression. All statistical tests were performed with the VassarStats Website for Statistical Computation (http://vassarstats.net, accessed on 4 February 2025).
4. Results
During 2021 and 2023, we measured chloride concentrations at Lake Winona sites during twelve (five snowmelt and seven rainfall) distinct runoff events (Table 1). Snowmelt runoff events spanned time periods ranging from three to sixteen days, whereas rainfall runoff events ranged only from one to three days. We collected and tested chloride in 25 samples from Lake Winona (at the lake outlet and the culvert connection between basins) and 143 samples from runoff outfall culverts, ditches, and the inflow from the channelized Gilmore Creek (Table 2). Lake water chloride levels commonly were in the 30 to 40 ppm range with little variability (the coefficient of variation [CV] = 32%), whereas runoff outfall samples typically were three to four times higher (100 to 200 ppm) and displayed high variability (CV = 100%; Figure 3). Outfall chloride concentrations in both basins were significantly higher than those of the lake (Kruskal–Wallis H = 14.26, p = 0.0008), but chloride concentrations did not differ significantly between eastern and western basin outfalls (Figure 2).

Table 1.
Run-off events (snowmelt, rainfall) during which chloride concentrations were measured entering Lake Winona via stormwater outfalls, late winter 2021 and 2023. Rainfall totals are shown in parentheses.

Table 2.
Summary statistics for chloride concentrations (ppm Cl−) in Lake Winona and runoff into the lake, late winter 2021 and 2023. Outfall samples included those collected from the Gilmore Creek inlet.
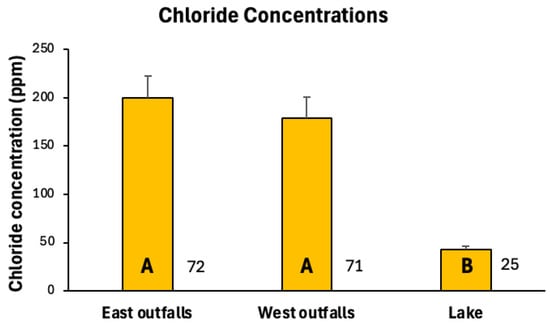
Figure 3.
Mean (+standard error) chloride concentrations of stormwater runoff entering Lake Winona via outfalls on eastern (seven sites) and western (five sites) basins, and chloride levels at two in-lake sites, 2021 and 2023. Numbers next to bars are sample sizes. Bars sharing a common letter are not significantly different from one another (Tukey’s HSD test).
Although approximately two-thirds of all runoff samples tested had chloride concentrations <200 ppm, outfalls in both basins frequently had chloride levels of 300 to 700 ppm in snowmelt and rainfall runoff (Figure 4). When concentration distributions were compared between the two basins, both basins displayed similar patterns that did not differ significantly (contingency table Chi-square = 4.72, df = 7, p = 0.694) from each other.
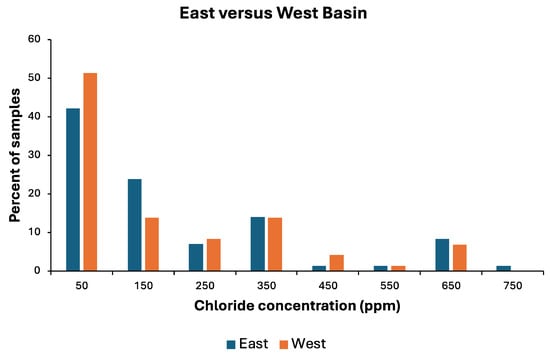
Figure 4.
Percentages of snowmelt and rainfall runoff samples collected entering eastern and western basins of Lake Winona (late winter, 2021 and 2023) falling into various 100 ppm chloride concentration bins. Numbers along X axis represent midpoints of each bin.
Highly variable chloride concentrations (both means and maxima) were evident among the 14 Lake Winona sites surveyed (one site was not sampled during 2021), and sites with the highest values during one year typically did not have high values during the other year (Figure 5). Only one outfall site (sELK19) that carried runoff from a section of highway into the eastern basin (Figure 1) had high chloride values during both years. Both in-lake sites (PIER and CULVERT) displayed consistently low chloride levels in both years. The creek inlet site (BRIDGE) had lower chloride concentrations than most other outfall sites in 2021 but had higher concentrations than most outfall sites in 2023 (Figure 5).
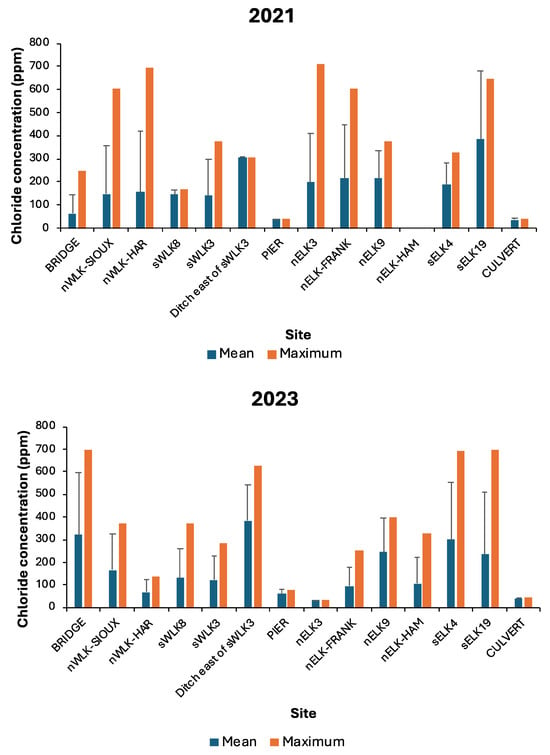
Figure 5.
Mean (+standard deviation) and maximum chloride concentrations of snowmelt and rainfall runoff from each of 14 sites on Lake Winona, late winter, 2021 and 2023. See Figure 1 for site locations.
Stormwater outfalls primarily draining the highway corridor into the southern shores of the lake had higher mean chloride concentrations than outfalls draining into the northern shores from mostly residential areas during both years (Figure 6). Differences in chloride levels were significant only during 2023. Chloride levels from highway runoff were similar during both years, whereas mean chloride concentrations from residential runoff were 33% lower in 2023 than in 2021.
