Phytochemical Profiling and Antioxidant Activity of True Leaves and Cotyledons of Adenocaulon himalaicum
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Apparatus and Chemicals
2.3. Sample Extraction
2.4. LC–MS/MS States
2.5. Sample Preparation and Standard Solutions for HPLC
2.6. HPLC/PDA Conditions
2.7. Calibration Curves
2.8. TPC Assay
2.9. TFC Assay
2.10. DPPH Assay
2.11. ABTS+ Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. Sample Extraction Yield
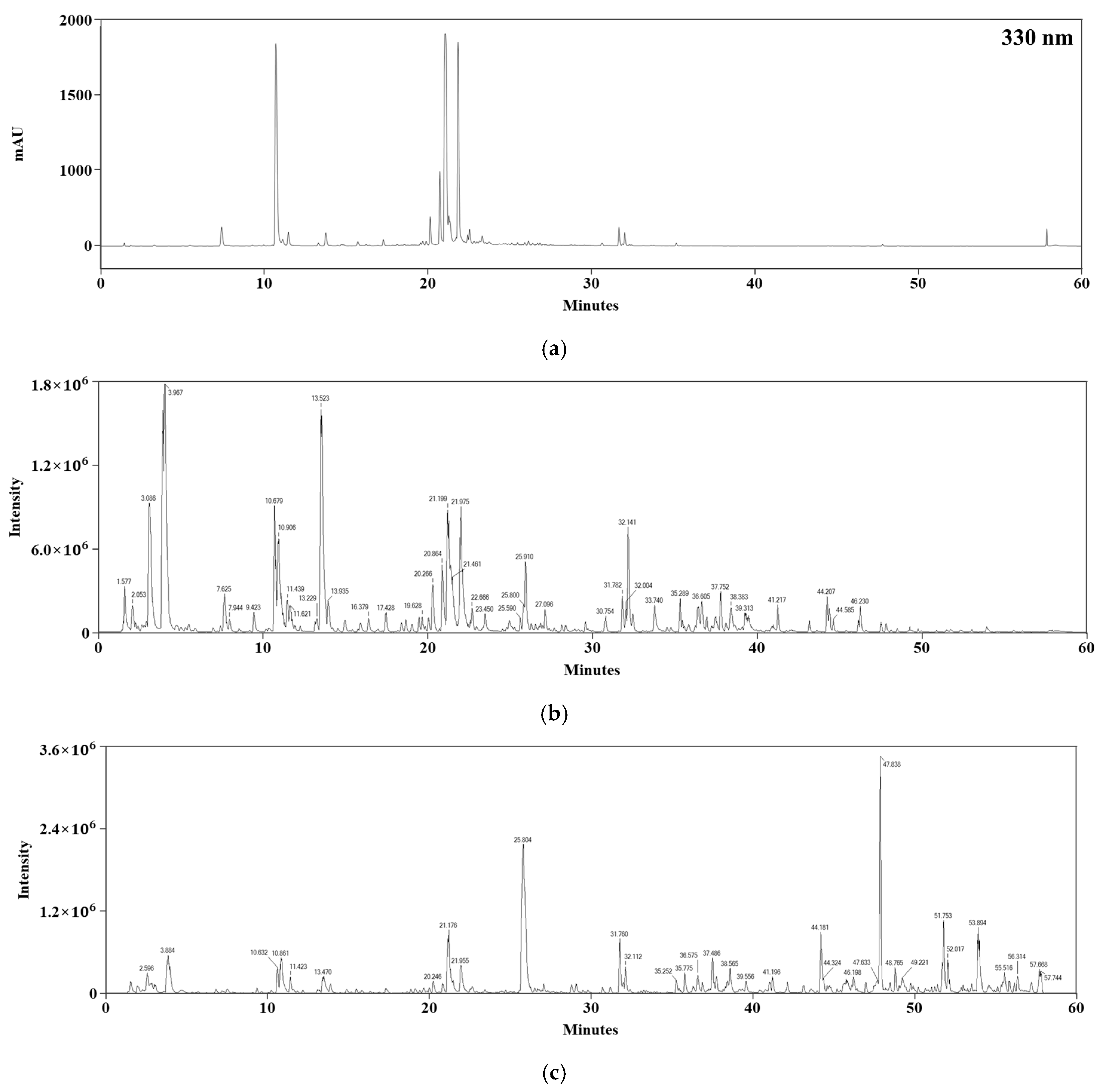
3.2. LC–MS/MS Analysis
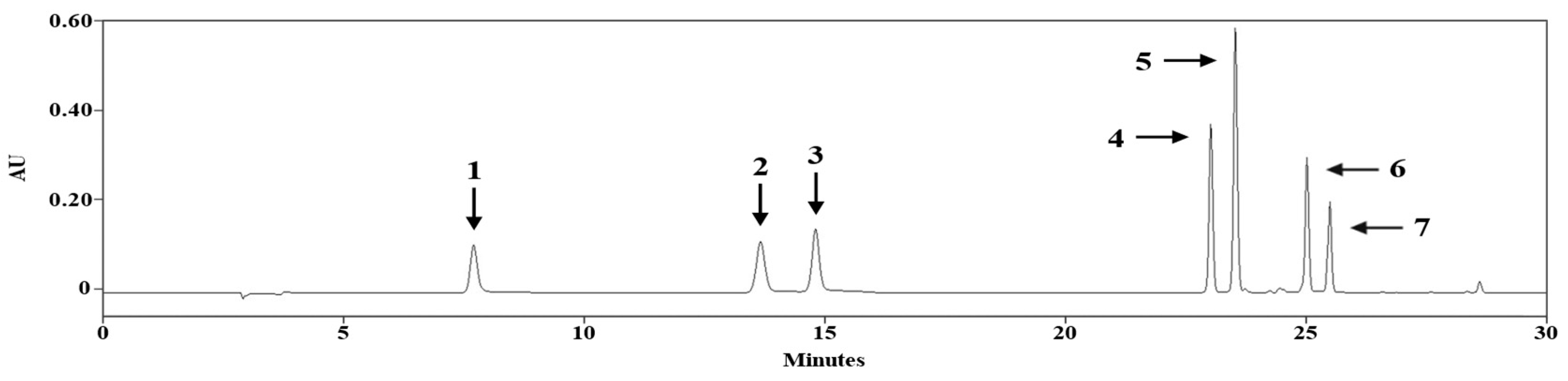
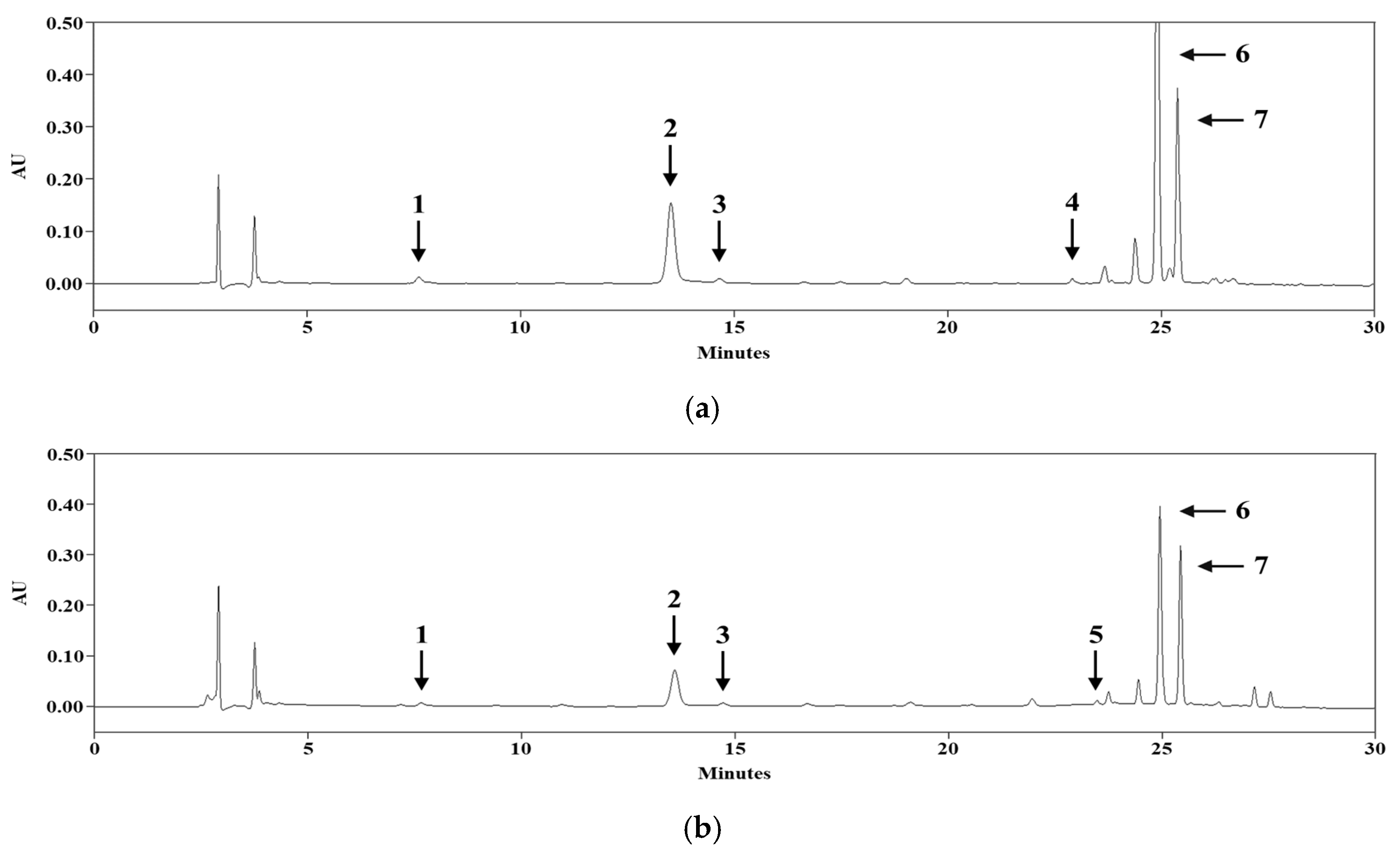
3.3. HPLC/PDA Analysis
3.4. TPC and TFC
3.5. DPPH and ABTS+ Radical-Scavenging Assays
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinogradova, Y.; Shelepova, O.; Vergun, O.; Ganina, A. Possibility of ssing an invasive species Adenocaulon adhaerescens Maxim. (Asteraceae) as a medicin plant. Agrobiodivers. Improv. Nutr. Health Life Qual. 2020, 4, 59–69. [Google Scholar] [CrossRef]
- Ahn, H.S.; Kim, H.J.; Na, C.; Jang, D.S.; Shin, Y.-K.; Lee, S.H. The protective effect of Adenocaulon himalaicum Edgew. and its bioactive compound neochlorogenic acid against UVB-induced skin damage in human dermal fibroblasts and epidermal keratinocytes. Plants 2021, 10, 1669. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.J.; Choi, J.W.; Im, S.W.; Jeong, J.B. Immunostimulatory and anti-obesity activities of Adenocaulon himalaicum leaves in RAW264.7 and 3T3-L1 Cells. Exp. Ther. Med. 2023, 25, 219. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.H.; Lee, S.B.; Kang, K.; Lee, E.H.; Lee, H.J.; Jung, S.H.; Nho, C.W. Bifunctional chemopreventive effects of Adenocaulon himalaicum through induction of detoxification enzymes and apoptosis. J. Med. Food 2013, 16, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Khoddami, A.; Wilkes, M.; Roberts, T. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Yoon, N.; Yu, H.; Tran, G.H.; Ko, C.H.; Kim, H.; Yoon, M.J.; Lee, S. Profiling the phytochemicals of Orostachys margaritifolia: Biological activities, LC-ESI/MS, and HPLC analyses. Pharmaceuticals 2024, 17, 290. [Google Scholar] [CrossRef]
