Preparation and Characterization of Supramolecular Bonding Polymers Based on a Pullulan Substrate Grafted with Acrylic Acid/Acrylamide by Microwave Irradiation
Abstract
1. Introduction
2. The Experimental Section
2.1. Materials and Methods
2.2. Preparation and Absorption Measurement of SAPs
2.2.1. Preparation of SAPs
- Mixing monomers step: The AA and AM monomer solutions were mixed, and MBA solution was added (0.04 g in 5 mL of DW). The AM monomer solution was prepared by dissolving 6 g in 12 mL of DW with continuous stirring. The AA solution was prepared in an ice bath to avoid polymerization by dissolving 6 g of AA monomer with partial neutralization (80% by weight) using 5 M NaOH.
- Substrate preparation step: The pullulan substrate concentration was prepared by dissolving 1.20 g of PUL in 35 mL of distilled water, at about 3.2 wt.%. The solution was mixed by a mechanical mixer at 60 °C for 15 min, and then aqueous solutions of SDBS surfactants at different concentrations (0, 1, 2, 3, and 4 mM) were continuously mixed until a homogeneous solution was obtained.
- Generation of free radicals: To facilitate the grafting step, free radicals were generated using a redox initiator system composed of two solutions: 0.118 g of KPS and 0.051 g of TEMED (each in 5 mL of DW). These solutions were added dropwise to the PUL solution at a temperature of 60 °C for 10 min in the presence of strong mechanical mixing.
- Grafting step: The monomer solution (AM/AA) was added to the PUL solution after it was cooled to 50 °C, with mechanical mixing at a rotational speed of 1050 rpm for 15 min. The final solution of the reaction mixture was supplemented with 100 mL of added DW, and the final solution was treated in a microwave oven at 475 watts for 4 min. The temperature and viscosity of the mixture gradually increased, and the gelation point was reached after 250 s; the product was a yellowish elastic gel. The SAP grafting polymerization reactions are explained in Figure 1.
- Treatment of the final product: To remove the remaining surfactant, the resulting elastic gel was cut into small pieces, and the resulting gel was washed several times with ethanol/water (10:1, v/v). Then, the unreacted materials (PUL, AA, AM, KPS, TEMED, and MBA) were completely removed after immersion in pure methanol for 24 h to dehydrate and dissolve without reacting. The final product was washed with ethanol and then ground and baked in an oven at 60 °C for 24 h [27,33].
2.2.2. Swelling Measurements
2.2.3. Measurement of Grafting Parameters
2.3. Characterization
3. Results and Discussions
3.1. Prepared SAPs
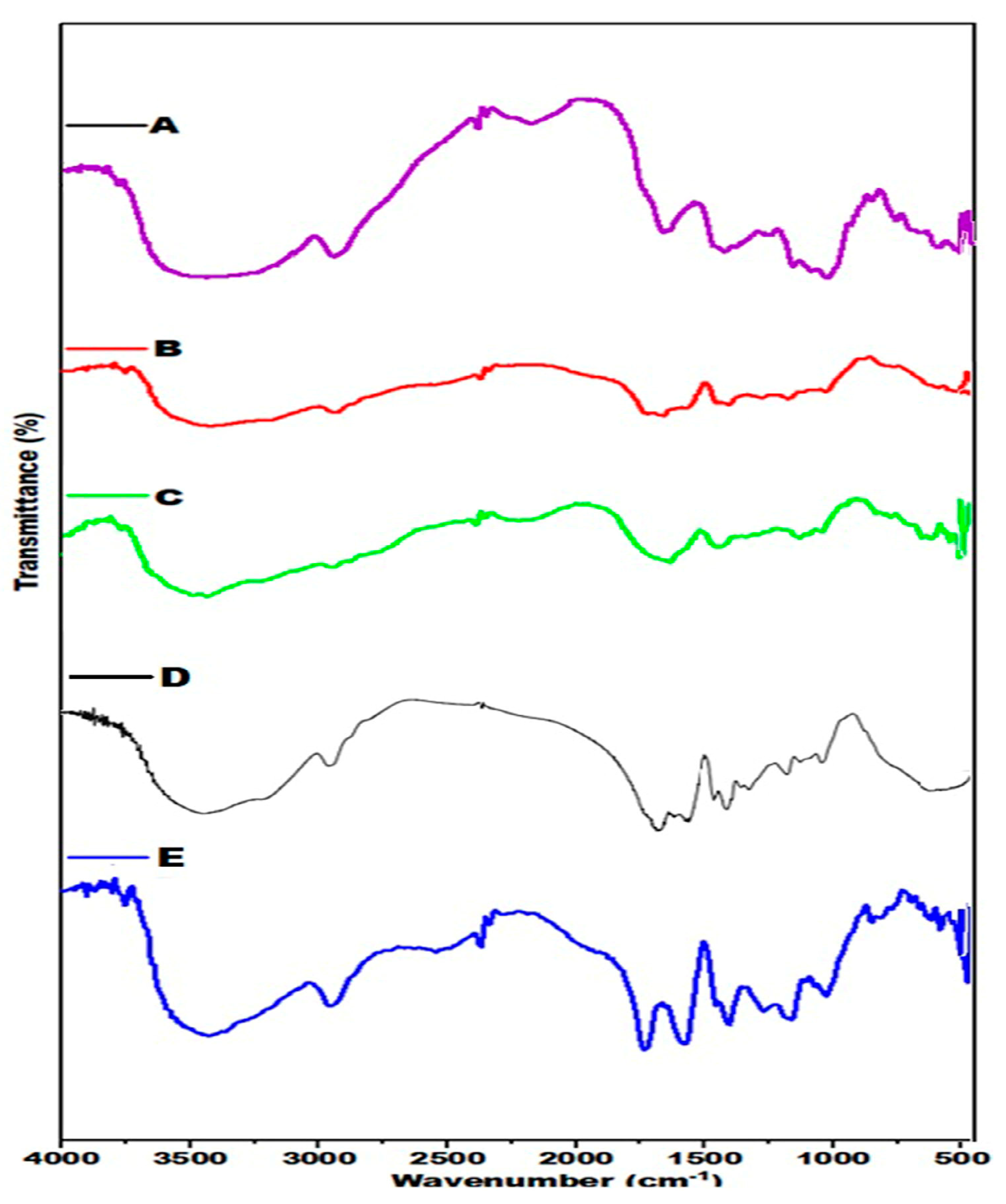
