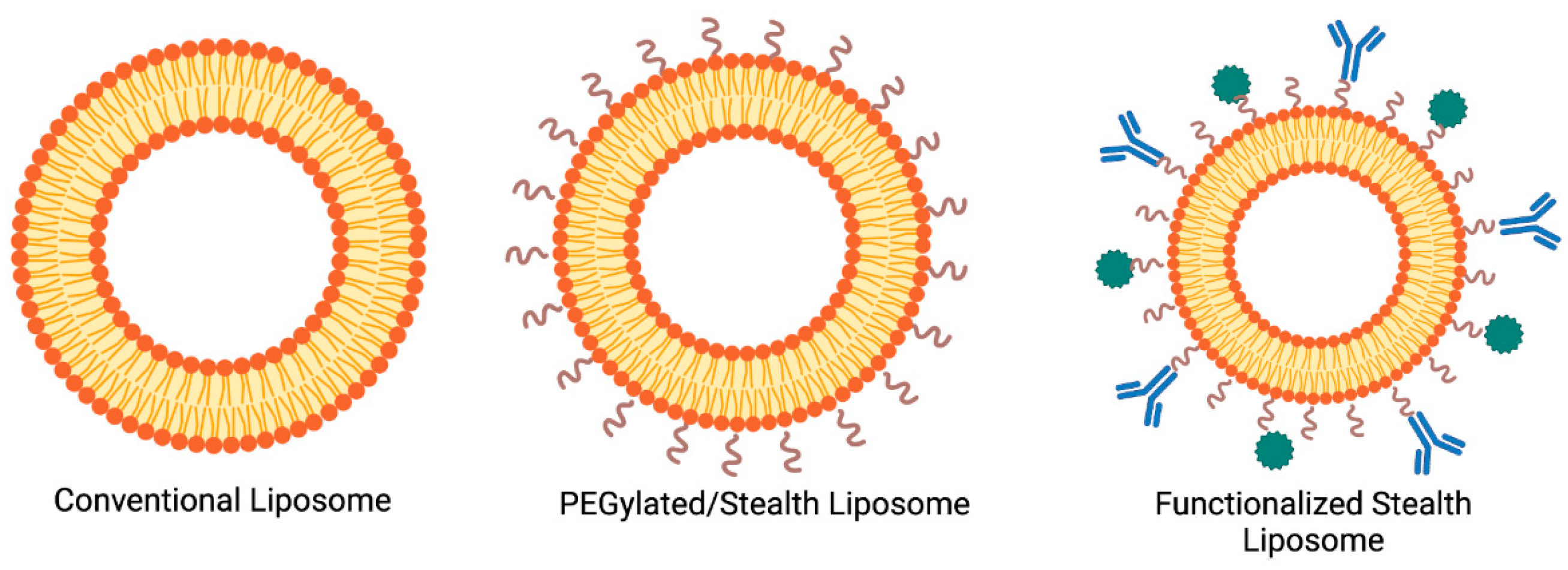
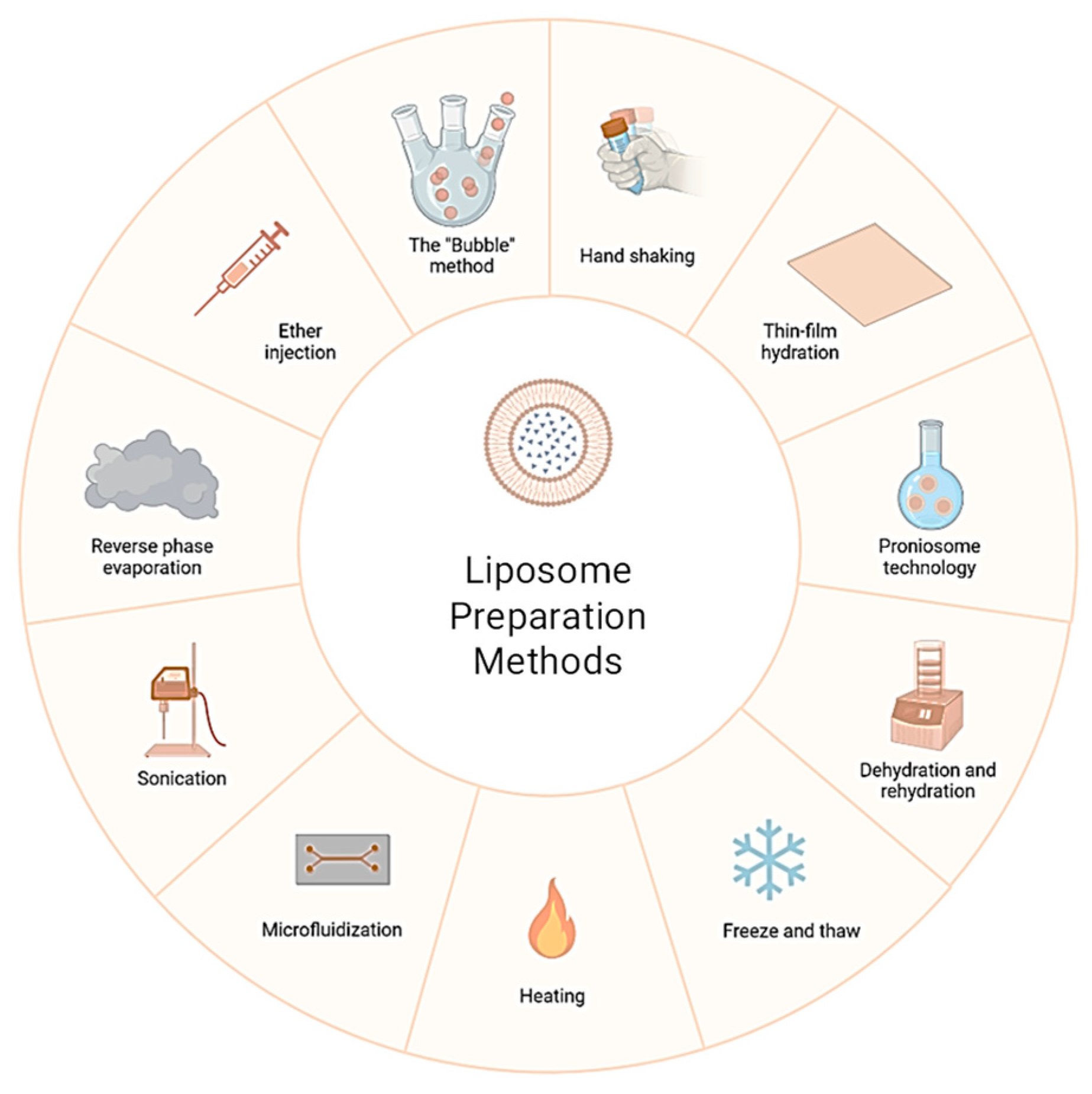
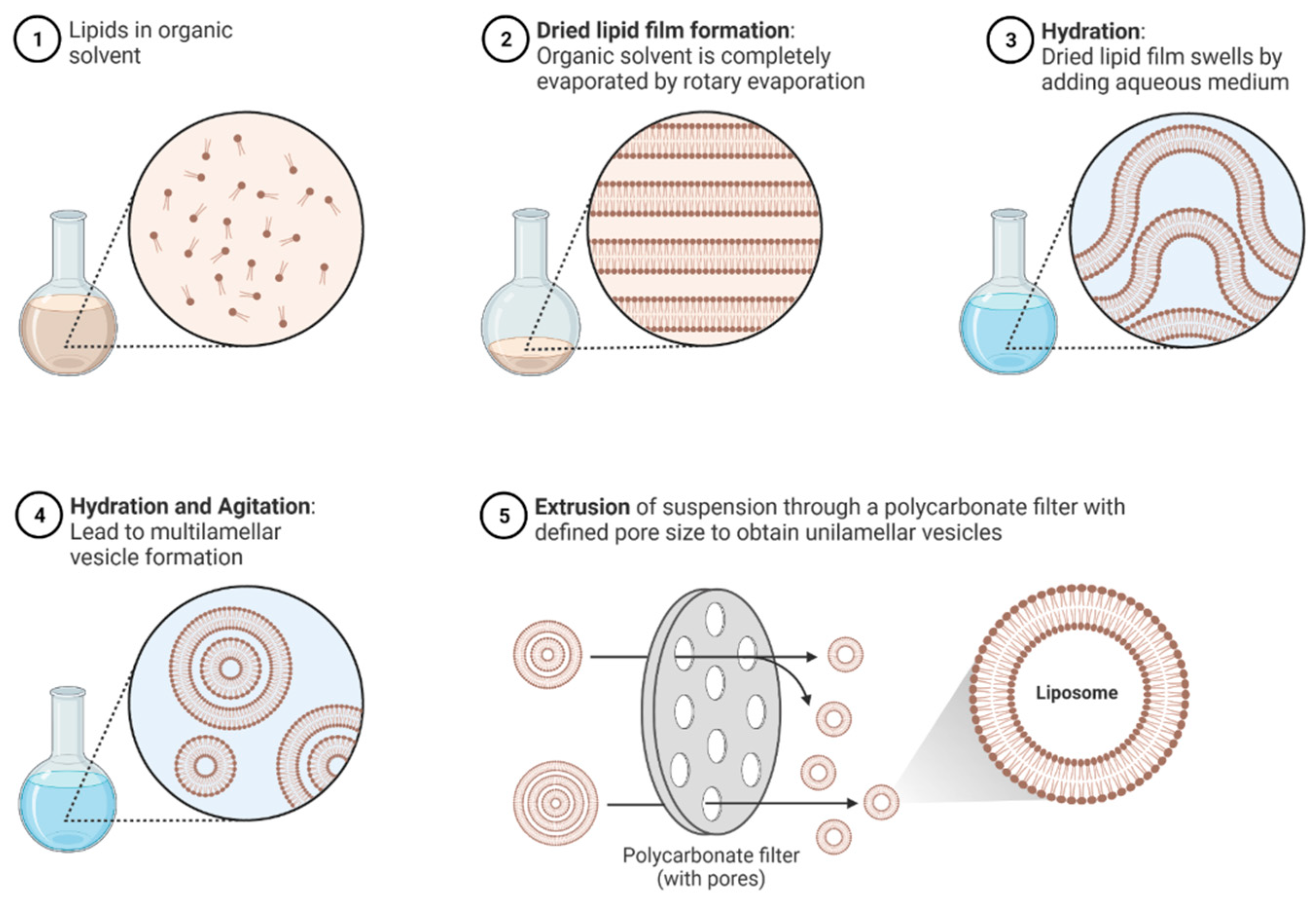
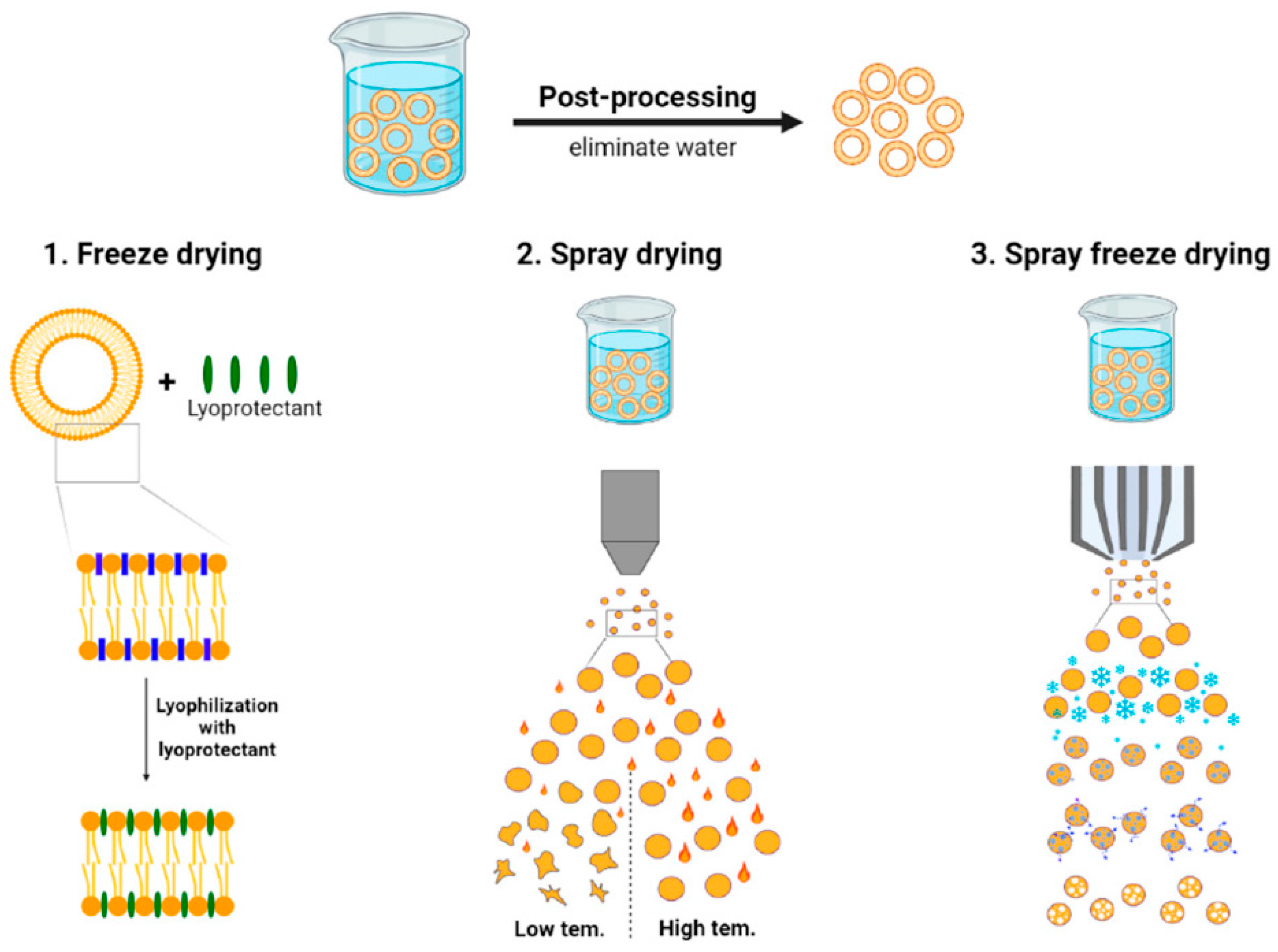
3.1. Importance in Modern Therapeutics
Liposomes have had a transformative impact on modern therapeutics by enhancing the delivery and efficacy of various drugs (
Figure 1). The clinical success of liposomal formulations such as Doxil
® (PEGylated liposomal doxorubicin) and AmBisome
® (liposomal amphotericin B) underscores their ability to improve treatment outcomes in cancer and fungal infections, respectively. By encapsulating drugs, liposomes can reduce systemic toxicity (e.g., Doxil
® significantly lowers cardiac toxicity of doxorubicin) and alter pharmacokinetics to favor accumulation in target tissues (for instance, PEGylation extends circulation half-life, allowing for liposomes to passively target tumor sites via the enhanced permeability and