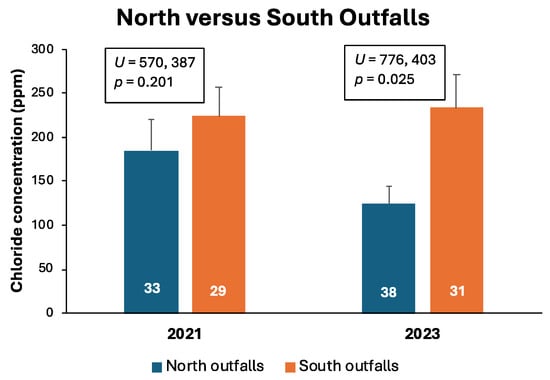
Figure 6.
Mean (+standard error) chloride concentrations of snowmelt and rainfall runoff entering Lake Winona via stormwater outfalls on the northern and southern shores during late winter, 2021 and 2023. Numbers on bars are sample sizes. Results of Mann–Whitney tests comparing chloride concentrations between northern and southern outfalls are displayed above the bars for each year separately.
When mean chloride concentrations in runoff during snowmelt events were compared to those during rainfall events, patterns appeared to change by year and by lake basin (Figure 7). During 2021, snowmelt events produced higher chloride levels than did rainfall events at outfalls in each lake basin. In contrast, rainfall events produced higher chloride levels than snowmelt events at outfalls in both lake basins in 2023. However, due to high variability in chloride levels among outfall sites, none of the snowmelt versus rainfall comparisons differed significantly (Table 3).
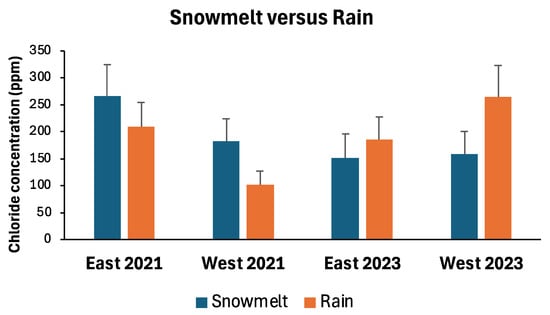
Figure 7.
Mean (+standard error) chloride concentrations of snowmelt versus rainfall runoff entering Lake Winona via stormwater outfalls in eastern and western basins, late winter 2021 and 2023. See Table 3 for statistical comparisons between snowmelt and rainfall concentrations for each lake basin/year combination.

Table 3.
Summary statistics comparing chloride concentrations of runoff from snowmelt and rainfall events flowing into eastern and western basins of Lake Winona during late winter 2021 and 2023. See Figure 7 for chloride values.
In-lake (PIER and CULVERT sites) chloride concentrations during winter 2021 and 2023 (mean ± SD: 43 ± 14 ppm) were more than three times higher (and significantly greater: Mann–Whitney U = 1399, 51; p < 0.0001, n = 25) than surface water chloride concentrations (14 ± 4 ppm, n = 16) measured through the ice during the early 1970s. Historical spring/summer chloride concentrations (16 ± 6 ppm, n = 30) were significantly lower (by 20%: Mann–Whitney U = 631, 1110; p = 0.036) than winter chloride concentrations (20 ± 8 ppm, n = 58), with winter chloride concentrations also increasing significantly with depth from which the sample was collected (Figure 8).
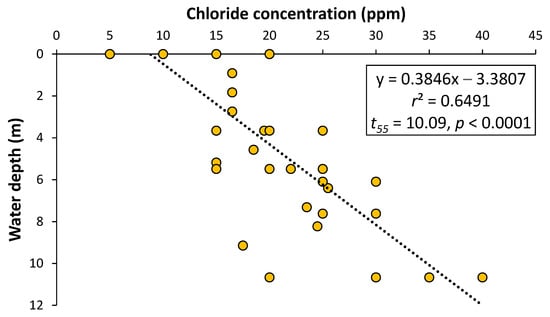
Figure 8.
Relationship between winter chloride concentrations and water depths (n = 57) for historical data [12] from Lake Winona, 1971–1975.
5. Discussion
5.1. Chloride Runoff
Road deicing salts applied to improve the safety of urban roadways and walkways in northern climates continue to contaminate surface and groundwaters via snowmelt and rainfall runoff [66,67]. Our observations of runoff entering Lake Winona indicated that local chloride concentrations (mean = 190 ppm, range = 6–711 ppm) exceeded the chronic toxicity standards for the protection of aquatic life in USA (230 ppm) and Canada (120 ppm) surface waters in 33% and 48% of samples collected, respectively [39,68]. No samples exceeded the acute toxicity chloride standard (860 ppm) for the USA, but 5% of runoff samples exceeded Canada’s acute toxicity chloride standard (640 ppm) [39,68]. Similarly, another study of chloride runoff into Lake Winona in 2021 and 2022 reported several samples exceeding chronic but not acute standards [60]. Snowmelt water samples (n = 4) collected from roadside gutters near Lake Winona (8 February 2023) all exceeded 600 ppm chloride (mean ± SD, 705 ± 76 ppm, range = 624–807 ppm) (N. Mundahl, unpublished data). By contrast, stormwater runoff outfall chloride levels in urban Poland were as high as 12,086 ppm during winter, with all February samples exceeding Poland’s 1000 ppm limit for discharge to surface waters [69]. Even worse, chloride concentrations in runoff leaving an urban parking lot in Canada exhibited short-term spikes up to 60,000 ppm [42]. However, unlike in the present study, chloride concentrations in winter rainfall runoff (7–24 ppm) in Poland were significantly lower than in snowmelt runoff (137–12,086 ppm) [69].
Our runoff chloride data displayed significant variation among stormwater outfalls, among runoff events, and between years. Such wide variation is not unusual in urban areas in northern climates such as Minnesota [33], Canada [34,43], and Europe [24,69]. The seasonal application of deicers inherently produces dramatic seasonal swings in chloride concentrations in both runoff and receiving surface waters [33,34]. During late winter, multiple factors likely affect the concentration of chloride in runoff delivered to surface waters, including the following: the volume of deicing salts applied to impervious surfaces within a drainage basin; the total area of impervious surfaces receiving salt applications; the volume of snowmelt or rainfall washing salts from those surfaces; and the directness of connection between impervious surface and the receiving surface water [24,33,34,43]. These variables, influenced by the frequency of precipitation events that can either require deicer applications or serve to flush those salts from impervious surfaces, may somewhat limit the potential for targeting specific stormwater drainages for future runoff control practices [24,69].