3.2. FTIR Analysis
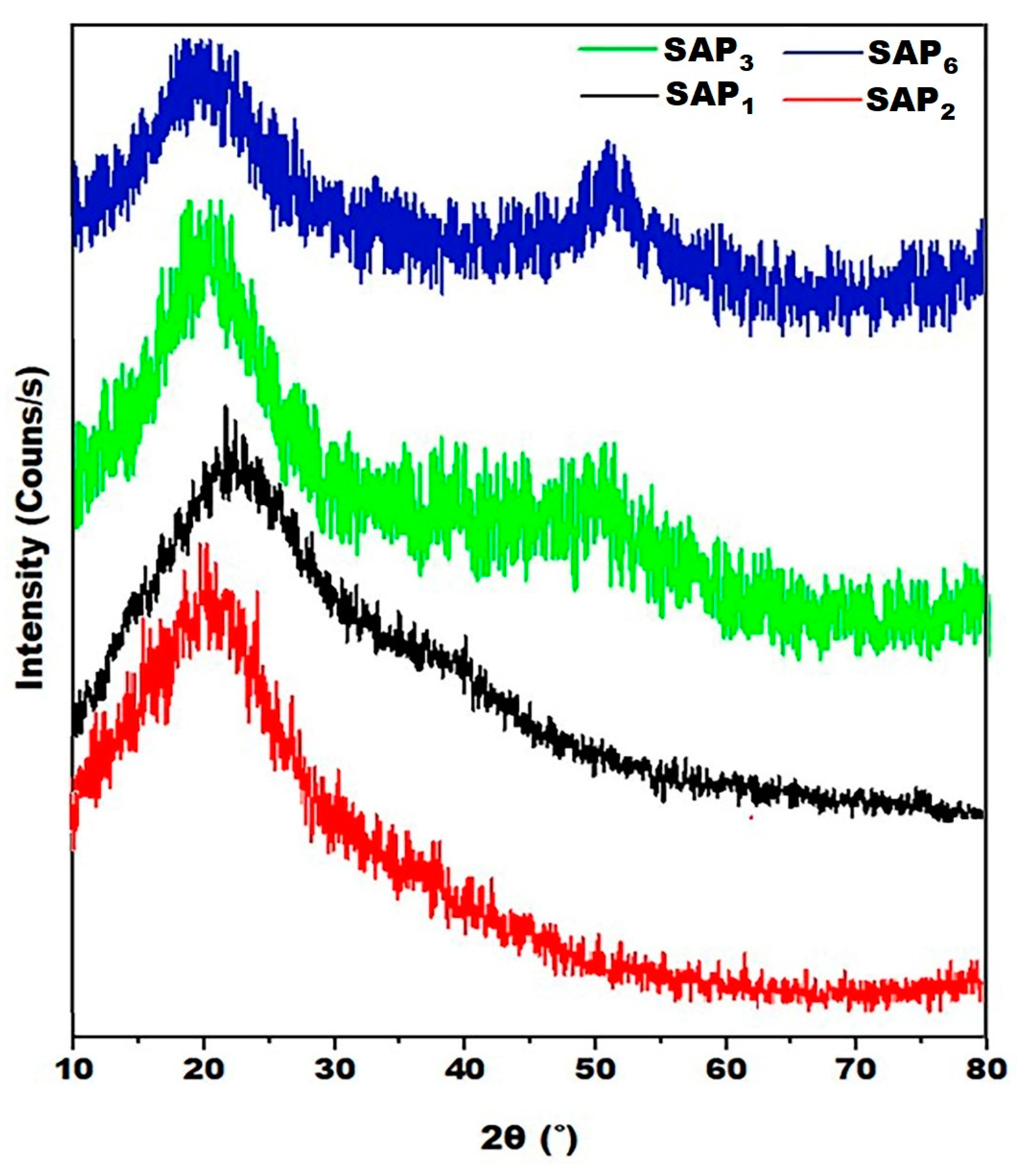
3.3. XRD Analysis
3.4. Thermal Properties
3.5. The Effect of SDBS on the Absorption and Grafting Parameters
3.6. The Effect of SDBS on the Absorption Capacity and Swelling Kinetics
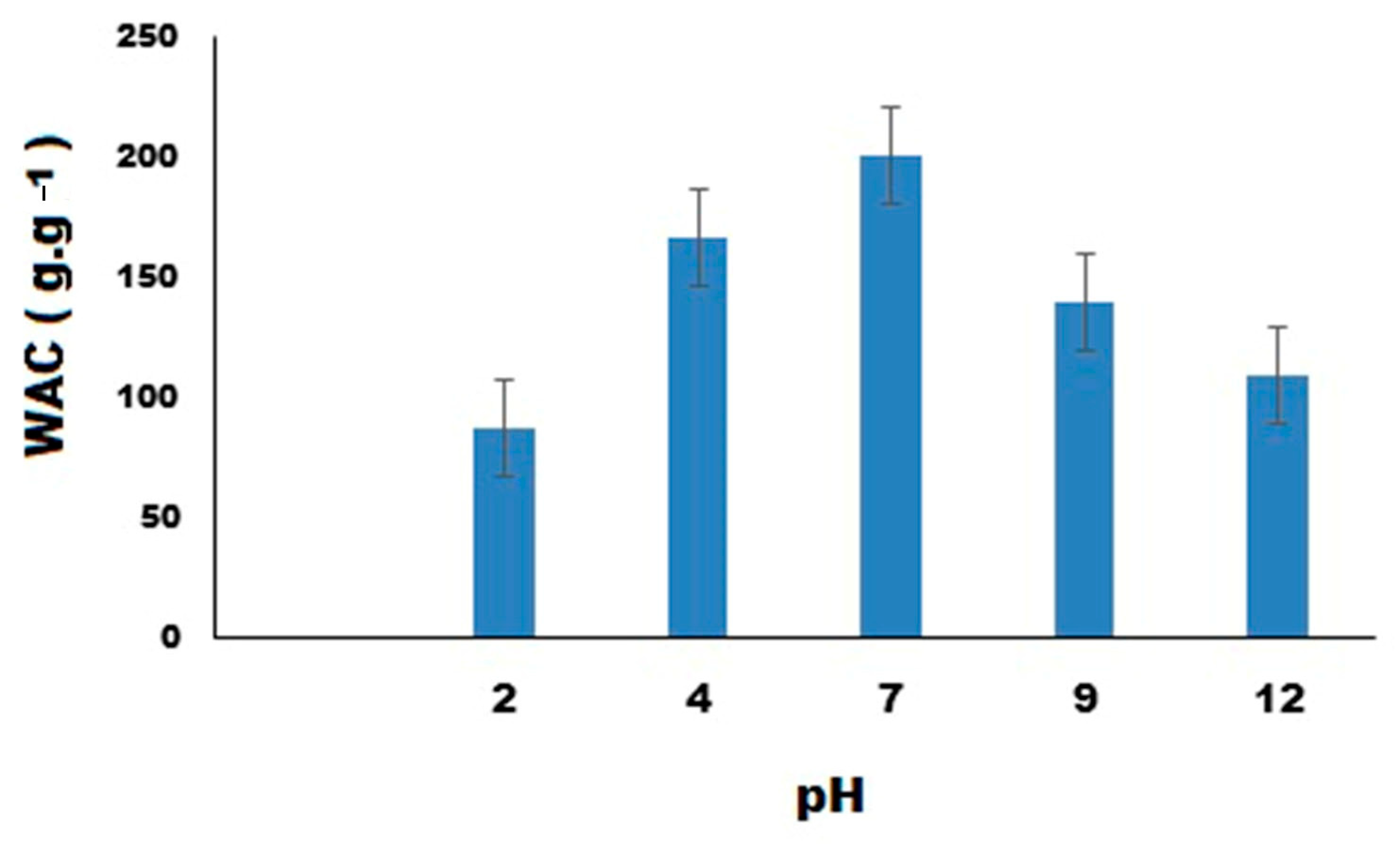
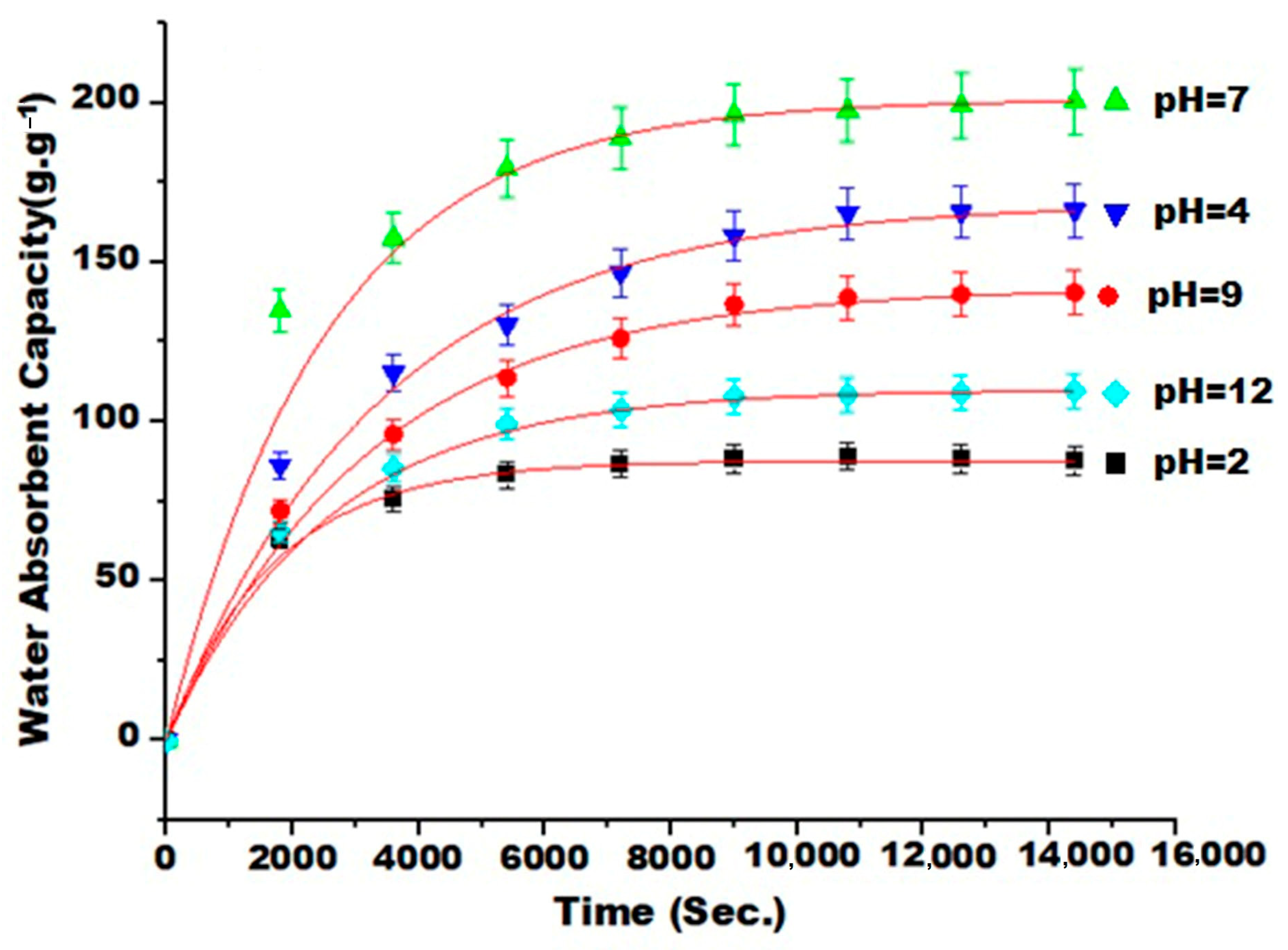
3.7. The Impact of pH on Water Absorbency and Swelling Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salih, S.I.; Hashem, F.A.; Braihi, A.J. Preparation and characterization of concrete reinforced by the super-absorbent hydrogel nanocomposites (SAHNCs) used for construction applications. Adv. Nat. Appl. Sci. 2016, 10, 112–125. [Google Scholar]
- Arredondo, R.; Yuan, Z.; Sosa, D.; Johnson, A.; Beims, R.F.; Li, H.; Xu, C.C. Performance of a novel, eco-friendly, cellulose-based superabsorbent polymer (Cellulo-SAP): Absorbency, stability, reusability, and biodegradability. Can. J. Chem. Eng. 2023, 101, 762–1771. [Google Scholar] [CrossRef]
- Braihi, A.J.; Salih, S.I.; Hashem, F.A.; Ahmed, J.A. Proposed cross-linking model for carboxymethyl cellulose/starch superabsorbent polymer blend. Int. J. Mater. Sci. Eng. 2014, 3, 363–369. [Google Scholar] [CrossRef]
- Wei, J.; Yang, H.; Cao, H.; Tan, T. Using poly aspartic acid hydro-gel as water retaining agent and its effect on plants under drought stress. Saudi J. Biol. Sci. 2016, 23, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Tubert, E.; Vitali, V.A.; Alvarez, M.S.; Tubert, F.A.; Baroli, I. Amodeo, Synthesis and evaluation of a superabsorbent-fertilizer composite for maximizing the nutrient and water use efficiency in forestry plantations. J. Environ. Manag. 2018, 210, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Inobeme, A.; Ajai, A.I.; Inobeme, J.; Adetunji, C.O.; Obar, A.; Mathew, J.T.; Nwakife, N. Superabsorbent Polymers for the Development of Nanofiltration. In Properties and Applications of Superabsorbent Polymers: Smart Applications with Smart Polymers; Springer Nature: Singapore, 2023; pp. 157–170. [Google Scholar]
- Rather, R.A.; Bhat, M.A.; Shalla, A.H. An insight into Synthetic, Physiological aspect of Superabsorbent Hydrogels based on Carbohydrate type polymers for various Applications: A Review. Carbohydr. Polym. Technol. Appl. 2022, 3, 100202. [Google Scholar] [CrossRef]
- Shi, X.; Wang, W.; Wang, A. Effect of surfactant on porosity and swelling behaviors of guar gum-poly(sodium acrylate-co-styrene)/attapulgite superabsorbent hydrogels. Colloids Surf. B 2011, 88, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Mistry, P.A.; Konar, M.N.; Latha, S.; Chadha, U.; Bhardwaj, P.; Eticha, T.K. Chitosan Superabsorbent Biopolymers in Sanitary and Hygiene Applications. Int. J. Polym. Sci. 2023, 2023, 4717905. [Google Scholar] [CrossRef]
