Synthesis and Characterization of Azo-Based Cyclotriphosphazene Compounds: Liquid Crystalline and Dielectric Properties
Abstract
1. Introduction
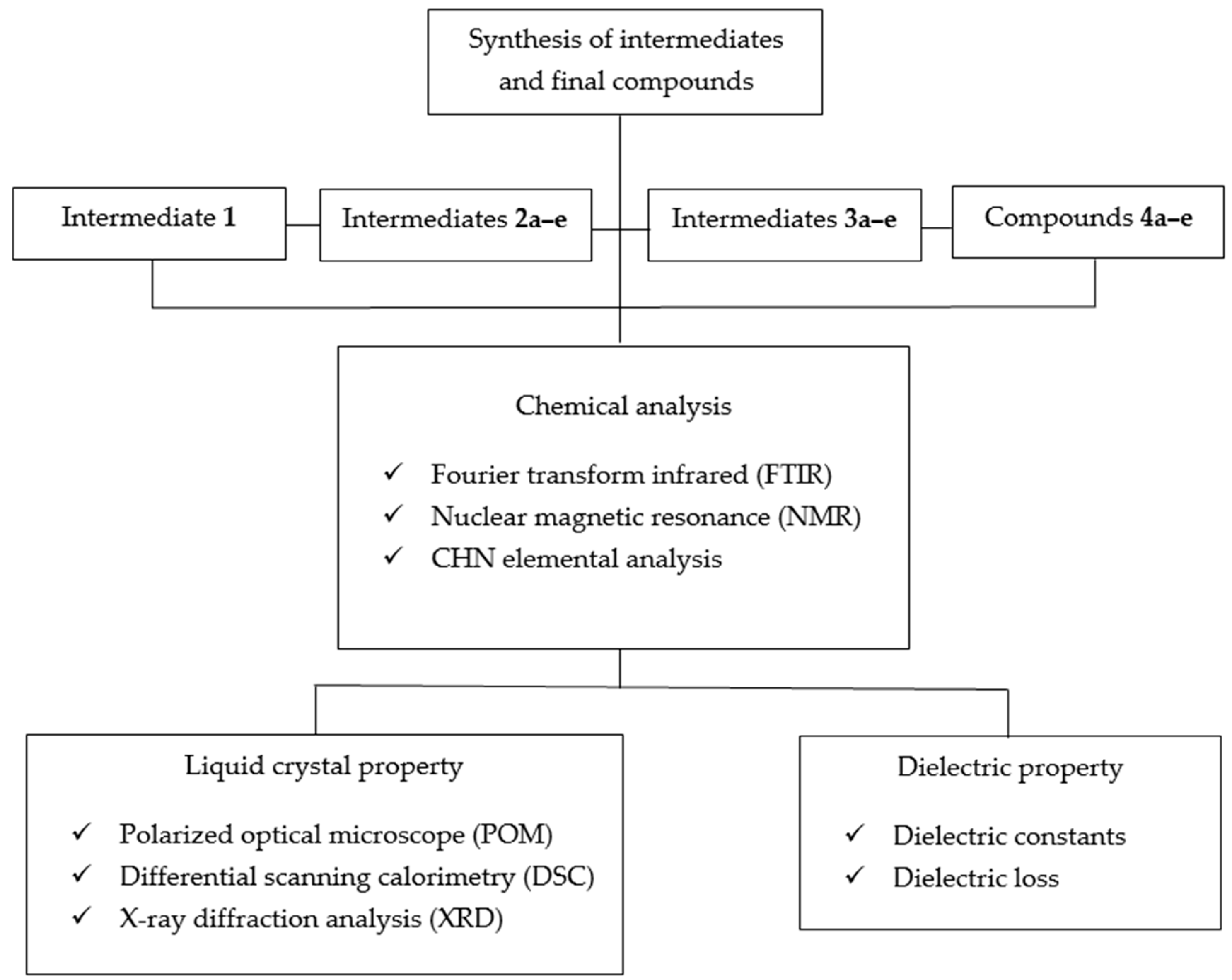
2. Materials and Methods
2.1. List of Chemicals
2.2. Characterization Methods
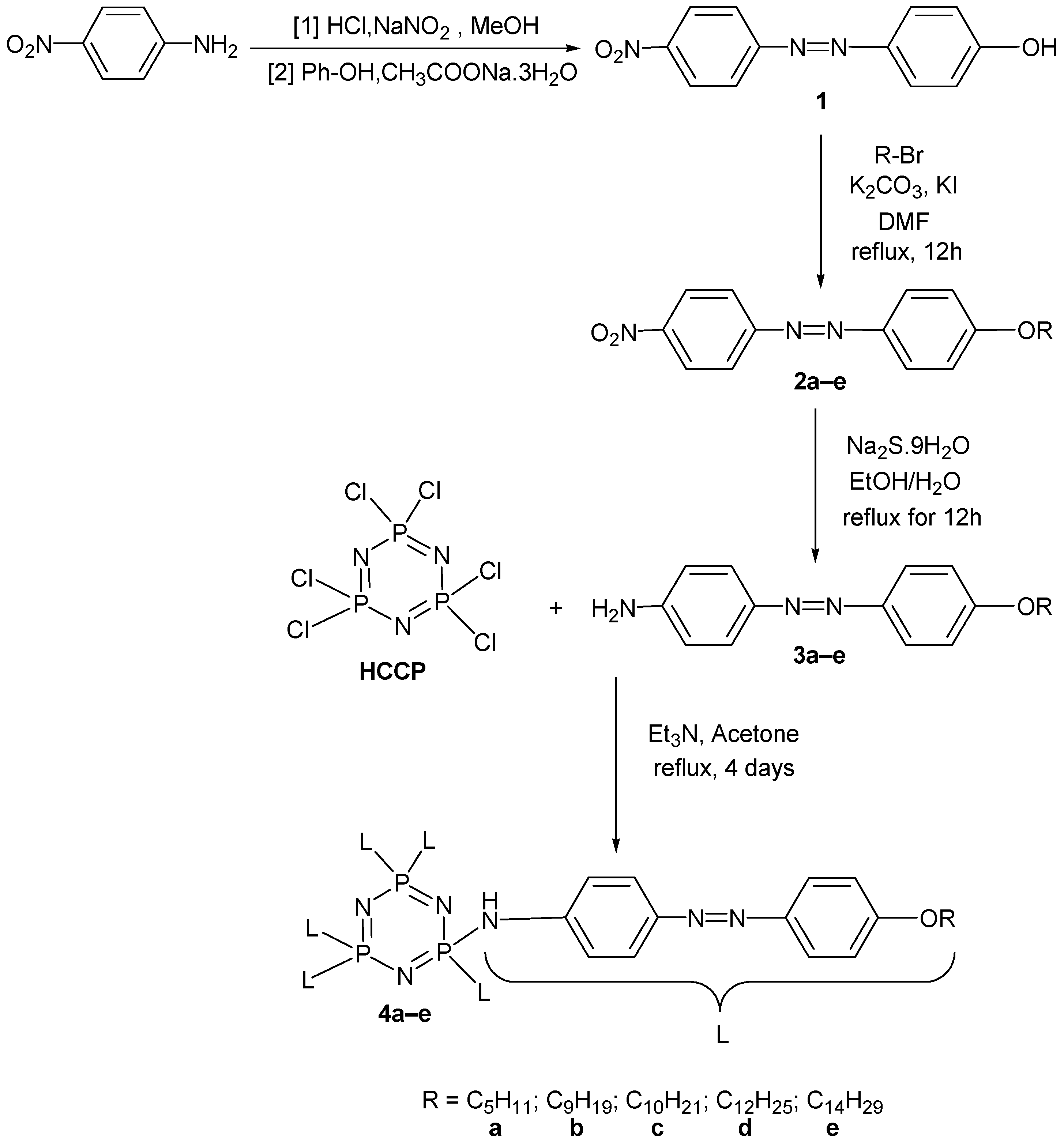
2.3. Synthesis Method
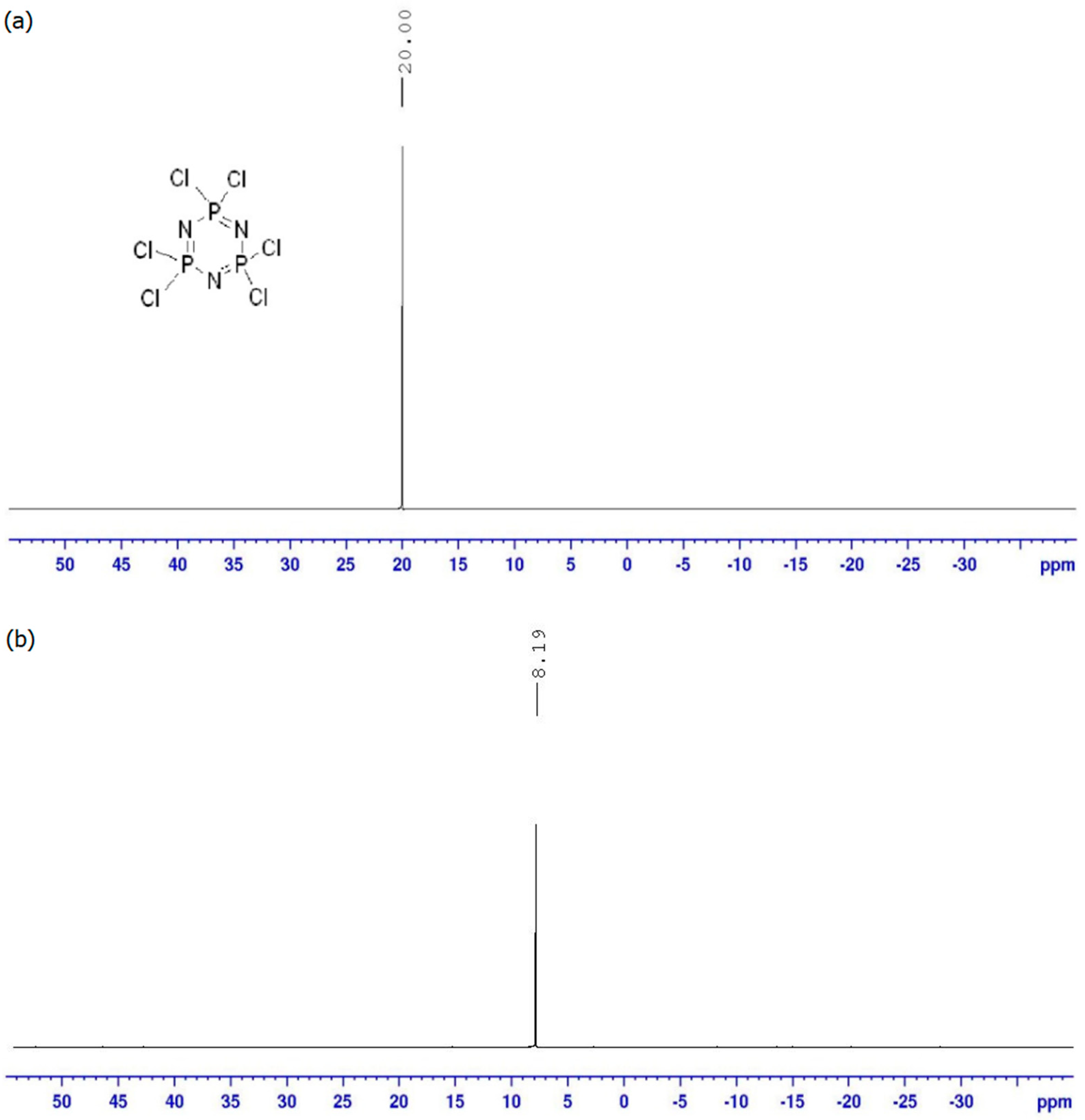
Synthesis of Hexakis(4-((4(substituted)phenyl)diazenyl)phenamino)triazaphosphazene
3. Results and Discussion
3.1. FTIR Spectral Discussion
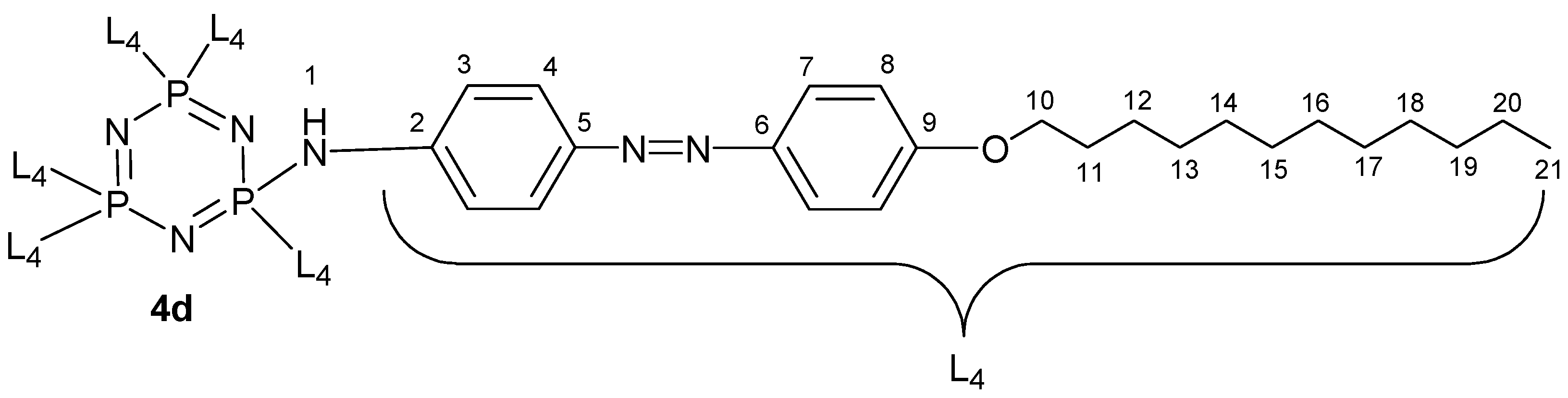
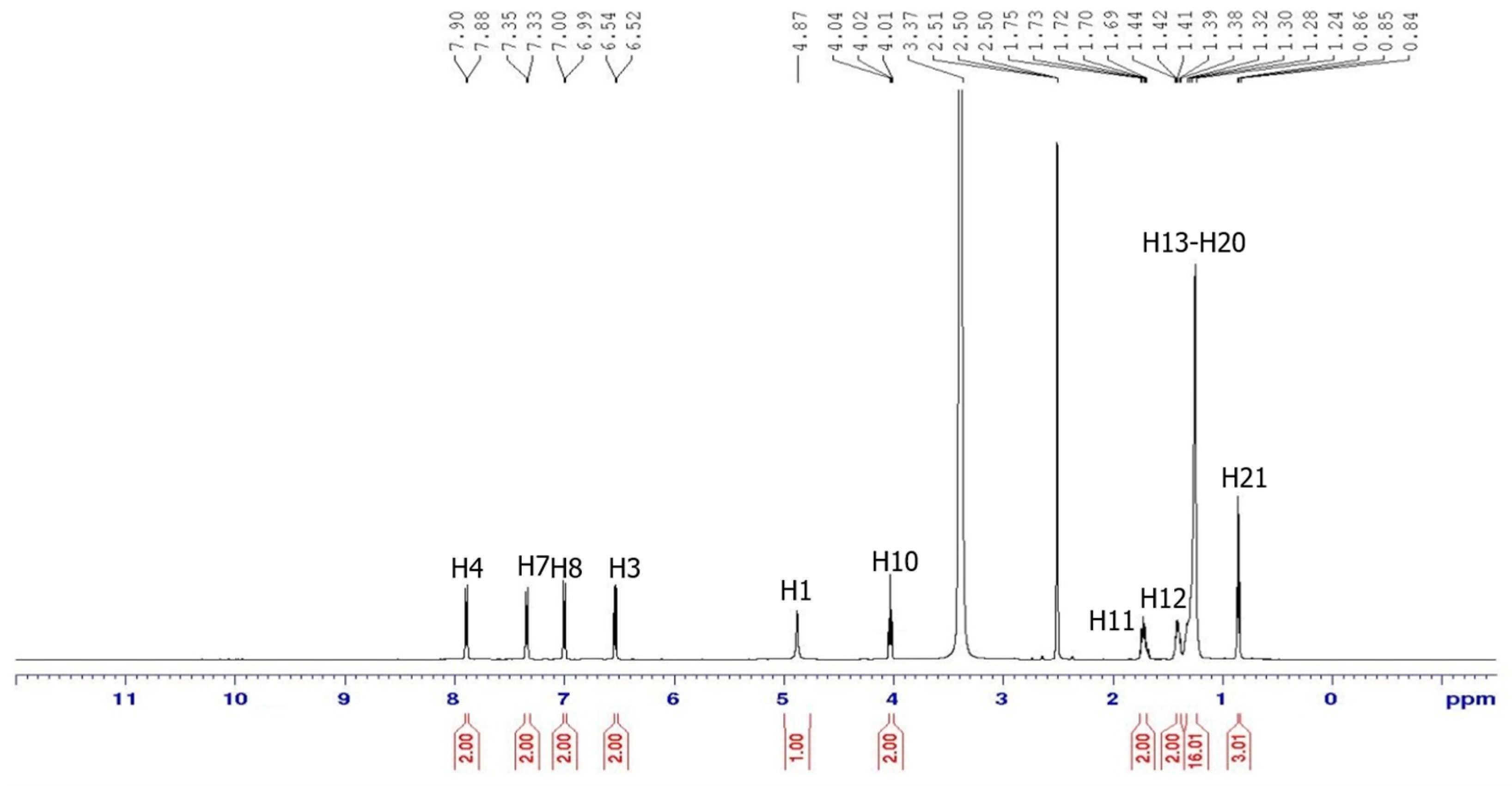
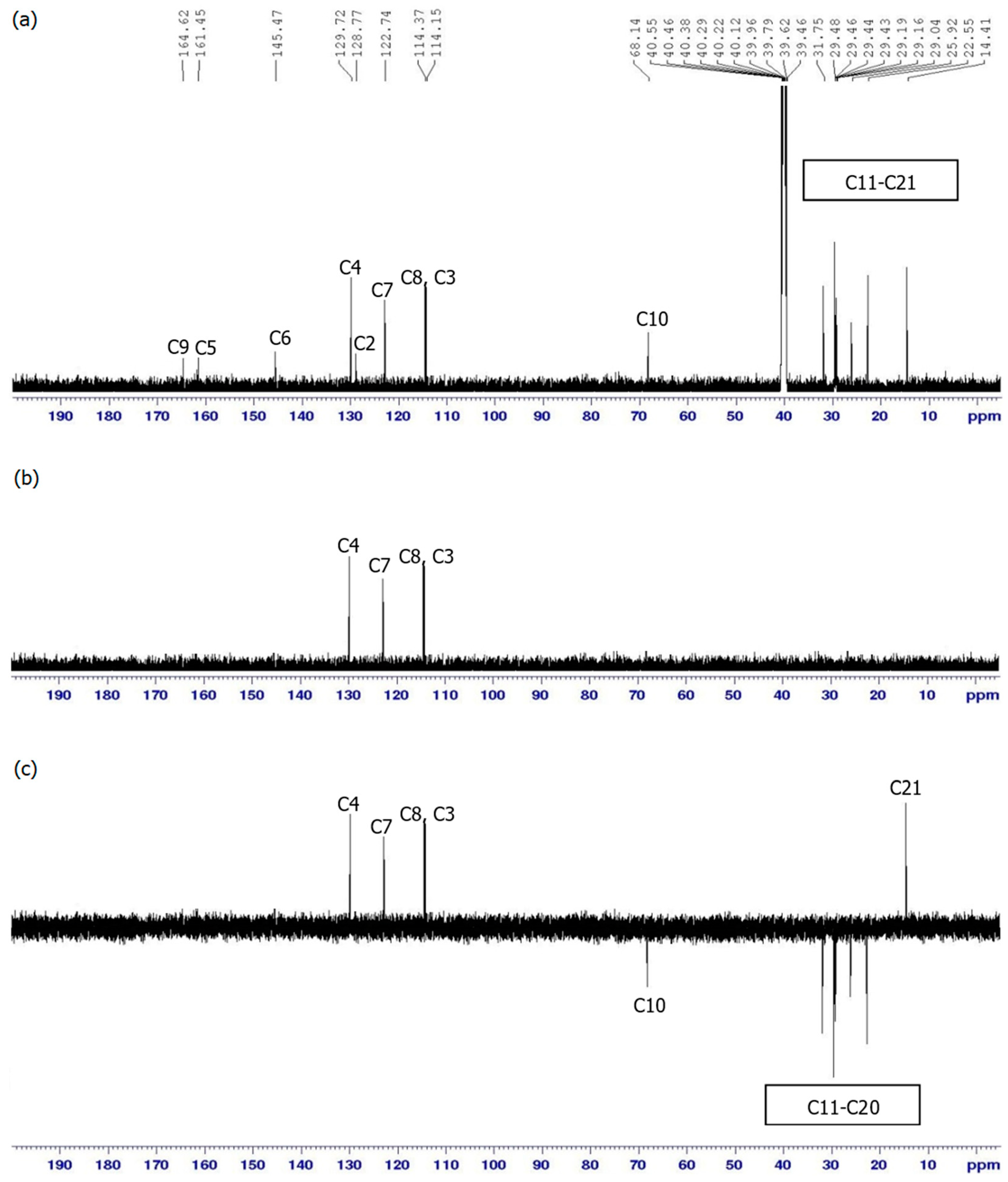
3.2. NMR Spectral Discussion
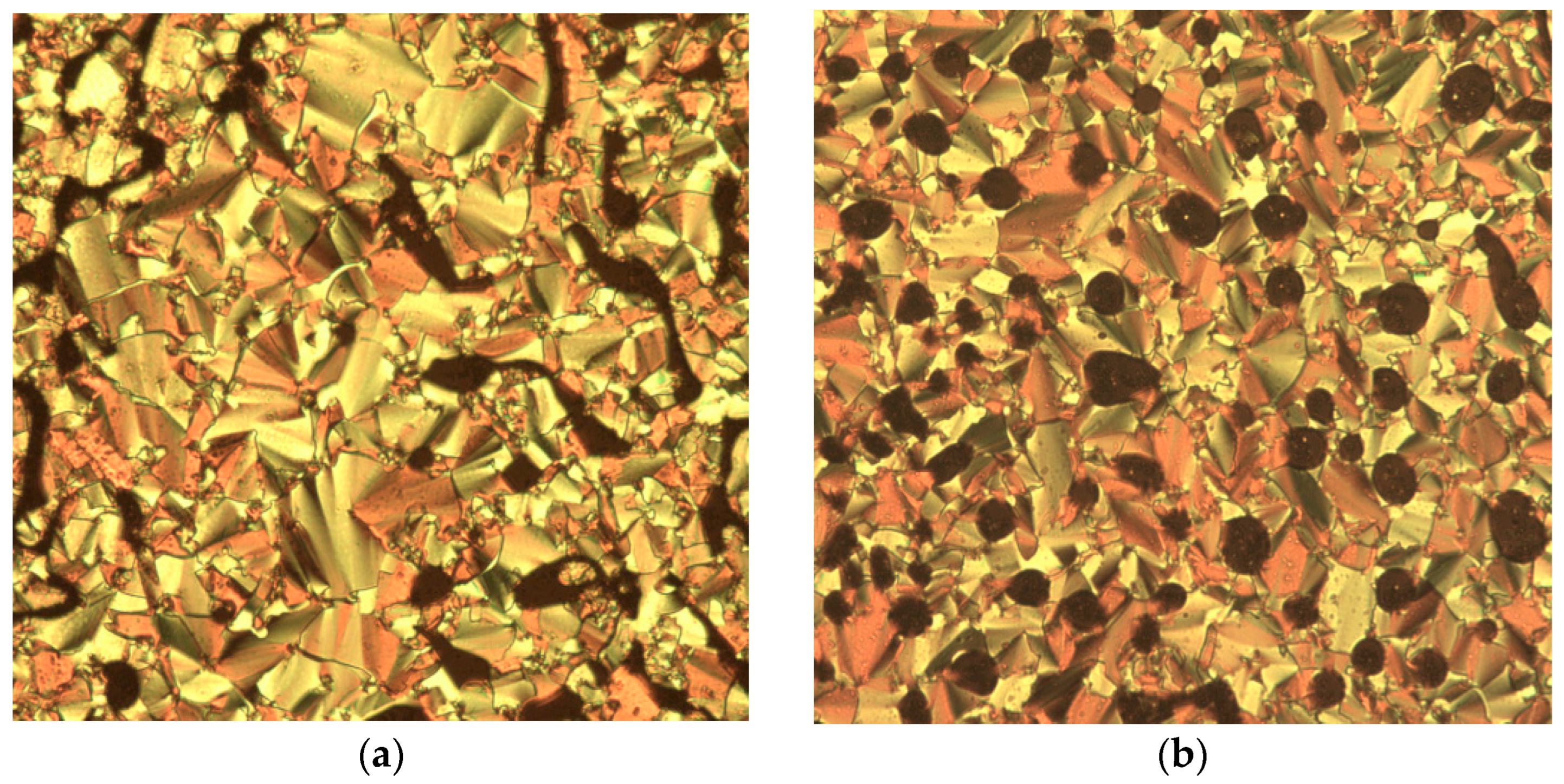
3.3. Determination of Mesophase Behavior Using POM
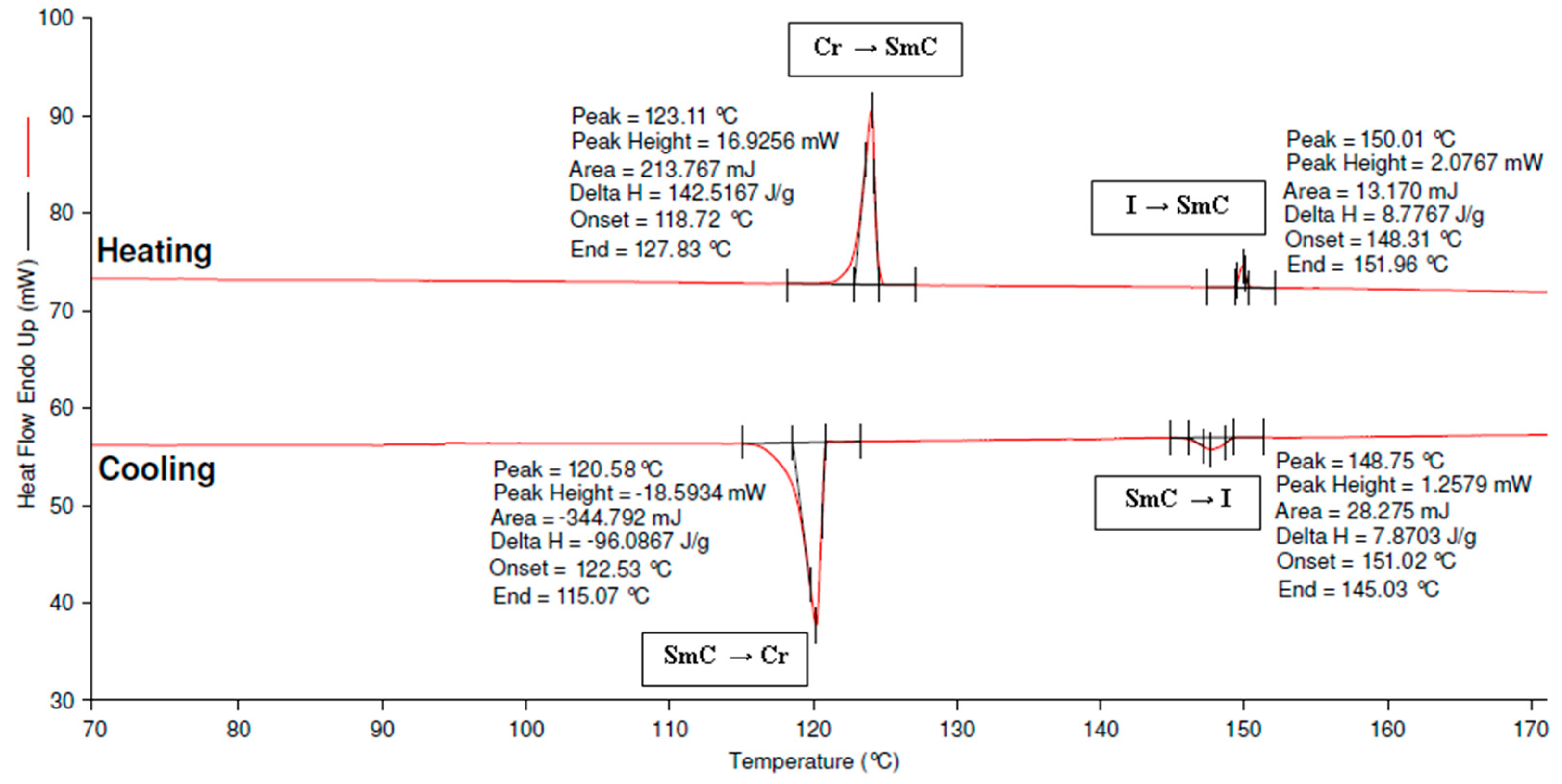
3.4. Determination of Thermal Transitions Using DSC
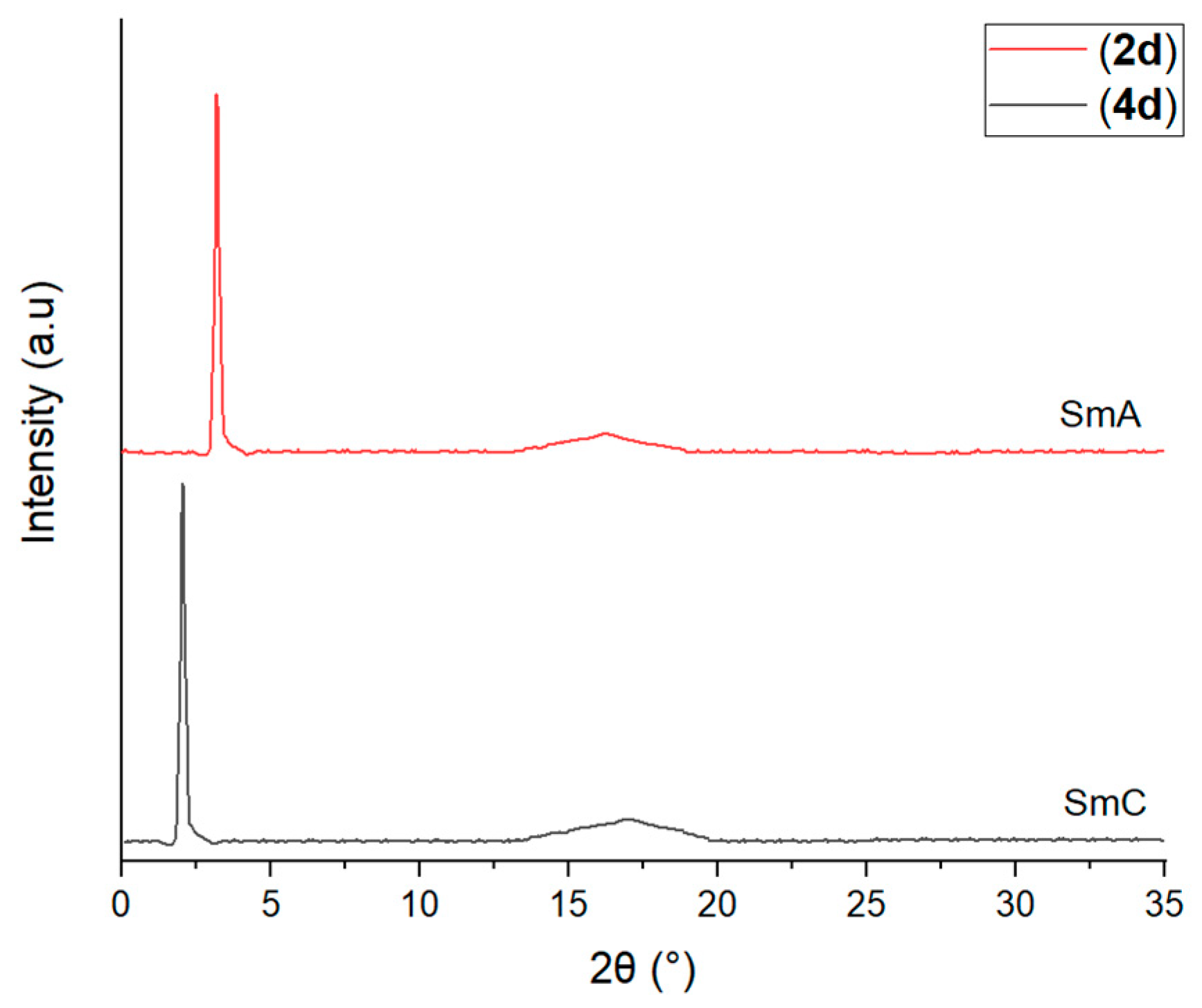
3.5. Determination of Liquid Crystal Behavior Using XRD
3.6. Structure–Properties Relationship in Liquid Crystal Study
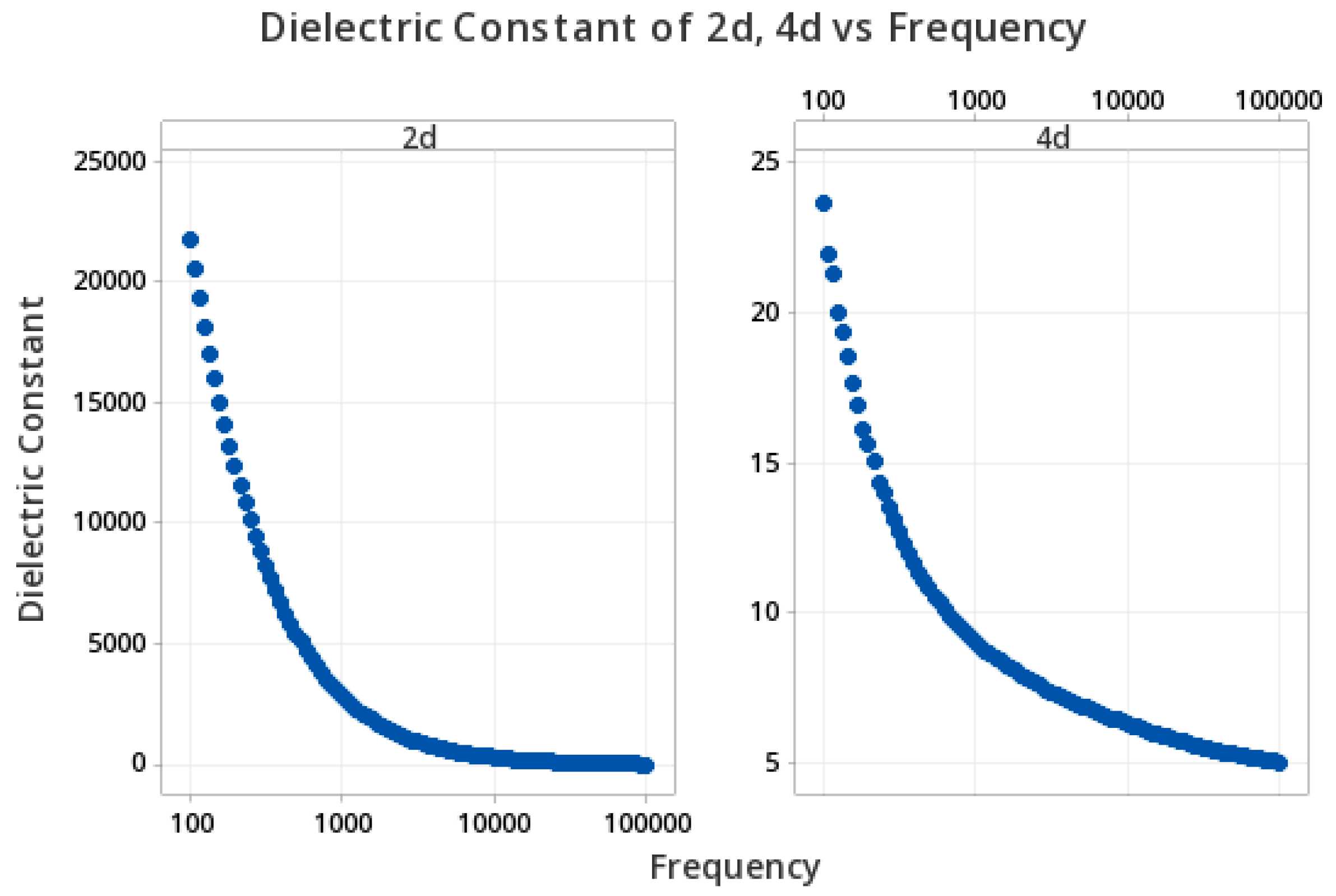
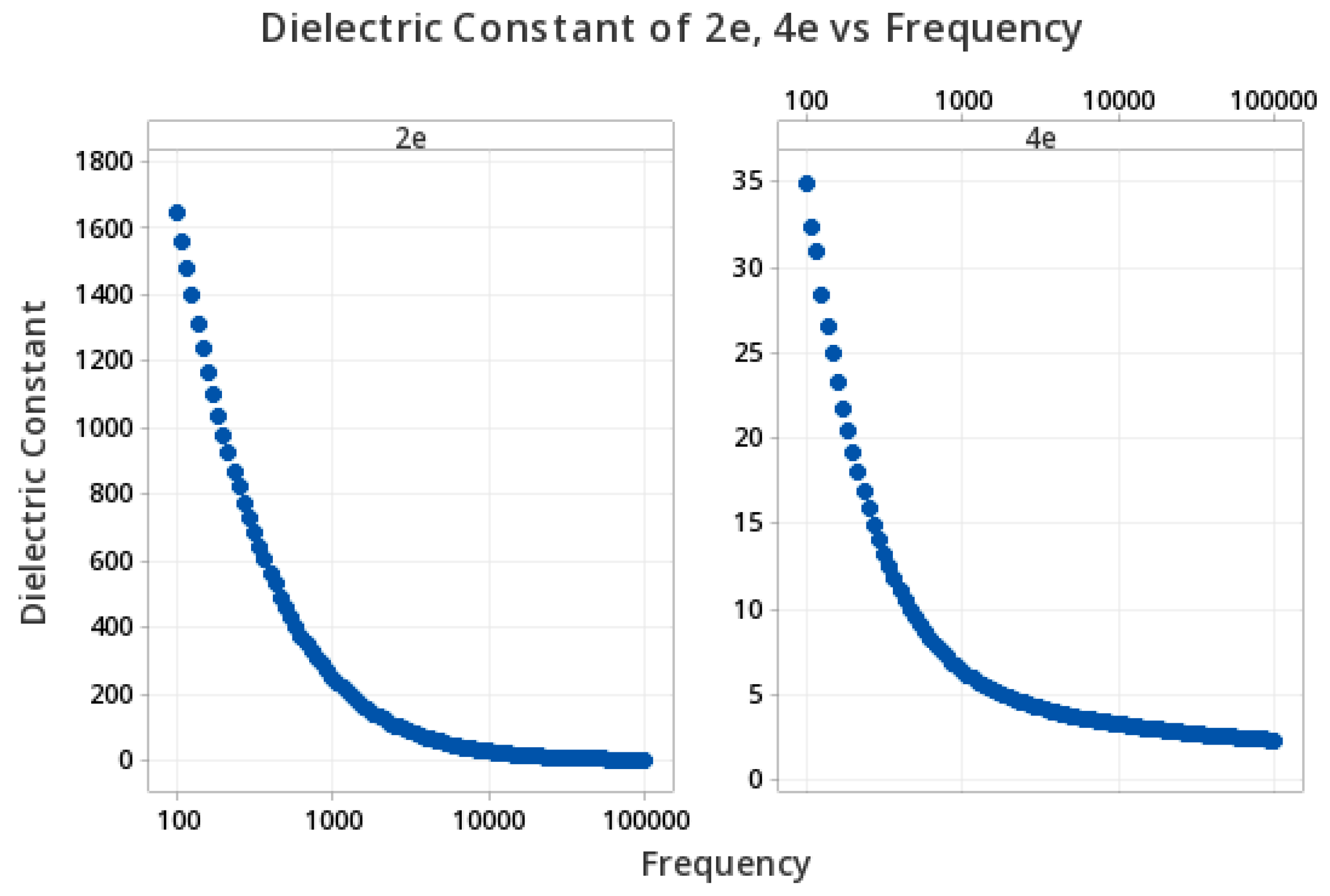
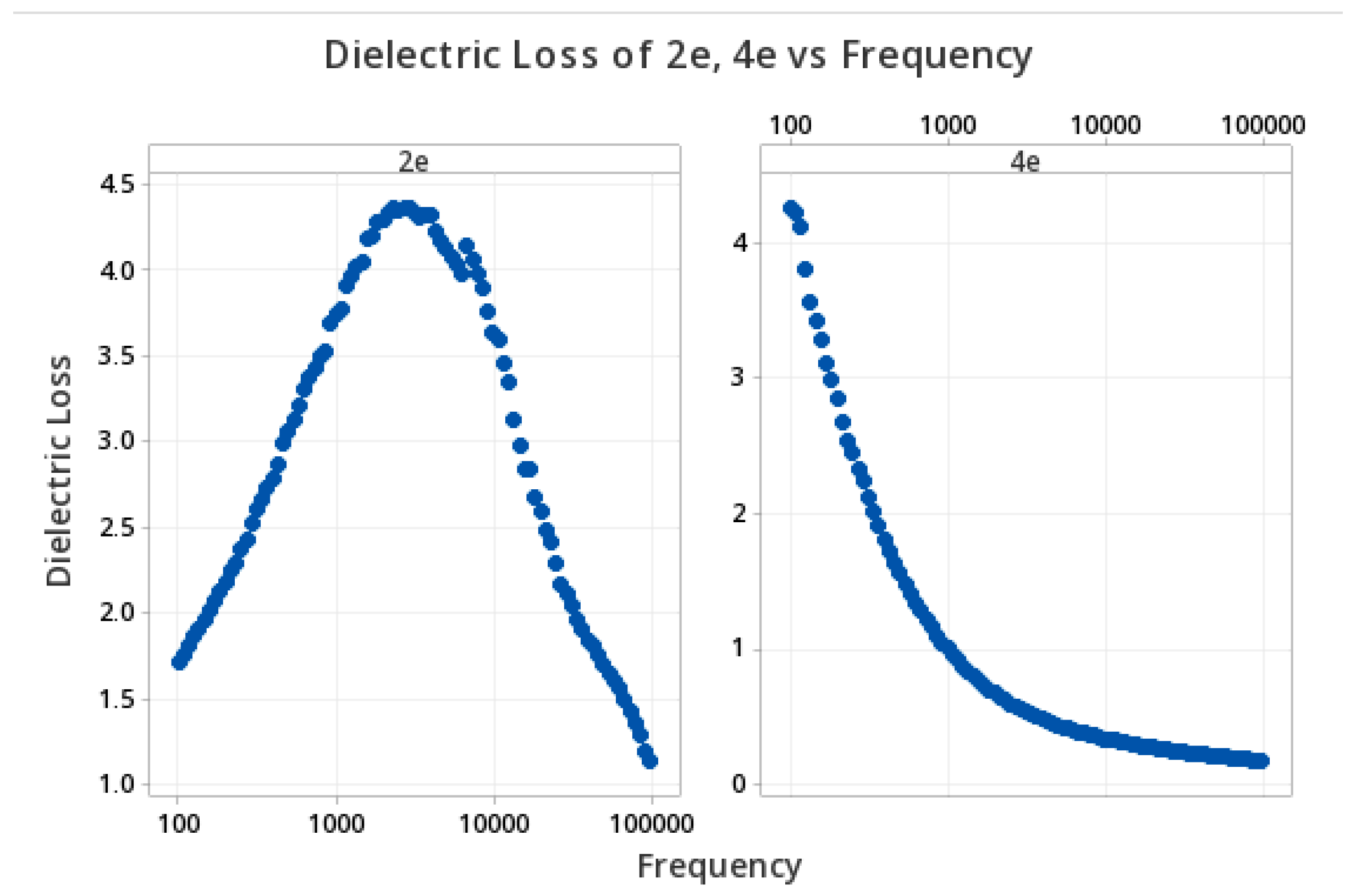
3.7. Determination of Dielectric Properties
3.8. Structure–Properties Relationship in Dielectric Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allcock, H.R.; Kugel, R.L. Synthesis of high polymeric alkoxy-and aryloxyphosphonitriles. J. Am. Chem. Soc. 1965, 87, 4216–4217. [Google Scholar] [CrossRef]
- Allcock, H.R. Recent advances in phosphazene (phosphonitrilic) chemistry. Chem. Rev. 1972, 72, 315–356. [Google Scholar] [CrossRef]
- Davarci, D.; Doganci, S. Liquid Crystal Phosphazenes. J. Mol. Struc. 2022, 1269, 1–18. [Google Scholar] [CrossRef]
- Palabıyık, D.; Balcı, C.M.; Tümay, S.O.; Sengul, I.F.; Beşli, S. New design of cyclotriphosphazene derivatives bearing carbazole units: The syntheses, characterization, and photophysical properties. Inorganica Chim. Acta 2022, 539, 121022. [Google Scholar] [CrossRef]
- Elkhalgi, H.H.M.; Khandka, S.; Singh, U.B.; Pandey, K.L.; Dabrowski, R.; Dhar, R. Dielectric and electro-optical properties of a nematic liquid crystalline material with gold nanoparticles. Liq. Cryst. 2018, 45, 1795–1801. [Google Scholar] [CrossRef]
- Mohan, M.M.L.N. Investigations on dielectric relaxations, memory and thermistor applications in a liquid crystal nematogen. Liq. Cryst. 2023, 50, 210–217. [Google Scholar]