Previous research has indicated that the percentage of impervious surfaces within various stormwater drainages were good indicators of chloride concentrations (mean and maximum) in receiving waters, whereas drainage areas were poorly correlated to chloride levels [33,67]. We found that runoff outfalls draining into Lake Winona from the major local highway typically had higher chloride levels (significantly higher in 2023) than runoff coming from the lake’s residential side. We suspect that the highway-dominated outfall drainages had relatively higher proportions of impervious surfaces than did residential areas, leading to higher chloride concentrations in runoff. We conservatively estimated that 3.2 km of a four-lane highway and a parallel two-lane street bordering the south shore of the lake received >120 metric tons of deicing salts each winter [36,37], a substantial amount of salt applied in close proximity to the lake.
During a “normal” winter, repeated snowfall events should lead to repeated deicing applications (both pre-treatment and post-snowfall removal), resulting in salt accumulations on or bordering impervious surfaces. Roadway and walkway surfaces often develop a whitish appearance as rock salt particles become crushed by motor vehicles and foot traffic. Deicing salts remain stockpiled in this manner until they are cleared off during the next snow removal activity, or until they are flushed off by late-winter snowmelt or spring rainfall. In recent years, “normal” winter weather has shifted to now include both snowfall and rainfall events plus multiple snowmelt events during typical winter months (December–February) in many northern urban areas [70]. Runoff events become intermixed with snowfall events that now produce alternating episodes of deicer application and chloride runoff (see Table 1), possibly reducing the total accumulation of deicers on and near roadways throughout the winter season but extending the potential salt runoff season.
We anticipated that gradual seasonal warming leading to snowmelt would gradually mobilize accumulated deicers into the stormwater system for delivery to Lake Winona. However, we expected that late-winter rainfall would more efficiently flush salts from the landscape [42], particularly from roadways, producing higher chloride concentrations in rainfall runoff than in snowmelt runoff. Our runoff data did not support this expectation, with no significant difference in chloride concentrations in snowmelt-generated versus rainfall-generated runoff. Again, the alternating pattern of snowmelt and rainfall that occurred during both years of our monitoring likely affected the quantity of deicing salts available for conveyance to the lake, with multiple runoff episodes each carrying a reduced load of chloride compared to what might be moved under an idealized single, gradual snowmelt followed by a single cleansing rainfall event. Alternatively, greater runoff flows during rainfall versus snowmelt events [42] potentially diluted chloride concentrations during rainfall events, reducing chloride concentrations in runoff to levels similar to those observed during snowmelt.
5.2. In-Lake Chloride Levels and Stormwater Management
Although chloride levels in runoff entering Lake Winona sometimes reached toxic levels, the lake itself had relatively low chloride levels (30–40 ppm) compared to those (100 s to 1000 s ppm) reported for lakes and streams in other urban areas in the USA, Canada, and Europe [24,33,34,38,69]. Despite Lake Winona’s low levels, historical data [12] indicate that in-lake chloride concentrations have increased at a rate of 0.6 ppm/year over the past 50 years, a rate of increase slower than that (1–2 ppm/year) reported for urban lakes in Wisconsin [38] and elsewhere in Minnesota [33]. However, like other regional urban lakes, historical chloride data for Lake Winona during the period of ice cover displayed significantly increasing chloride concentrations with increasing depth resulting from saline waters with higher density sinking to the lake bottom [33].
Local efforts to manage stormwater flows into Lake Winona have focused mainly on methods to reduce inflows of nutrients, as the lake is currently impaired by excessive phosphorus [71]. The City of Winona has mandated the inclusion of stormwater retention ponds and/or underground retention basins for new developments with new streets, roadways, and parking lots to slow the delivery of runoff to local surface waters. In addition, the city has provided partial funding for established residential and commercial property owners to install rain gardens to capture runoff and encourage infiltration. Seasonal street maintenance practices (e.g., mechanized street sweeping) have also been altered to prevent fallen leaves (and their nutrients) from entering storm sewers during fall and to collect any sand and salt remaining on streets after winter has ended.
Many urban areas have taken measures to reduce the impacts of stormwater runoff on local surface waters, including many of the same approaches being used in the City of Winona [51,52,54,57,58,59,60,61,62]. In addition, sophisticated modeling and computer simulations of urban runoff management have led to altered delivery patterns (i.e., changed hydrology) of stormwater to lakes and streams that have slowed rates of delivery and reduced peak flows via engineered re-routing, retention, and infiltration [47,48,50,53,55,56]. Similarly, stormwater retention and infiltration also have led to improved methods aimed to remove or treat stormwater contaminants such as suspended sediments, oils, heavy metals, and a variety of chemical ions [9,10,11,22].
5.3. Chloride Infiltration
Unfortunately for northern urban areas, many of the engineered solutions to manage the hydrology and contamination chemistry of stormwater flows and their eventual delivery to surface water systems [47,48,50,51,52,53,54,55,56,57,58,59,60,61] may not be appropriate or suitable for managing deicer concentrations in urban runoff. For example, the use of retention ponds, bioswales, grassed roadside ditches, and rain gardens designed to filter and treat sediments, nutrients, other organic chemicals, and heavy metals in runoff via infiltration [24,43] may have little effect on deicer salts unless reactive media (e.g., anthracite coal, dolomite) are used [43,72]. Chlorides can accumulate in unsaturated soils and groundwaters >800 m from treated roadways [73] and remain unchanged for extended time periods, eventually moving into lakes, streams, and rivers via groundwater discharges [24,67]. Although the stormwater delivery of deicing salts to surface waters may be most noticeable during snowmelt and early spring rains [33,42], groundwater seepage can deliver elevated chloride levels into lakes and streams year round, resulting in chloride levels in these systems that can exceed toxic levels during all seasons [33,34].
5.4. Chloride Reduction and Management
Alternatives to using salt deicers to clear ice and snow from roads and walkways are limited (e.g., acetates, nitrates, beet juice, proprietary distillation by-products, technical roadway installations), with some (e.g., roadway heating) being exceedingly cost-prohibitive to use on a large scale [24]. Consequently, most northern municipalities continue to focus on reducing and/or optimizing the use of deicing salts and attempting to manage the side effects of deicer runoff [24,34,42,43]. Improved deicing devices and practices, education and reward programs for commercial and residential applicators, and a combination of roadway temperature sensors and access to real-time meteorological data have significantly reduced salt use (by up to 35%) in some localities [74]. Routing deicer-laden runoff into basins for re-use or recycling, through wetlands with halophyte plants (to use as phtyodesalinators) or through various absorptive media (anthracite coal, pozzolan, dolomite, limestone, biochar, or charcoal), may be effective and economical approaches to reduce the amount of salts delivered (by 4–68%) to surface waters via stormwater systems [24,43,72]. Of these possibilities, the City of Winona could reduce salt use with more modern and efficient application equipment, continuing education for applicators, and more pre-treatment to reduce post-snowfall salt use. However, phytodesalination likely would be ineffective in Winona and other northern cities during winter/early spring (i.e., non-growing seasons) when deicer runoff is highest, and all or most stormwater outfalls would need to be directed through absorptive media filters prior to delivery to surface waters to make the process effective.
