Copper Recovery from Copper Sulfide Ore by Combined Method of Collectorless Flotation and Additive Roasting Followed by Acid Leaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Experimental Procedure
2.2.1. Flotation
2.2.2. Collectorless Flotation
2.2.3. Flotation Kinetics
2.2.4. Roasting
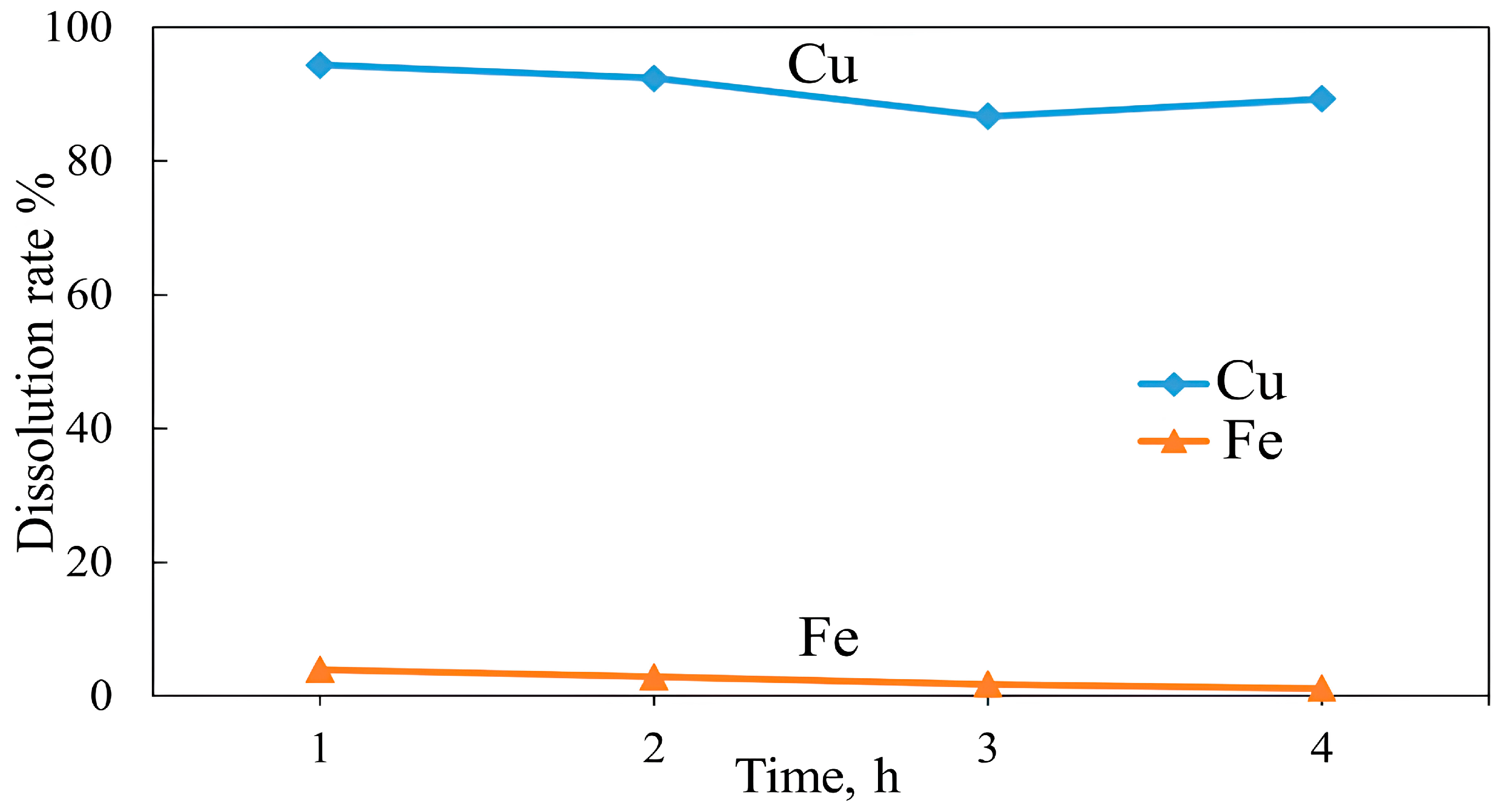
2.2.5. Atmospheric Acid Leaching
3. Results and Discussion
3.1. Flotation
3.1.1. Effect of Flotation Time
3.1.2. Effect of Pulp pH
3.1.3. Effect of Collector Dosage
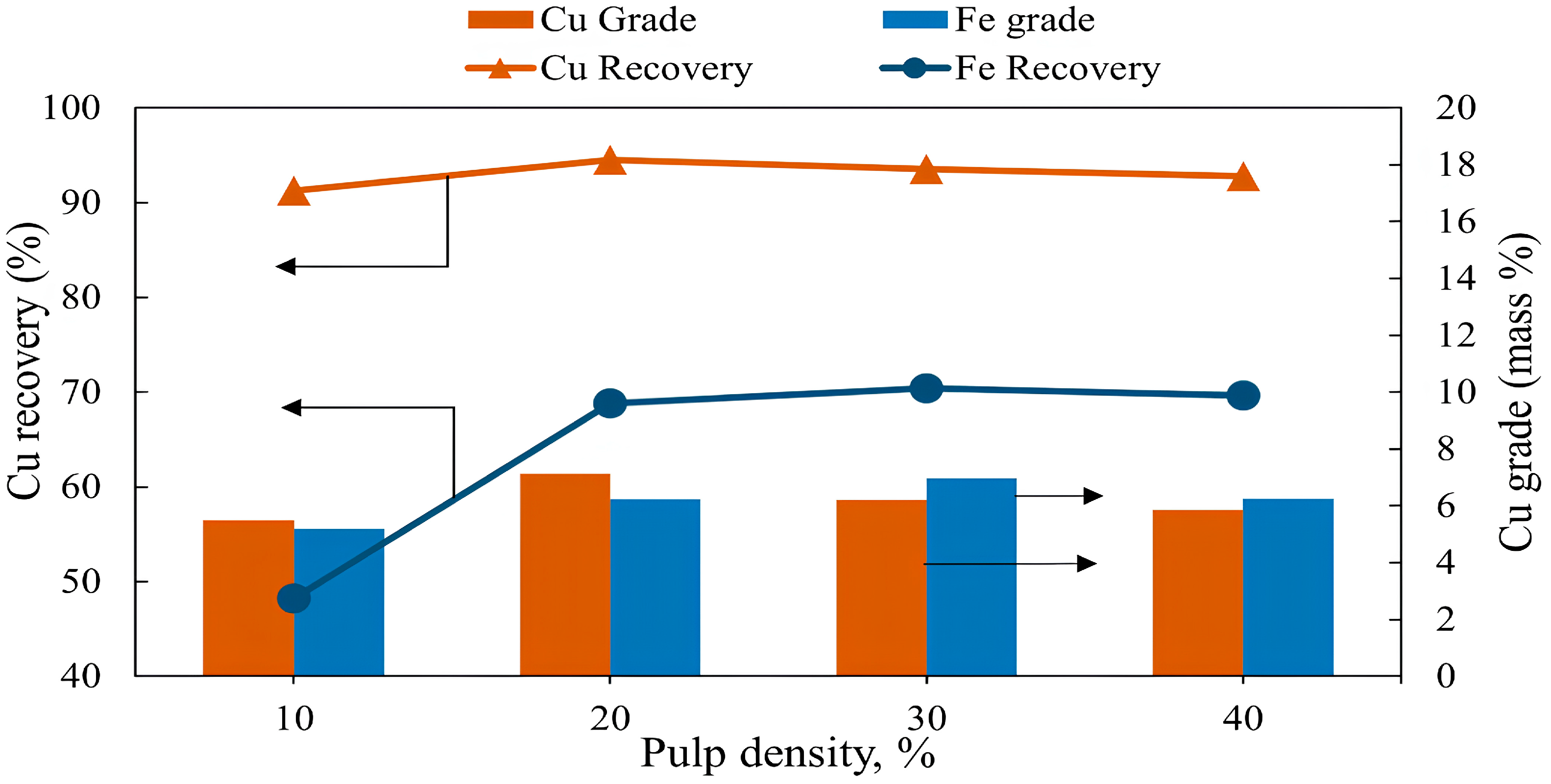
3.1.4. Effect of Pulp Density
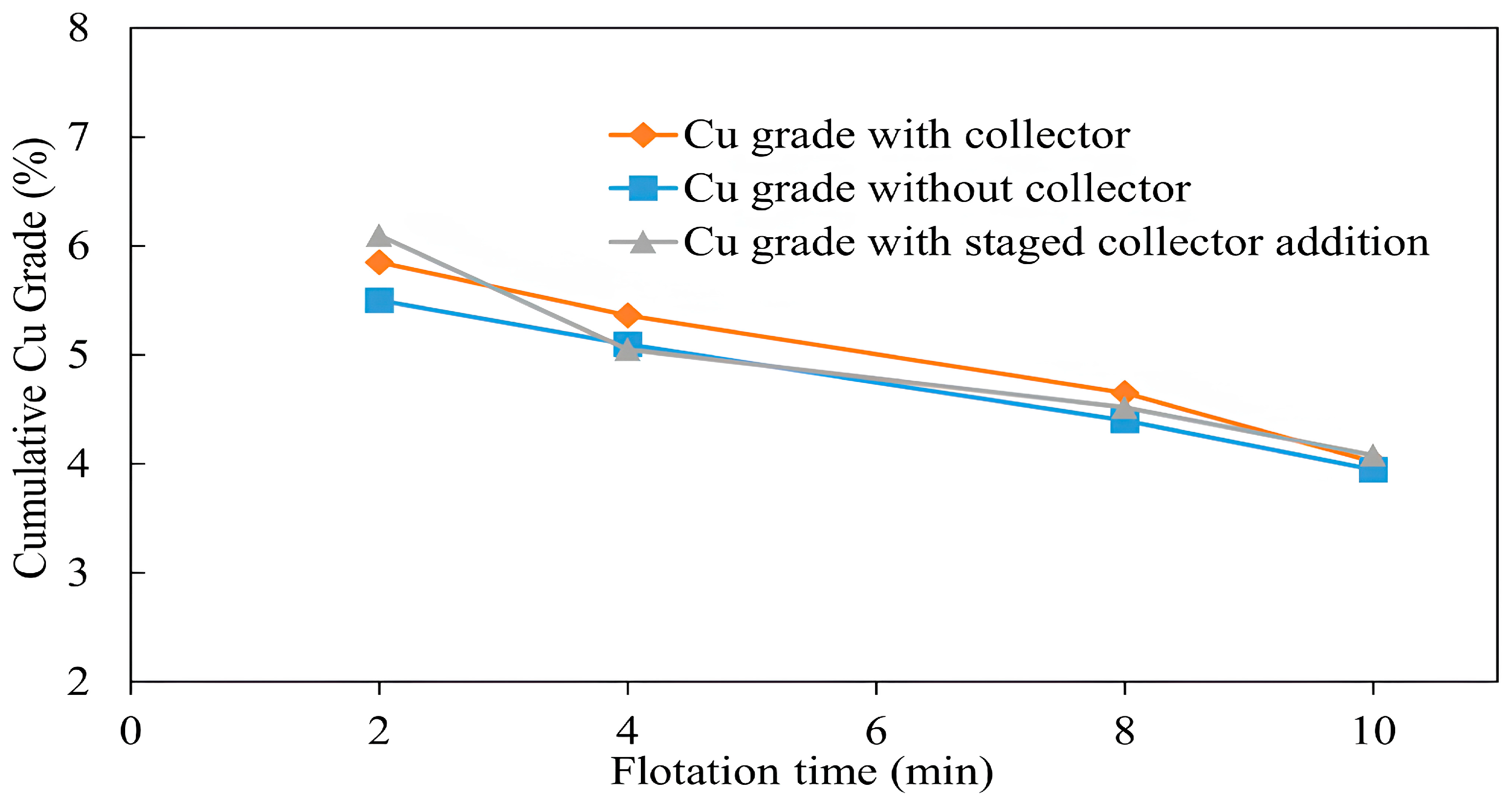
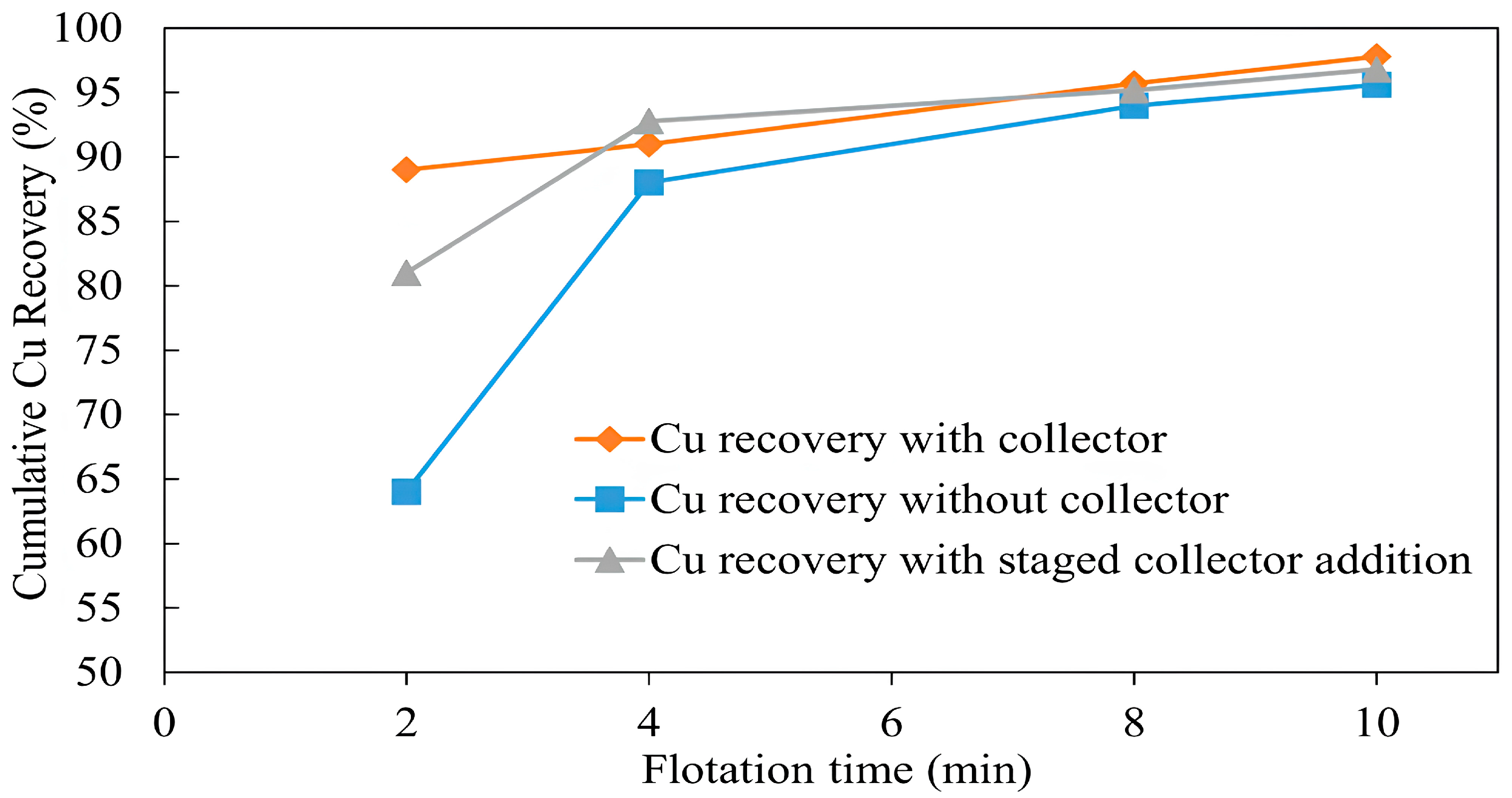
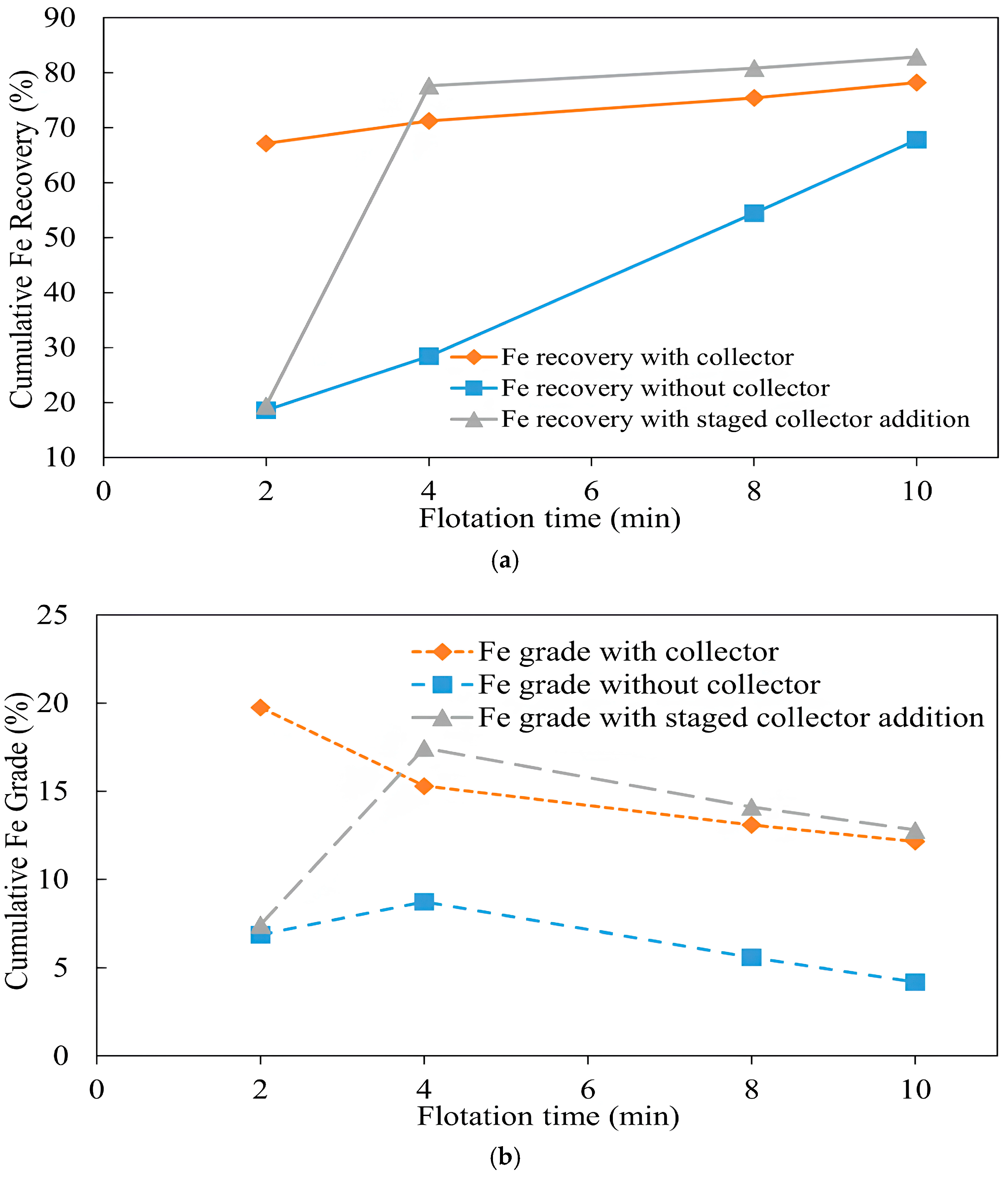
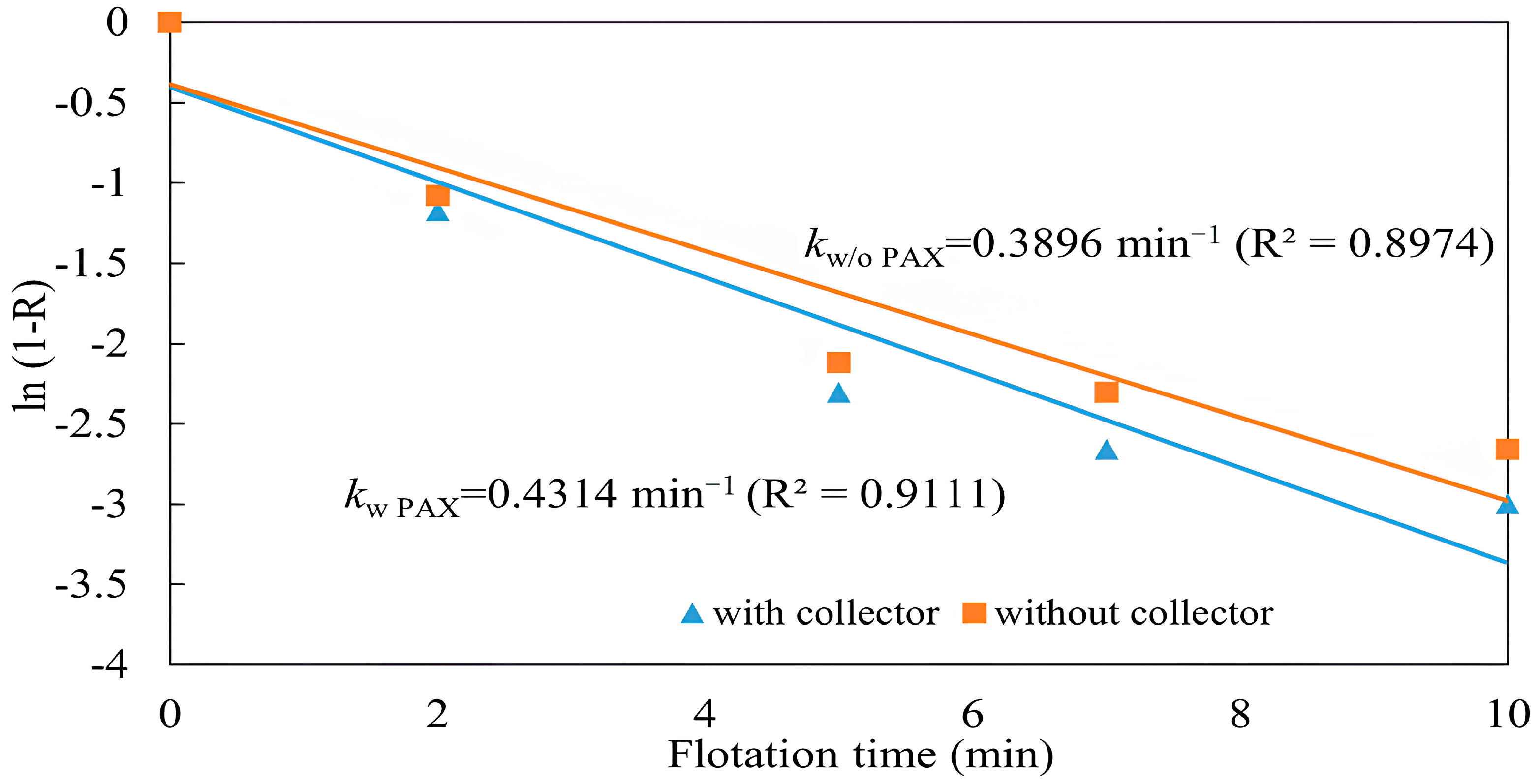
3.1.5. Copper Recovery with and Without a Collector
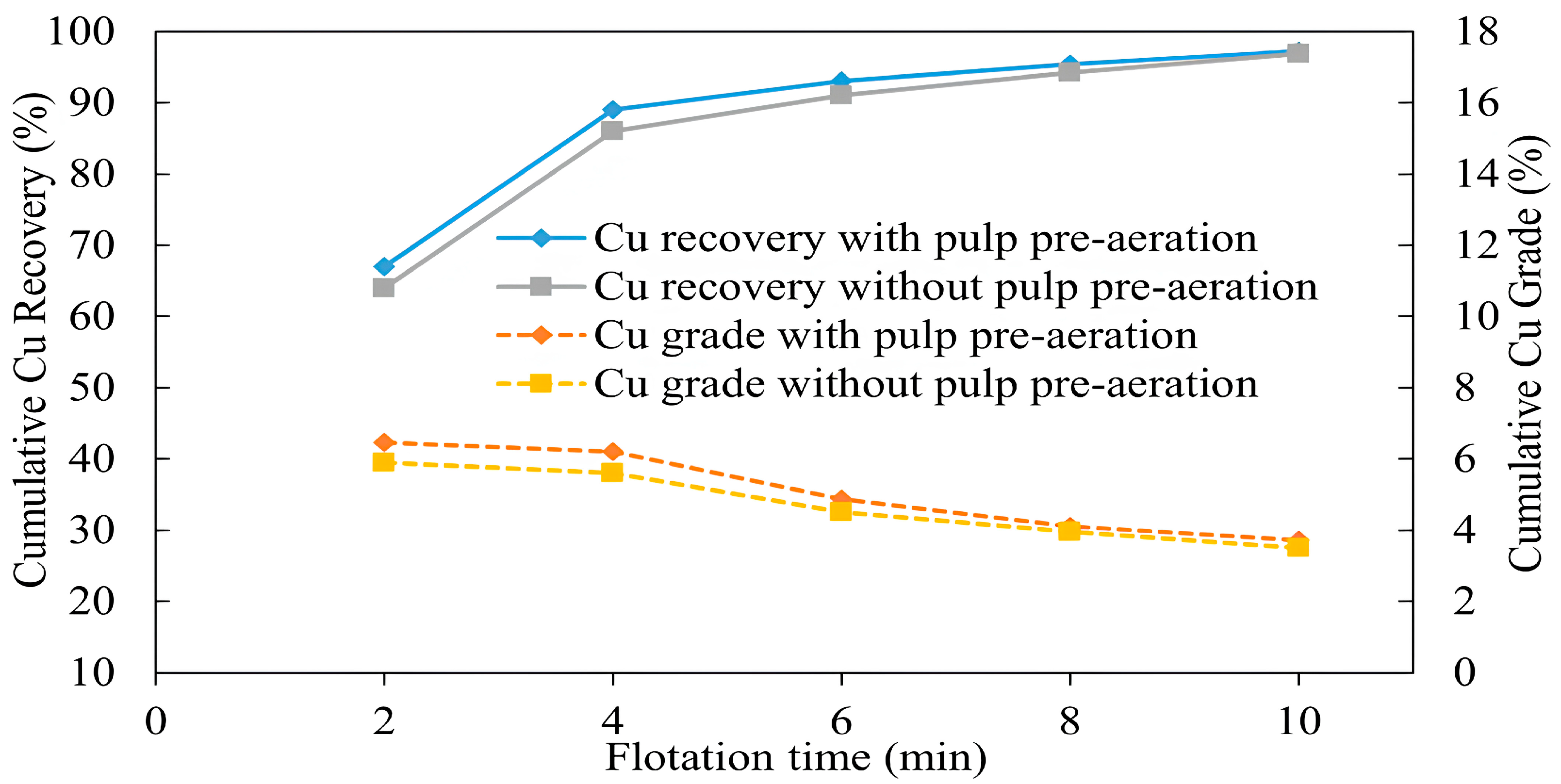
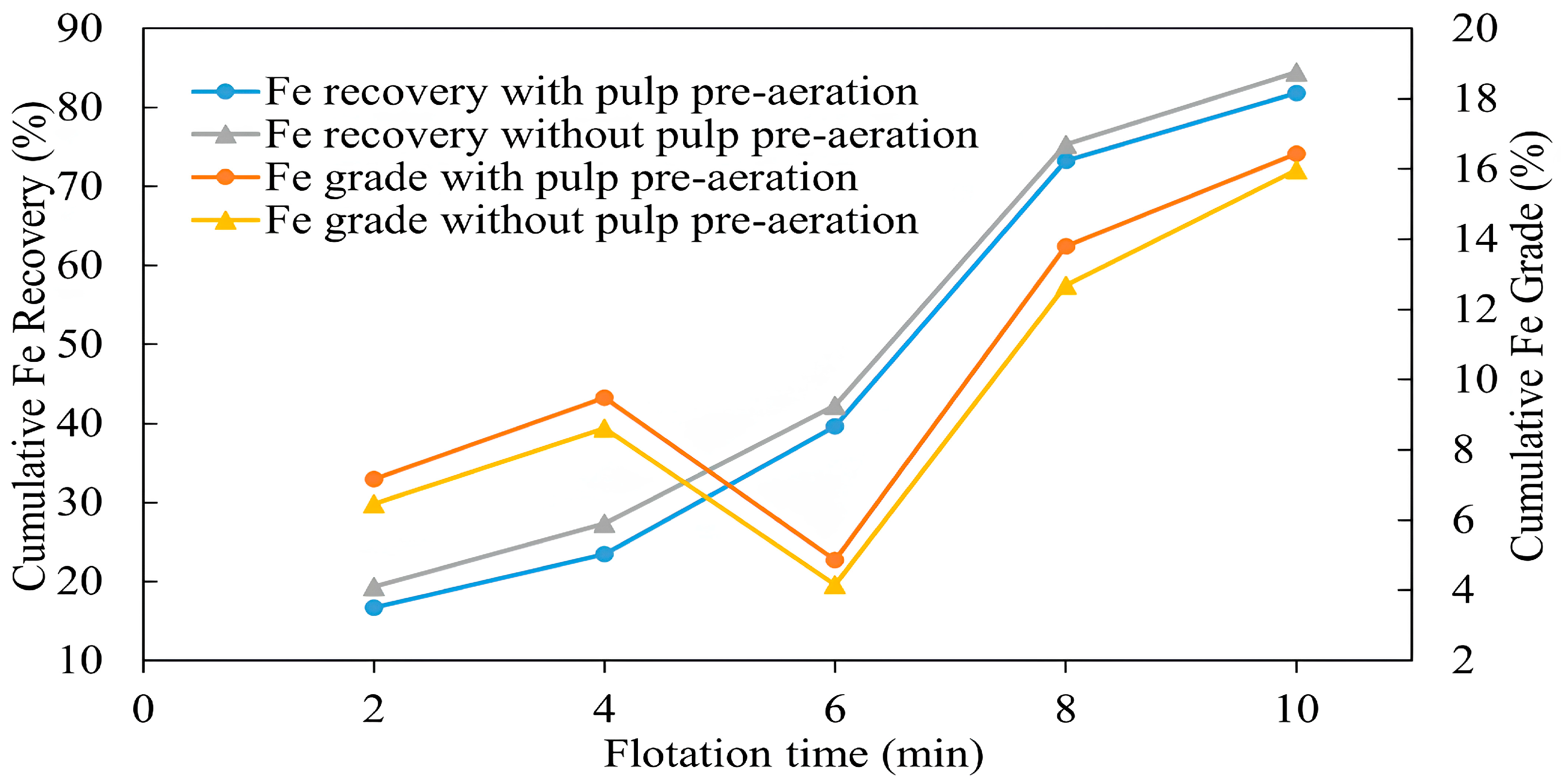
3.1.6. Effect of Aeration on Collectorless Flotation
3.1.7. The Beneficiation of Copper from Copper Sulfide Ore Under Optimum Flotation Conditions
3.2. Flotation Kinetics
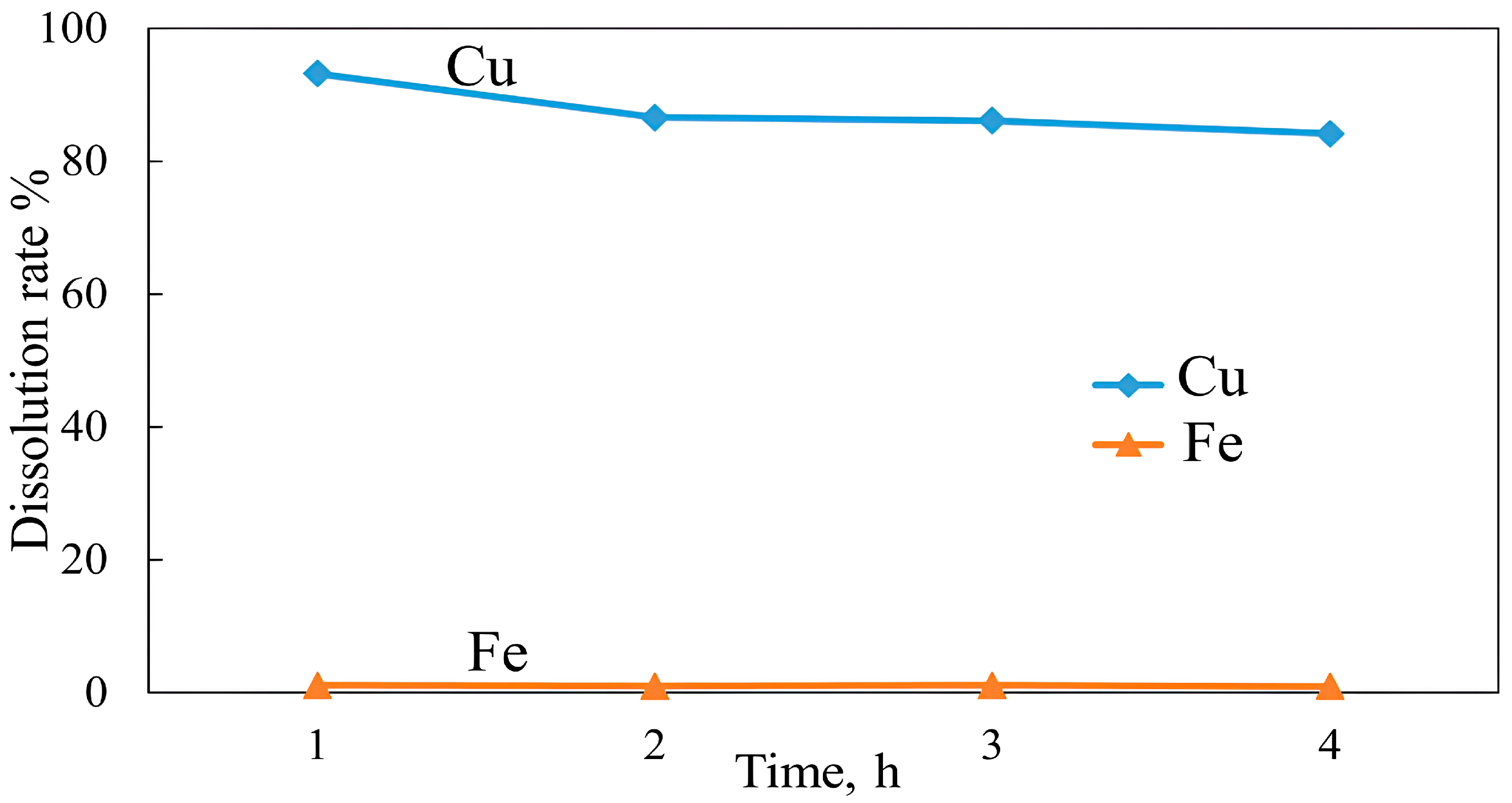
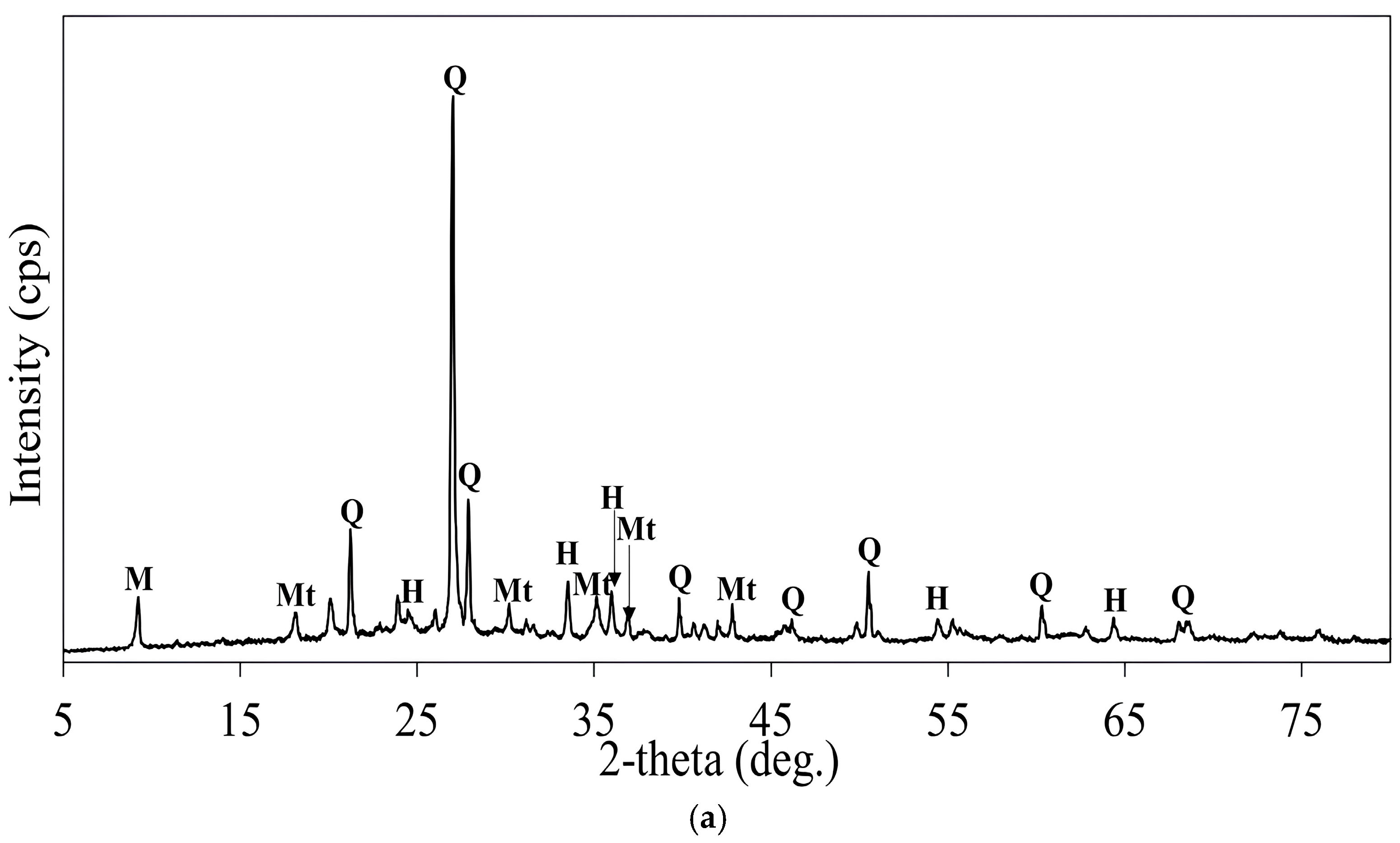
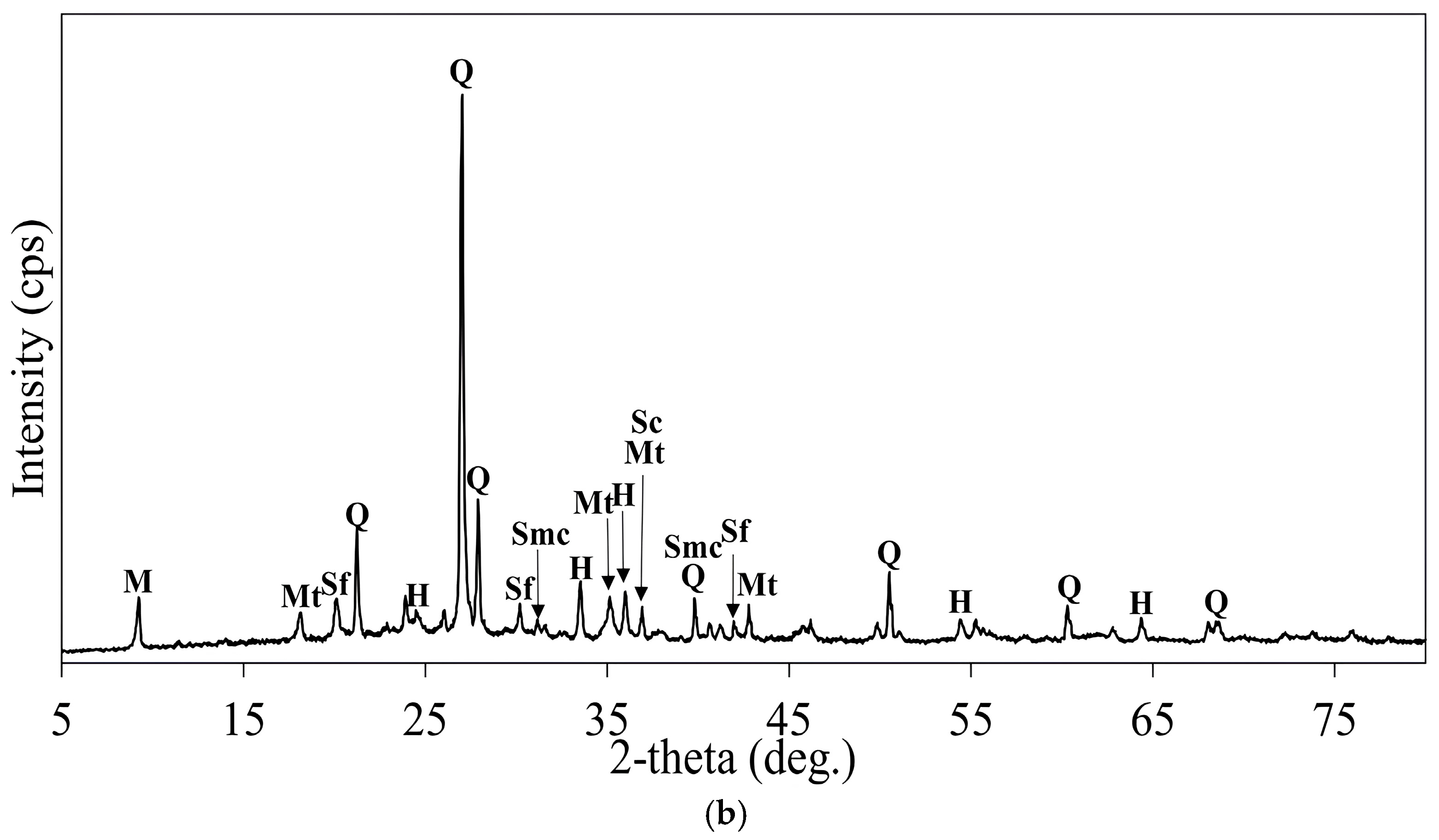
3.3. Roasting and Leaching
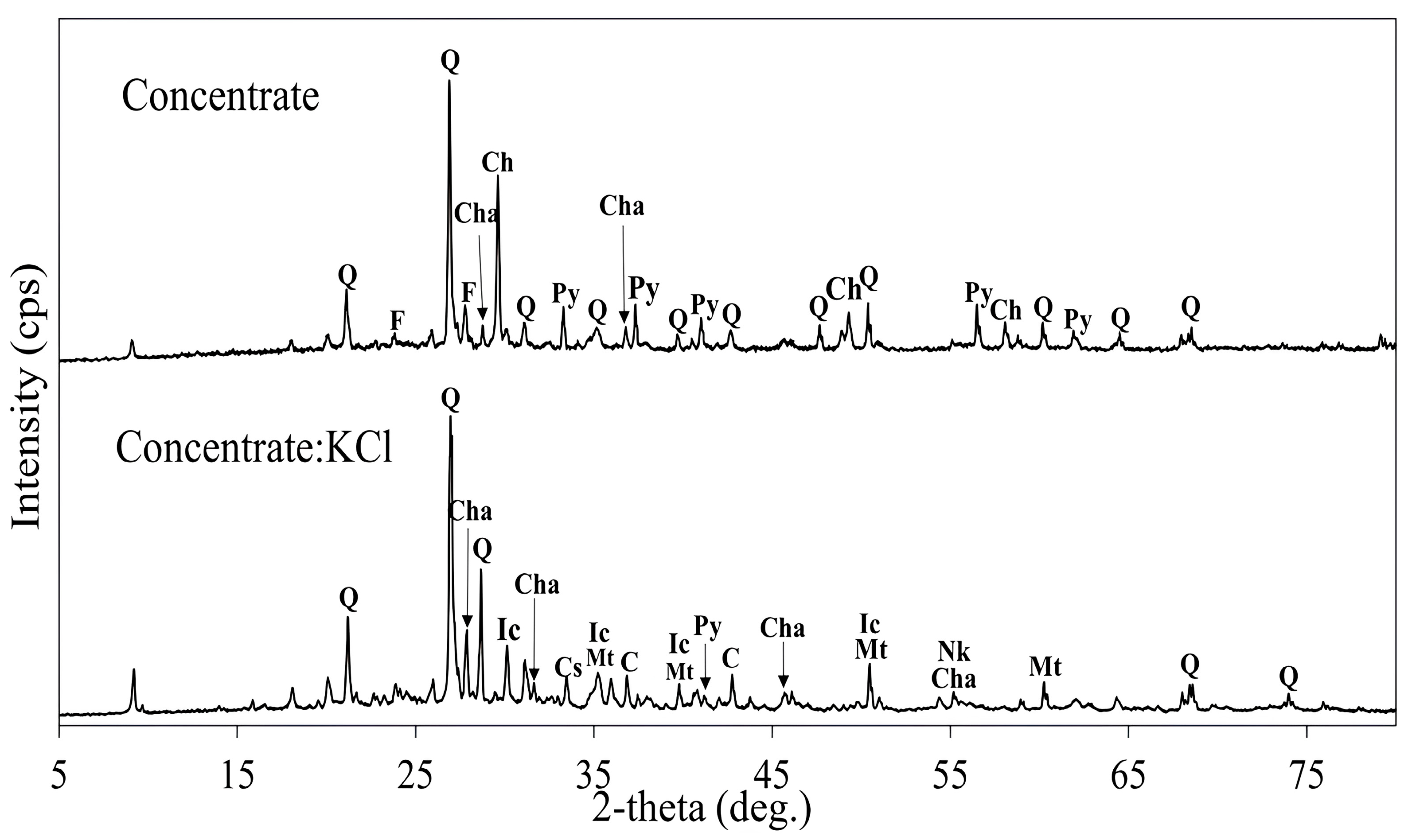
3.3.1. Effect of Concentrate:KCl Mass Ratio
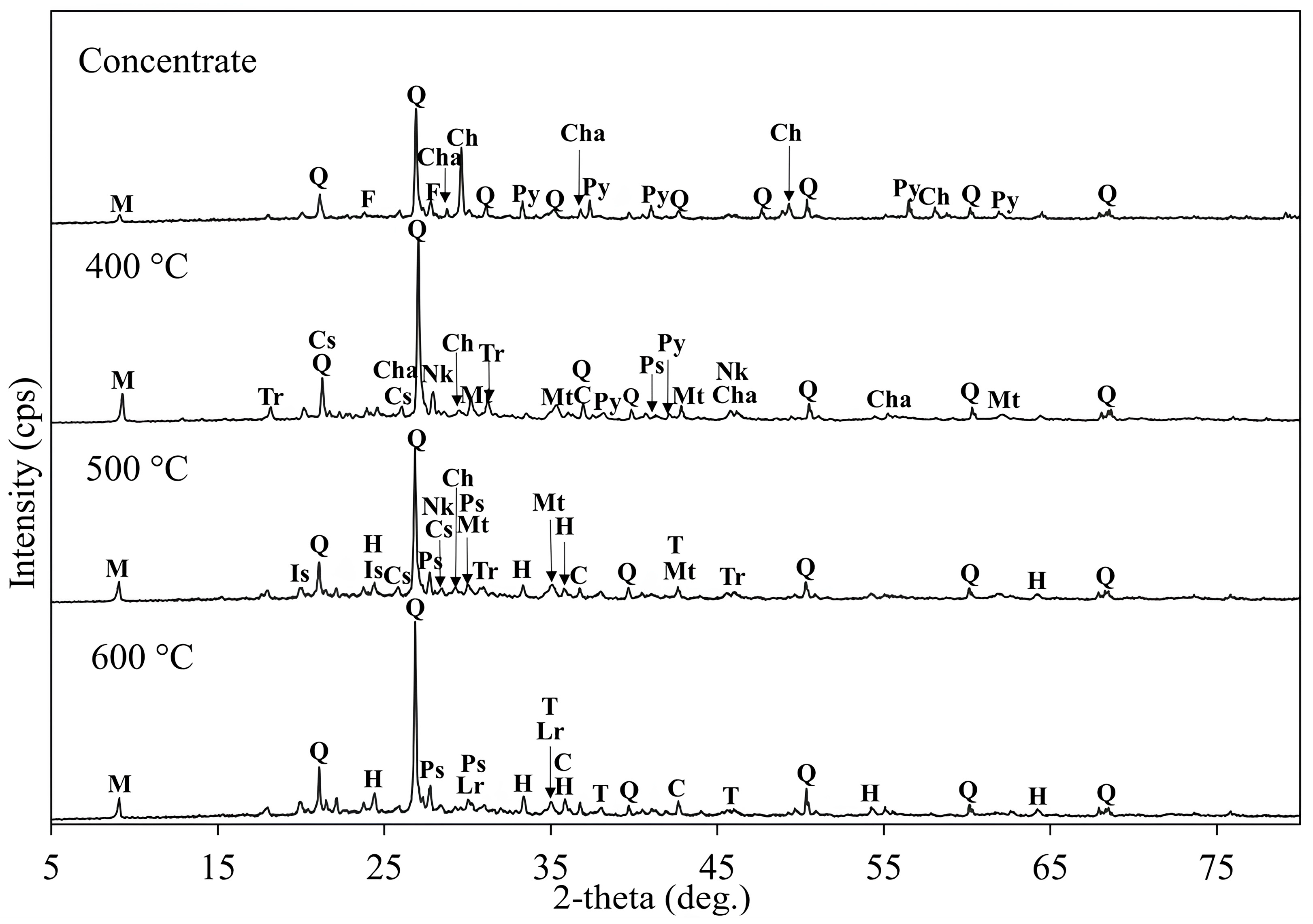
3.3.2. Effect of Roasting Temperature Using KCl Additive
3.3.3. Effect of Roasting Time Using KCl Additive
3.3.4. Effect of Concentrate:NaOH Mass Ratio
3.3.5. Effect of Roasting Temperature Using NaOH Additive
3.3.6. Effect of Roasting Time Using NaOH Additive
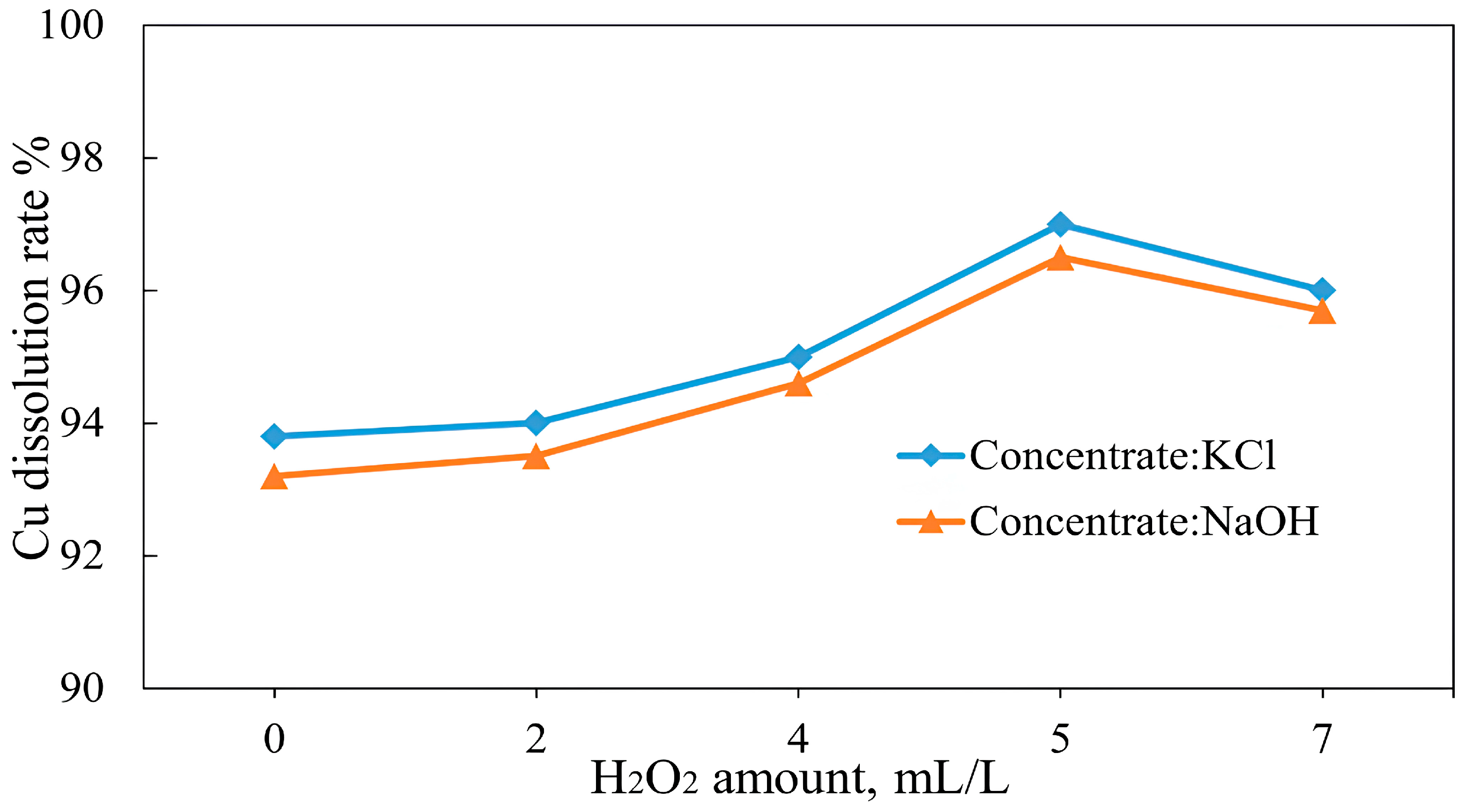
3.4. Effect of an Oxidizing Reagent on Copper Recovery
3.5. A Flowchart for Copper Recovery from Copper Sulfide Ore Using Collectorless Flotation and Additive Roasting
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ndoro, T.O.; Witika, L.K. A review of the flotation of copper minerals. Int. J. Sci. Basic Appl. Res. 2017, 34, 145–165. [Google Scholar]
- Peng, Y.; Bhambhani, T. Preface to the MME special focus issue on managing gangue minerals. Min. Metall. Explor. 2021, 38, 669–671. [Google Scholar] [CrossRef]
- Calvo, G.; Mudd, G.; Valero, A.; Valero, A. Decreasing ore grades in global metallic mining: A Theoretical Issue or a Global Reality? Resources 2016, 5, 36. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Wills’ Mineral Processing Technology: An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Butterworth-Heinemann: London, UK; Elsevier: Waltham, MA, USA, 2015; p. 265. [Google Scholar]
- Liu, J.; Xie, R.; Zhu, Y.; Li, Y.; Liu, C. Flotation behavior and mechanism of styrene phosphonic acid as collector on the flotation separation of fluorite from calcite. J. Mol. Liq. 2021, 326, 115261. [Google Scholar] [CrossRef]