- Das, M.K.; Pramanik, A.; Das, B.; Szezuenski, L.; Dabrowski, R. A comparative study of the mesomorphic properties of fluoro-isothiocyanated and fluorinated terphenyl liquid crystals from birefringence, dielectric permittivity, splay elastic constant and rotational viscocity measurements. J. Phys. D Appl. Phys. 2012, 45, 415304. [Google Scholar] [CrossRef]
- Pramanik, A.; Das, B.; Das, M.K.; Garbat, K.; Urban, S.; Dabrowski, R. Mesomorphic. optical, dielectric, elastic and viscous properties of multicomponent isothiocyanato mixtures. Liq. Cryst. 2013, 40, 149–158. [Google Scholar] [CrossRef]
- Lee, M.-J.; Lee, W. Liquid crystal-based capacitive, electro-optical and dielectric biosensors for protein quantitation. Liq. Cryst. 2020, 47, 1145–1153. [Google Scholar] [CrossRef]
- Das Gupta, S.; Roy, S.K. Splay and bend elastic constants and rotational viscocity coefficient in a mixture of 4-4’-n-pentyl-cyanobiphenyl and 4-4’-n-decyl-cyanobiphenyl. Phys. Lett. A 2003, 306, 235–242. [Google Scholar] [CrossRef]
- Sharma, A.; Mishra, R.; Tripathi, P.K. The overview of dielectric properties of liquid crystals with the function of frequency and temperature. Int. J. Adv. Res. 2022, 10, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Jamain, Z.; Khairuddean, M.; Kamaruddin, K.; Rui, Y. Synthesis, structural elucidation and mesophase behaviour of hexasubstituted cyclotriphosphazene molecules with amide linking unit. Malays. J. Chem. 2021, 23, 213–225. [Google Scholar]
- Alidaği, H.A.; Girgiç, Ö.M.; Zorlu, Y.; Hacivelioğlu, F.; Çelik, S.Ü.; Bozkurt, A.; Kiliç, A.; Yeȿilot, S. Synthesis and proton conductivity of azole-substituted cyclic and polymeric phosphazenes. Polymer 2013, 54, 2250–2256. [Google Scholar] [CrossRef]
- Inoue, K.; Yamauchi, T.; Itoh, T.; Ihara, E. Ionic conductivity of cross-linked polymethacrylate derivatives/cyclophosphazenes/ Li+ Salt Complexes. J. Inorg. Organomet. Polym. Mater. 2007, 17, 367–375. [Google Scholar] [CrossRef]
- Koran, K.; Özen, F.; Biryan, F.; Görgülü, A.O. Synthesis, structural characterization and dielectric behaviour of new oxime-cyclotriphosphazene derivatives. J. Mol. Struc. 2016, 1105, 135–141. [Google Scholar] [CrossRef]