- Faris, D.; Hadi, N.J.; Habeeb, S.A. Effect of rheological properties of (Poly vinyl alcohol/Dextrin/Naproxen) emulsion on the performance of drug encapsulated nanofibers. Mater. Today Proc. 2021, 42, 2725–2732. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, A.; Dhaliwal, A.S. Superabsorbent Polymers Application in Agriculture Sector. In Properties and Applications of Superabsorbent Polymers: Smart Applications with Smart Polymers, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Sankar, P.R. Superabsorbent Polymer Sponges for the Design of Saliva Absorption Pad. Ph.D. Thesis, Sree Chitra Tirunal Institute for Medical Sciences & Technology, Kerala, India, 2021. [Google Scholar]
- Villa, A.J.C. Novel Superabsorbent Materials Obtained from Plant Proteins; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2017. [Google Scholar]
- Shi, X.; Wang, W.; Wang, A. pH-responsive sodium alginate-based super porous hydrogel generated by an anionic surfactant micelle templating. Carbohydr. Polym. 2013, 94, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Poursamara, S.A.; Azamib, M.; Mozafari, M. Controllable synthesis and characterization of porous polyvinyl alcohol/hydroxyapatite nanocomposite scaffolds via an in situ colloidal technique. Colloids Surf. B Biointerfaces 2011, 84, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, D. Swelling and mechanical properties of glycol chitosan/poly (vinyl alcohol) IPN-type super porous hydrogels. J. Boimed. Mater. Res. Part A 2006, 78, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Delaney, J.T.; Liberski, A.R.; Perelaer, J.; Schubert, U.S. Reactive inkjet printing of calcium alginate hydrogel porogen—A new strategy to open-pore structure matrices with controlled geometry. Soft Matter 2010, 6, 866–869. [Google Scholar] [CrossRef]
- Elbert, D.L. Liquid-liquid two-phase systems for the production of porous hydrogels and hydrogel microspheres for biomedical application: A tutorial review. Acta Biomater. 2011, 7, 31–56. [Google Scholar] [CrossRef] [PubMed]
- Partrap, S.; Muthutantri, A.; Rehman, I.U.; Davis, G.R.; Darr, J.A. Preparation and characterization of controlled porosity alginate hydrogels made via a simultaneous micelle templating and internal gelation process. J. Mater. Sci. 2007, 42, 3502–3507. [Google Scholar] [CrossRef]