retention effect). Furthermore, liposomes enable combination therapy approaches by co-delivering multiple agents and can be surface-functionalized (with PEG or targeting ligands) to achieve active targeting (
Figure 1). These advantages position liposomes as a cornerstone of nanomedicine, addressing unmet needs in drug delivery for complex diseases [
5,
6,
7,
8]. Their success in the clinic validates the laboratory concepts of liposomal drug delivery and has driven interest in translating more liposomal formulations from bench to bedside. In summary, liposomal systems provide a versatile platform that improves the therapeutic index of drugs, making treatments safer and more effective.
3.3. Scaling up: Transition to Industrial Production
3.3.1. Challenges in Scaling up Liposome Production
Translating liposome preparation from bench-top scale to industrial scale introduces numerous challenges. Processes that are efficient in small volumes often encounter difficulties when volume, batch size, and regulatory requirements increase. Major challenges include the following:
Reproducing liposome characteristics (size distribution, drug loading, and release profile) consistently across production batches is critical. Slight deviations in mixing times, temperature, or batch composition can lead to significant differences in liposomal properties. Such fluctuations in batch quality can affect therapeutic efficacy—for instance, if one batch of liposomal drug has a higher proportion of large vesicles, it may clear faster from circulation, reducing drug exposure at the target site. Ensuring tight control over process parameters is therefore necessary to maintain product uniformity. Automated processes and in-line monitoring (discussed later in
Section 3.3.2) are often employed in manufacturing to minimize inter-batch variability [
21].