- Adkins, S.J.; Pearse, M.J. The Influences of Collector Chemistry on Kinetics and Selectivity in Base-Metal Sulphide Flotation. Miner. Eng. 1992, 5, 295–310. [Google Scholar] [CrossRef]
- Xu, B.; Zhong, S.; Wu, J.; Zhou, Y.; Yang, Y.; Li, Q.; Jiang, T. A comprehensive recovery process for selective separation and enrichment of copper, zinc and iron minerals from a polymetallic ore and the adsorption mechanism of collector Z–200. Minerals 2022, 12, 384. [Google Scholar] [CrossRef]
- Qiu, X.; Huang, Z.; Cao, F.; Sun, D.; Wang, P.; Chen, C. Flotation separation of chalcopyrite from pyrite using a novel O-n-butyl-N-isobutyl thionocarbamate as the selective collector. Colloids Surf. A Physicochem. Eng. Asp. 2023, 661, 130890. [Google Scholar] [CrossRef]
- Fairthorne, G.; Fornasiero, D.; Ralston, J. Effect of oxidation on the collectorless flotation of chalcopyrite. Int. J. Miner. Process. 1997, 49, 31–48. [Google Scholar] [CrossRef]
- Khoso, S.A.; Hu, Y.; Liu, R.; Tian, M.; Sun, W.; Gao, Y.; Han, H.; Gao, Z. Selective depression of pyrite with a novel functionally modified biopolymer in a Cu–Fe flotation system. Miner. Eng. 2019, 135, 55–63. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice: Volume 1: Flotation of Sulfide Ores; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 0080471374. [Google Scholar]
- Castellón, C.I.; Toro, N.; Gálvez, E.; Robles, P.; Leiva, W.H.; Jeldres, R.I. Froth Flotation of Chalcopyrite/Pyrite Ore: A Critical Review. Materials 2022, 15, 6536. [Google Scholar] [CrossRef]
- Heyes, G.W.; Trahar, W.J. The natural flotability of chalcopyrite. Int. J. Miner. Process. 1977, 4, 317–344. [Google Scholar] [CrossRef]
- Gardner, J.R.; Woods, R. An electrochemical investigation of the natural floatability of chalcopyrite. Int. J. Miner. Process. 1979, 6, 1–16. [Google Scholar] [CrossRef]
- Luttrell, G.H.; Yoon, R.H. Surface studies of the collectorless flotation of chalcopyrite. Colloids Surf. 1984, 12, 239–254. [Google Scholar] [CrossRef]
- Smart, R.S.C.; Amarantidis, J.; Skinner, W.; Prestidge, C.A.; LaVanier, L.; Grando, S. Surface analytical studies of oxidation and collector adsorption in sulphide mineral flotation. Scanning Microsc. 1998, 12, 553–583. [Google Scholar]
- Hu, Y.; Sun, W.; Wang, D. Natural Floatability and Collectorless Flotation of Sulphide Minerals. In Electrochemistry of Flotation of Sulphide Minerals; Hu, Y., Sun, W., Wang, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 20–52. [Google Scholar]
- Chakravortty, M.; Srikanth, S. Kinetics of salt roasting of chalcopyrite using KCl. Thermochim. Acta 2000, 362, 25–35. [Google Scholar] [CrossRef]
- Prasad, S.; Pandey, B.D. Alternative processes for treatment of chalcopyrite—A review. Miner. Eng. 1998, 11, 763–781. [Google Scholar] [CrossRef]
