Modifying Superparamagnetic Iron Oxide Nanoparticles as Methylene Blue Adsorbents: A Review
Abstract
1. Introduction
- How to synthesize SPION-based adsorbents;
- How to characterize the adsorbents;
- How to perform the adsorption and desorption experiments;
- How to calculate the adsorption kinetics, adsorption isotherms, and adsorption thermodynamics properties;
- How to calculate the desorption kinetics;
- Comparing the MB adsorption capacity, kinetics, isotherms, and thermodynamic properties of the most recent adsorbents;
- The future research of methylene blue adsorption by using the SPION-based composite, including recyclability, antimicrobial activities, cost–benefit analysis, and optimization.
1.1. Methylene Blue
1.2. Superparamagnetic Iron Oxide Nanoparticles
2. SPION Synthesis
3. Modifications of SPION
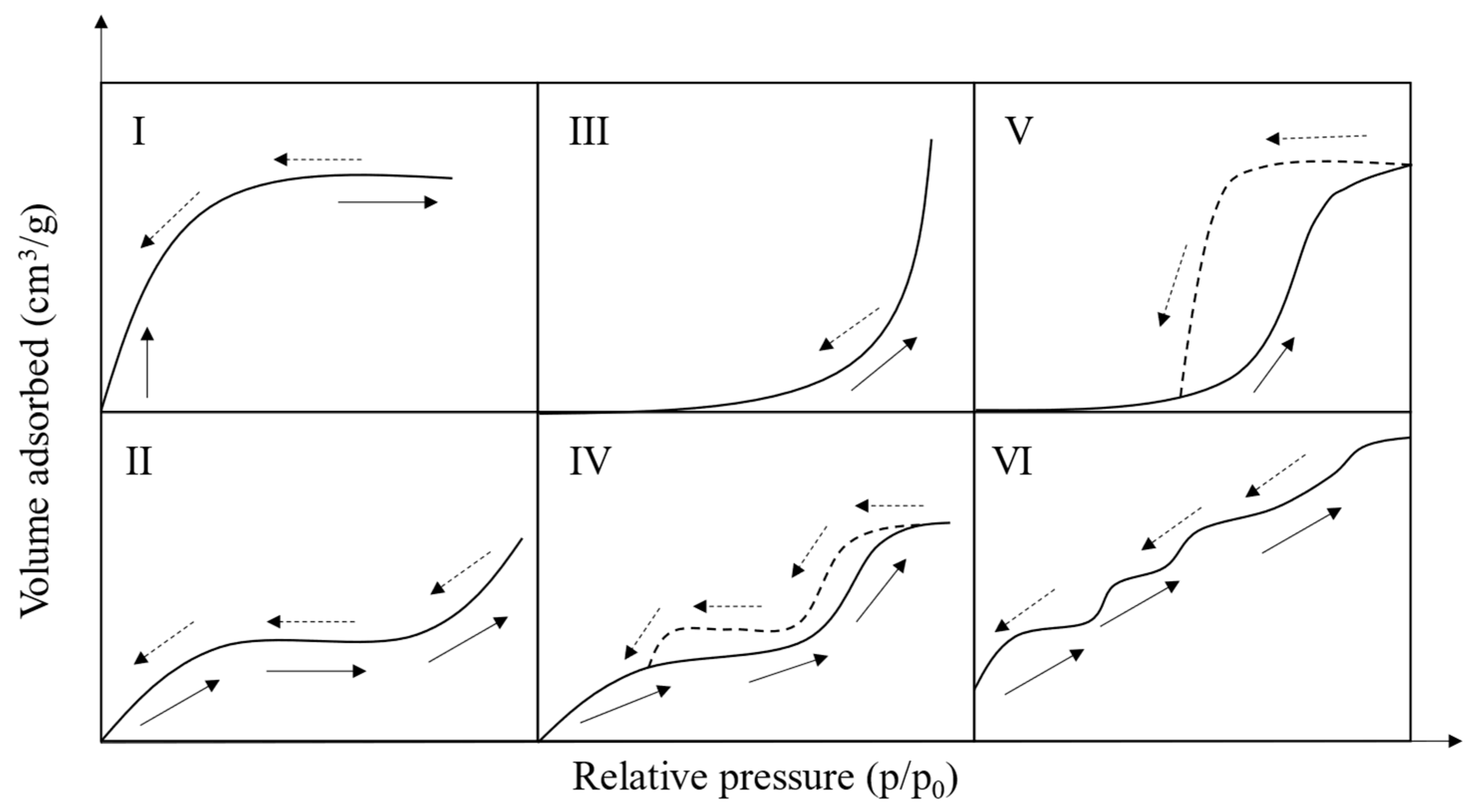
4. Characterization
5. Adsorption
5.1. Adsorption Methods
5.2. Adsorption Mechanism
5.3. Effects on Temperature
5.4. Effects on pH
5.5. Effects on Initial Concentration
5.6. Adsorption Comparison Studies
6. Desorption
6.1. Desorption Methods [4]
6.2. Desorption Mechanism
7. Future Research
7.1. Recyclability
7.2. Antibacterial Properties
7.3. Optimization
8. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C0 | Initial concentration (mg/mL) | Constant of the relative adsorption capacity of the adsorbent | |
| Concentration at time t (mg/mL) | Theoretical saturation capacity | ||
| V | Reaction volume (mL) | The activity coefficient related to the mean free energy of adsorption | |
| m | Nanoparticles mass (g) | The Polanyi potential | |
| The amounts of adsorbate (MB) adsorbed at the equilibrium (mg/g) | R | Universal gas constant | |
| MB mass adsorbed at time t (mg/g) | T | Temperature | |
| The equilibrium aqueous-phase concentration adsorbate (mg/L) | Equilibrium binding constant | ||
| The theoretical adsorption capacity or the monolayer adsorption capacity (mg/g) | B1 | Related to the heat of adsorption | |
| Constant related to the free adsorption energy and the reciprocal of the concentration at which half saturation of the adsorbent is reached | The Halsey isotherm constant | ||
| The quantity of adsorbate adsorbed in a single monolayer | Intraparticle diffusion rate constant | ||
| The fractional surface coverage | I | Constant | |
| The respective rate constant for adsorption | The theoretical initial adsorption rate | ||
| The respective rate constant for desorption | The theoretical desorption constant | ||
| The intensity of the adsorption | Gibbs free energy change | ||
| Entropy change | Standard enthalpy change | ||
| K0 | Thermodynamic equilibrium constant in the adsorption process | Mt | Released mass fraction at a time (t) |
| Released fraction mass | KH | Higuchi release rate constant | |