With 35 separate stormwater outfalls plus Gilmore Creek entering Lake Winona, managing the delivery of chloride-containing deicers to the system will not be a simple undertaking. We had expected that some outfalls would display consistently higher chloride levels in runoff than others, allowing the city to target specific stormwater drainages for more directed stormwater management activities. The higher chloride levels entering Lake Winona from highway drainages may provide a starting point, although stormwater management of these drainages would require collaboration with the state agency (Minnesota Department of Transportation) with jurisdiction over the highway, its deicing practices, and its runoff within the highway easement. Wetlands within several of these highway drainages may allow for some degree of outfall consolidation (currently 17 outfalls enter the lake from the highway) and stormwater retention and treatment (halophytes or absorptive media) to reduce the amount of chloride reaching the lake. A similar scenario (directing waters through an engineered wetland) has been recommended to reduce nutrient levels of waters flowing into the lake from Gilmore Creek [71]. A modification of this treatment also could serve to reduce chloride levels in creek inflows, since the creek also receives stormwater runoff from at least nine outfalls along its route through the city to the lake [12].
Stormwaters draining into Lake Winona from residential areas also could be managed differently to reduce chloride levels during winter/early spring. Again, some consolidation of the 18 outfalls currently entering into the north side of the lake could be performed to reduce the number of inputs needing control or treatment. The entire north shore and adjacent lands lie within a city park, potentially allowing for some flexibility in siting possible treatment ponds, basins, or absorptive media structures. Some locations currently are federally designated wetlands, and rerouting stormwater inflows into wetland sites for nutrient management and infiltration already has been proposed. As previously mentioned for other bordering wetlands, these could be modified to accomplish some degree of chloride removal via plantings and absorption, possibly using regionally abundant sources of dolomite or limestone. Also, other outfalls potentially could be equipped with combinations of reactive media to remove chloride and iron-enhanced sand filters to remove phosphorus from stormwaters [71]. The installation of five such sand filters for phosphorus capture already has been proposed for the north side of the eastern basin [71].
Ultimately, the reduced use of deicing salts in urban areas may require obtaining public acceptance of roadways and parking lots being less than totally clear of ice and snow shortly after each snowfall event. As the use of deicers has increased steadily, the general public has come to expect that roadways will be cleared quickly down to bare pavement, allowing for safe travel [75]. Winter maintenance practitioners now feel pressure from residents to remove snow and ice as quickly as possible. Rather than waiting out bad weather and venturing out on roads only for absolute necessities (as used to be typical before the widespread use of deicers), the general public has come to expect clear and safe roadways virtually at all times. If these expectations could be changed, the amount of road salts could be reduced significantly, resulting in reduced runoff and fewer environmental impacts [75].
5.5. Chloride in Groundwater
A significant unknown for Lake Winona is the chloride level of groundwaters connecting to the lake. Other northern localities have reported increasing chloride concentrations in shallow groundwaters (sometimes up to toxic levels) due to application of deicers during winter and their infiltration into soils and aquifers [29,34,43,67]. Infiltration can result from runoff capture and seepage in ponds or other types of permeable basins or via pervious road shoulders, vegetated roadside ditches, or other open stormwater channels [34,43]. Elevated chloride levels in soils and shallow groundwaters can lead to a sustained, year-round delivery of chloride into lakes and streams [33,34]. If these surface waters are large in total volume or have high flushing rates relative to groundwater chloride inputs, chlorides can be removed rapidly and elevated concentrations can be prevented [33]. However, smaller lakes and those with low flushing rates are particularly vulnerable to groundwater chloride inputs [33].
The City of Winona sits on a porous, 1.75 km-wide sand bar that separates Lake Winona from the Mississippi River. The water table beneath the city is controlled primarily by the river level and secondarily by a pumping station that prevents the city from flooding at high river stages [12]. Consequently, groundwater movements into Lake Winona can be substantial during periods of high river flow. Whether such groundwater movements carry chloride contamination into the lake or flush accumulated chlorides out of the system is unknown. The three-fold increase in chloride levels in Lake Winona over the past 50 years suggests that more chloride is entering than leaving, indicating a need to better manage the use of deicing salts within the city and begin to integrate chloride capture techniques into future stormwater management plans.
5.6. Limitations of the Present Study
Several factors beyond the scope of the present study limit our ability to fully estimate the potential impact of chloride runoff into Lake Winona. These include a lack of current year-round data on chloride levels in both Lake Winona and its inflows, limited information on flow rates of stormwater runoff into the lake, complicated groundwater movements and unknown groundwater chemistry in the vicinity of the lake, few data on runoff water quality except for chloride and phosphorus, and an incomplete picture of how climate change may alter winter precipitation patterns and precipitation type (snow versus rain). Filling these data gaps in the future will lead to a more complete understanding of chloride dynamics in the Lake Winona system and surrounding area and allow for improved management to limit the harmful impacts of chloride on the aquatic environment.
Unfortunately, no current on-going water quality programs measure chloride levels in Lake Winona or its inflows (i.e., Gilmore Creek, stormwater outfalls) on a systematic, year-round basis. Seasonal changes in chloride levels in regional lakes can be significant, with the highest levels during winter deicer applications and salt runoff and the lowest levels during summer and fall when freshwater rainfall runoff enters lakes and flushes away or dilutes the chloride [33]. The only year-round chloride data available for Lake Winona are now >50 years old [12], so more current information for the lake is needed. Even worse, we were unable to locate (and did not have funding to collect ourselves) any monitoring data on chloride levels of water flowing into the lake during late spring, summer, or fall. Increasing levels of chloride in urban lakes within the region [33,38] suggest that either freshwater runoff in spring/summer/fall is insufficient to flush out salts accumulated during winter or that spring/summer/fall runoff still contains significant levels of chloride. Lacking information for Lake Winona and its inflows, we cannot know if the in-lake chloride levels change seasonally and if chloride inputs via runoff decline during the open water period.