- Kumbar, S.G.; Bhattacharyya, S.; Nukavarapu, S.P.; Khan, Y.M.; Nair, L.S.; Laurencin, C.T. In vitro and in vivo characterization of biodegradable poly(organophosphazenes) for biomedical applications. J. Inorg. Organomet. Polym. Mater. 2006, 16, 365–385. [Google Scholar] [CrossRef]
- Perkowski, P. The parasitic effects in high-frequency dielectric spectroscopy of liquid crystals—The review. Liq. Cryst. 2021, 48, 767–793. [Google Scholar] [CrossRef]
- Dotelli, G.; Galazzi, M.C.; Mari, C.M.; Greppi, F.; Montoneri, E.; Manueli, A. Polyalkylphosphazenes as solid proton conducting electrolytes. J. Mater. Sci. 2004, 39, 6937–6943. [Google Scholar] [CrossRef]
- Akbaş, H.; Karadağ, A.; Destegül, A.; Çakırlar, Ç.; Yerli, Y.; Tekin, K.C.; Malayoglu, U.; Kılıç, Z. Synthesis, and spectroscopic, thermal and dielectric properties of phosphazene based ionic liquids: OFET application and tribological behavior. New J. Chem. 2019, 43, 2098–2110. [Google Scholar] [CrossRef]
- Koran, K.; Özen, F.; Torğut, G.; Pıhtılı, G.; Çil, E.; Görgülü, A.O.; Arslan, M. Synthesis, characterization and dielectric properties of phosphazenes containing chalcones. Polyhedron 2014, 79, 213–220. [Google Scholar] [CrossRef]
- Usri, S.N.K.; Jamain, Z.; Makmud, M.Z.H. A review on Synthesis, Structural, Flame Retardancy and Dielectric Properties of Hexasubstituted Cyclotriphosphazene. Polymers 2021, 13, 2916. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Jiao, L.; Deng, Y. Cellulose-and nanocellulose-based dielectric materials. Nanocellulose Based Composites for Electronics. In Micro and Nano Technologies, Nanocellulose Based Composites for Electronics; Elsevier: Amsterdam, The Netherlands, 2021; pp. 73–100. [Google Scholar]
- Koran, K.; Özen, F.; Biryan, F.; Demirelli, K.; Görgülü, A.O. Eu+3-doped chalcone substituted cyclotriphosphazenes: Synthesis, characterizations, thermal and dielectrical properties. Inorg. Chim. Acta 2016, 450, 162–169. [Google Scholar] [CrossRef]
- Koran, K.; Tekin, Ç.; Biryan, F.; Tekin, S.; Sandal, S.; Görgülü, A.O. Synthesis, structural and thermal characterizations, dielectric properties and in vitro cytotoxic activities of new 2,2,4,4-tetra(4′-oxy-substituted-chalcone)-6,6-diphenyl cyclotri-phosphazene derivatives. Med. Chem. Res. 2017, 26, 962–974. [Google Scholar] [CrossRef]