- Hua, Y.; Cai, C.; Cui, Y. Microwave-enhanced roasting of copper sulfide concentrate in the presence of CaCO3. Sep. Purif. Technol. 2006, 50, 22–29. [Google Scholar] [CrossRef]
- Tumen-Ulzii, N.; Garnaad, A.; Silam, A.; Tumendelger, A.; Gunchin, B.; Shirchinnamjil, N.; Haga, K.; Shibayama, A.; Batnasan, A. Copper recovery from chalcopyrite concentrate by oxidative roasting and acid leaching. Int. J. Soc. Mater. Eng. Resour. 2022, 25, 56–62. [Google Scholar] [CrossRef]
- Prater, J.D.; Queneau, P.B.; Hudson, T.J. The sulphation of copper-iron sulphides with concentrated sulphuric acid. J. Met. 1970, 22, 23–27. [Google Scholar]
- Dutrizac, J.E.; MacDonald, J.C. Ferric ion as a leaching medium. Min. Sci. Eng. 1974, 6, 59–100. [Google Scholar]
- Haver, F.P.; Wang, M.M. Recovery of Cu, Fe and S from chalcopyrite concentrate using a ferric chloride leach. J. Met. 1971, 23, 25–29. [Google Scholar]
- Roman, R.J.; Benner, B.R. The dissolution of copper concentrates. Miner. Sci. Eng. 1973, 5, 3–24. [Google Scholar]
- Bjorling, G.; Faldt, I.; Lindgren, E.; Toromanov, I. A nitric acid route in combination with solvent extraction for hydrometallurgical treatment of chalcopyrite. In Proceedings of the International Symposium on Copper Extraction and Refining, Las Vegas, NV, USA, 22–26 February 1976. [Google Scholar]
- Han, B.; Batnasan, A.; Haga, K.; Takasaki, Y.; Shibayama, A. Copper recovery from silicate-containing low-grade copper ore using flotation followed by high-pressure oxidative leaching. Resour. Process. 2017, 64, 3–14. [Google Scholar] [CrossRef]
- Marcuson, S.W. Roasting of copper concentrate in presence of additives. Miner. Sci. Eng. 1980, 12, 21–36. [Google Scholar]
- Medvedev, A.S.; So, T.; Ptitsyn, A.M. Combined processing technology of the Udokan sulfide copper concentrate. Russ. J. Non-Ferr. Met. 2012, 53, 125–128. [Google Scholar] [CrossRef]
- Medvedev, A.S.; Tu, S. Characteristics of electrochemical reactions accompanying chlorinating annealing of copper sulfide concentrates. Russ. J. Non-Ferr. Met. 2013, 54, 1–4. [Google Scholar] [CrossRef]
- Li, G.; Zou, X.; Cheng, H.; Geng, S.; Xiong, X.; Xu, Q.; Zhou, Z.; Lu, X. A novel ammonium chloride roasting approach for the high-efficiency co-sulfation of nickel, cobalt, and copper in polymetallic sulfide minerals. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2020, 51, 2769–2784. [Google Scholar] [CrossRef]
- Ma, H.F.; Liao, Y.L.; Wu, M.; Jia, X.B.; Yang, S.Y. Phase transformation mechanism of oxidation roasting of low-grade polymetallic chalcopyrite ore in the presence of CaO. J. Min. Metall. Sect. B-Metall. 2023, 59, 375–382. [Google Scholar] [CrossRef]
- Eksteen, J.J.; Oraby, E.A.; Tanda, B.C. A conceptual process for copper extraction from chalcopyrite in alkaline glycinate solutions. Miner. Eng. 2017, 108, 53–66. [Google Scholar] [CrossRef]