Previous studies [71,76] have attempted to model the hydrology of Lake Winona to better understand lake nutrient dynamics, and these models may be used to partially inform the role of stormwater inflows in affecting lake chloride levels. We did not attempt to measure the flow rates at our stormwater outfall sites when we collected our water samples for chloride analysis. Based on our field observations, flow rates were highly variable among outfalls during the same runoff event and at the same outfall during different stages of a runoff event. Rapid versus slow snowmelt and intense rainfall versus steady rain also produced widely variable inflows. However, on an annual basis, stormwater outfalls are estimated to contribute 13.6% of the volume of water entering and flowing through the lake, with annual stormwater outfall runoff volume equivalent to 32% of total lake volume [12,71]. Determining what proportion of annual stormwater runoff is generated by snowmelt and late-winter rains is a necessary next step toward estimating chloride loading for Lake Winona and evaluating the impacts that these inputs may have on the lake ecosystem.
The hydrology of groundwaters associated with Lake Winona is poorly understood (see Section 5.5) and has not been addressed in previous studies of water quality in the lake [71,76]. Anecdotal evidence from shallow sandpoint (or driven point) wells (typically used to water residential or commercial landscaping) and building basement sumps (to manage groundwater infiltration) indicates that chloride has contaminated the shallow (water table at 3 m or less [37]) groundwaters beneath the City of Winona (N.D. Mundahl, unpublished data). Ambient groundwater levels of chloride in Winona range from 25 to 75 ppm in the sand and gravel aquifers beneath the city [37], similar to the chloride levels that we observed for the lake itself during our study. While deicing salts are the most likely source of this groundwater contamination [29,34,37], its potential movement between the Mississippi River and Lake Winona is not known. The City of Winona actively maintains a few piezometers near the river to monitor for potential flooding, and these likely also could be employed to monitor chloride levels through time. Estimating groundwater chloride inputs into the lake and/or mapping chloride levels likely would require the identification and reactivation of currently inactive piezometers scattered elsewhere throughout the city, plus multiple sandpoint wells and sumps that could be targeted for regular monitoring.
For the present study, we did not measure the typical water quality parameters (e.g., pH, temperature, turbidity, dissolved oxygen, nutrient levels) of stormwater runoff when we measured chloride levels, so we were unable to assess whether elevated chloride levels were correlated to other water quality measures. Few studies have examined the water chemistry of stormwater runoff into Lake Winona, and those studies have been limited to only chloride and phosphorus [[60,71,76], present study]. The City of Winona’s stormwater discharge permit (from the Minnesota Pollution Control Agency: Phase II of the National Pollutant Discharge Elimination System {NPDES} MS4 general permit) currently does not require any type of water quality monitoring or assessment of stormwaters, so there has been no systematic assessment of any water quality parameter of waters discharged to Lake Winona. Any future monitoring of chloride levels on stormwater runoff in Winona ideally should be paired with simultaneous measurements of typical water quality parameters to allow for an assessment of potential correlations between chloride levels and the other variables.
Changing climate patterns within the region of our study [70] have led to an increased frequency of freeze–thaw cycles during the normal winter period (December–February). Whereas a period of prolonged sub-freezing temperatures typically might result in deicer applications around the time of each snowfall event, multiple freeze–thaw cycles might produce a need for repeated deicer applications to keep meltwaters from re-freezing on roadways, even if no snow or rain event occurs. This scenario potentially could lead to heavier use of salt to keep roads and sidewalks safe for travel. Increased extreme precipitation events [70] also could result in increased salt use if that precipitation falls as snow, or it could lessen the need for salt use if more precipitation falls as rain rather than snow. Consequently, predicting how salt use might change with a changing climate, and in turn how this might impact chloride loading to Lake Winona, is probably speculative at best at this point in time.
To summarize, the present study detected several important patterns related to chloride runoff into Lake Winona, but many question marks and data gaps still remain. Investigating these questions and improving our understanding of chloride dynamics in and around Lake Winona should be the focus of future studies to protect the lake environment and prevent it from becoming negatively impacted by chloride.
6. Conclusions
Chloride runoff to Lake Winona via municipal stormwater outfalls was highly variable among outfalls, among runoff events, and between years. The highest chloride concentrations were produced from highway runoff, but concentrations also reached chronic toxicity levels in runoff from residential drainages. In-lake chloride concentrations have tripled over the last 50 years, suggesting increased deicer use and/or increased accumulation in Lake Winona. Various opportunities are available within the city to reduce deicer use, capture and treat salt-laden runoff, and prevent or reduce the delivery of chlorides to the lake. If conducted in conjunction with other projects aimed at reducing nutrient flows to the lake, cost-effective chloride reductions can be achieved and lead to improved water quality in Lake Winona.
Author Contributions
Conceptualization, J.H. and N.D.M.; methodology, N.D.M. and J.H.; software, N.D.M.; validation, N.D.M.; formal analysis, N.D.M.; investigation, N.D.M. and J.H.; resources, N.D.M. and J.H.; data curation, N.D.M.; writing—original draft preparation, N.D.M.; writing—review and editing, N.D.M. and J.H.; visualization, N.D.M.; supervision, N.D.M.; project administration, N.D.M. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by N.D.M. on reasonable request.