- Jamain, Z.; Khairuddean, M.; Guan-Seng, T. Synthesis of new star-shaped liquid crystalline cyclotriphosphazene derivatives with fire retardancy bearing amide-azo and azo-azo linking units. Int. J. Mol. Sci. 2020, 21, 4267. [Google Scholar] [CrossRef] [PubMed]
- Andrienko, D. Introduction to liquid crystals. J. Mol. Liq. 2018, 267, 520–541. [Google Scholar] [CrossRef]
- Moriya, K.; Masuda, T.; Suzuki, T.; Yano, S.; Kajiwara, M. Liquid crystalline phase transition in hexakis(4-(N-(4’-alkoxyphenyl)iminomethyl)phenoxy) cyclotriphosphazene. Mol. Cryst. Liq. Cryst. 2006, 318, 267–277. [Google Scholar] [CrossRef]
- Rong, Y.; Bo, W.; Xiaofeng, H.; Binbin, M.; Jinchun, L. Synthesis and characterisation of flame retardant rigid polyurethane foam based on a reactive flame retardant containing phosphazene and cyclotriphosphazene. Polym. Degrad. Stab. 2017, 144, 62–69. [Google Scholar]
- Pociecha, D.; Kardas, D.; Gorecka, E.; Szydlowska, J.; Mieczkowski, J.; Guillon, D. Modulated and intercalated smectic phases formed by dimeric molecules. J. Mater. Chem. 2003, 13, 34–37. [Google Scholar] [CrossRef]
- Zhang, B.Y.; Jia, Y.G.; Yao, D.S.; Dong, X.W. Preparation and properties of siloxane liquid crystalline elastomers with a mesogenic crosslinking agent. Liq. Cryst. 2010, 31, 339–345. [Google Scholar] [CrossRef]
- Jamain, Z.; Azman, A.N.A.; Razali, N.A.; Makmud, M.Z.H. A review on mesophase and physical properties of cyclotriphosphazene derivatives with Schiff base linkage. Crystals 2022, 12, 1174. [Google Scholar] [CrossRef]
- Darvaci, D. Liquid crystalline cyclotriphosphazene: Full substitute trimer derivatives with 12-carbon-chain mesogen moiety. CBU J. Sci. 2016, 12, 535–542. [Google Scholar]
- Jeong, M.J.; Park, J.H.; Lee, C.; Chang, J.Y. Discotic Liquid Hydrazone Compounds: Synthesis and Mesomorphic Properties. Org. Lett. 2006, 8, 2221–2224. [Google Scholar] [CrossRef] [PubMed]
- Jamain, Z.; Khairuddean, M. Synthesis and Mesophase behaviour of benzylidene-based molecules containing two azomethine units. J. Phys. Conf. Ser. 2021, 1882, 012120. [Google Scholar] [CrossRef]
- Soni, R.; Nakum, K.J.; Katariya, K.D.; Soman, S.S.; Nada, N.; Hagar, M. Synthesis, mesomorphic properties and DFT calculations of new coumarin Schiff base-ester liquid crystals. Liq. Cryst. 2023, 50, 636–651. [Google Scholar] [CrossRef]