- Dhahir, S.A.; Braihi, A.J.; Habeeb, S.A. Comparative Analysis of Hydrogel Adsorption/Desorption with and without Surfactants. Gels 2024, 10, 251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, S.; Chen, Z.; Wang, M.; Cao, J.; Wang, R. Preparation and characterization of superabsorbent polymers based on sawdust. Polymers 2019, 11, 1891. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wan, L.; Huang, X. Surface Modification by Graft Polymerization. In Surface Engineering of Polymer Membranes; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Chaudhary, S.; Jain, V.P.; Jaiswar, G. The composition of polysaccharides: Monosaccharides and binding, group decorating, polysaccharides chains. In Innovation in Nano-Polysaccharides for Eco-Sustainability; Elsevier: Amsterdam, The Netherlands, 2022; pp. 83–118. [Google Scholar]
- Rimando, S.; Perale, G.; Rossi, F. Polysaccharide-based scaffold for tissue regeneration. In Functional Polysaccharides for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2019; pp. 189–212. [Google Scholar]
- Habeeb, S.A.; Abdulkadhim, M.K. Natural Biopolymer-hydrogels Nanofibers for Antibacterial Applications. J. Eng. Mater. Technol. 2023, 146, 011008. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Zhou, X.; Liu, H.; Xu, B. Synergistic interactions between zwitterionic surfactants derived from olive oil and an anionic surfactant. J. Dispers. Sci. Technol. 2019, 40, 1308–1316. [Google Scholar] [CrossRef]
- Tally, M.; Atassi, Y. Synthesis and characterization of pH-sensitive superabsorbent hydrogels based on sodium alginate-g-poly (acrylic acid-co-acrylamide) obtained via an anionic surfactant micelle templating under microwave irradiation. Polym. Bull. 2016, 73, 3183–3208. [Google Scholar]
- Tally, M.; Atassi, Y. Optimized synthesis and swelling properties of a pH-sensitive semi-IPN superabsorbent polymer based on sodium alginate-g-poly (acrylic acid-co-acrylamide) and polyvinylpyrrolidone and obtained via microwave irradiation. J. Polym. Res. 2015, 22, 181. [Google Scholar] [CrossRef]
- Rizwan, M.; Gilani, S.R.; Durani, A.I.; Naseem, S. Materials diversity of hydrogel: Synthesis, polymerization process and soil conditioning properties in agricultural field. J. Adv. Res. 2021, 33, 15–40. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Vats, T.; Clark, J.H. Microwave-Assisted Polymerization; Royal Society of Chemistry: London, UK, 2015; ISBN 978-1-78262-317-5. [Google Scholar]
- Li, Y.; Xiao, H.; Pan, Y.; Zhang, M.; Jin, Y. Thermal and pH dual-responsive cellulose microfilament spheres for dye removal in single and binary systems. J. Hazard. Mater. 2019, 377, 88–97. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, T.G.; Karimi, F.; Ashokkumar, M.; Qiao, G.G. Ultrasound and sonochemistry for radical polymerization: Sound synthesis. Chem. Eur. J. 2019, 25, 5372–5388. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, N.; Atassi, Y.; Tally, M. Synthesis and swelling behavior of metal-chelating superabsorbent hydrogels based on sodium alginate-g-poly (AMPS-co-AA-co-AM) obtained under microwave irradiation. Polym. Bull. 2017, 74, 4453–4481. [Google Scholar] [CrossRef]