Not all lab-scale methods scale up gracefully. Thin film hydration is difficult to scale because uniformly hydrating a large lipid film is inefficient, whereas ethanol injection and homogenization techniques are more readily adapted to large volumes. However, even scalable methods like high-pressure homogenization must be optimized to prevent issues such as foaming, overheating, or shear-induced product degradation when operated continuously at large scales. Equipment design differences (e.g., mixing geometry in a small microfluidic chip versus a large T-mixer) can result in different mixing dynamics, so processes often require re-optimization during scale-up. Additionally, what works in a sterile, small-batch setting may introduce contamination risks or reproducibility issues in a production environment if not carefully controlled [
22].
At larger scales, maintaining control over critical quality attributes (CQAs) such as particle size, lamellarity, and encapsulation efficiency becomes more challenging. Processes tend to have increased variability, and minor distributional inhomogeneities (for example, gradients of solvent or temperature in a large tank) can lead to heterogeneity in the product. Ensuring uniform mixing and energy input throughout the batch is key. Industrial manufacturers often use techniques like multiple sequential extrusion or microfluidic mixing in parallel to ensure all liposome batches meet the same size specifications determined at the lab scale. The use of real-time analytical tools (
Section 3.3.2) can help in adjusting process parameters on the fly to keep properties within spec [
19].
Achieving high drug loading on a large scale sometimes requires process modifications. Active loading techniques (like ammonium sulfate gradients) that work in small batches must be scaled such that gradient formation and maintenance are uniform across a much larger volume. Inefficient scaling could result in some liposomes with suboptimal drug content, affecting potency. Furthermore, losses during scale-up (e.g., drug or lipid adhering to reactor surfaces, filters, or tubing) can reduce overall encapsulation yield. Process engineers need to account for such losses and may increase initial drug feed or improve equipment design (low-binding materials, optimized flow paths) to maximize loading efficiency at scale [
23].
Producing liposomes at industrial scale must adhere to Good Manufacturing Practice (GMP) regulations. This means stringent requirements for sterility (aseptic processing or terminal sterilization), endotoxin levels, solvent residues, and overall quality control. Some reagents or conditions used at lab scale might not be acceptable at manufacturing scale (for example, chloroform is generally avoided in GMP manufacturing due to toxicity, even though it might be used in small-scale lipid preparations). The process must be designed to ensure trace solvents or byproducts are below regulatory limits. Documentation and validation demands are high: every step must be validated to consistently produce a product meeting predefined specifications. Scaling up often uncovers hidden issues; for instance, a buffer that was stable on the lab bench may precipitate on long hold times in a large tank. Such issues need to be resolved, and the solutions vetted under regulatory guidelines [
24,
25].
Scaling up liposome production is resource-intensive. Specialized equipment (high-pressure homogenizers, large-volume extruders, microfluidic production units, etc.) and their operation (energy, maintenance) can significantly increase production costs. For example, manufacturing liposomal doxorubicin incurs higher costs per unit than conventional doxorubicin due to the need for remote loading processes and sterile nano-filtration steps. Additionally, materials like pharmaceutical-grade lipids and cholesterol are expensive, and losses during scale-up directly translate to cost inefficiency. Companies must optimize processes to improve yield (maximize the fraction of drug that ends up encapsulated and recovered) and scale operations in a way that drives down the cost per dose. This often involves process innovations like continuous manufacturing (to reduce downtime and labor) and adopting larger batch sizes to gain economies of scale, all while ensuring that quality is not compromised [
26].
In summary, scaling up liposome production requires carefully bridging the gap between the controlled conditions of the laboratory and the rigorous, large-scale environment of pharmaceutical manufacturing. Each challenge—consistency, scalability of methods, property control, loading efficiency, regulatory compliance, and cost management—must be addressed through a combination of engineering solutions and process innovations to achieve a successful industrial production process for liposomal formulations.
3.3.2. Strategies for Process Optimization
To overcome the above challenges, various strategies and technologies are employed in process development and optimization for liposome manufacturing:
Where possible, methods inherently suited to scale-up are chosen or developed. For instance, instead of large batch hydration, manufacturers use continuous solvent injection systems or microfluidic mixers that can operate in a flow-through manner [
10]. In such systems, lipid in solvent and aqueous buffer are mixed under precisely controlled flow rates (e.g., in a staggered herringbone micromixer), producing liposomes continuously. This approach minimizes batch variability because conditions (flow, concentration) remain constant once the process is at steady state. Notably, microfluidic-based production of lipid nanoparticles (as used for mRNA vaccine manufacturing) has demonstrated that scaling out (running many channels in parallel) can achieve industrially relevant throughputs while maintaining particle consistency, an approach that is now being applied to liposome production. Additionally, high-pressure homogenization can be run in recirculation mode, where liposome dispersion is cycled through the homogenizer until the desired size is achieved, ensuring uniform processing of the entire batch [
27,
28].
The application of PAT allows for real-time monitoring and control of key parameters during manufacturing. For liposome production, in-line particle size analyzers (e.g., dynamic light scattering probes) can measure vesicle size distribution continuously, enabling immediate adjustments to homogenization time or extrusion cycles. Similarly, in-line detectors can monitor drug concentration (via UV-Vis spectroscopy) in filtrate to assess if encapsulation is complete or if drug is leaking. By employing feedback loops, the process can automatically adjust (for example, perform an additional extrusion pass if the size is above target, or cool the product if real-time stability indicates potential aggregation) to maintain quality. This reduces reliance on end-product testing and increases the likelihood that each batch will meet specifications without extensive reprocessing. PAT implementation is encouraged by regulatory agencies as part of a Quality by Design framework, and liposomal drug manufacturers have been integrating tools like in-line HPLC sampling or turbidity meters (for monitoring clarity during solvent removal) to tighten process control [
29].