- Shanker, G.; Srinatha, M.K.; Ocak, H. Effect of polar group on the 2,3,4-trihydroxy benzonitrile based liquid crystals trimers. Liq. Cryst. 2023, 50, 195–202. [Google Scholar] [CrossRef]
- Tomma, J.H. Synthesis and study the mesomorphic behaviour of some new 1,3,4-thiadiazoline derivatives. Liq. Cryst. 2023, 50, 998–1006. [Google Scholar] [CrossRef]
- Jamain, Z.; Habil, S.; Makmud, M.Z.H.; Khairuddean, M. Synthesis, Structural and Dielectric Characteristics of Liquid Crystalline Azo-Based Compounds with Different Terminal Length. In Proceedings of the 2021 IEEE International Conference on the Properties and Applications of Dielectric Materials (ICPADM), Johor Bahru, Malaysia, 12–14 July 2021; pp. 49–52. [Google Scholar]
- Wang, Q.; Chen, H.; Xing, H.; Deng, Y.; Luo, Z.-W.; Xie, H.-L. Long rod-like liquid crystal containing azobenzene and the applications in phase-transition regulation and orientation of nematic liquid crystal. Crystals 2021, 11, 418. [Google Scholar] [CrossRef]
- Jamain, Z.; Khairuddean, M.; Loh, M.L.; Manaff, N.L.A.; Makmud, M.Z.H. Synthesis and characterization of hexasubstituted cyclotriphosphazene derivatives with azo linking units. Malaysian. J. Chem. 2020, 22, 125–140. [Google Scholar]
- Aldahri, T.H.; Alaasar, M.; Ahmed, H.A. The influence of core fluorination on the phase behaviour of rod-like mesogens. Liq. Cryst. 2023, 50, 1059–1068. [Google Scholar] [CrossRef]
- Heeley, E.L.; Hughes, D.J.; El Aziz, Y.; Williamson, I.; Taylor, P.G.; Bassindale, A.R. Properties and self-assembled packing morphology of long alkyl-chained substituted polyhedral oligomeric silsesquioxanes (POSS) cages. Phys. Chem. Chem. Phys. 2013, 15, 5518–5529. [Google Scholar] [CrossRef]
- Jamain, Z.; Khairuddean, M.; Zulbaharen, N.N.; Chung, T.K. Synthesis, characterization and determination of mesophase transition of azo-azomethine derivatives with different terminal chain lengths. Malays. J. Chem. 2019, 21, 73–85. [Google Scholar]
- Lim, Y.W.C.; Ha, S.T.; Yeap, G.W.; Sastry, S.S. Synthesis and mesomorphic properties of new heterocyclic liquid crystals with central ester-chalcone linkages. J. Taibah Univ. Sci. 2017, 11, 133–140. [Google Scholar] [CrossRef]