- Zohourian, M.M.; Kabiri, K. Superabsorbent polymers materials: A review. Iran Polym. J. 2008, 17, 451–477. [Google Scholar]
- El-Sayed, M.; Sorour, M.; ElMoneem, N.A.; Talaat, H.; Shalaan, H.; ElMarsafy, S. Synthesis and properties of natural polymers—Grafted-acrylamide. World Appl. Sci. J. 2011, 13, 360–368. [Google Scholar]
- Ghasemzadeh, H.; Ghanaat, F. Antimicrobial alginate/PVA silver nanocomposite hydrogel, synthesis, and characterization. J. Polym. Res. 2014, 21, 355–368. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Aminabhavi, T.M. Novel interpenetrating network chitosan-poly (ethylene oxide-g-acrylamide) hydrogel mi crospheres for the controlled release of capecitabine. Int. J. Pharm. 2006, 324, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Ma, J.; Li, N. Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly(AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym. 2011, 84, 76–82. [Google Scholar] [CrossRef]
- Zhang, L.M.; Yang, C.; Yan, L. Perspectives on: Strategies to fabricate starch-based hydrogels with potential biomedical applications. J. Bioact. Compat. Pol. 2005, 20, 297–314. [Google Scholar] [CrossRef]
- Hirschberg, J.H.K.K.; Beijer, F.H.; van Aert, H.A.; Magusin, P.C.M.M.; Sijbesma, R.P.; Meijer, E.W. Supramolecular polymers from linear telechelic siloxanes with quadruple-hydrogen-bonded units. Macromolecules 1999, 32, 2696–2705. [Google Scholar] [CrossRef]
- Chen, Y.; Kushner, A.M.; Williams, G.A.; Guan, Z. Multiphase design of autonomic self-healing thermoplastic elastomers. Nat. Chem. 2012, 4, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.R.; Hunter, J.H.; Nguyen, N.A.; Harries, J.L.; Greenland, B.W.; Mackay, M.E.; Colquhoun, H.M.; Hayes, W. Multivalency in healable supramolecular polymers: The effect of supramolecular cross-link density on the mechanical properties and healing of non-covalent polymer networks. Polym. Chem. 2014, 5, 3680–3688. [Google Scholar] [CrossRef]
- Sonmez, B.; Celikkol, A.N. Pullulan-based hydrogels for the removal of various metal ions from aqueous solutions. J. Environ. Chem. Eng. 2021, 9, 106188. [Google Scholar] [CrossRef]
- Mustfa, W.M.; Habeeb, S.A. Evaluation of the Physical Properties and Filtration Efficiency of PVDF/PAN Nanofiber Membranes by Using Dry Milk Protein. Mater. Res. Express 2023, 10, 095306. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani-Farahani, S.; Vasheghani-Farahani, E. Theoretical description of hydrogelswelling: A review. Iran Polym. J. 2010, 19, 375–398. [Google Scholar]