- Parkes, S.; Wang, P.; Galvin, K.P. Investigating the system flotation kinetics of fine chalcopyrite in a REFLUX™ flotation cell using a standardised flotation cell reference method. Miner. Eng. 2022, 178, 107411. [Google Scholar] [CrossRef]
- Han, H.; Yin, W.; Yang, B.; Wang, D.; Yao, J.; Zhu, Z. Adsorption behavior of sodium oleate on iron minerals and its effect on flotation kinetics. Colloids Surf. A Physicochem. Eng. Asp. 2022, 647, 129108. [Google Scholar] [CrossRef]
- Zhao, J.; Godirilwe, L.L.; Haga, K.; Yamada, M.; Shibayama, A. Flotation behavior and surface analytical study of synthesized (octylthio) aniline and bis(octylthio)benzene as novel collectors on sulfide minerals. Miner. Eng. 2023, 204, 108422. [Google Scholar] [CrossRef]
- Bu, X.; Xie, G.; Peng, Y.; Ge, L.; Ni, C. Kinetics of flotation. Order of process, rate constant distribution and ultimate recovery. Physicochem. Probl. Miner. Process. 2017, 53, 342–365. [Google Scholar]
- Reyes, M.; Patino, F.; Escudero, R.; P’erez, M.; Flores, M.U.; Reyes, I.A. Kinetics and hydrodynamics of silver ion flotation. J. Mex. Chem. Soc. 2012, 56, 408–416. [Google Scholar]
- Buckley, A.N.; Woods, R. An x-ray photoelectron spectroscopic study of the oxidation of chalcopyrite. Aust. J. Chem. 1984, 37, 2403–2413. [Google Scholar] [CrossRef]
- Hu, Y.; Sun, W.; Wang, D. Corrosive Electrochemistry of Oxidation-Reduction of Sulphide Minerals. In Electrochemistry of Flotation of Sulphide Minerals; Springer: Berlin/Heidelberg, Germany, 2009; pp. 167–200. [Google Scholar]
- Leppinen, J.O. FTIR and Flotation Investigation of the Adsorption of Ethyl Xanthate on Activated and Non-Activated Sulfide Minerals. Int. J. Miner. Process. 1990, 30, 245–263. [Google Scholar] [CrossRef]
- Miao, Y.; Zhao, G.; Fang, X.; Yan, H.; Qiu, X.; Yang, C.; Zhang, S.; Qiu, T. Investigation the effect of filling materials on chalcopyrite flotation. Powder Technol. 2024, 431, 119049. [Google Scholar] [CrossRef]
- Zheng, X.; Manton, P. A potential application of collectorless flotation in a copper/gold operation. Miner. Eng. 2010, 23, 895–902. [Google Scholar] [CrossRef]
- Paria, S.; Khilar, K.C. A Review on Experimental Studies of Surfactant Adsorption at the Hydrophilic Solid–Water Interface. Adv. Colloid. Interface Sci. 2004, 110, 75–95. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant Adsorption Isotherms: A Review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef]
- Lynch, A.J.; Johnson, N.W.; Manlapig, E.V.; Thorne, C.G. Mineral and Coal Flotation Circuits; Their Simulation and Control; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1981; pp. 21–56. [Google Scholar]