- Uy, N.P.; Kim, H.; Ku, J.; Lee, S. Regional variations in Peucedanum japonicum antioxidants and phytochemicals. Plants 2024, 13, 377. [Google Scholar] [CrossRef]
- Lee, C.-D.; Uy, N.P.; Lee, Y.; Lee, D.-H.; Lee, S. Comparative analysis of phytochemical composition and antioxidant properties of Smilax china rhizome from different regions. Horticulturae 2024, 10, 850. [Google Scholar] [CrossRef]
- Lee, Y.M.; Bae, J.H.; Jung, H.Y.; Kim, J.H.; Park, D.S. Antioxidant activity in water and methanol extracts from Korean edible wild plants. J. Korean Soc. Food Sci. Nutr. 2011, 40, 29–36. [Google Scholar] [CrossRef]
- Liigand, P.; Kaupmees, K.; Haav, K.; Liigand, J.; Leito, I.; Girod, M.; Antoine, R.; Kruve, A. Think negative: Finding the best electrospray Ionization/MS mode for your analyte. Anal. Chem. 2017, 89, 5665–5668. [Google Scholar] [CrossRef]
- Han, Y.K.; Vinh, L.B.; Nam, M.; Lee, K.Y. Identification of compounds using HPLC-QTOF-MS online antioxidant activity mapping from aerial parts of Ligularia stenocephala. Appl. Biol. Chem. 2023, 66, 53. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Harborne, J.B. Nature, distribution and function of plant flavonoids. Prog. Clin. Biol. Res. 1986, 213, 15–24. [Google Scholar] [PubMed]
- Javed, H.; Khan, M.M.; Ahmad, A.; Vaibhav, K.; Ahmad, M.E.; Khan, A.; Ashafaq, M.; Islam, F.; Siddiqui, M.S.; Safhi, M.M.; et al. Rutin prevents cognitive impairments by ameliorating oxidative stress and neuroinflammation in rat model of sporadic dementia of alzheimer type. Neuroscience 2012, 210, 340–352. [Google Scholar] [CrossRef]
- Richetti, S.K.; Blank, M.; Capiotti, K.M.; Piato, A.L.; Bogo, M.R.; Vianna, M.R.; Bonan, C.D. Quercetin and rutin prevent scopolamine-induced memory impairment in zebrafish. Behav. Brain Res. 2011, 217, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Nassiri-Asl, M.; Mortazavi, S.-R.; Samiee-Rad, F.; Zangivand, A.-A.; Safdari, F.; Saroukhani, S.; Abbasi, E. The effects of rutin on the development of pentylenetetrazole kindling and memory retrieval in rats. Epilepsy Behav. 2010, 18, 50–53. [Google Scholar] [CrossRef]