- Wu, J.; Zhong, F.; Li, Y.; Shoemaker, C.F.; Xia, W. Preparation and characterization of pullulan–chitosan and pullulan–carboxymethyl chitosan blended films. Food Hydrocoll. 2013, 30, 82–91. [Google Scholar] [CrossRef]
- Saber-Samandari, S.; Gulcan, H.O.; Saber-Samandari, S.; Gazi, M. Efficient removal of anionic and cationic dyes from an aqueous solution using pullulan-graft-polyacrylamide porous hydrogel. Water Air Soil Pollut. 2014, 225, 2177. [Google Scholar] [CrossRef]
- Chen, M.; Ni, Z.; Shen, Y.; Xiang, G.; Xu, L. Reinforced swelling and water-retention properties of super-absorbent hydrogel fabricated by a dual stretchable single network tactic. Colloids Surf. A Physicochem. 2020, 602, 125133. [Google Scholar] [CrossRef]
- Wei, J.; Zhao, Y.; Yu, S.; Du, J.; Hu, X.; Bai, G.; Wang, Z. Environment-friendly dual-network hydrogel dust suppressant based on xanthan gum, polyvinyl alcohol, and acrylic acid. J. Environ. Manag. 2021, 295, 113139. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zeng, W.; Zhao, J.; Qiu, X.; Xiong, H.; Liang, Y.; Ye, X.; Lei, Z.; Chen, D. Preparation and anti-leakage properties of hydroxyethyl cellulose-g-poly (butyl acrylate-co-vinyl acetate) emulsion. Carbohydr. Polym. 2021, 255, 117467. [Google Scholar] [CrossRef] [PubMed]
- Işıklan, N.; Küçükbalcı, G. Microwave-induced synthesis of alginate–graft-poly (N-isopropylacrylamide) and drug release properties of dual pH-and temperature-responsive beads. Eur. J. Pharm. Biopharm. 2012, 82, 316–331. [Google Scholar] [CrossRef] [PubMed]
- Kabiri, K.; Zouhiriaan-Mehr, M.J.; Kheirabadi, M.M. Superalcohol-absorbent gels of sulfonic acid-contained poly(acrylic acid). J. Polym. Res. 2011, 18, 449–458. [Google Scholar] [CrossRef]
- Khoshkho, S.M.; Tanhaei, B.; Ayati, A.; Kazemi, M. Preparation and characterization of ionic and non-ionic surfactants impregnated κ-carrageenan hydrogel beads for investigation of the adsorptive mechanism of cationic dye to develop for biomedical applications. J. Mol. Liq. 2021, 324, 115118. [Google Scholar] [CrossRef]
- Warkar, S.G.; Kumar, A. Synthesis and assessment of carboxymethyl tamarind kernel gum-based novel superabsorbent hydrogels for agricultural applications. Polymer 2019, 182, 121823. [Google Scholar]
- Sihama, I.S.; Fadhel, A.H.; Braihi, A.J. Optimization of Nano Graphite Oxide Concentration for Super Absorbent Nano Composites Polymeric Materials. J. Eng. Appl. 2018, 13, 8228. [Google Scholar]