A systematic approach (often using design of experiments—DOE) is taken to optimize the combination of formulation and processing parameters for robust output. For example, a DOE might examine the influence of lipid-to-drug ratio, hydration temperature, and extrusion pressure on encapsulation efficiency and particle size. By modeling responses, one can identify an optimal operating space that yields high encapsulation and the desired size with minimal variability. This can reveal non-obvious interactions, e.g., slightly higher cholesterol content might require a higher extrusion temperature to achieve the same size. Manufacturers also optimize scale-specific parameters—the speed of stirring during lipid hydration on a large scale, or the nozzle diameter in solvent injection—to maximize consistency. Concrete examples of process optimization include implementing an intermediate size-reduction step during large-scale hydration to avoid the formation of extremely large multilamellar vesicles or the adjustment of buffer exchange procedures (tangential flow filtration parameters) to improve drug retention after remote loading. Each incremental optimization contributes to a more efficient and controlled process [
26].
Traditional batch manufacturing is gradually being supplemented or replaced by continuous processes in the pharmaceutical industry, and liposome production is part of this trend. Continuous manufacturing might involve a series of connected steps such as lipid mixing, size homogenization, drug loading, and purification flowing one into the next. This eliminates hold times between steps (reducing opportunities for degradation or aggregation) and can improve reproducibility by maintaining constant processing conditions. For liposomes, a continuous process could entail solvent injection followed immediately by an in-line solvent removal unit (like a dialysis or TFF module) and then in-line drug loading. Although implementing a fully continuous liposome production line is complex, some companies have reported semi-continuous processes that significantly cut down production time and variability. These process innovations, once validated, will likely become standard as demand for liposomal formulations grows and the industry seeks to improve efficiency.
By leveraging these strategies scalable methods, PAT tools, rigorous optimization, and continuous processing the transition from lab-scale protocols to industrial production can be managed more effectively. Indeed, several modern liposomal products are now manufactured using highly optimized and controlled processes that were unimaginable a few decades ago. The result is improved batch quality, lower production costs, and greater confidence in the product’s consistency and performance in the clinic.
3.4. Instruments and Techniques in Liposome Production
The production of liposomes at both laboratory and industrial scales relies on a suite of specialized instruments and techniques. These can be broadly categorized into formulation equipment (to create liposomes) and analytical/quality control instruments (to characterize them). Key instruments and their roles include the following:
In lab settings, round-bottom flasks with rotary evaporators are used to prepare thin lipid films under vacuum. For scale-up, this may translate to larger rotary evaporators or industrial thin film dryers that can remove solvent from lipid mixtures in pans or on heated belts. Controlled temperature and vacuum ensure a uniform lipid film, which is essential for subsequent hydration [
9].
High-pressure homogenizers force lipid suspensions through narrow valves at pressures often above 100 MPa, breaking down multilamellar liposomes into smaller vesicles. This technique (e.g., Microfluidizer
®) is widely used in industrial production due to its scalability and consistency. Membrane extruders, on the other hand, push liposome dispersions through polycarbonate membranes of defined pore sizes. Lab-scale extruders (hand-held or bench-top units) typically handle a few milliliters to liters, whereas industrial extruders have large filter cartridges and pumps to process tens of liters. Both methods require temperature control (extrusion often performed at 50–60 °C for lipid membranes) and can be iterated to reach the target size [
30].
Microfluidic mixers, such as staggered herringbone or T-junction microfluidic chips, have gained prominence for liposome formation. In these devices, streams of lipid-in-solvent and aqueous buffer meet in microchannels, and the rapid mixing leads to nucleation of liposomes. The small dimensions ensure a very fast and uniform mixing, resulting in homogeneous liposome populations. For industrial scale, numbering-up strategies are used (multiple chips or parallel channels) to achieve higher throughput. These devices exemplify how precision engineering at a small scale can be applied to larger scale manufacturing by parallelization [
31].
To address stability concerns, liposomal formulations are sometimes freeze-dried with cryoprotectants (e.g., sucrose or trehalose) to create a dry product that is reconstituted before use. Industrial lyophilizers with shelf volumes capable of handling large batch vials are utilized for approved products (for example, AmBisome® is supplied as a lyophilized powder). The process requires careful optimization of freezing and drying cycles to avoid damaging liposome structure and to ensure the cryoprotectant adequately preserves liposome integrity during drying.
Most liposome formulations are intended for parenteral administration, requiring sterile final products (
Figure 5). Large-scale liposome production lines include sterile filtration units (typically 0.22 µm filters) to remove any microbial contaminants, which also serve to remove any oversized liposomes. The filtration step can be challenging since highly concentrated or large liposomes may clog filters; thus, sometimes liposomes are produced under aseptic conditions to avoid the need for terminal sterile filtration. Equipment like clean-in-place (CIP) and steam-in-place (SIP) systems attached to reactors and piping allow for maintenance of aseptic conditions during production [
32].
A range of analytical techniques are employed for in-process and final product testing. Dynamic light scattering (DLS) and laser diffraction instruments measure particle size and polydispersity. Zeta potential analyzers assess surface charge, which is important for stability predictions. UV–visible spectrophotometers and HPLC systems quantify drug content (both encapsulated and unencapsulated, after separation techniques). Cryo-electron microscopy and atomic force microscopy, while more research-oriented, are occasionally used to visualize liposome morphology and lamellarity in development stages. For routine quality control, however, techniques like transmission electron microscopy can confirm vesicle structure on a small sample of a batch. Additionally, differential scanning calorimetry (DSC) might be used to verify lipid phase transition behavior, ensuring that the formulation has the expected thermal properties (indirectly confirming composition and drug incorporation) [
33].