- Schubert, H. On the optimization of hydrodynamics in flotation process. In Proceedings of the 13th International Mineral Processing Congress, Warsaw, Poland, 4–9 June 1979. [Google Scholar]
- Runge, K.; Tabosa, E.; Crosbie, R.; McMaster, J. Effect of flotation feed density on the operation of a flotation cell. In Proceedings of the 11th AusIMM Mill Operators’ Conference, Hobart, TAS, Australia, 29–31 October 2012. [Google Scholar]
- Buckley, A.N.; Woods, R. An X-ray photoelectron spectroscopic study of the oxidation of galena. Appl. Surf. Sci. 1984, 17, 401–414. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Cases, J.M.; Alnot, M.; Ehrhardt, J.J. XPS characterization of chalcopyrite, tetrahedrite, and tennantite surface products after different conditioning. 1. Aqueous solution at pH 10. Langmuir 1996, 12, 2519–2530. [Google Scholar] [CrossRef]
- Chander, S. Electrochemistry of sulfide flotation: Growth characteristics of surface coatings and their properties, with special reference to chalcopyrite and pyrite. Int. J. Miner. Process. 1991, 33, 121–134. [Google Scholar] [CrossRef]
- Buckley, A.N.; Hamilton, I.C.; Woods, R. Investigation of the surface oxidation of bornite by linear potential sweep voltammetry and X-ray photoelectron spectroscopy. J. Appl. Electrochem. 1984, 14, 63–74. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. The surface oxidation of pyrite. Appl. Surf. Sci. 1987, 27, 347–452. [Google Scholar] [CrossRef]
- Goktepe, F.; Williams, K.P. Electrochemical effects in flotation of a Turkish complex sulfide ore. Miner. Eng. 1995, 8, 1035–1048. [Google Scholar] [CrossRef]
- Xu, M.; Finch, J.A.; Rao, S.R.; Lin, D. Reverse flotation of pyrite from a zinc concentrate using nitrogen. Miner. Eng. 1995, 8, 1159–1173. [Google Scholar] [CrossRef]
- Forson, P.; Zanin, M.; Skinner, W.; Asamoah, R. Differential flotation of pyrite and Arsenopyrite: Effect of pulp aeration and the critical importance of collector concentration. Miner. Eng. 2022, 178, 107421. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The depression of pyrite in selective flotation by different reagent systems—A Literature review. Miner. Eng. 2016, 96-97, 143–156. [Google Scholar] [CrossRef]
- Kuopanportti, H.; Suorsa, T.; Dahl, O.; Niinimaki, J. A model of conditioning in the flotation of a mixture of pyrite and chalcopyrite ores. Int. J.Miner. Process. 2000, 59, 327–338. [Google Scholar] [CrossRef]
- Owusu, C.; Fornasiero, D.; Addai-Mensah, J.; Zanin, M. Influence of pulp aeration on the flotation of chalcopyrite with xanthate in chalcopyrite/pyrite mixtures. Int. J. Miner. Process. 2015, 134, 50–57. [Google Scholar] [CrossRef]
- Agorhom, E.A.; Skinner, W.; Zanin, M. Post-regrind selective depression of pyrite in pyritic copper–gold flotation using aeration and diethylenetriamine. Miner. Eng. 2015, 72, 36–46. [Google Scholar] [CrossRef]