- Qian, G.; Hu, K.; Gong, X.; Li, N.; Yu, H. Real-time flow behavior of hot mix asphalt (HMA) compaction based on rheological constitutive theory. Materials 2019, 12, 1711. [Google Scholar] [CrossRef] [PubMed]
- Sannino, A.; Pappadà, S.; Madaghiele, M.; Maffezzoli, A.; Ambrosio, L.; Nicolais, L. Crosslinking of cellulose derivatives and hyaluronic acid with water-soluble carbodiimide. Polymer 2005, 46, 11206–11212. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Sadeghzadeh, M.; Babazadeh, M. Preparation and properties of carrageenan—G-poly (acrylic acid)/bentonite superabsorbent composite. J. Boimater. Nanobiotechol. 2011, 2, 311–317. [Google Scholar] [CrossRef]
- Batouti, M.F.; Sadik, W.; Eldemerdash, A.G.; Hanafy, E.; Fetouh, H.A. New and innovative microwave-assisted technology for synthesis of guar gum-grafted acrylamide hydrogel superabsorbent for the removal of acid red 8 dye from industrial wastewater. Polym. Bull. 2023, 80, 4965–4989. [Google Scholar] [CrossRef]
- Haidari, H.; Kopecki, Z.; Sutton, A.T.; Garg, S.; Cowin, A.J.; Vasilev, K. PH-responsive “smart” hydrogel for controlled delivery of silver nanoparticles to infected wounds. Antibiotics 2021, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Jamali, F.; Etminani-Esfahani, N.; Rahmati, A. Maleic acid as an important monomer in synthesis of stimuli-responsive poly (acrylic acid-co-acrylamide-co-maleic acid) superabsorbent polymer. Sci. Rep. 2023, 13, 3511. [Google Scholar] [CrossRef] [PubMed]
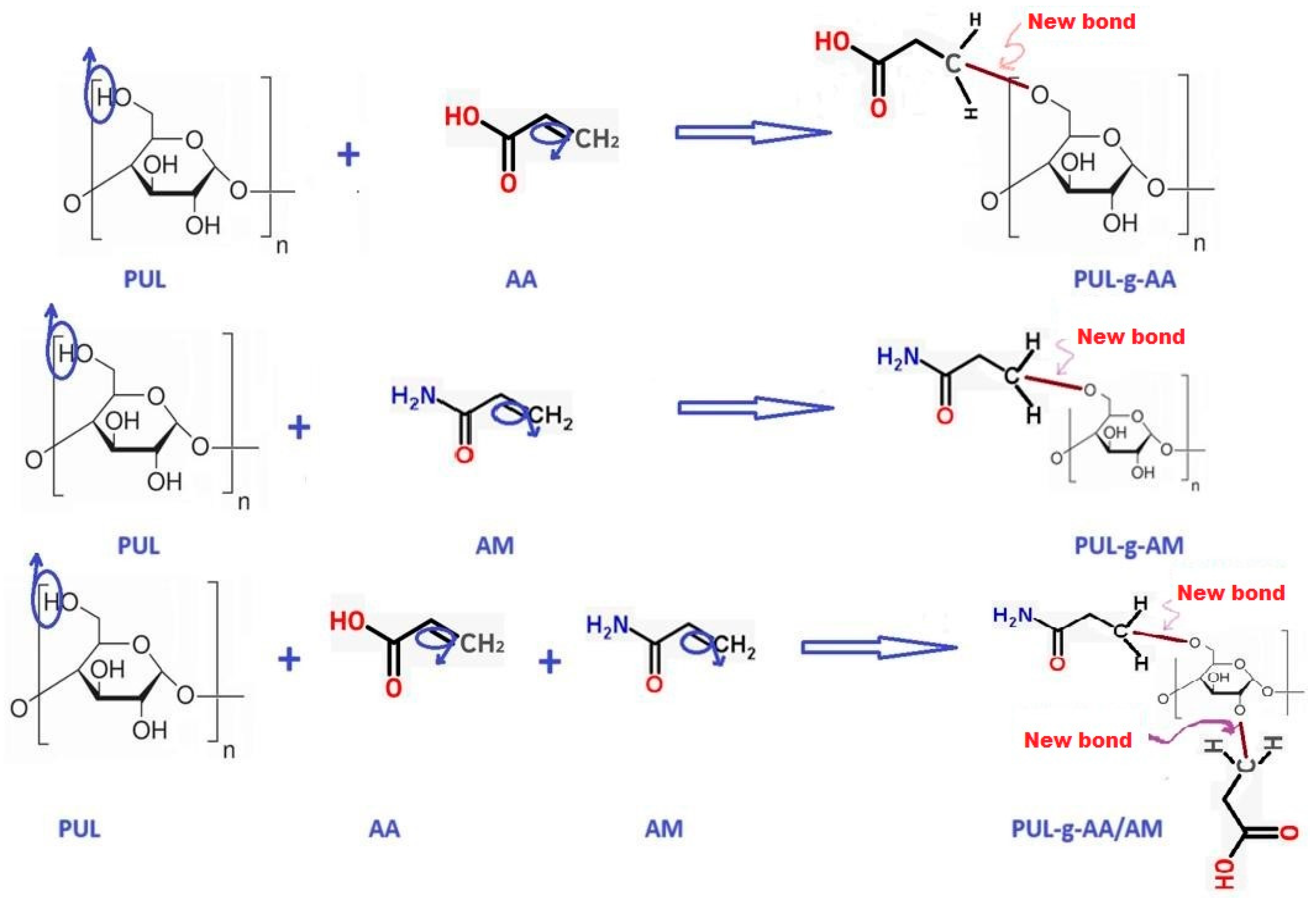



| Samples | Composition of SAPs |
|---|---|
| SAP1 | PUL-g-AA |
| SAP2 | PUL-g-AM |
| SAP3 | PUL-g-AM/AA |
| SAP4 | PUL-g-AM/AA-1 mM SDBS |
| SAP5 | PUL-g-AM/AA-2 mM SDBS |
| SAP6 | PUL-g-AM/AA-3 mM SDBS |
| SAP7 | PUL-g-AM/AA-4 mM SDBS |
| SAP8 | PUL-g-AM/AA-5 mM SDBS |
| Samples | Pore Volume (µm3) | Porosity % | Pore Volume (µm3/g) | Surface Area (m2/g) |
|---|---|---|---|---|
| SAP1 | 0.0225 ± 0.0113 | 5.318 | 6.39 | 2.25 |
| SAP2 | 0.0329 ± 0.0103 | 9.223 | 7.18 | 1.83 |
| SAP3 | 0.0626 ± 0.0102 | 11.822 | 8.87 | 2.19 |
| SAP6 | 2.276 ± 0.0818 | 15.903 | 23.47 | 3.80 |
| SAPs | Pos. [°2Th.] | d-Spacing [Å] | FWHM [°2Th.] | (h k l) | Crystallinity (%) | Crystallite Size (nm) |
|---|---|---|---|---|---|---|
| SAP1 | 21.59 | 3.569 | 0.16593 | 110 | 15.02 | 48.46 |
| SAP2 | 21.94 | 4.115 | 0.15884 | 110 | 11.35 | 40.86 |
| SAP3 | 18.78 49.83 | 4.725 2.378 | 0.35080 0.16320 | 110 220 | 22.61 | 51.60 |
| SAP6 | 19.73 50.43 | 3.335 1.809 | 0.17640 0.16211 | 110 211 | 23.87 | 54.30 |
| SAPs | Tw (%) | Step 1 | Step 2 | Step 3 | Step 4 | Step 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | A | B | ||
| SAP1 | 81.78 | 150 | 1.88 | 220 | 8.38 | 370 | 14.7 | 480 | 35.00 | 800 | 21.82 |