- Mellou, F.; Loutrari, H.; Stamatis, H.; Roussos, C.; Kolisis, F.N. Enzymatic esterification of flavonoids with unsaturated fatty acids: Effect of the novel esters on vascular endothelial growth factor release from K562 cells. Process Biochem. 2006, 41, 2029–2034. [Google Scholar] [CrossRef]
- Trumbeckaite, S.; Bernatoniene, J.; Majiene, D.; Jakstas, V.; Savickas, A.; Toleikis, A. The effect of flavonoids on rat heart mitochondrial function. Biomed. Pharmacother. 2006, 60, 245–248. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Maas, R.; Troost, R.; Böger, R.H. Clinical pharmacokinetics of antioxidants and their impact on systemic oxidative stress. Clin. Pharmacokinet. 2003, 42, 437–459. [Google Scholar] [CrossRef]
- Janbaz, K.H.; Saeed, S.A.; Gilani, A.H. Protective effect of rutin on paracetamol- and CCl4-induced hepatotoxicity in rodents. Fitoterapia 2002, 73, 557–563. [Google Scholar] [CrossRef]
- La Casa, C.; Villegas, I.; Alarcón de la Lastra, C.; Motilva, V.; Martıín Calero, M.J. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol. 2000, 71, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Shoji, O.; Ono, E.; Hiromi, T.; Watanabe, N.; Toshiyuki, O. Identification and characterization of Camellia sinensis glucosyltransferase, UGT73A17: A possible role in flavonol glucosylation. Plant Biotechnol. 2014, 31, 573–578. [Google Scholar] [CrossRef]
- Liu, Z.; Bruins, M.E.; de Bruijn, W.J.C.; Vincken, J.P. A comparison of the phenolic composition of old and young tea leaves reveals a decrease in flavanols and phenolic acids and an increase in flavonols upon tea leaf maturation. J. Food Compos. Anal. 2020, 86, 103385. [Google Scholar] [CrossRef]
- Fabjan, N.; Rode, J.; Košir, I.J.; Wang, Z.; Zhang, Z.; Kreft, I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem. 2003, 51, 6452–6455. [Google Scholar] [CrossRef]
- Hua, S.; Liu, J.; Zou, L.; Li, P. Hyperoside: A review of resources, synthesis pathways, physicochemical properties, metabolism, pharmacological activities, clinical trials and human studies, toxicology and safety, marketed products, and patents. In Handbook of Dietary Flavonoids; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–103. [Google Scholar] [CrossRef]