- Zieja, P.; Tykarska, M.; Morawiak, P.; Piecek, W. Influence of the molecular structure of calamitic compounds with trifluoromethoxy terminal group on the induction of the smectic a phase. Part I. Liq. Cryst. 2023, 50, 363–378. [Google Scholar] [CrossRef]
- Vaupotič, N.; Pociecha, D.; Rybak, P.; Matraszek, J.; Čepič, M.; Wolska, J.M.; Gorecka, E. Dielectric response of a ferroelectric nematic liquid crystalline phase in thin cells. Liq. Cryst. 2023, 50, 584–595. [Google Scholar] [CrossRef]
- Krishnadevi, K.; Grace, A.N.; Alagar, M.; Selvaraj, V. Development of hexa (aminophenyl)cyclotriphosphazene-modified cyanate ester composites for high-temperature applications. High. Perform. Polym. 2013, 26, 89–96. [Google Scholar] [CrossRef]
- Sarjeant, W.J.; Clelland, I.W.; Price, R.A. Capacitive components for power electronics. Proc. IEEE 2001, 89, 846–855. [Google Scholar] [CrossRef]
- Sarjeant, W.J.; Zirnheld, J.; MacDougall, F.W. Capacitors. IEEE Trans. Plasm. Sci. 1998, 26, 1368–1392. [Google Scholar] [CrossRef]
- Tan, Q.; Irwin, P.; Cao, Y. Advance dielectrics for capacitors. IEEJ Trans. Fundam. Mater. 2006, 126, 1153–1159. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Q. Novel ferroelectric polymers for high energy density and low loss dielectrics. Micromolecules 2012, 45, 2937–2954. [Google Scholar] [CrossRef]
- Zhang, Q.M.; Huang, C.; Xia, F.; Su, J. Electric EAP. In Electroactive Polymer (EAP) Actuators as Artificial Muscles: Reality, Potential and Challenges, 2nd ed.; Bar-Cohen, Y., Ed.; SPIE Press: Bellingham, WA, USA, 2014; Volume 136, pp. 89–139. [Google Scholar]
- Carpi, F.; De Rossi, D.R.; Kornbluh, R.; Pelrine, R.; Sommer-Larsen, P. Dielectric Elastomers as Electromechanical Transducers: Fundamentals Materials Device Models and Application of an Emerging Electroactive Polymer Technology; Elsevier: Boston, MA, USA, 2008. [Google Scholar]
- Brochu, P.; Pei, Q. Advances in dielectric elastomers for actuators and artificial muscles. Macromol. Rapid Commun. 2010, 31, 10–36. [Google Scholar] [CrossRef]
- Pelrine, R.; Kornbluh, R.; Joseph, J.; Heydt, R.; Pei, Q.B.; Chiba, S. High-field deformation of elastomeric dielectrics for actuators. Mater. Sci. Eng. 2000, 11, 89–100. [Google Scholar] [CrossRef]