| SAP2 | 90.92 | 125 | 4.23 | 300 | 53.53 | 470 | 22.2 | 800 | 10.96 | - | - |
| SAP3 | 86.46 | 150 | 1.56 | 370 | 42.60 | 800 | 42.3 | - | - | - | - |
| SAP6 | 72.59 | 220 | none | 260 | 5.03 | 370 | 23.5 | 470 | 18.20 | 800 | 25.86 |
| Sample | C (%) | G (%) | E (%) | Gel (%) | WAC (g/g) | WR (%) |
|---|---|---|---|---|---|---|
| SAP1 | 72.07 | 325.0 | 65.00 | 51.70 | 32.0 | 63.70 |
| SAP2 | 73.19 | 333.3 | 66.67 | 61.80 | 58.0 | 68.00 |
| SAP3 | 83.57 | 743.3 | 74.33 | 73.00 | 74.1 | 73.44 |
| SAP4 | 87.03 | 760.0 | 76.00 | 74.39 | 123.5 | 74.93 |
| SAP5 | 88.32 | 783.3 | 78.33 | 78.72 | 143.6 | 76.00 |
| SAP6 | 89.95 | 838.3 | 83.83 | 81.40 | 200.5 | 79.50 |
| SAP7 | 89.13 | 820.0 | 82.00 | 74.81 | 137.8 | 73.09 |
| SAP8 | 88.86 | 800.0 | 80.00 | 78.88 | 128.7 | 71.54 |
| Parameters for Different SAPs and a pH of 7 | Parameters for Different pHs and 3 mM SDBS | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SBDS (mM) | pH | ||||||||
| 0 | 0.935 | 0.016 | 95.24 | 74.3 | 2 | 0.987 | 0.067 | 92.59 | 87.6 |
| 1 | 0.987 | 0.050 | 139.86 | 101.3 | 4 | 0.986 | 0.079 | 197.24 | 168.7 |
| 2 | 0.993 | 0.075 | 150.38 | 137.8 | 7 | 0.994 | 0.183 | 217.39 | 201.5 |
| 3 | 0.997 | 0.148 | 212.77 | 202.5 | 9 | 0.985 | 0.068 | 166.39 | 142.0 |
| 4 | 0.997 | 0.104 | 152.44 | 143.5 | 12 | 0.982 | 0.086 | 120.63 | 109.9 |
| 5 | 0.998 | 0.121 | 134.41 | 116.9 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhahir, S.A.; Braihi, A.J.; Habeeb, S.A. Preparation and Characterization of Supramolecular Bonding Polymers Based on a Pullulan Substrate Grafted with Acrylic Acid/Acrylamide by Microwave Irradiation. ChemEngineering 2024, 8, 77. https://doi.org/10.3390/chemengineering8040077
Dhahir SA, Braihi AJ, Habeeb SA. Preparation and Characterization of Supramolecular Bonding Polymers Based on a Pullulan Substrate Grafted with Acrylic Acid/Acrylamide by Microwave Irradiation. ChemEngineering. 2024; 8(4):77. https://doi.org/10.3390/chemengineering8040077
Chicago/Turabian StyleDhahir, Salam Abdulla, Auda Jabbar Braihi, and Salih Abbas Habeeb. 2024. "Preparation and Characterization of Supramolecular Bonding Polymers Based on a Pullulan Substrate Grafted with Acrylic Acid/Acrylamide by Microwave Irradiation" ChemEngineering 8, no. 4: 77. https://doi.org/10.3390/chemengineering8040077
APA StyleDhahir, S. A., Braihi, A. J., & Habeeb, S. A. (2024). Preparation and Characterization of Supramolecular Bonding Polymers Based on a Pullulan Substrate Grafted with Acrylic Acid/Acrylamide by Microwave Irradiation. ChemEngineering, 8(4), 77. https://doi.org/10.3390/chemengineering8040077