| KKP | Korsmeyer–Peppas release rate constant | nKP | Korsmeyer–Peppas release exponent factor |
| ko | Constant mass fraction at a time (t) release | Pseudo-first-order rate constant (s−1) | |
| Chi-square value | Pseudo-second-order rate constant (s−1) | ||
| The amount of MB at equilibrium state | Modified Langmuir constant (dm3/g) | ||
| Redlich–Peterson constant (dm3/g) | Redlich–Peterson constant (dm3/g) | ||
| h0 | The initial adsorption rate (mg g−1 min−1) | EA | Arrhenius activation energy (kJ/mol) |
| R | Universal gas constant (8.314 J mol−1 K−1) |
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Ali, H. Biodegradation of Synthetic Dyes—A Review. Water Air Soil Pollut. 2010, 213, 251–273. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Quach, T.P.T.; Doan, L. Surface Modifications of Superparamagnetic Iron Oxide Nanoparticles with Polyvinyl Alcohol, Chitosan, and Graphene Oxide as Methylene Blue Adsorbents. Coatings 2023, 13, 1333. [Google Scholar] [CrossRef]
- Damasceno, B.S.; da Silva, A.F.V.; de Araújo, A.C.V. Dye Adsorption onto Magnetic and Superparamagnetic Fe3O4 Nanoparticles: A Detailed Comparative Study. J. Environ. Chem. Eng. 2020, 8, 103994. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, X.; Quan, J.; Xing, G.; Yang, L.; Zhao, C.; Wu, P.; Zhao, F.; Hu, B.; Hu, Y. Application of Magnetic Fields to Wastewater Treatment and Its Mechanisms: A Review. Sci. Total Environ. 2021, 773, 145476. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.P.; Olatunya, A.M.; Dada, O. Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. IOSR J. Appl. Chem. IOSR JAC 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Chhetri, T.; Cunningham, G.; Suresh, D.; Shanks, B.; Kannan, R.; Upendran, A.; Afrasiabi, Z. Wastewater Treatment Using Novel Magnetic Nanosponges. Water 2022, 14, 505. [Google Scholar] [CrossRef]
- Santos, T.R.T.; Silva, M.F.; Nishi, L.; Vieira, A.M.S.; Klein, M.R.F.; Andrade, M.B.; Vieira, M.F.; Bergamasco, R. Development of a Magnetic Coagulant Based on Moringa Oleifera Seed Extract for Water Treatment. Environ. Sci. Pollut. Res. 2016, 23, 7692–7700. [Google Scholar] [CrossRef]
- Yusop, M.F.M.; Ahmad, M.A.; Rosli, N.A.; Manaf, M.E.A. Adsorption of Cationic Methylene Blue Dye Using Microwave-Assisted Activated Carbon Derived from Acacia Wood: Optimization and Batch Studies. Arab. J. Chem. 2021, 14, 103122. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Strobel, R.; Pratsinis, S.E. Direct Synthesis of Maghemite, Magnetite and Wustite Nanoparticles by Flame Spray Pyrolysis. Adv. Powder Technol. 2009, 20, 190–194. [Google Scholar] [CrossRef]
- Machala, L.; Zboril, R.; Gedanken, A. Amorphous Iron(III) OxideA Review. J. Phys. Chem. B 2007, 111, 4003–4018. [Google Scholar] [CrossRef]