Acknowledgments
We thank the many Winona State University Environmental Biology students who assisted with sample collection and measurement. The City of Winona provided geospatial mapping services.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Goldman, C.R.; Horne, A.J. Limnology, 2nd ed.; McGraw-Hill Education: New York, NY, USA, 1994. [Google Scholar]
- Wetzel, R.G. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001. [Google Scholar]
- Mundahl, N.; Borsari, B.; Meyer, C.; Wheeler, P.; Siderius, N.; Harmes, S. Sustainable management of water quality in southeastern Minnesota, USA: History, citizen attitudes, and future implications. In Sustainable Water Use and Management: Examples of New Approaches and Perspectives; Filho, W.L., Sümer, V., Eds.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Allan, J.D.; Castillo, M.M.; Capps, K.A. Stream Ecology: Structure and Function of Running Waters, 3rd ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2021. [Google Scholar]
- Bhaduri, B.; Grove, M.; Lowry, C.; Harbor, J. Assessing long-term hydrologic effects of land use change. J. Am. Water Work. Assoc. 1997, 89, 94–106. [Google Scholar]
- Mao, D.; Cherkauer, K.A. Impacts of land-use on hydrologic responses in the Great Lakes region. J. Hydrol. 2009, 374, 71–82. [Google Scholar]
- Prudencio, L.; Null, S.E. Stormwater management and ecosystem services: A review. Environ. Res. Lett. 2018, 13, 033002. [Google Scholar]
- Cañedo-Argüelles, M.; Kefford, B.; Schäfer, R. Salt in freshwaters: Causes, effects and prospects—Introduction to the theme issue. Philos. Trans. R. Soc. B 2019, 374, 20180002. [Google Scholar]
- Maas, C.M.; Kaushal, S.S.; Rippy, M.A.; Mayer, P.M.; Grant, S.B.; Shatkay, R.R.; Malin, J.T.; Bhide, S.V.; Vikesland, P.; Krauss, L.; et al. Freshwater salinization syndrome limits management efforts to improve water quality. Front. Environ. Sci. 2023, 11, 1106581. [Google Scholar]
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Utz, R.M.; Haq, S.; Gorman, J.; Grese, M. Freshwater salinization syndrome on a continental scale. Proc. Natl. Acad. Sci. USA 2018, 115, E574–E583. [Google Scholar]
- Kaushal, S.S.; Reimer, J.E.; Mayer, P.M.; Shatkay, R.R.; Maas, C.M.; Nguyen, W.D.; Boger, W.L.; Yaculak, A.M.; Doody, T.R.; Pennino, M.J.; et al. Freshwater salinization syndrome alters retention and release of ‘chemical cocktails’ along flowpaths: From stormwater management to urban streams. Freshw. Sci. 2022, 41, 420–441. [Google Scholar] [CrossRef]
- Fremling, C.R.; Heins, G.A. A Lake Winona Compendium: Information Concerning the Reclamation of an Urban Winter-Kill Lake at Winona, Minnesota, 2nd ed.; Winona State University: Winona, MN, USA, 1986. [Google Scholar]
- Trimble, S.W. Historical Agriculture and Soil Erosion in the Upper Mississippi Valley Hill Country; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Søndergaard, M.; Jeppesen, E.; Lauridsen, T.L.; Skov, C.; Van Nes, E.H.; Roijackers, R.; Lammens, E.; Portielje, R. Lake restoration: Successes, failures and long-term effects. J. Appl. Ecol. 2007, 44, 1095–1105. [Google Scholar] [CrossRef]
- Hunt, R.L. Trout Stream Therapy; University of Wisconsin Press: Madison, WI, USA, 1993. [Google Scholar]
- Hunter, C.J. Better Trout Habitat: A Guide to Stream Restoration and Management; Island Press: Washington, DC, USA, 1991. [Google Scholar]
- Roni, P.; Hanson, K.; Beechie, T. Global review of the physical and biological effectiveness of stream rehabilitation techniques. N. Am. J. Fish. Manag. 2008, 28, 856–890. [Google Scholar]
- Bakker, E.S.; Van Donk, E.; Immers, A.K. Lake restoration by in-lake iron addition: A synopsis of iron impact on aquatic organisms and shallow lake ecosystems. Aquat. Ecol. 2016, 50, 121–135. [Google Scholar]
- Tammeorg, O.; Chorus, I.; Spears, B.; Nõges, P.; Nürnberg, G.K.; Tammeorg, P.; Søndergaard, M.; Jeppesen, E.; Paerl, H.; Huser, B.; et al. Sustainable lake restoration: From challenges to solutions. WIREs Water 2024, 11, e1689. [Google Scholar]
- Dunalska, J.A.; Wiśniewski, G. Can we stop the degradation of lakes? Innovative approaches in lake restoration. Ecol. Eng. 2016, 95, 714–722. [Google Scholar]
- Riley, A.L. Restoring Streams in Cities: A Guide for Planners, Policymakers, and Citizens; Island Press: Washington, DC, USA, 1998. [Google Scholar]
- Kaushal, S.S.; Likens, G.E.; Pace, M.L.; Reimer, J.E.; Maas, C.M.; Galella, J.G.; Utz, R.M.; Duan, S.; Kryger, J.R.; Yaculak, A.M.; et al. Freshwater salinization syndrome: From emerging global problem to managing risks. Biogeochemistry 2021, 154, 255–292. [Google Scholar]
- Kaushal, S.S.; Mayer, P.M.; Likens, G.E.; Reimer, J.E.; Maas, C.M.; Rippy, M.A.; Grant, S.B.; Hart, I.; Utz, R.M.; Shatkay, R.R.; et al. Five state factors control progressive stages of freshwater salinization syndrome. Limnol. Oceanogr. Lett. 2023, 8, 190–211. [Google Scholar] [CrossRef]
- Szklarek, S.; Górecka, A.; Wojtal-Frankiewicz, A. The effects of road salt on freshwater ecosystems and solutions for mitigating chloride pollution—A review. Sci. Total Environ. 2022, 805, 150289. [Google Scholar]
- Evans, M.; Frick, C. The Effects of Road Salts on Aquatic Ecosystems; Environment Canada Water Science and Technology Directorate Contribution No. 02-308; National Water Research Institute: Saskatoon, SK, Canada, 2001. [Google Scholar]
- Schuler, M.S.; Relyea, R.A. A review of the combined threats of road salts and heavy metals to freshwater systems. BioScience 2018, 68, 327–335. [Google Scholar]
- Corsi, S.R.; Graczyk, D.J.; Geis, S.W.; Booth, N.L.; Richards, K.D. A fresh look at road salt: Aquatic toxicity and water-quality impacts on local, regional, and national scales. Environ. Sci. Technol. 2010, 44, 7376–7382. [Google Scholar]
- Jones, D.K.; Mattes, B.M.; Hintz, W.D.; Schuler, M.S.; Stoler, A.B.; Lind, L.A.; Cooper, R.O.; Relyea, R.A. Investigation of road salts and biotic stressors on freshwater wetland communities. Environ. Pollut. 2017, 221, 159–167. [Google Scholar]
- Minnesota Pollution Control Agency. Statewide Chloride Management Plan: Effectively Managing Salt Use to Protect Minnesota’s Lakes and Streams; Minnesota Pollution Control Agency: Saint Paul, MN, USA, 2020; Available online: https://www.pca.state.mn.us/water/draft-statewide-chloride-management-plan (accessed on 21 January 2025).