- Cai, W.; Chen, Y.; Xie, L.; Zhang, H.; Hou, C. Characterization and density functional theory study of the antioxidant activity of quercetin and its sugar-containing analogues. Eur. Food Res. Technol. 2013, 238, 121–128. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Hu, W.; Sarengaowa; Guan, Y.; Feng, K. Biosynthesis of phenolic compounds and antioxidant activity in fresh-cut fruits and vegetables. Front. Microbiol. 2022, 13, 906069. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Alcázar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Hamed, Y.S.; Abdin, M.; Chen, G.; Akhtar, H.M.S.; Zeng, X. Effects of impregnate temperature on extraction of caffeoylquinic acid derivatives from Moringa oleifera leaves and evaluation of inhibitory activity on digestive enzyme, antioxidant, anti-proliferative and antibacterial activities of the extract. Int. J. Food Sci. Technol. 2020, 55, 3082–3090. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Angeloni, S.; Navarini, L.; Angeloni, C.; Freschi, M.; Hrelia, S.; Vitali, L.A.; Sagratini, G.; Vittori, S.; Caprioli, G. Coffee silverskin extracts: Quantification of 30 bioactive compounds by a new HPLC-MS/MS method and evaluation of their antioxidant and antibacterial activities. Food Res. Int. 2020, 133, 109128. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, A.; Ivanova, V.; Rangelov, M.; Todorova, M.; Ozek, G.; Your, S.; Ozek, T.; Aneva, I.; Veleva, R.; Moskova-Doumanova, V.; et al. Caffeoylquinic acids, cytotoxic, antioxidant, acetylcholinesterase and tyrosinase enzyme inhibitory activities of Six Inula species from Bulgaria. Chem. Biodivers. 2020, 17, e2000051. [Google Scholar] [CrossRef]
- Soumaya, B.; Iness, B.R.; Khaoula, M.H.; Isoda, H.; Riadh, K.; Wided, M.K. LC-ESI-TOF-MS and GC-MS profiling of Artemisia herba-alba and evaluation of its bioactive properties. Food. Res. Int. 2017, 99, 702–712. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Vereshchagina, Y.V.; Veremeichik, G.N. Anticancer polyphenols from cultured plant cells: Production and new bioengineering strategies. Curr. Med. Chem. 2018, 25, 4671–4692. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current advances in naturally occurring caffeoylquinic acids: Structure, bioactivity, and synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef] [PubMed]
- Murad, L.D.; da Costa Pereira Soares, N.; Brand, C.; Monteiro, M.C.; Teodoro, A.J. Effects of caffeic and 5-caffeoylquinic acids on cell viability and cellular uptake in human colon adenocarcinoma cells. Nutr. Cancer 2015, 67, 532–542. [Google Scholar] [CrossRef]
- Taira, J.; Uehara, M.; Tsuchida, E.; Wakana, O. Inhibition of the β-catenin/Tcf signaling by caffeoylquinic acids in sweet potato leaf through down regulation of the Tcf-4 transcription. J. Agric. Food Chem. 2013, 62, 167–172. [Google Scholar] [CrossRef]
- Dimitrina, Z.D.; Reneta, G.; Zaharieva, M.M.; Hristo, N.; Silviya, R.; Lozanov, V.; Vessela, B.; Yagi, S.; Georgi, M.; Vanio, M. HPLC-UV and LC–MS analyses of acylquinic acids in Geigeria alata (DC) Oliv. & Hiern. and their contribution to antioxidant and antimicrobial capacity. Phytochem. Anal. 2016, 28, 176–184. [Google Scholar] [CrossRef]
- Matthews, D.G.; Caruso, M.; Alcazar, M.A.; Wright, K.M.; Maier, C.S.; Stevens, J.F.; Gray, N.E.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic acids in Centella asiatica reverse cognitive deficits in male 5XFAD Alzheimer’s disease model mice. Nutrients 2020, 12, 3488. [Google Scholar] [CrossRef]
- Gray, N.E.; Morré, J.; Kelley, J.; Maier, C.S.; Stevens, J.F.; Quinn, J.F.; Soumyanath, A. Caffeoylquinic acids in Centella asiatica protect against amyloid-β toxicity. J. Alzheimers Dis. 2014, 40, 359–373. [Google Scholar] [CrossRef]
- Sasaki, K.; Davies, J.; Doldán, N.G.; Arao, S.; Ferdousi, F.; Szele, F.G.; Isoda, H. 3,4,5-Tricaffeoylquinic acid induces adult neurogenesis and improves deficit of learning and memory in aging model senescence-accelerated prone 8 mice. Aging 2019, 11, 401–422. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Nemzer, B.; Ou, B.; Stalmach, A.; Hunter, J.; Clifford, M.N.; Combet, E. The antioxidant and chlorogenic acid profiles of whole coffee fruits are influenced by the extraction procedures. J. Agric. Food Chem. 2011, 59, 3754–3762. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]