- Ekmekçi, Z.; Demirel, H. Effects of Galvanic Interaction on Collectorless Flotation Behaviour of Chalcopyrite and Pyrite. Int. J. Miner. Process. 1997, 52, 31–48. [Google Scholar] [CrossRef]
- You, L.Q.; Heping, L.; Li, Z. Study of Galvanic Interactions between Pyrite and Chalcopyrite in a Flowing System: Implications for the Environment. Environ. Geol. 2007, 52, 11–18. [Google Scholar]
- Sokić, M.; Ilić, I.; Živković, D.; Vučković, N. Investigation of mechanism and kinetics of chalcopyrite concentrate oxidation process. Metalurgija 2008, 47, 109–113. [Google Scholar]
- Mitovski, A.; Štrbac, N.; Manasijević, D.; Sokić, M. Thermal analysis and kinetics of the chalcopyrite-pyrite concentrate oxidation process. Metalurgija 2015, 54, 311–314. [Google Scholar]
- Atesoglu, G.; Atilgan, İ. Effect of roasting temperature on the leaching of chalcopyrite concentrate in sulphuric acid. Min. Metall. Explor. 2022, 39, 2199–2208. [Google Scholar] [CrossRef]
- Dunn, J.; Ginting, A.; O’Connor, B. A thermoanalytical study of the oxidation of chalcocite. J. Therm. Anal. 1994, 41, 671–686. [Google Scholar] [CrossRef]
- Dunn, J.G.; Muzenda, C. Thermal oxidation of covellite (CuS). Thermochim. Acta 2001, 369, 117–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Liu, X.; Xu, B.; Yang, Y.; Jiang, T.A. Thermodynamic analysis on the roasting of pyrite. Minerals 2019, 9, 220. [Google Scholar] [CrossRef]
- Mohammadabad, F.K.; Hejazi, S.; Khaki, J.V.; Babakhani, A. Mechanochemical leaching of chalcopyrite concentrate by sulfuric acid. Int. J. Miner. 2016, 23, 380–388. [Google Scholar] [CrossRef]
- Petrović, S.J.; Bogdanović, G.D.; Antonijević, M.M.; Vukčević, M.; Kovačević, R. The extraction of copper from chalcopyrite concentrate with hydrogen peroxide in sulfuric acid solution. Metals 2023, 13, 1818. [Google Scholar] [CrossRef]
- Xiao, Y.; Yang, Y.; van den Berg, J.; Sietsma, J.; Agterhuis, H.; Visser, G.; Bol, D. Hydrometallurgical recovery of copper from complex mixtures of end-of-life shredded ICT products. Hydrometallurgy 2013, 140, 128–134. [Google Scholar] [CrossRef]
- Antonijević, M.M.; Dimitrijević, M.; Janković, Z. Leaching of pyrite with hydrogen peroxide in sulphuric acid. Hydrometallurgy 1997, 46, 71–83. [Google Scholar] [CrossRef]
- Mahajan, V.; Misra, M.; Zhong, K.; Fuerstenau, M.C. Enhanced leaching of copper from chalcopyrite in hydrogen peroxide–glycol system. Miner. Eng. 2007, 20, 670–674. [Google Scholar] [CrossRef]