Modern industrial production employs computerized control systems (SCADA systems) to automate the operation of pumps, valves, and monitors (
Figure 5). These systems help maintain the precise timings (for mixing or hydration), temperatures (for lipid dissolution and handling), and pressures (for homogenization) required by the liposome production process. Automation reduces human error and improves reproducibility a critical factor when scaling up [
32].
Instruments and techniques must be selected and integrated into a production process with a view toward regulatory compliance, reliability, and product quality. Continuous improvements in equipment design (such as newer extruders with less dead volume, or homogenizers capable of higher pressures without generating excessive heat) directly contribute to better liposome products. By using the appropriate tools for each step of liposome fabrication and characterization, manufacturers can ensure that the transition from lab bench to factory floor does not compromise the intricate quality attributes that define a liposomal drug delivery system.
3.5. Industrial Challenges in Liposome Production
At the industrial scale, several overarching challenges persist despite advances in technology and process optimization. Many of these issues echo the scale-up challenges discussed earlier, but in a full production environment, they take on additional dimensions of complexity, regulation, and economic pressure. In this section, we contextualize those challenges and elaborate on a few critical aspects such as stability and regulatory compliance.
3.5.1. Batch Consistency and Reproducibility
The requirement that every batch of a pharmaceutical product must meet the same specifications is stringent for liposomal products. As discussed in
Section 3.3.1, maintaining batch-to-batch consistency in liposome size, encapsulation efficiency, and release profile is difficult. In an industrial setting, this consistency is monitored through quality control testing of each batch. Failure to meet criteria can result in batch rejection, which is extremely costly. Thus, companies implement robust Standard Operating Procedures (SOPs) and employee training to ensure each manufacturing run follows the validated process exactly. One strategy to enhance reproducibility is the use of large batch manufacturing, where a single batch can supply the market for a longer time, reducing the frequency of scale-up cycles. However, this places a high burden on making that one batch correctly. Another strategy is statistical process control: data from each batch (size, pH, lipid content, etc.) are recorded and analyzed for trends, so that drifts can be detected and corrected proactively [
34].
3.5.2. Strategies to Address Scale-Related Challenges
Many of the strategies covered in
Section 3.3.2 (process optimization and PAT) are implemented at industrial scale as part of the overall manufacturing control strategy. For example, if microfluidic mixers are used in development, an industrial equivalent might be a multi-jet injection system that mimics micro-scale mixing. If real-time particle size monitoring proved useful at pilot scale, it will be included in the industrial process to allow for on-line adjustments. Qualities by Design (QbD) principles are applied: the manufacturing process is developed with a deep understanding of how inputs affect outputs, building in controls to ensure consistent product quality. For instance, manufacturers often establish an acceptable “design space” for critical parameters (such as lipid concentration and homogenization pressure) within which the process can vary without impacting product quality. This provides some operational flexibility while maintaining confidence in product consistency. Additionally, contingency protocols (like reprocessing steps) are defined: e.g., if a batch initially fails to meet the size specification, it may be passed through an additional extrusion cycle under defined conditions rather than scrapped, provided it can be demonstrated that this does not adversely affect the liposome or drug integrity [
31].
3.5.3. Stability and Shelf-Life Concerns
Stability is a major industrial challenge for liposomal formulations. Many liposomes, especially those carrying hydrophilic drugs, can suffer from drug leakage or particle size growth (aggregation or fusion) during storage. Physical instability (such as fusion of liposomes leading to increased mean size) and chemical instability (like peroxidation of unsaturated lipid components, or hydrolysis of lipids) both threaten shelf-life. To address this, formulations are often optimized with stabilizers (e.g., antioxidants like α-tocopherol to prevent lipid oxidation, or buffering agents to maintain pH). Lyophilization (freeze-drying) with cryoprotectants is a common approach to enhance stability, as seen with products like AmBisome
®, which is stable as a dry powder for reconstitution. However, lyophilization introduces its own challenges—the process must be carefully tailored to avoid damaging the liposomes (e.g., using a slow freezing rate and including adequate cryoprotectant to prevent lamellar fusion). Another consideration is temperature control in the supply chain. Liposomal products often require refrigerated storage (2–8 °C) to remain stable over their shelf-life. Excursions outside recommended temperatures can cause rapid degradation; therefore, manufacturers invest in cold-chain logistics and stability-indicating packaging. From an industrial perspective, demonstrating a suitable shelf-life (often 18–24 months for commercial viability) is crucial. Stability testing under ICH guidelines (accelerated conditions at elevated temperature and humidity) is carried out to ensure the product remains within specifications (size, potency, etc.) throughout its shelf-life. If a formulation fails these tests, reformulation might be necessary (for example, switching to fully saturated lipids that are less prone to oxidation, or adding lyoprotectants). Achieving a stable liposomal product is a fine balance one that often distinguishes a successful commercial product from an academic prototype [
35].