- Werker, E. Trichome Diversity and development. Adv. Bot. Res. 2000, 31, 1–35. [Google Scholar] [CrossRef]
- Tissier, A. Glandular trichomes: What comes after expressed sequence tags? Plant J. 2012, 70, 51–68. [Google Scholar] [CrossRef]
- Perveen, A.; Khan, M.; Mansuri, S.; Tabassum, T. Morphological studies on trichome of family Asteraceae. Int. J. Biol. Biotechnol. 2016, 13, 172–182. [Google Scholar]
- Aschenbrenner, A.K.; Amrehn, E.; Bechtel, L.; Spring, O. Trichome differentiation on leaf primordia of Helianthus annuus (Asteraceae): Morphology, gene expression and metabolite profile. Planta 2014, 241, 837–846. [Google Scholar] [CrossRef]
- Karabourniotis, G.; Liakopoulos, G.; Nikolopoulos, D.; Bresta, P. Protective and defensive roles of non-glandular trichomes against multiple stresses: Structure–function coordination. J. For. Res. 2019, 31, 1–12. [Google Scholar] [CrossRef]
- Spring, O.; Bienert, U.; Klemt, V. Sesquiterpene lactones in glandular trichomes of sunflower leaves. J. Plant Physiol. 1987, 130, 433–439. [Google Scholar] [CrossRef]
- Spring, O.; Rodon, U.; Macias, F.A. Sesquiterpenes from noncapitate glandular trichomes of Helianthus annuus. Phytochemistry 1992, 31, 1541–1544. [Google Scholar] [CrossRef]
- Göpfert, J.; Conrad, J.; Spring, O. 5-Deoxynevadensin, a novel flavone in sunflower and aspects of biosynthesis during trichome development. Nat. Prod. Commun. 2006, 1, 1934578X0600101. [Google Scholar] [CrossRef]
- Schilling, E.E.; Panero, J.L.; Storbeck, T.A. Flavonoids of Helianthus series Microcephali. Biochem. Syst. Ecol. 1987, 15, 671–672. [Google Scholar] [CrossRef]
- Kumar, K.; Debnath, P.; Singh, S.; Kumar, N. An overview of plant phenolics and their involvement in abiotic stress tolerance. Stresses 2023, 3, 570–585. [Google Scholar] [CrossRef]