- Pereira, O.C.; Bernardin, A.M. Ceramic colorant from untreated iron ore residue. J. Hazard. Mater. 2012, 233–234, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Prim, S.R.; Folgueras, M.V.; de Lima, M.A.; Hotza, D. Synthesis and characterization of hematite pigment obtained from a steel waste industry. J. Hazard. Mater. 2011, 192, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Meena; Gumber, K. Magnetic nanoparticles as emerging sorbents for efficient wastewater treatment. Mater. Today Proc. 2022, 68, 817–823. [Google Scholar] [CrossRef]
- Handayani, I.; Rasyid, M.A.; Fadhilah, R.; Gusti, H.I.K. Beneficiation processing of magnetite ore from Lampung as dense media for dense medium separator in coal washing plant. In Proceedings of the IPMC 2023, Bandung, Indonesia, 12–13 September 2023. [Google Scholar]
- Ersoy, L.; Akhoundnejad, Y.; Daşgan, H.Y.; Temur, B. The effect of potassium sulphate applications on plant growth and nutrient content of pepper plants grown under high temperature stress. Dergipark Akad. 2024, 13, 51–64. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Lin, X.; Mao, T.; Zhu, Z.; Lv, J.; Fu, C.; Chen, S.; Wu, A.; Li, X.; et al. Study on three-stage counter-current water washing desalination characteristics and mechanism of high chlorine waste incineration fly ash. Processes 2022, 10, 2540. [Google Scholar] [CrossRef]
- Lv, X.; Song, K.; Xin, Y.; Lv, X. Novel process for deep removal of chlorine and recycling of chlorinated tailings from titanium-bearing blast-furnace slag. Process Saf. Environ. Prot. 2022, 159, 842–849. [Google Scholar] [CrossRef]
- Zhao, K.; Hu, Y.; Tian, Y.; Chen, D.; Feng, Y. Chlorine removal from MSWI fly ash by thermal treatment: Effects of iron/aluminum additives. J. Environ. Sci. 2020, 88, 112–121. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, Y.; Xia, Z.; Zhao, X.; Zhou, Z.; Ye, L. Green and circular method for chloride separation from acid wastewater: Application in zinc smelter. Sep. Purif. Technol. 2022, 283, 120221. [Google Scholar] [CrossRef]
- Heponiemi, A.; Pesonen, J.; Tynjälä, P.; Lassi, U. Use of waste sodium sulfate from battery chemical production in the preparation of alkali-activated materials. ACS Sustain. Res. Man. 2024, 1, 1400–1407. [Google Scholar] [CrossRef]
- Ogedengbe, A.; Achiobu, K.; Scoccimarro, S.; Brunet, S.; Gagnon, G.; Fabrik, M.; Ibrahim, H. Valorization of sodium sulfate waste to potassium sulfate fertilizer: Experimental studies, process modeling, and optimization. Int. J. Green Ener. 2020, 17, 605–618. [Google Scholar] [CrossRef]
- Chemical & Engineering News. Available online: https://cen.acs.org/energy/battery-industrys-sodium-sulfate-waste/102/i21 (accessed on 9 July 2024).
- Takasu, H.; Hoshino, H.; Tamura, Y.; Kim, S.T.; Kato, Y. Sodium ferrite/carbon dioxide reactivity for high temperature thermochemical energy storage. ISIJ Inter. 2019, 59, 715–720. [Google Scholar] [CrossRef]