- Kaushal, S.S.; Groffman, P.M.; Likens, G.E.; Belt, K.T.; Stack, W.P.; Kelly, V.R.; Band, L.E.; Fisher, G.T. Increased salinization of fresh water in the northeastern United States. Proc. Natl. Acad. Sci. USA 2005, 102, 13517–13520. [Google Scholar]
- United States Environmental Protection Agency. Winter Is Coming! And with It, Tons of Salt on Our Roads; United States Environmental Protection Agency: Washington, DC, USA, 2024. Available online: https://www.epa.gov/snep/winter-coming-and-it-tons-salt-our-roads (accessed on 22 January 2025).
- Marsalek, J. Road salts in urban stormwater: An emerging issue in stormwater management in cold climates. Water Sci. Technol. 2003, 48, 61–70. [Google Scholar]
- Novotny, E.V.; Murphy, D.; Stefan, H.G. Increase of urban lake salinity by road deicing salt. Sci. Total Environ. 2008, 406, 131–144. [Google Scholar] [PubMed]
- Perera, N.; Gharabaghi, B.; Howard, K. Groundwater chloride response in the Highland Creek watershed due to road salt application: A re-assessment after 20 years. J. Hydrol. 2013, 479, 159–168. [Google Scholar]
- Anning, D.W.; Finn, M.E. Dissolved-Solids Sources, Loads, Yields, and Concentrations in Streams of the Conterminous United States; Scientific Investigations Report 2014–5012; U.S. Geological Survey: Tucson, AZ, USA, 2014. Available online: https://pubs.usgs.gov/sir/2014/5012/ (accessed on 19 January 2025).
- Procell, C. Road Salt Use. USA Today Network. 2025. Available online: https://infogram.com/road-salt-use-by-state-1h8n6m1971nm6xo (accessed on 19 January 2025).
- Minnesota Pollution Control Agency. Minnesota Stormwater Manual, Chloride Management Plan Combined; Minnesota Pollution Control Agency: Saint Paul, MN, USA, 2022; Available online: https://stormwater.pca.state.mn.us/index.php?title=Chloride_Management_Plan_combined (accessed on 19 January 2025).
- City of Madison. Road Salt in Our Lakes, Waterways, and Drinking Water; City of Madison: Madison, WI, USA, 2025; Available online: https://www.cityofmadison.com/live-work/winter/snow-removal/salt-sustainability/our-lakes-drinking-water (accessed on 19 January 2025).
- United States Environmental Protection Agency. National Recommended Water Quality Criteria—Aquatic Life Criteria Table; United States Environmental Protection Agency: Washington, DC, USA, 2024. Available online: https://www.epa.gov/wqc/national-recommended-water-quality-criteria-aquatic-life-criteria-table (accessed on 21 January 2025).
- Jackson, R.B.; Jobbágy, E.G. From icy roads to salty streams. Proc. Natl. Acad. Sci. USA 2005, 201, 14487–14488. [Google Scholar]
- Kelly, V.R.; Lovett, G.M.; Weathers, K.C.; Findlay, S.E.G.; Strayer, D.L.; Burns, D.J.; Likens, G.E. Long-term sodium chloride retention in a rural watershed: Legacy effects of road salt on streamwater concentration. Environ. Sci. Technol. 2008, 42, 410–415. [Google Scholar]
- Trenouth, W.R.; Gharabaghi, B.; Perera, N. Road salt application planning tool for winter de-icing operations. J. Hydrol. 2015, 524, 401–410. [Google Scholar]
- Gardeshi, M.E.; Arab, H.; Benguit, A.; Drogui, P. Runoff water loaded with road de-icing salts: Occurrence, environmental impact and treatment processes. Water Environ. J. 2024, 38, 20–31. [Google Scholar]
- City of Madison. Road Salt Background and History; City of Madison: Madison, WI, USA, 2025; Available online: https://www.cityofmadison.com/live-work/winter/snow-removal/salt-sustainability/background-history (accessed on 18 March 2025).
- Government of Canada. Road Salts Overview; Government of Canada: Ottawa, ON, Canada, 2025. Available online: https://www.canada.ca/en/environment-climate-change/services/pollutants/road-salts/overview.html (accessed on 18 March 2025).
- United States Environmental Protection Agency. National Pollutant Discharge Elimination System (NPDES): Stormwater Discharges from Municipal Sources; United States Environmental Protection Agency: Washington, DC, USA, 2024. Available online: https://www.epa.gov/npdes/stormwater-discharges-municipal-sources (accessed on 20 January 2025).
- Burns, M.J.; Fletcher, T.M.; Walsh, C.J.; Ladson, A.R.; Hatt, B.E. Hydrologic shortcomings of conventional urban stormwater management and opportunities for reform. Landsc. Urban Plan. 2012, 105, 230–240. [Google Scholar]
- Jefferson, A.J.; Bhaskar, A.S.; Hopkins, K.G.; Fanelli, R.; Avellaneda, P.M.; McMillan, S.K. Stormwater management network effectiveness and implications for urban watershed function: A critical review. Hydrol. Process. 2017, 31, 4056–4080. [Google Scholar]
- Costa, C.S.; Norton, C.; Domene, E.; Hoyer, J.; Marull, J.; Salminen, O. Water as an element of urban design: Drawing lessons from four European case studies. In Sustainable Water Use and Management: Examples of New Approaches and Perspectives; Filho, W.L., Sümer, V., Eds.; Springer International Publishing: Cham, Switzerland, 2015. [Google Scholar]
- Zhang, Y.; Zhao, W.; Jun, C.; Hao, J.; Tang, X.; Zhai, J. Assessment on the effectiveness of urban stormwater management. Water 2021, 13, 4. [Google Scholar]
- Walsh, C.J.; Booth, D.B.; Burns, M.J.; Fletcher, T.D.; Hale, R.L.; Hoang, L.N.; Livingston, G.; Rippy, M.A.; Roy, A.H.; Scoggins, M.; et al. Principles for urban stormwater management to protect stream ecosystems. Freshw. Sci. 2016, 35, 398–411. [Google Scholar]
- Hernández-Hernández, M.; Olcina, J.; Morote, Á.-F. Urban stormwater management, a tool for adapting to climate change: From risk to resource. Water 2020, 12, 2616. [Google Scholar] [CrossRef]
- Zeng, F.; Ma, M.-G.; Di, D.-R.; Shi, W.-Y. Separating the impacts of climate change and human activities on runoff: A review of method and application. Water 2020, 12, 2201. [Google Scholar] [CrossRef]
- Liao, K.-H.; Deng, S.; Tan, P.Y. Blue-green infrastructure: New frontier for sustainable urban stormwater management. In Greening Cities: Forms and Functions; Tan, P.Y., Jim, C.Y., Eds.; Springer Nature: Singapore, 2017. [Google Scholar]
- Khurelbaatar, G.; van Afferden, M.; Ueberham, M.; Stefan, M.; Geyler, S.; Müller, R.A. Management of urban stormwater at block-level (MUST-B): A new approach for potential analysis of decentralized stormwater management systems. Water 2021, 13, 378. [Google Scholar] [CrossRef]
- Maiolo, M.; Palermo, S.A.; Brusco, A.C.; Pirouz, B.; Turco, M.; Vinci, A.; Spezzano, G.; Piro, P. On the use of a real-time control approach for urban stormwater management. Water 2020, 12, 2842. [Google Scholar] [CrossRef]
- Boller, M. Towards sustainable urban stormwater management. Water Sci. Technol. Water Supply 2004, 4, 55–65. [Google Scholar] [CrossRef]
- Cettner, A.; Ashley, R.; Viklander, M.; Nilsson, K. Stormwater management and urban planning: Lessons from 40 years of innovation. J. Environ. Plan. Manag. 2013, 56, 786–801. [Google Scholar] [CrossRef]
- Jusić, S.; Hadžić, E.; Milišić, H. Urban stormwater management—New technologies. In New Technologies, Development and Application II. NT 2019. Lecture Notes in Networks and Systems; Karabegović, I., Ed.; Springer International Publishing: Cham, Switzerland; Volume 76, 2020; Available online: https://link.springer.com/chapter/10.1007/978-3-030-18072-0_90 (accessed on 22 January 2025).