- Martinez, V.; Mestre, T.C.; Rubio, F.; Girones-Vilaplana, A.; Moreno, D.A.; Mittler, R.; Rivero, R.M. Accumulation of flavonols over hydroxycinnamic acids favors oxidative damage protection under abiotic stress. Front. Plant Sci. 2016, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- Agati, G.; Tattini, M. Multiple functional roles of flavonoids in photoprotection. New Phytol. 2010, 186, 786–793. [Google Scholar] [CrossRef]
- Kolb, C.A.; Käser, M.A.; Kopecký, J.; Zotz, G.; Riederer, M.; Pfündel, E.E. Effects of natural intensities of visible and ultraviolet radiation on epidermal ultraviolet screening and photosynthesis in grape leaves. Plant Physiol. 2001, 127, 863–875. [Google Scholar] [CrossRef]
- Degfie, T.; Endale, M.; Tafese, T.; Dekebo, A.; Shenkute, K. In vitro antibacterial, antioxidant activities, molecular docking, and ADMET analysis of phytochemicals from roots of Hydnora johannis. Appl. Biol. Chem. 2022, 65, 76. [Google Scholar] [CrossRef]
- So, J.; Lee, H.D.; Kim, J.H.; Lee, S.; Lim, J.H. Antioxidant, antimicrobial, and skin-whitening effects and quantitative analysis of phenolic compounds in Korean wild Chrysanthemum flowers via HPLC/UV. Hortic. Environ. Biotechnol. 2024, 65, 215–227. [Google Scholar] [CrossRef]
- Doan, T.T.M.; Tran, G.H.; Nguyen, T.K.; Lim, J.H.; Lee, S. Antioxidant activity of different cultivars of Chrysanthemum morifolium and quantitative analysis of phenolic compounds by HPLC/UV. Appl. Biol. Chem. 2024, 67, 17. [Google Scholar] [CrossRef]


| Sample | Dry Sample (g) | Extract (g) | Yield (%) |
|---|---|---|---|
| TLA | 10 | 3.8 | 38.0 |
| CLA | 4.0 | 1.6 | 40.0 |
| tR (min) | MW | Tentative Identification |
|---|---|---|
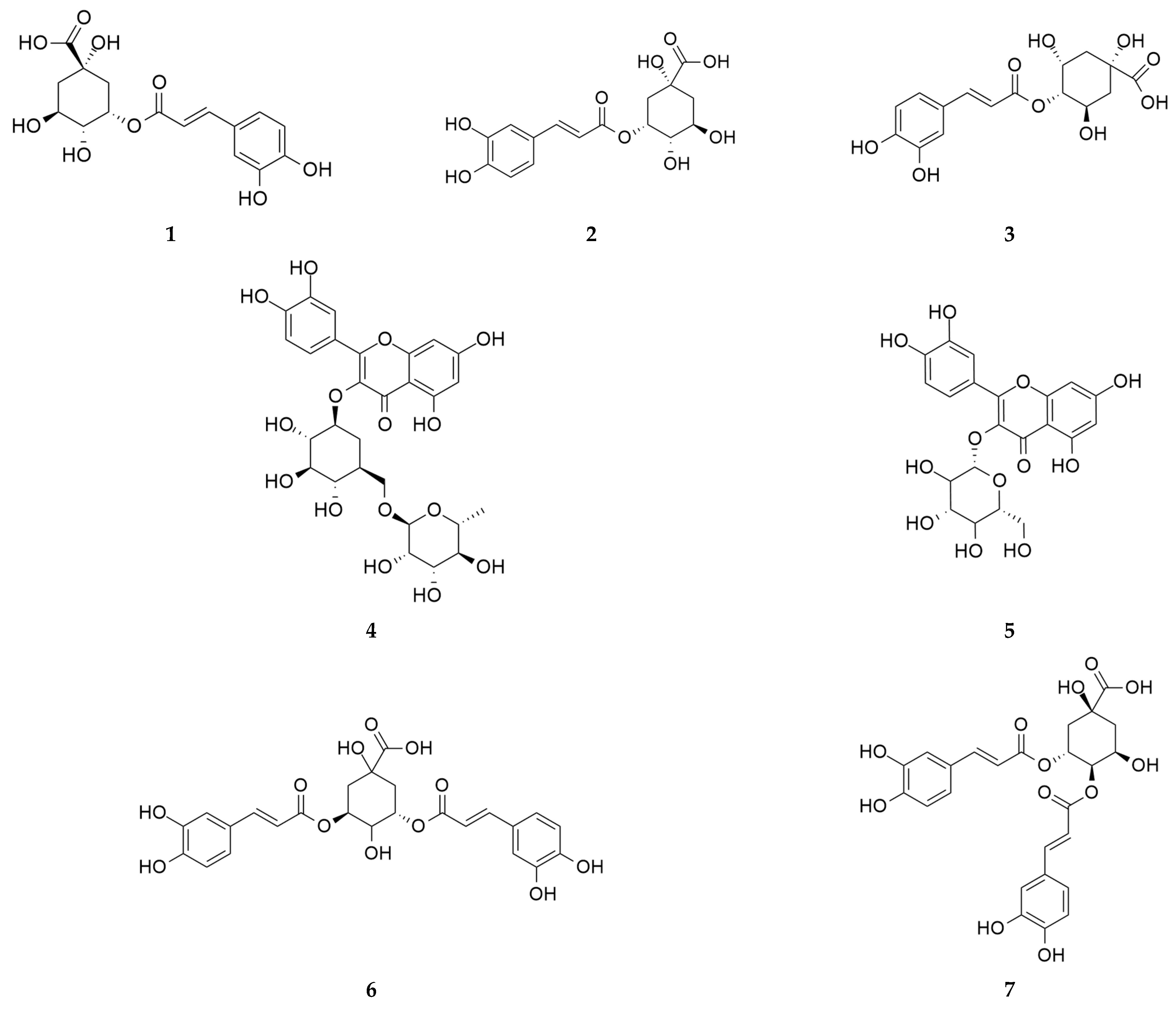
| 7.63 | 354.1 | Neochlorogenic acid 1 |
| 10.89 | 354.1 | Chlorogenic acid 1,2 |
| 11.62 | 354.1 | Cryptochlorogenic acid 1 |
| 13.48 | 354.1 | 1-O-Caffeoylquinic acid 1 |
| 13.91 | 338.1 | 4-O-Coumaroylquinic acid 1,2 |
| 15.84 | 368.1 | 5-O-Feruloylquinic acid 1,2 |
| 16.38 | 338.1 | 1-O-Coumaroylquinic acid 1 |
| 19.68 | 610.2 | Rutin 1,2 |
| 19.70 | 564.1 | Kaempferol 3-arabinofuranoside 7-rhamnofuranoside 1 |
| 19.99 | 464.1 | Hyperin 1,2 |
| 20.88 | 516.1 | Cynarin 1 |
| 21.19 | 516.1 | Isochlorogenic acid A 1,2 |