- Ma, J.; Jing, Y.; Gao, L.; Chen, J.; Wang, Z.; Weng, L.; Li, H.; Chen, Y.; Li, Y. Hetero-Aggregation of Goethite and Ferrihydrite Nanoparticles Controlled by Goethite Nanoparticles with Elongated Morphology. Sci. Total Environ. 2020, 748, 141536. [Google Scholar] [CrossRef] [PubMed]
- Can, M.M.; Coşkun, M.; Fırat, T. A Comparative Study of Nanosized Iron Oxide Particles; Magnetite (Fe3O4), Maghemite (γ-Fe2O3) and Hematite (α-Fe2O3), Using Ferromagnetic Resonance. J. Alloys Compd. 2012, 542, 241–247. [Google Scholar] [CrossRef]
- Seabra, A.B.; Pelegrino, M.T.; Haddad, P.S. Antimicrobial Applications of Superparamagnetic Iron Oxide Nanoparticles. In Nanostructures for Antimicrobial Therapy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 531–550. ISBN 978-0-323-46152-8. [Google Scholar]
- Wahajuddin; Arora, S. Superparamagnetic Iron Oxide Nanoparticles: Magnetic Nanoplatforms as Drug Carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef]
- Jabbar, K.Q.; Barzinjy, A.A.; Hamad, S.M. Iron Oxide Nanoparticles: Preparation Methods, Functions, Adsorption and Coagulation/Flocculation in Wastewater Treatment. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100661. [Google Scholar] [CrossRef]
- Caruntu, D.; Caruntu, G.; O’Connor, C.J. Magnetic Properties of Variable-Sized Fe3O4 Nanoparticles Synthesized from Non-Aqueous Homogeneous Solutions of Polyols. J. Phys. D Appl. Phys. 2007, 40, 5801–5809. [Google Scholar] [CrossRef]
- Ejderyan, N.; Sanyal, R.; Sanyal, A. Chapter 6—Stimuli-Responsive Polymer-Coated Iron Oxide Nanoparticles as Drug Delivery Platforms. In Stimuli-Responsive Nanocarriers; Gajbhiye, V., Gajbhiye, K.R., Hong, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 133–169. ISBN 978-0-12-824456-2. [Google Scholar]
- Basuki, J.S.; Jacquemin, A.; Esser, L.; Li, Y.; Boyer, C.; Davis, T.P. A Block Copolymer-Stabilized Co-Precipitation Approach to Magnetic Iron Oxide Nanoparticles for Potential Use as MRI Contrast Agents. Polym. Chem. 2014, 5, 2611–2620. [Google Scholar] [CrossRef]
- Herranz, F.; Salinas, B.; Groult, H.; Pellico, J.; Lechuga-Vieco, A.V.; Bhavesh, R.; Ruiz-Cabello, J. Superparamagnetic Nanoparticles for Atherosclerosis Imaging. Nanomaterials 2014, 4, 408–438. [Google Scholar] [CrossRef]
- Jensen, K.M.Ø.; Andersen, H.L.; Tyrsted, C.; Bøjesen, E.D.; Dippel, A.-C.; Lock, N.; Billinge, S.J.L.; Iversen, B.B.; Christensen, M. Mechanisms for Iron Oxide Formation under Hydrothermal Conditions: An in Situ Total Scattering Study. ACS Nano 2014, 8, 10704–10714. [Google Scholar] [CrossRef]
- Kurzhals, S.; Zirbs, R.; Reimhult, E. Synthesis and Magneto-Thermal Actuation of Iron Oxide Core–PNIPAM Shell Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 19342–19352. [Google Scholar] [CrossRef]