- Dusbabek, I.; Franz, J. Chloride Contamination in Natural Water Sources; Winona State University: Winona, MN, USA, 2023; Available online: https://openriver.winona.edu/cgi/viewcontent.cgi?article=1083&context=wsurrc (accessed on 18 March 2025).
- Roy, A.H.; Wenger, S.J.; Fletcher, T.D.; Walsh, C.J.; Ladson, A.R.; Shuster, W.D.; Thurston, H.W.; Brown, R.R. Impediments and solutions to sustainable, watershed-scale urban stormwater management: Lessons from Australia and the United States. Environ. Manag. 2008, 42, 344–359. [Google Scholar] [CrossRef]
- Rentachintala, L.R.N.P.; Reddy, M.G.M.; Mohapatra, P.K. Urban stormwater management for sustainable and resilient measures and practices: A review. Water Sci. Technol. 2022, 85, 1120. [Google Scholar] [CrossRef]
- City of Winona. Stormwater; City of Winona: Winona, MN, USA, 2025; Available online: https://www.cityofwinona.com/341/Stormwater (accessed on 23 January 2025).
- Izaak Walton League of America. Salt Watch; Izaak Walton League of America: Gaithersburg, MD, USA, 2025; Available online: https://www.iwla.org/water/stream-monitoring/salt-watch (accessed on 13 March 2025).
- Water Rangers. Chloride; Water Rangers: Ottawa, ON, Canada, 2025; Available online: https://waterrangers.com/testkits/tests/chloride/?v=0b3b97fa6688 (accessed on 13 March 2025).
- Laceby, J.P.; Kerr, J.G.; Zhu, D.; Chung, C.; Situ, Q.; Abbasi, S.; Orwin, J.F. Chloride inputs to the North Saskatchewan River watershed: The role of road salts as a potential driver of salinization downstream of North America’s northern most major city (Edmonton, Canada). Sci. Total Environ. 2019, 688, 1056–1068. [Google Scholar] [CrossRef]
- Soper, J.J.; Guzman, C.D.; Kumpel, E.; Tobiason, J.E. Long-term analysis of road salt loading and transport in a rural drinking water reservoir watershed. J. Hydrol. 2021, 603, 127005. [Google Scholar] [CrossRef]
- Canadian Council of Ministers. Canadian Water Quality Guidelines: Chloride Ion Scientific Criteria Document; Canadian Council of Ministers of the Environment: Winnipeg, ON, Canada, 2011. [Google Scholar]
- Szklarek, S.; Stolarska, M.; Wagner, I.; Mankiewicz-Boczek, J. The microbiotest battery as an important component in the assessment of snowmelt toxicity in urban watercourses—Preliminary studies. Environ. Monit. Assess. 2015, 187, 16. [Google Scholar] [CrossRef] [PubMed]
- Minnesota Department of Natural Resources. Climate Trends; Minnesota Department of Natural Resources: Saint Paul, MN, USA, 2025; Available online: https://www.dnr.state.mn.us/climate/climate_change_info/climate-trends.html (accessed on 31 January 2025).
- Barr Engineering. Lake Winona Water Quality Improvement Plan: A Targeted, Prioritized, and Measurable Implementation Plan to Effectively Restore Lake Winona; Barr Engineering: Minneapolis, MN, USA, 2020. [Google Scholar]
- de Santiago-Martin, A.; Michaux, A.; Guesdon, G.; Constantin, B.; Despréaux, M.; Galvez-Cloutier, R. Potential of anthracite, dolomite, limestone and pozzolan as reactive media for de-icing salt removal from road runoff. Int. J. Environ. Sci. Technol. 2016, 13, 2313–2324. [Google Scholar] [CrossRef][Green Version]
- Schweiger, A.H.; Audorff, V.; Beierkuhnlein, C. Salt in the wound: The interfering effect of road salt on acidified forest catchments. Sci. Total Environ. 2015, 532, 595–604. [Google Scholar] [CrossRef]
- Salminen, J.M.; Nystén, T.H.; Tuominen, S. Review of approaches to reducing adverse impacts of road deicing on groundwater in Finland. Water Qual. Res. J. Can. 2011, 46, 166–173. [Google Scholar] [CrossRef]
- Patenaude, J. Changing Winter Driving Habits Could Reduce Salt Use, Ill Effects of Road Salt; Wisconsin Public Radio: Madison, WI, USA, 2025; Available online: https://www.wpr.org/environment/winter-driving-reduce-ill-effects-road-salt (accessed on 21 February 2025).
- Minnesota Pollution Control Agency. Mississippi River—Winona Watershed Pollutant Reduction Project (Total Maximum Daily Load Study) for Nutrients, Sediment and Bacteria; wq-iw9-18e; Minnesota Pollution Control Agency: Saint Paul, MN, USA, 2016. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).