| 21.96 | 516.1 | Isochlorogenic acid B 1,2 |
| 23.44 | 516.1 | Dicaffeoylquinic acid 1 |
| Compound | tR (min) | Calibration Equation | Correlation Factor, r2 |
|---|---|---|---|
| 1 | 7.61 | - | - |
| 2 | 13.51 | Y = 10,830X − 14,226 | 0.9996 |
| 3 | 14.73 | - | - |
| 4 | 23.04 | - | - |
| 5 | 23.50 | - | - |
| 6 | 24.96 | Y = 10,251X + 53,292 | 0.9998 |
| 7 | 25.45 | Y = 6733.6X + 99,840 | 0.9991 |
| Sample | Content (mg/g DW) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | Total | |
| TLA | tr | 9.002 ± 0.055 **** | tr | tr | ND | 28.512 ± 0.217 **** | 12.223 ± 0.074 **** | 49.737 **** |
| CLA | tr | 4.422 ± 0.032 **** | tr | ND | tr | 10.062 ± 0.070 **** | 11.255 ± 0.049 **** | 25.739 **** |
| Standard | Calibration Equation | Correlation Factor, r2 |
|---|---|---|
| Tannic acid | Y = 0.0147X + 0.0883 | 0.9995 |
| Quercetin | Y = 0.0167X − 0.0329 | 0.9999 |
| Sample | DPPH (IC50, mg/mL) | ABTS+ (IC50, mg/mL) |
|---|---|---|
| TLA | 1.59 ± 0.02 a | 1.43 ± 0.04 a |
| CLA | 2.82 ± 0.12 b | 2.53 ± 0.05 b |
| AA | 0.12 ± 0.00 c | 0.13 ± 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-Y.; Yoon, N.; Uy, N.P.; Choi, C.-H.; Lee, S. Phytochemical Profiling and Antioxidant Activity of True Leaves and Cotyledons of Adenocaulon himalaicum. ChemEngineering 2025, 9, 31. https://doi.org/10.3390/chemengineering9020031
Lee S-Y, Yoon N, Uy NP, Choi C-H, Lee S. Phytochemical Profiling and Antioxidant Activity of True Leaves and Cotyledons of Adenocaulon himalaicum. ChemEngineering. 2025; 9(2):31. https://doi.org/10.3390/chemengineering9020031
Chicago/Turabian StyleLee, Sang-Yun, Nari Yoon, Neil Patrick Uy, Chung-Ho Choi, and Sanghyun Lee. 2025. "Phytochemical Profiling and Antioxidant Activity of True Leaves and Cotyledons of Adenocaulon himalaicum" ChemEngineering 9, no. 2: 31. https://doi.org/10.3390/chemengineering9020031
APA StyleLee, S.-Y., Yoon, N., Uy, N. P., Choi, C.-H., & Lee, S. (2025). Phytochemical Profiling and Antioxidant Activity of True Leaves and Cotyledons of Adenocaulon himalaicum. ChemEngineering, 9(2), 31. https://doi.org/10.3390/chemengineering9020031