- Chin, A.B.; Yaacob, I.I. Synthesis and Characterization of Magnetic Iron Oxide Nanoparticles via w/o Microemulsion and Massart’s Procedure. J. Mater. Process. Technol. 2007, 191, 235–237. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Sun, Q.; Chen, Q. One-Step Hydrothermal Process to Prepare Highly Crystalline Fe3O4 Nanoparticles with Improved Magnetic Properties. Mater. Res. Bull. 2003, 38, 1113–1118. [Google Scholar] [CrossRef]
- Mao, B.; Kang, Z.; Wang, E.; Lian, S.; Gao, L.; Tian, C.; Wang, C. Synthesis of Magnetite Octahedrons from Iron Powders through a Mild Hydrothermal Method. Mater. Res. Bull. 2006, 41, 2226–2231. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Lassenberger, A.; Grünewald, T.A.; van Oostrum, P.D.J.; Rennhofer, H.; Amenitsch, H.; Zirbs, R.; Lichtenegger, H.C.; Reimhult, E. Monodisperse Iron Oxide Nanoparticles by Thermal Decomposition: Elucidating Particle Formation by Second-Resolved in Situ Small-Angle X-Ray Scattering. Chem. Mater. 2017, 29, 4511–4522. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Guardia, P.; Pérez-Juste, J.; Labarta, A.; Batlle, X.; Liz-Marzán, L.M. Heating Rate Influence on the Synthesis of Iron Oxide Nanoparticles: The Case of Decanoic Acid. Chem. Commun. 2010, 46, 6108–6110. [Google Scholar] [CrossRef]
- Doan, L.; Lu, Y.; Karatela, M.; Phan, V.; Jeffryes, C.; Benson, T.; Wujcik, E.K. Surface Modifications of Superparamagnetic Iron Oxide Nanoparticles with Polylactic Acid-Polyethylene Glycol Diblock Copolymer and Graphene Oxide for a Protein Delivery Vehicle. Eng. Sci. 2019, 7, 10–16. [Google Scholar] [CrossRef]
- Doan, L.; Nguyen, L.T.; Nguyen, N.T.N. Modifying Superparamagnetic Iron Oxides Nanoparticles for Doxorubicin Delivery Carriers: A Review. J. Nanopart. Res. 2023, 25, 73. [Google Scholar] [CrossRef]
- Marinin, A. Synthesis and Characterization of Superparamagnetic Iron Oxide Nanoparticles Coated with Silica. Master’s Thesis, Royal Institute of Technology, Stockholm, Sweden, 2012. [Google Scholar]
- Riaz, S.; Bashir, M.; Naseem, S. Iron Oxide Nanoparticles Prepared by Modified Co-Precipitation Method. IEEE Trans. Magn. 2014, 50, 40105586. [Google Scholar] [CrossRef]
- Kang, Y.S.; Risbud, S.; Rabolt, J.F.; Stroeve, P. Synthesis and Characterization of Nanometer-Size Fe3O4 and γ-Fe2O3 Particles. Chem. Mater. 1996, 8, 2209–2211. [Google Scholar] [CrossRef]
- Bruce, I.J.; Taylor, J.; Todd, M.; Davies, M.J.; Borioni, E.; Sangregorio, C.; Sen, T. Synthesis, Characterisation and Application of Silica-Magnetite Nanocomposites. J. Magn. Magn. Mater. 2004, 284, 145–160. [Google Scholar] [CrossRef]
- Laurent, S.; Forge, D.; Port, M.; Roch, A.; Robic, C.; Vander Elst, L.; Muller, R.N. Magnetic Iron Oxide Nanoparticles: Synthesis, Stabilization, Vectorization, Physicochemical Characterizations, and Biological Applications. Chem. Rev. 2008, 108, 2064–2110. [Google Scholar] [CrossRef]