- Jabeen, T.; Khan, M.S.; Javaid, S.; Azeem, W.; Ayoub, R.; Motola, M. Synergistic effects of β-NaFeO2 ferrite nanoparticles for photocatalytic degradation, antibacterial, and antioxidant applications. RSC Adv. 2024, 14, 12513–12527. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Asari, T.; Yoshida, H.; Yaabuuchi, N.; Shiiba, H.; Nakayama, M.; Komaba, S. Understanding the structural evolution and redox mechanism of a NaFeO2–NaCoO2 solid solution for sodium-ion batteries. Adv. Funct. Mater. 2016, 26, 6047–6059. [Google Scholar] [CrossRef]
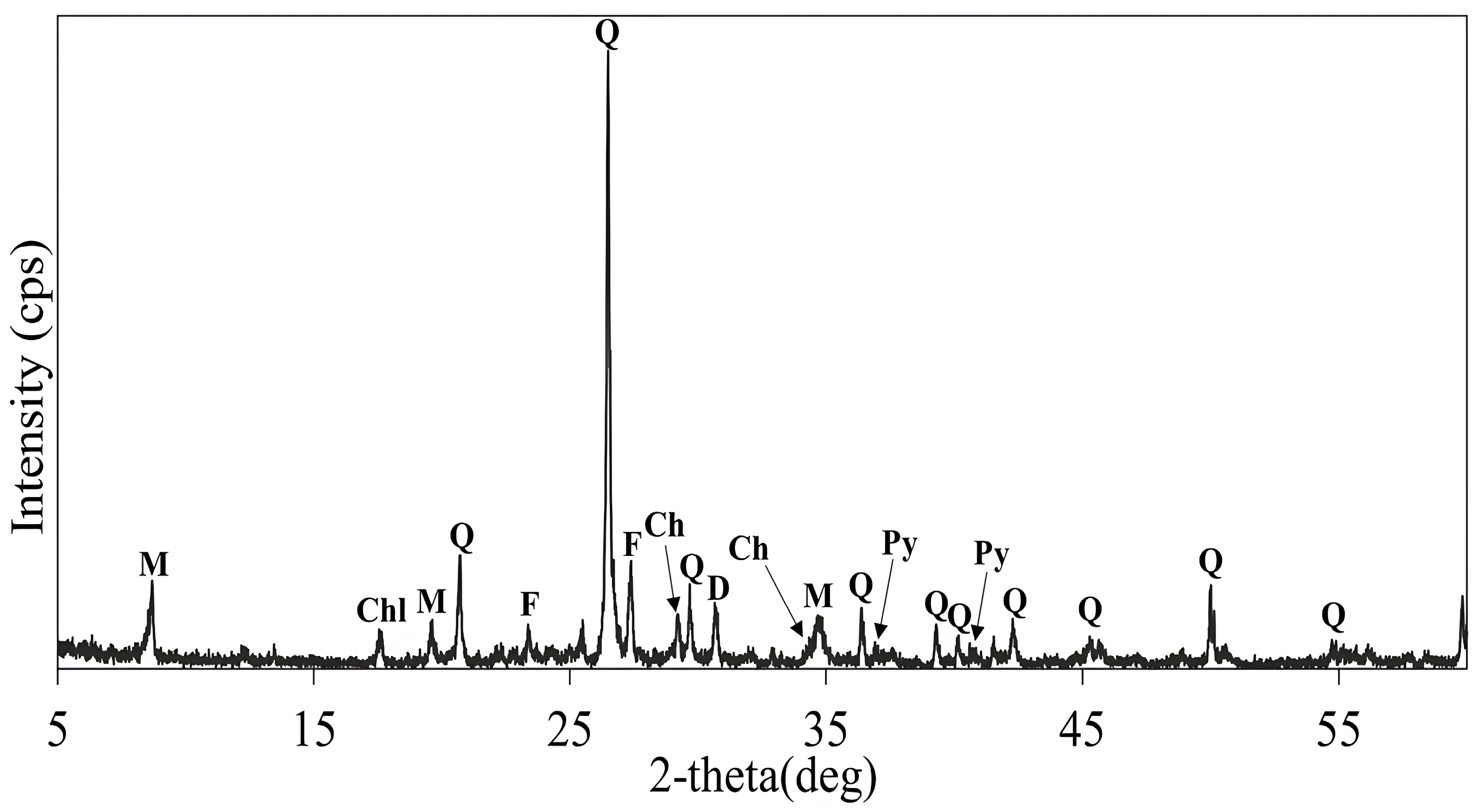


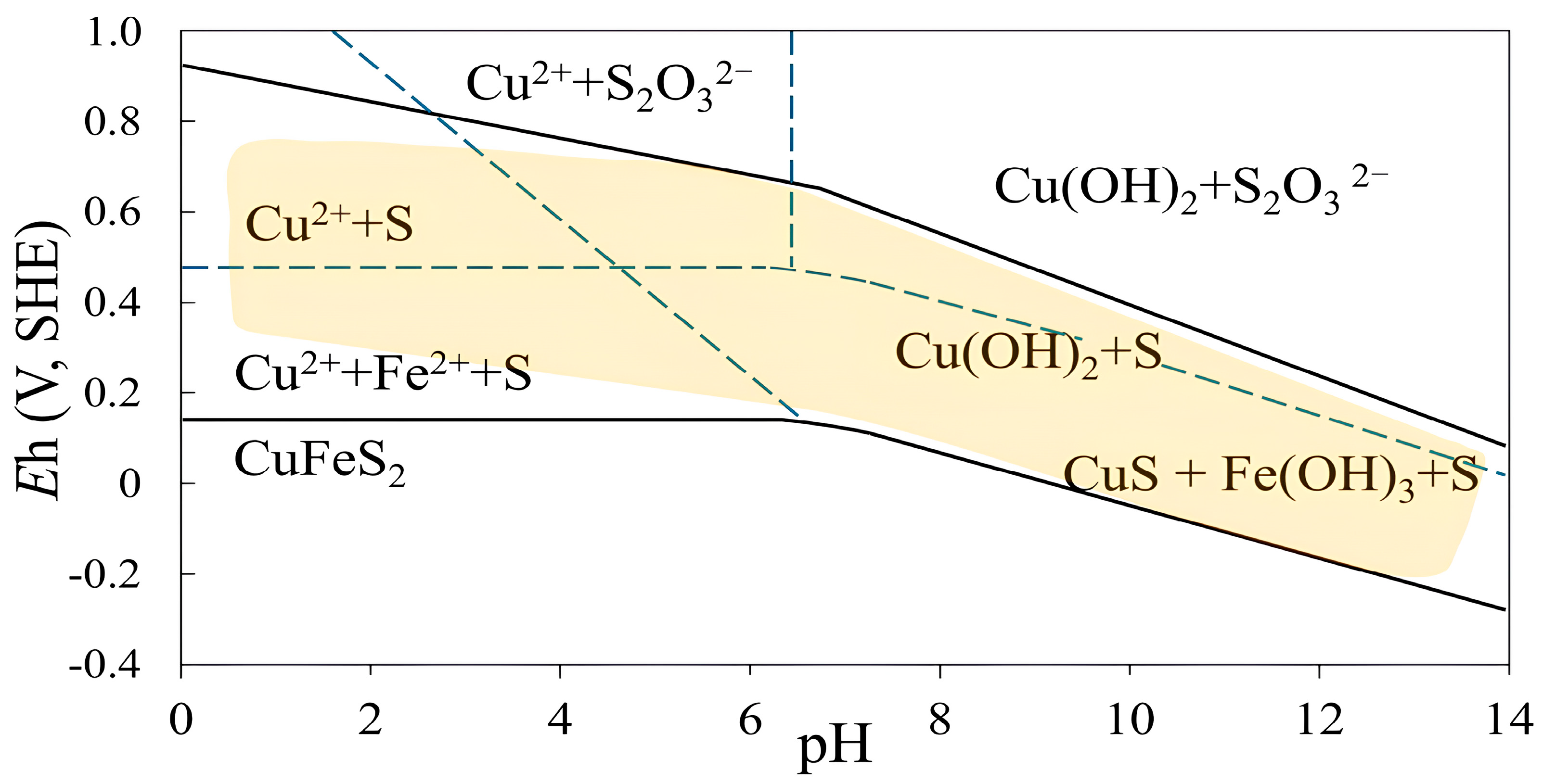
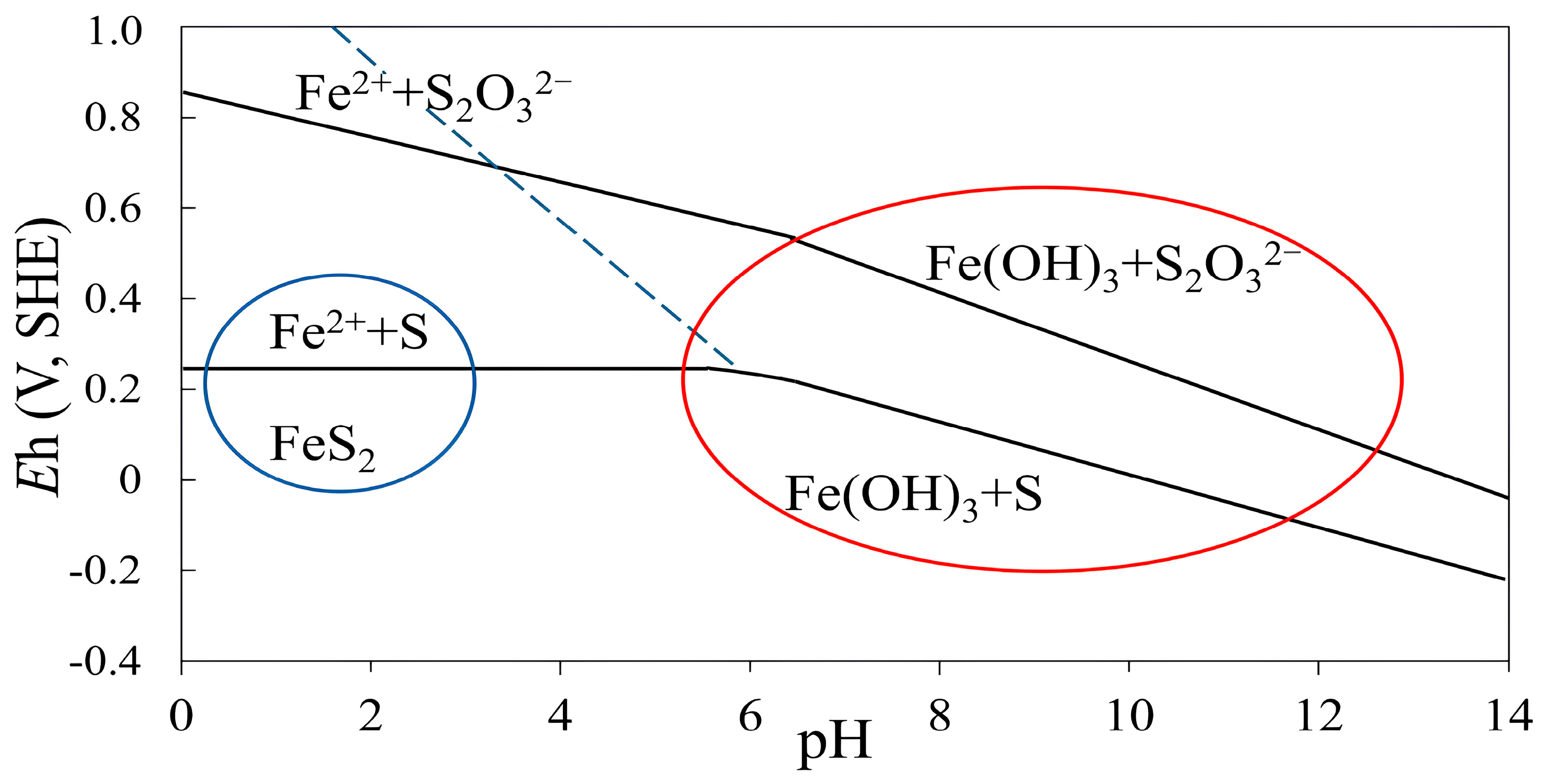
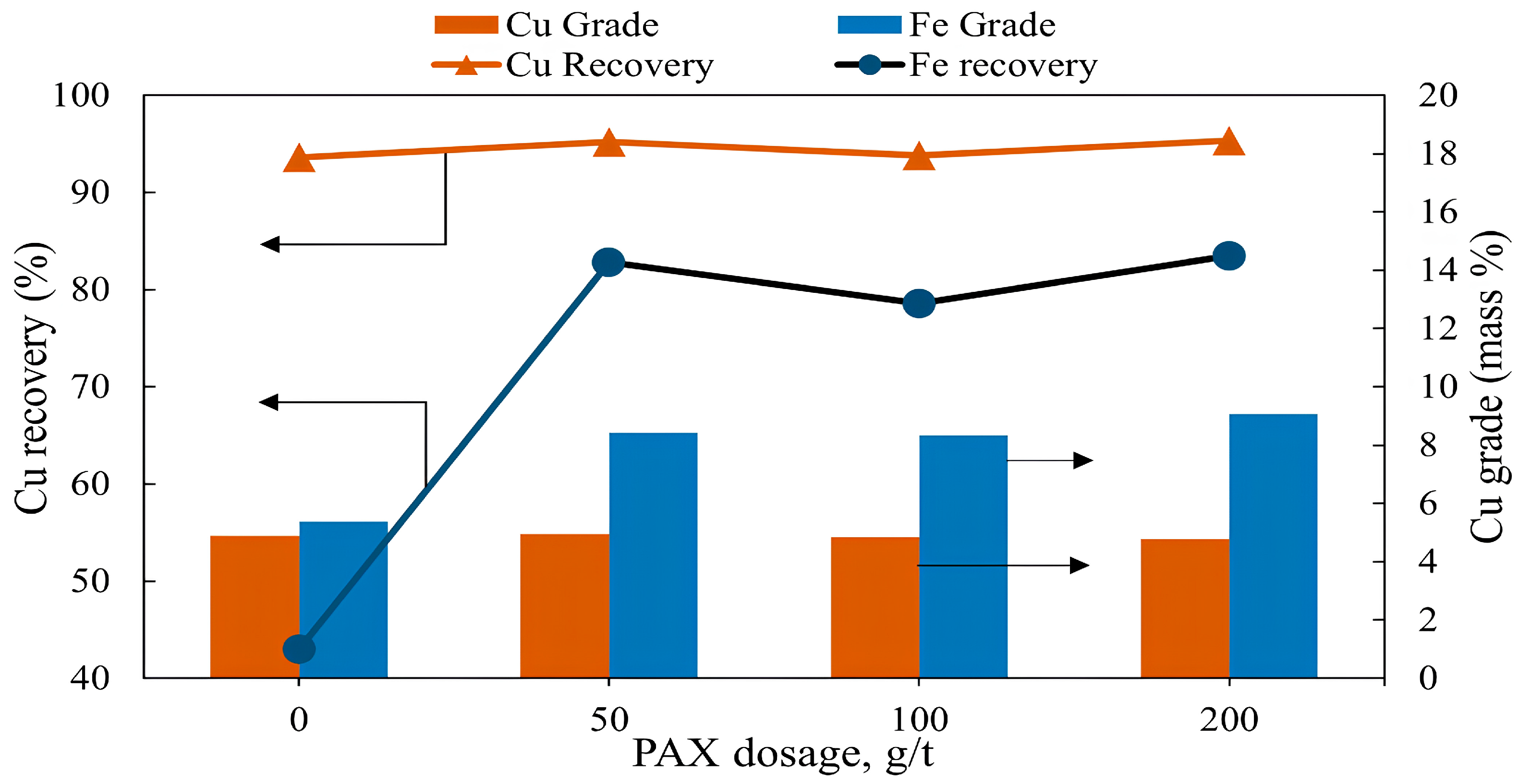




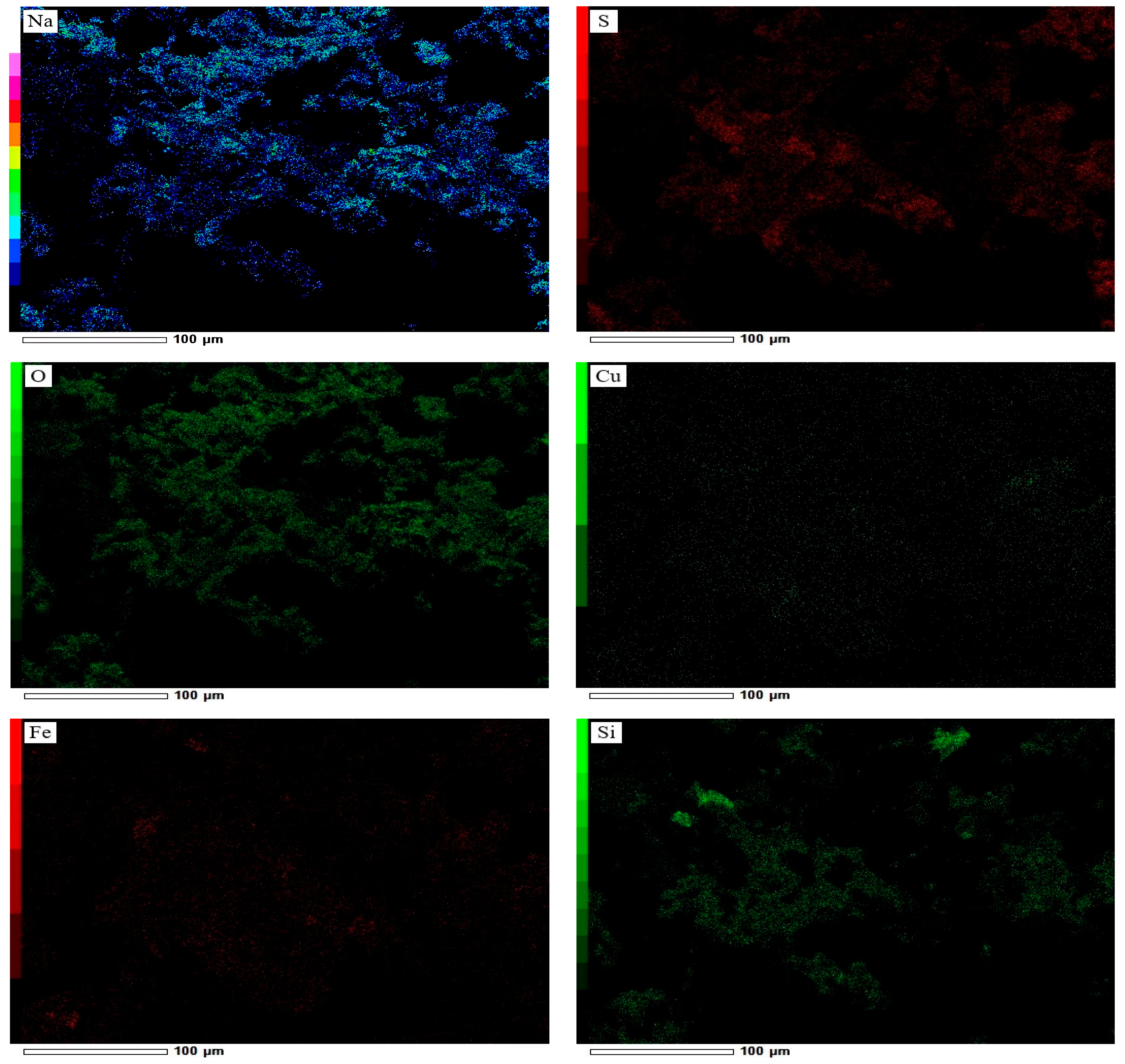
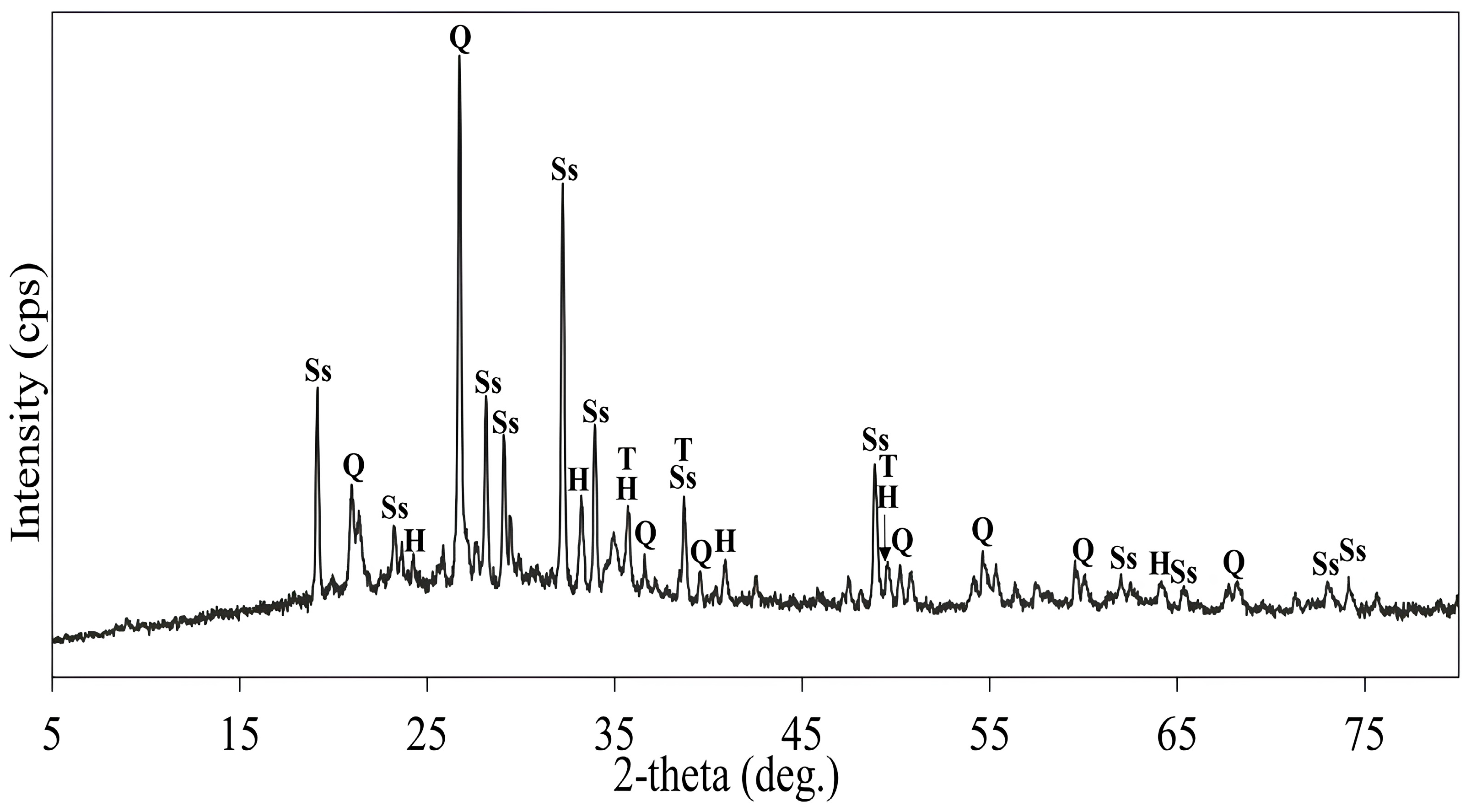

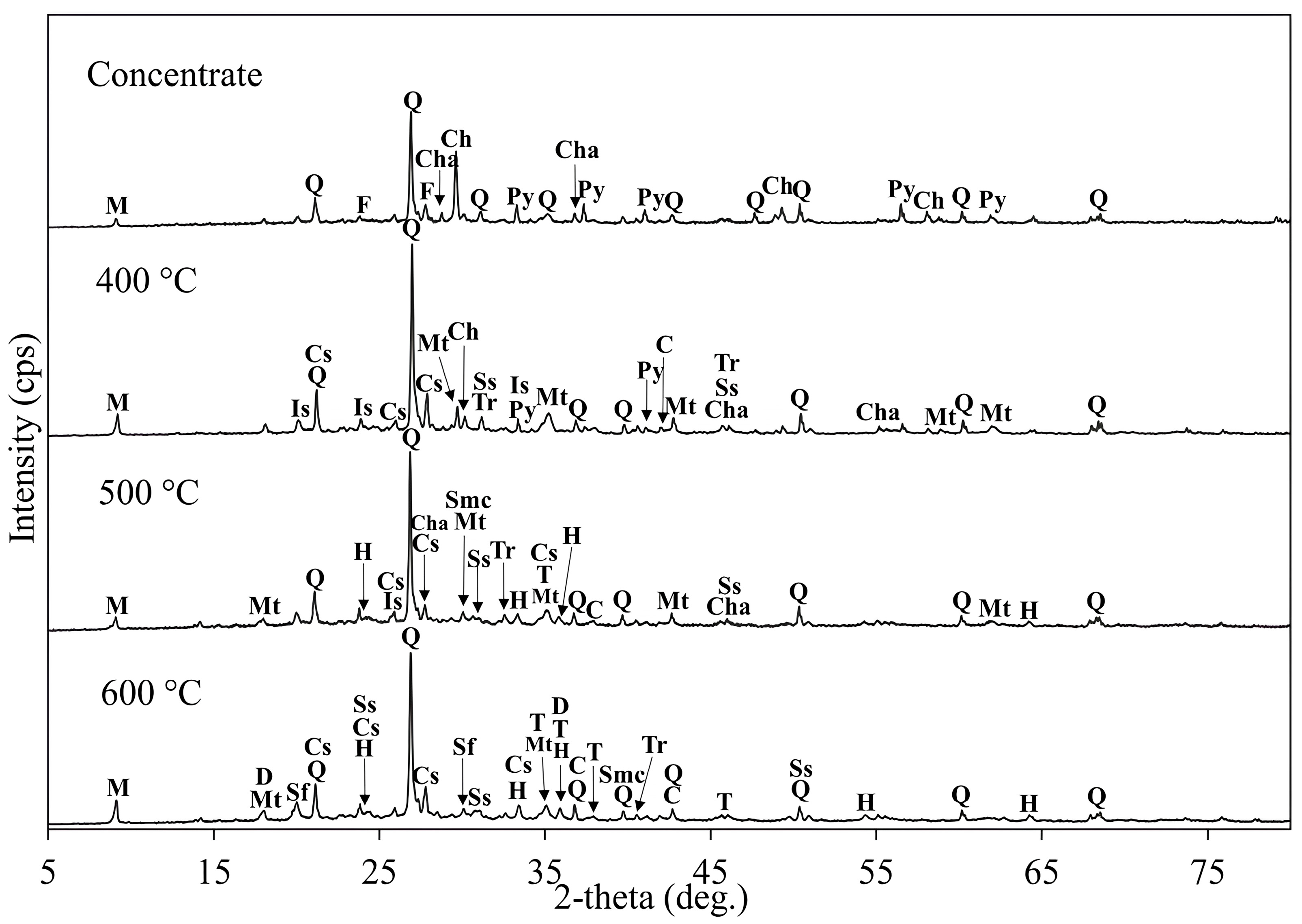

| Cu | Fe | S | Al | SiO2 | K | Mg | Ca |
| 0.94 | 4.14 | 11.17 | 9.85 | 43.54 | 5.76 | 1.37 | 1.14 |
| Parameters | Conditions | Cu Recovery,% | Cu Grade, Mass% |
|---|---|---|---|
| Flotation time | 7 min | ||
| Pulp pH | 10 | 94.5 | 7.12 |
| Pulp density | 20% | ||
| Collector dosage | 0 g/t ore |
| Sample | Grade, Mass% | |||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Fe | S | Al | SiO2 | K | Mg | Ca | |
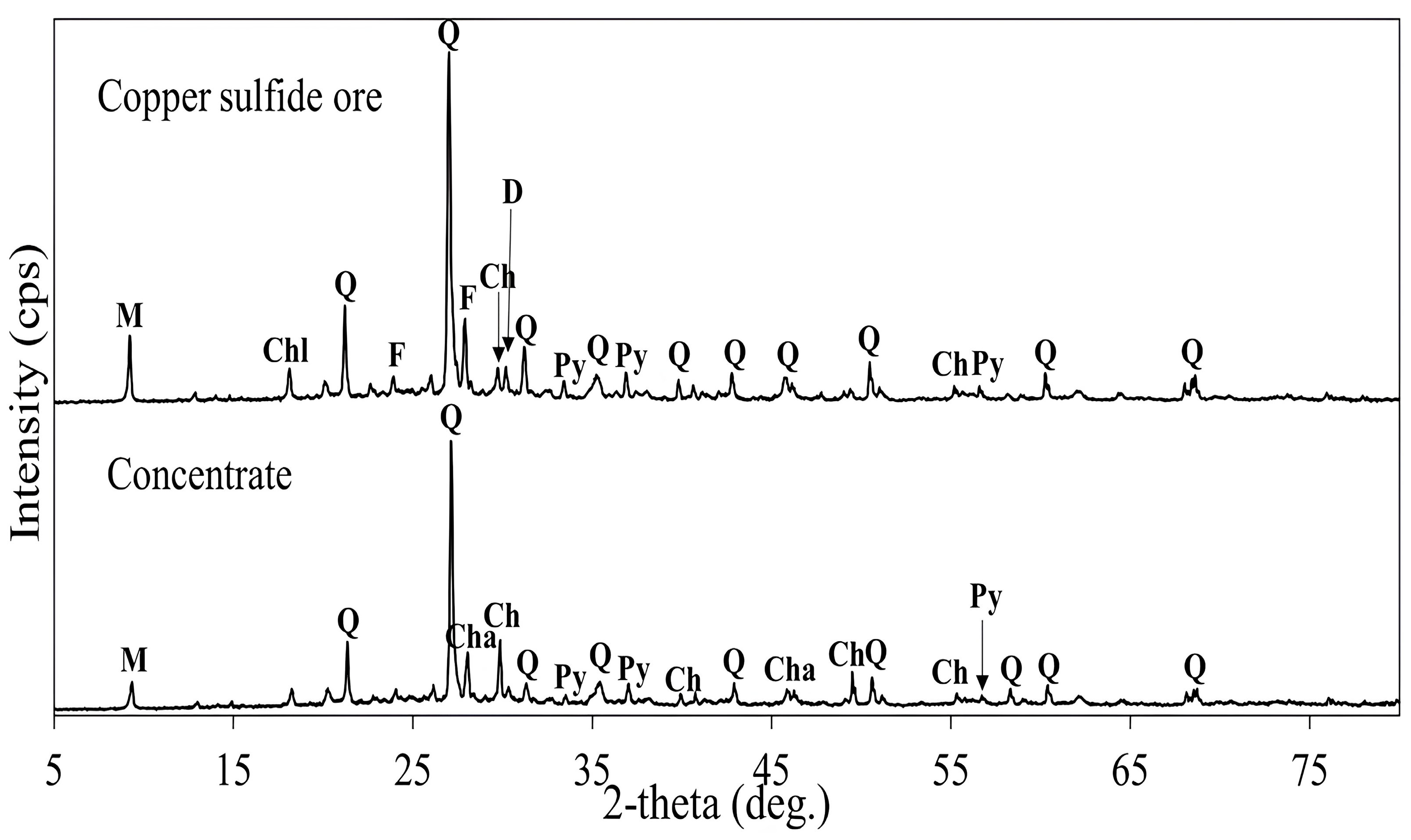
| Copper sulfide ore | 0.94 | 4.14 | 11.17 | 9.85 | 43.54 | 5.76 | 1.37 | 1.14 |
| Concentrate | 7.12 | 6.23 | 2.37 | 10.48 | 25.57 | 4.23 | 1.14 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gayratov, B.; Gayratov, B.; Godirilwe, L.L.; Jeon, S.; Saynazarov, A.; Mutalibkhonov, S.; Shibayama, A. Copper Recovery from Copper Sulfide Ore by Combined Method of Collectorless Flotation and Additive Roasting Followed by Acid Leaching. ChemEngineering 2025, 9, 117. https://doi.org/10.3390/chemengineering9060117
Gayratov B, Gayratov B, Godirilwe LL, Jeon S, Saynazarov A, Mutalibkhonov S, Shibayama A. Copper Recovery from Copper Sulfide Ore by Combined Method of Collectorless Flotation and Additive Roasting Followed by Acid Leaching. ChemEngineering. 2025; 9(6):117. https://doi.org/10.3390/chemengineering9060117
Chicago/Turabian StyleGayratov, Bekhzod, Bobur Gayratov, Labone L. Godirilwe, Sanghee Jeon, Abduqahhor Saynazarov, Saidalokhon Mutalibkhonov, and Atsushi Shibayama. 2025. "Copper Recovery from Copper Sulfide Ore by Combined Method of Collectorless Flotation and Additive Roasting Followed by Acid Leaching" ChemEngineering 9, no. 6: 117. https://doi.org/10.3390/chemengineering9060117
APA StyleGayratov, B., Gayratov, B., Godirilwe, L. L., Jeon, S., Saynazarov, A., Mutalibkhonov, S., & Shibayama, A. (2025). Copper Recovery from Copper Sulfide Ore by Combined Method of Collectorless Flotation and Additive Roasting Followed by Acid Leaching. ChemEngineering, 9(6), 117. https://doi.org/10.3390/chemengineering9060117