- Neese, B.; Chu, B.; Lu, S.G.; Wang, Y.; Furman, E.; Zhang, Q.M. Large electrocaloric effect in ferroelectric temperature. Science 2008, 321, 821–823. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.G.; Zhang, Q. Electrocaloric materials for solid-state refrigeration. Adv. Mater. 2009, 21, 1983–1987. [Google Scholar]
- Liu, Y.; Zhao, X.-Y.; Sun, Y.-G.; Li, W.-Z.; Zhang, X.-S.; Luan, J. Synthesis and applications of low dielectric polyimide. Resour. Chem. Mater. 2023, 2, 49–62. [Google Scholar] [CrossRef]
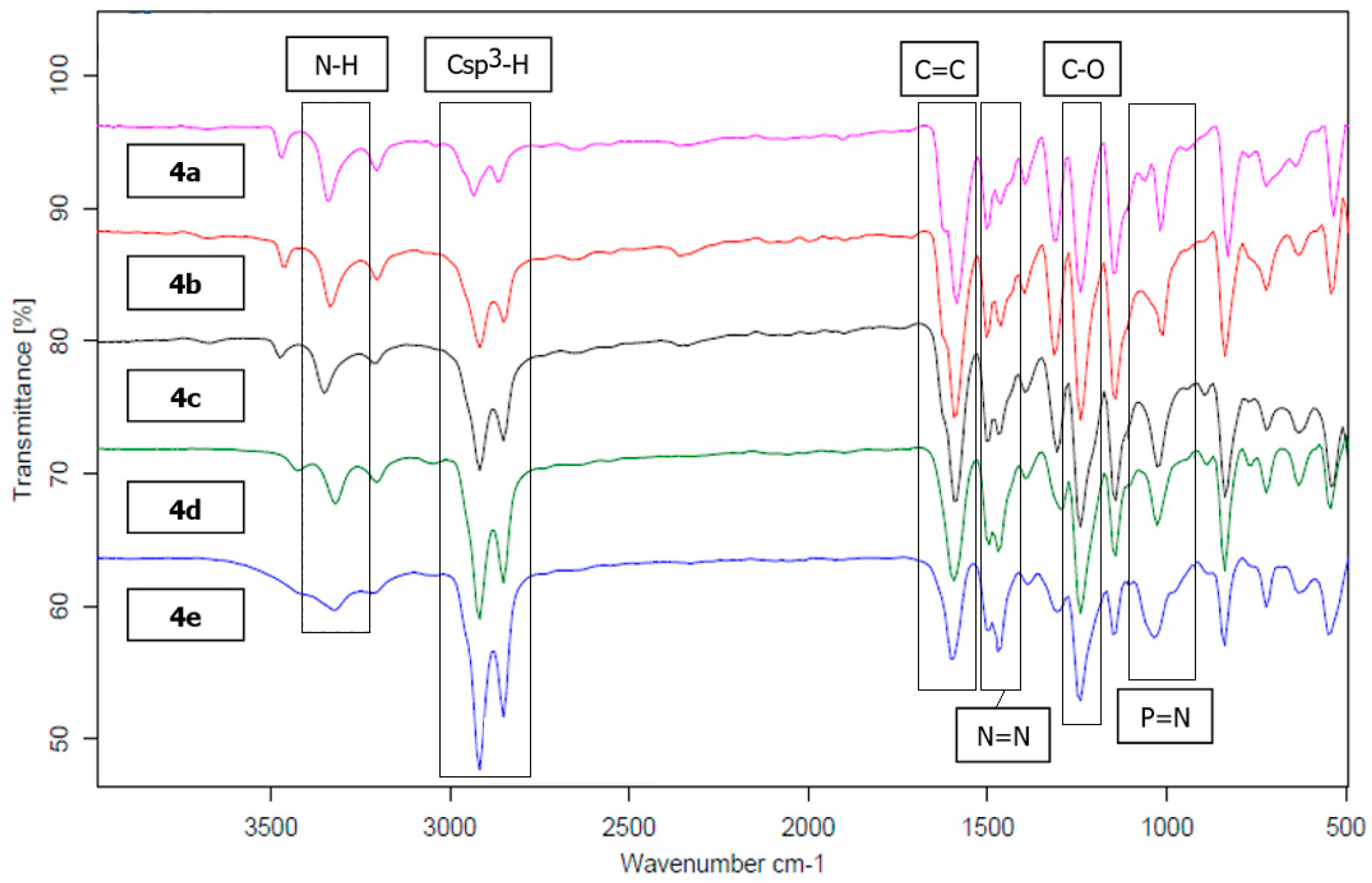



| Compound | Vibrational Stretching (cm−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N–H | NH2 | OH | Csp3–H | C=C | N=N | C–O | P=N | C–N | |
| 1 | - | - | 3281 | - | 1502 | 1455 | 1340 | - | 1163 |
| 2a | - | - | - | 2857, 2943 | 1602 | 1469 | 1253 | - | 1158 |
| 2b | - | - | - | 2850, 2924 | 1602 | 1467 | 1250 | - | 1142 |
| 2c | - | - | - | 2849, 2918 | 1601 | 1499 | 1250 | - | 1137 |
| 2d | - | - | - | 2849, 2919 | 1604 | 1465 | 1252 | - | 1140 |
| 2e | - | - | - | 2848, 2916 | 1602 | 1466 | 1248 | - | 1141 |
| 3a | - | 3338, 3471 | - | 2868, 2936 | 1621 | 1470 | 1240 | - | 1143 |
| 3b | - | 3334, 3464 | - | 2847, 2917 | 1621 | 1467 | 1240 | - | 1143 |
| 3c | - | 3351, 3476 | - | 2849, 2918 | 1621 | 1468 | 1247 | - | 1143 |
| 3d | - | 3319, 3429 | - | 2851, 2920 | 1599 | 1473 | 1249 | - | 1148 |
| 3e | - | 3316, 3430 | - | 2850, 2918 | 1600 | 1467 | 1249 | - | 1147 |
| 4a | 3338 | - | - | 2868, 2916 | 1622 | 1433 | 1239 | 1191 | 1144 |
| 4b | 3332 | - | - | 2847, 2918 | 1621 | 1468 | 1241 | 1180 | 1143 |
| 4c | 3351 | - | - | 2848, 2917 | 1621 | 1468 | 1246 | 1182 | 1143 |
| 4d | 3321 | - | - | 2851, 2920 | 1599 | 1473 | 1247 | 1181 | 1143 |
| 4e | 3321 | - | - | 2850, 2918 | 1601 | 1467 | 1249 | 1183 | 1148 |
| Proton | 1H [δ (ppm), Multiplicity, Coupling Constant (Hz)] | COSY (1H–1H) Correlation | HSQC (1H–13C) Correlation (ppm) |
|---|---|---|---|
| H3 | 6.54 (d, J = 10.0 Hz) | H4 | C3 (114.15) |
| H4 | 7.90 (d, J = 10.0 Hz) | H3 | C4 (129.72) |
| H7 | 7.35 (d, J = 10.0 Hz) | H8 | C7 (122.74) |
| H8 | 7.00 (d, J = 5.0 Hz) | H7 | C8 (114.37) |
| H10 | 4.04 (t, J = 10.00 Hz) | H11 | C10 (68.14) |
| H11 | 1.75–2.50 (m) | H10, H12 | C11 (31.75) |
| H12–H20 | 1.24–1.72 (m) | H11–H21 | C12 (29.48), |
| C13 (29.46), | |||
| C14 (29.44) | |||
| C15 (29.43) | |||
| C16 (29.19) | |||
| C17 (29.16) | |||