- Gupta, A.K.; Curtis, A.S.G. Lactoferrin and Ceruloplasmin Derivatized Superparamagnetic Iron Oxide Nanoparticles for Targeting Cell Surface Receptors. Biomaterials 2004, 25, 3029–3040. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Zhang, Y.; Voit, W.; Rao, K.V.; Muhammed, M. Synthesis and Characterization of Surfactant-Coated Superparamagnetic Monodispersed Iron Oxide Nanoparticles. J. Magn. Magn. Mater. 2001, 225, 30–36. [Google Scholar] [CrossRef]
- Zhu, N.; Ji, H.; Yu, P.; Niu, J.; Farooq, M.; Akram, M.; Udego, I.; Li, H.; Niu, X. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef]
- Massart, R.; Cabuil, V. Effect of some parameters on the formation of colloidal magnetite in alkaline-medium-yield and particle-size control. J. Chim. Phys. Phys. Chim. Biol. 1987, 84, 967–973. [Google Scholar] [CrossRef]
- Muthiah, M.; Park, I.-K.; Cho, C.-S. Surface Modification of Iron Oxide Nanoparticles by Biocompatible Polymers for Tissue Imaging and Targeting. Biotechnol. Adv. 2013, 31, 1224–1236. [Google Scholar] [CrossRef]
- Hadjipanayis, G.C.; Siegel, R.W. (Eds.) Nanophase Materials; Springer: Dordrecht, The Netherlands, 1994; ISBN 978-94-010-4469-1. [Google Scholar]
- Sjøgren, C.E.; Briley-Saebø, K.; Hanson, M.; Johansson, C. Magnetic Characterization of Iron Oxides for Magnetic Resonance Imaging. Magn. Reson. Med. 1994, 31, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Tartaj, P.; Morales, M.P.; Veintemillas-Verdaguer, S.; Gonzalez-Carreño, T.; Serna, C.J. Chapter 5 Synthesis, Properties and Biomedical Applications of Magnetic Nanoparticles. In Handbook of Magnetic Materials; Elsevier: Amsterdam, The Netherlands, 2006; Volume 16, pp. 403–482. ISBN 978-0-444-51850-7. [Google Scholar]
- Hyeon, T.; Lee, S.S.; Park, J.; Chung, Y.; Na, H.B. Synthesis of Highly Crystalline and Monodisperse Maghemite Nanocrystallites without a Size-Selection Process. J. Am. Chem. Soc. 2001, 123, 12798–12801. [Google Scholar] [CrossRef]
- Chen, D.; Xu, R. Hydrothermal Synthesis and Characterization of Nanocrystalline Fe3O4 Powders. Mater. Res. Bull. 1998, 33, 1015–1021. [Google Scholar] [CrossRef]
- Sato, S.; Murakata, T.; Yanagi, H.; Miyasaka, F.; Iwaya, S. Hydrothermal Synthesis of Fine Perovskite PbTiO3 Powders with a Simple Mode of Size Distribution. J. Mater. Sci. 1994, 29, 5657–5663. [Google Scholar] [CrossRef]
- Park, J.; Lee, E.; Hwang, N.-M.; Kang, M.; Kim, S.C.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; et al. One-Nanometer-Scale Size-Controlled Synthesis of Monodisperse Magnetic Iron Oxide Nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 2872–2877. [Google Scholar] [CrossRef]
- Dai, Z.; Meiser, F.; Möhwald, H. Nanoengineering of Iron Oxide and Iron Oxide/Silica Hollow Spheres by Sequential Layering Combined with a Sol–Gel Process. J. Colloid Interface Sci. 2005, 288, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Durães, L.; Costa, B.F.O.; Vasques, J.; Campos, J.; Portugal, A. Phase Investigation of As-Prepared Iron Oxide/Hydroxide Produced by Sol–Gel Synthesis. Mater. Lett. 2005, 59, 859–863. [Google Scholar] [CrossRef]