3.5.4. Regulatory and Quality Control
The regulatory landscape for liposome-based therapeutics involves multiple jurisdictions each with specific requirements. In the United States, the FDA has issued detailed guidance on liposome drug products (e.g., addressing Chemistry, Manufacturing, and Controls considerations), which calls for extensive characterization of liposome size, lamellarity, drug loading, release kinetics, and stability, as well as demonstration of batch reproducibility [
36]. In the European Union, liposomal formulations are reviewed by the EMA with attention to quality and safety aspects under guidelines for nanomedicines; they require, for instance, evidence that any novel excipients (like PEGylated lipids not previously used in approved products) are safe and that the manufacturing process is validated to consistently produce a product of intended quality. Different countries might have specific nuances. For example, some regulatory agencies may require additional toxicity studies for the liposome carrier itself or insist on real-time release testing for certain attributes if post-production testing is deemed insufficient. Manufacturers of liposomal drugs must navigate these requirements by building quality into the product from the ground up. This means extensive documentation: detailed records of raw material quality (lipids often need to be of “liposome grade”, with low peroxide values and specific purity criteria), in-process controls, validation of sterilization methods, and comprehensive final product testing. Batch release testing typically includes assays for drug content, encapsulation efficiency, particle size distribution (often by DLS), zeta potential (for informational purposes), sterility, endotoxins, and sometimes in vitro release (e.g., percent drug released in serum after X hours to ensure formulation integrity). Ensuring compliance also means preparing for regulatory inspections of manufacturing facilities, where auditors will confirm that the production is performed exactly as described in regulatory filings. The complexity of liposome products has led regulators to scrutinize them as “complex injectables”, and any post-approval changes to the process (even improvements) can trigger requirements for new comparability data. Therefore, industry tends to lock in a highly controlled process and then maintain it, with a robust change control system to evaluate potential impacts of any change. Overall, navigating the regulatory landscape is challenging but essential; successful approval and production of a liposomal drug implies that the manufacturer has achieved a high standard of quality and can sustain it consistently.
In summary, industrial production of liposomal drug delivery systems requires not only scientific and engineering solutions to technical hurdles but also a thorough approach to quality and regulatory compliance. Stability must be ensured through formulation and process choices, and regulatory expectations must be met with exhaustive characterization and control. By proactively addressing these industrial challenges, manufacturers can reliably deliver the therapeutic benefits of liposomes to patients at scale.
3.6. Applications in Drug Delivery
Liposome-based delivery systems have been applied across a broad range of therapeutic areas, turning laboratory innovations into life-saving medicines. Below are key domains where liposomal formulations have made significant impact:
Cancer treatment has greatly benefited from liposomal drug delivery. Doxil
® (liposomal doxorubicin) was the first FDA-approved nano-drug and is a prime example: encapsulating doxorubicin in PEGylated liposomes alters its biodistribution, greatly reducing cardiac toxicity and enhancing tumor accumulation of the drug [
37,
38,
39].
Liposomal daunorubicin/cytarabine (Vyxeos®) is another notable example, approved for acute myeloid leukemia; it co-encapsulates two chemotherapeutics at a fixed synergistic ratio, improving therapeutic outcomes compared to free drugs. Liposomal formulations of irinotecan (Onivyde®) and vincristine (Marqibo®) have also been developed to improve the pharmacokinetics of these agents. By delivering chemotherapeutics more selectively to tumors and shielding normal tissues, liposomes have increased the efficacy and tolerability of anticancer regimens, addressing a long-standing challenge in oncology.
Systemic fungal infections (such as invasive aspergillosis or candidiasis) often require potent drugs that can have serious side effects. Liposomal amphotericin B (AmBisome
®) encapsulates amphotericin B, a highly effective but nephrotoxic antifungal, in liposomes, thereby reducing kidney exposure and toxicity [
40].
This has allowed for higher dosing and prolonged treatment courses with improved safety. Liposomal formulations provide a reservoir that releases amphotericin B in a controlled manner, maintaining efficacy while sparing critical organs. The success of AmBisome® has made it a gold standard for treating certain severe fungal infections, especially in patients who cannot tolerate conventional amphotericin B. Research into liposomal delivery is ongoing for other antifungals (like nystatin or itraconazole) to similarly enhance their therapeutic index.
Liposomes have been utilized to extend the duration of local anesthetics and to provide sustained pain relief. A notable product is Exparel
® (liposomal bupivacaine), used for post-surgical analgesia. In Exparel
®, bupivacaine is encapsulated in multivesicular liposomes (DepoFoam technology) that slowly release the drug over 2–3 days [
41].
This extended release provides prolonged local numbness and pain control from a single injection, reducing the need for systemic analgesics (including opioids). Another example was DepoDur™ (liposomal morphine) for epidural administration, designed to provide long-lasting pain relief after surgery with one epidural dose. Although DepoDur™ is no longer marketed, it demonstrated the feasibility of liposomal encapsulation in sustaining opioid release. These examples illustrate how liposomal delivery can improve pain management by maintaining therapeutic drug levels at the site of action for extended periods, thereby improving patient comfort and reducing dosing frequency.
While antifungal liposomes are well established, liposomal approaches for other infections are being explored. Liposomal formulations of antivirals (e.g., acyclovir or tenofovir) have been investigated to enhance delivery to viral reservoirs. Liposomal antibiotics (such as liposomal amikacin, Arikayce
®, for inhalation in mycobacterial lung infections) have achieved approval, showing improved penetration into lung tissues and infection sites. These demonstrate that liposomes can target sites that are otherwise difficult for drugs to reach, such as intracellular pathogens or biofilms, by improving drug uptake into infected cells or prolonging drug presence at the infection site [
42].
Liposomes are also employed as vaccine adjuvants and delivery vehicles. They can present antigens in a way that elicits strong immune responses or deliver nucleic acids for genetic vaccines. For example, some research-stage and clinical trial vaccines use liposomal carriers for peptide or mRNA delivery (though lipid nanoparticles for mRNA vaccines are a closely related technology). Additionally, liposomes can carry immune modulators (like cytokines or Toll-like receptor agonists) to modulate the immune system in cancer immunotherapy or autoimmunity. These applications leverage the biocompatibility and versatility of liposomes to safely deliver potent immune-stimulating agents [
43,
44].