| C18 (29.04) | |||
| C19 (25.92) | |||
| C20 (22.55) | |||
| H21 | 0.86 (t, J = 5.00 Hz) | H20 | C21 (14.41) |
| Compounds | Mode | Transition Temperature (°C) Enthalpy, ΔH (kJ/mol) | ||||
|---|---|---|---|---|---|---|
| 4d | Heating | Cr | SmC | I | ||
| • | 123.11 344.64 | • | 150.01 21.22 | • | ||
| Cooling | I | SmC | Cr | |||
| • | 148.75 −232.36 | • | 120.58 19.03 | • | ||
| 4e | Heating | Cr | SmC | I | ||
| • | 120.55 252.50 | • | 144.56 21.68 | • | ||
| Cooling | I | SmC | Cr | |||
| • | 142.60 −254.34 | • | 118.42 19.10 | • | ||
| XRD Data Analysis | Intermediate 2d | Compound 4d |
|---|---|---|
| Value | Value | |
| 2 theta | 1.45 | 2.03 |
| d-Layer spacing | 29.86 | 43.52 |
| Molecular length (L) | 26.44 | 57.07 |
| Calculated d/L | 1.13 | 0.76 |
| Arrangement of smectic phase | Monolayer | Monolayer |
| Cpd | POM Observation | Cpd | POM Observation | Cpd | POM Observation |
|---|---|---|---|---|---|
| 2a | Smectic A | 3a | Non-mesogenic | 4a | Non-mesogenic |
| 2b | Smectic A | 3b | Non-mesogenic | 4b | Non-mesogenic |
| 2c | Smectic A | 3c | Non-mesogenic | 4c | Non-mesogenic |
| 2d | Smectic A | 3d | Non-mesogenic | 4d | Smectic C |
| 2e | Smectic A | 3e | Non-mesogenic | 4e | Smectic C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habil, S.; Jamain, Z.; Makmud, M.Z.H. Synthesis and Characterization of Azo-Based Cyclotriphosphazene Compounds: Liquid Crystalline and Dielectric Properties. ChemEngineering 2024, 8, 71. https://doi.org/10.3390/chemengineering8040071
Habil S, Jamain Z, Makmud MZH. Synthesis and Characterization of Azo-Based Cyclotriphosphazene Compounds: Liquid Crystalline and Dielectric Properties. ChemEngineering. 2024; 8(4):71. https://doi.org/10.3390/chemengineering8040071
Chicago/Turabian StyleHabil, Samerah, Zuhair Jamain, and Mohamad Zul Hilmey Makmud. 2024. "Synthesis and Characterization of Azo-Based Cyclotriphosphazene Compounds: Liquid Crystalline and Dielectric Properties" ChemEngineering 8, no. 4: 71. https://doi.org/10.3390/chemengineering8040071
APA StyleHabil, S., Jamain, Z., & Makmud, M. Z. H. (2024). Synthesis and Characterization of Azo-Based Cyclotriphosphazene Compounds: Liquid Crystalline and Dielectric Properties. ChemEngineering, 8(4), 71. https://doi.org/10.3390/chemengineering8040071