- Ismail, A.A. Synthesis and Characterization of Y2O3/Fe2O3/TiO2 Nanoparticles by Sol–Gel Method. Appl. Catal. B Environ. 2005, 58, 115–121. [Google Scholar] [CrossRef]
- Liu, X.Q.; Tao, S.W.; Shen, Y.S. Preparation and Characterization of Nanocrystalline α-Fe2O3 by a Sol-Gel Process. Sens. Actuators B Chem. 1997, 40, 161–165. [Google Scholar] [CrossRef]
- Kojima, K.; Miyazaki, M.; Mizukami, F.; Maeda, K. Selective Formation of Spinel Iron Oxide in Thin Films by Complexing Agent-Assisted Sol-Gel Processing. J. Sol Gel Sci. Technol. 1997, 8, 77–81. [Google Scholar] [CrossRef]
- Cannas, C.; Gatteschi, D.; Musinu, A.; Piccaluga, G.; Sangregorio, C. Structural and Magnetic Properties of Fe2O3 Nanoparticles Dispersed over a Silica Matrix. J. Phys. Chem. B 1998, 102, 7721–7726. [Google Scholar] [CrossRef]
- Ennas, G.; Musinu, A.; Piccaluga, G.; Zedda, D.; Gatteschi, D.; Sangregorio, C.; Stanger, J.L.; Concas, G.; Spano, G. Characterization of Iron Oxide Nanoparticles in an Fe2O3−SiO2 Composite Prepared by a Sol−Gel Method. Chem. Mater. 1998, 10, 495–502. [Google Scholar] [CrossRef]
- da Costa, G.M.; De Grave, E.; de Bakker, P.M.A.; Vandenberghe, R.E. Synthesis and Characterization of Some Iron Oxides by Sol-Gel Method. J. Solid State Chem. 1994, 113, 405–412. [Google Scholar] [CrossRef]
- del Monte, F.; Morales, M.P.; Levy, D.; Fernandez, A.; Ocaña, M.; Roig, A.; Molins, E.; O’Grady, K.; Serna, C.J. Formation of γ-Fe2O3 Isolated Nanoparticles in a Silica Matrix. Langmuir 1997, 13, 3627–3634. [Google Scholar] [CrossRef]
- Chanéac, C.; Tronc, E.; Jolivet, J.P. Thermal Behavior of Spinel Iron Oxide-Silica Composites. Nanostruct. Mater. 1995, 6, 715–718. [Google Scholar] [CrossRef]
- Niznansky, D.; Rehspringer, J.L.; Drillon, M. Preparation of Magnetic Nanoparticles (/Spl Gamma/-Fe/Sub2/O/Sub3/) in the Silica Matrix. IEEE Trans. Magn. 1994, 30, 821–823. [Google Scholar] [CrossRef]
- Bentivegna, F.; Nyvlt, M.; Ferré, J.; Jamet, J.P.; Brun, A.; Visnovsky, S.; Urban, R. Magnetically Textured γ-Fe2O3 Nanoparticles in a Silica Gel Matrix: Optical and Magneto-Optical Properties. J. Appl. Phys. 1999, 85, 2270–2278. [Google Scholar] [CrossRef]
- Solinas, S.; Piccaluga, G.; Morales, M.P.; Serna, C.J. Sol-Gel Formation of γ-Fe2O3/SiO2 Nanocomposites. Acta Mater. 2001, 49, 2805–2811. [Google Scholar] [CrossRef]
- Fievet, F.; Lagier, J.P.; Blin, B.; Beaudoin, B.; Figlarz, M. Homogeneous and Heterogeneous Nucleations in the Polyol Process for the Preparation of Micron and Submicron Size Metal Particles. Solid State Ion. 1989, 32–33, 198–205. [Google Scholar] [CrossRef]