Each of these application areas capitalizes on a different strength of liposome technology whether it is reduced toxicity, extended release, targeted delivery, or immune modulation. The diversity in successful liposomal products reflects the adaptability of liposome-based systems to solve various drug delivery problems. Ongoing research is expanding these applications, exploring liposomes for gene therapy (delivering DNA or RNA), for enzyme replacement therapies (encapsulating enzymes to protect them from degradation), and even for imaging (liposomal contrast agents). The continued development and industrialization of liposome technology in these domains promise to yield new therapies that can better meet clinical needs.
3.7. Future Perspectives
The field of liposome-based drug delivery continues to evolve rapidly, with research and development opening new frontiers that could further enhance their clinical utility. Looking ahead, several emerging trends and technologies are poised to shape the future of liposomal therapeutics:
Next-generation liposomes are being engineered to release their payload in response to specific physiological triggers present at disease sites. These triggers include pH (e.g., slightly acidic tumor microenvironments or endosomal compartments), enzymes (matrix metalloproteinases or other proteases upregulated in cancers and inflamed tissues), or external stimuli like temperature (thermosensitive liposomes that release drug when heated) and ultrasound. For instance, thermosensitive liposomal doxorubicin (under investigation) can be triggered to release drug upon mild hyperthermia at the tumor, allowing for spatial and temporal control of drug delivery. While some of these systems have shown promise in clinical trials, bringing them to market will require overcoming additional challenges in reproducible manufacturing and demonstrating clear therapeutic advantages. Nevertheless, stimuli-responsive designs represent an exciting advance that could make drug delivery even more precise [
45].
Researchers are exploring hybrid nanoparticles where liposomes are combined with other materials to impart new functionalities. One example is liposome–polymer hybrids, where a polymer shell can stabilize the liposome and provide stealth or targeting functions beyond what PEGylation alone offers [
46,
47,
48,
49]. Another is ligand-functionalized liposomes that carry targeting moieties (antibodies, peptides, aptamers) on their surface to actively seek out and bind to specific cell types (such as cancer cells overexpressing a certain receptor). Early studies show that such targeted liposomes can improve uptake by target cells and enhance efficacy in models. For example, a folate receptor-targeted liposomal doxorubicin demonstrated higher tumor uptake in folate receptor-positive tumor models compared to non-targeted liposomes, translating into improved tumor growth inhibition [
27].
Combining targeting with stimuli-responsiveness (e.g., a liposome that homes to a tumor and then releases drug in response to tumor-specific enzymes) is a current research frontier. These multi-functional liposomes blur the line between drug and biologic, and their successful development will hinge on sophisticated design and thorough understanding of in vivo behavior.
The adaptable nature of liposome formulation makes it amenable to personalized medicine approaches. In the future, we may see patient-specific liposomal therapies, for instance, loading a patient’s own tumor antigens into a liposomal vaccine for personalized cancer immunotherapy. Liposomes could also be used to deliver CRISPR/Cas9 components or siRNAs tailored to an individual’s genetic profile of disease. The concept of theranostics combining therapy and diagnostics is another area where liposomes are expected to play a key role. Liposomes can be dual-loaded with a drug and an imaging agent (such as a radioisotope or MRI contrast agent), allowing real-time tracking of drug distribution and accumulation by imaging while concurrently treating the disease. This could be particularly useful in cancer, to non-invasively monitor how well a therapy is reaching the tumor. Some theranostic liposome formulations are in the experimental stages, and as imaging technology and nanomedicine design progress hand-in-hand, the feasibility of such approaches will improve [
50,
51].
On the production side, the next decade is likely to bring even more streamlined and scalable manufacturing techniques for liposomes. Continuous manufacturing processes, potentially coupled with modular production units, might allow on-demand production of liposomal drugs (which could be transformative for personalized medicine and hospitals). Improvements in lipid raw material synthesis (yielding more uniform and pure lipids at lower cost) will also benefit liposome availability and cost-effectiveness. Moreover, as regulatory bodies become more familiar with liposomal products, clearer guidelines and standards specific to liposomes will emerge, smoothing the path for new entrants. Regulatory science is catching up with nanomedicine, which should ease some hurdles by providing well-defined expectations for what data and controls are needed for approval [
22].
Finally, liposomes may be integrated with other cutting-edge drug delivery systems. For example, liposome-coated drug-eluting implants or stents could locally release drugs over time, or liposomal formulations might be incorporated into 3D-printed pills for complex release profiles. The versatility of liposomes means they can synergize with other technologies—like combining liposomal drugs with checkpoint inhibitors in oncology for a one-two punch or using liposomes as part of tissue engineering scaffolds that release growth factors to promote regeneration [
52,
53].
Despite the remarkable progress in the field, certain hurdles must be overcome for these future directions to fully materialize in commercial products. Achieving the sophisticated designs of stimuli-responsive or targeted liposomes requires ensuring they can be manufactured reproducibly at scale and remains stable until they reach the desired site of action. Regulatory pathways for combination products (e.g., a liposome with an imaging agent) need further development. Cost-effectiveness will also be scrutinized, as more complex liposomes may be expensive to produce. Nonetheless, the trajectory of research and recent successes in related areas (such as lipid nanoparticles for nucleic acid delivery) provide optimism. It is anticipated that in the coming years, liposome-based drug delivery systems will continue to proliferate and diversify, solidifying their role in precision medicine.