- Tzitzios, V.K.; Petridis, D.; Zafiropoulou, I.; Hadjipanayis, G.; Niarchos, D. Synthesis and Characterization of L10 FePt Nanoparticles from Pt–Fe3O4 Core-Shell Nanoparticles. J. Magn. Magn. Mater. 2005, 294, e95–e98. [Google Scholar] [CrossRef]
- Chow, G.M.; Kurihara, L.K.; Kemner, K.M.; Schoen, P.E.; Elam, W.T.; Ervin, A.; Keller, S.; Zhang, Y.D.; Budnick, J.; Ambrose, T. Structural, Morphological, and Magnetic Study of Nanocrystalline Cobalt-Copper Powders Synthesized by the Polyol Process. J. Mater. Res. 1995, 10, 1546–1554. [Google Scholar] [CrossRef]
- Viau, G.; Ravel, F.; Acher, O.; Fiévet-Vincent, F.; Fiévet, F. Preparation and Microwave Characterization of Spherical and Monodisperse Co20Ni80 Particles. J. Appl. Phys. 1994, 76, 6570–6572. [Google Scholar] [CrossRef]
- Viau, G.; Ravel, F.; Acher, O.; Fiévet-Vincent, F.; Fiévet, F. Preparation and Microwave Characterization of Spherical and Monodisperse CoNi Particles. J. Magn. Magn. Mater. 1995, 140–144, 377–378. [Google Scholar] [CrossRef]
- Viau, G.; Fiévet-Vincent, F.; Fiévet, F. Monodisperse Iron-Based Particles: Precipitation in Liquid Polyols. J. Mater. Chem. 1996, 6, 1047–1053. [Google Scholar] [CrossRef]
- Viau, G.; Fiévet-Vincent, F.; Fiévet, F. Nucleation and Growth of Bimetallic CoNi and FeNi Monodisperse Particles Prepared in Polyols. Solid State Ion. 1996, 84, 259–270. [Google Scholar] [CrossRef]
- Viau, G.; Fiévet-Vincent, F.; Fiévet, F.; Toneguzzo, P.; Ravel, F.; Acher, O. Size Dependence of Microwave Permeability of Spherical Ferromagnetic Particles. J. Appl. Phys. 1997, 81, 2749–2754. [Google Scholar] [CrossRef]
- Toneguzzo, P.; Acher, O.; Viau, G.; Fiévet-Vincent, F.; Fiévet, F. Observations of Exchange Resonance Modes on Submicrometer Sized Ferromagnetic Particles. J. Appl. Phys. 1997, 81, 5546–5548. [Google Scholar] [CrossRef]
- Toneguzzo, P.; Viau, G.; Acher, O.; Fiévet-Vincent, F.; Fiévet, F. Monodisperse Ferromagnetic Particles for Microwave Applications. Adv. Mater. 1998, 10, 1032–1035. [Google Scholar] [CrossRef]
- Toneguzzo, P.; Acher, O.; Viau, G.; Pierrard, A.; Fievet-Vincent, F.; Fievet, F.; Rosenman, I. Static and Dynamic Magnetic Properties of Fine CoNi and FeCoNi Particles Synthesized by the Polyol Process. IEEE Trans. Magn. 1999, 35, 3469–3471. [Google Scholar] [CrossRef]
- Yu, S.; Chow, G.M. Synthesis of Monodisperse Iron Oxide and Iron/Iron Oxide Core/Shell Nanoparticles via Iron-Oleylamine Complex. J. Nanosci. Nanotechnol. 2006, 6, 2135–2140. [Google Scholar] [CrossRef]
- Hegde, M.S.; Larcher, D.; Dupont, L.; Beaudoin, B.; Tekaia-Elhsissen, K.; Tarascon, J.-M. Synthesis and Chemical Reactivity of Polyol Prepared Monodisperse Nickel Powders. Solid State Ion. 1996, 93, 33–50. [Google Scholar] [CrossRef]
- Saravanan, P.; Jose, T.A.; Thomas, P.J.; Kulkarni, G.U. Submicron Particles of Co, Ni and Co-Ni Alloys. Bull. Mater. Sci. 2001, 24, 515–521. [Google Scholar] [CrossRef]
- Jungk, H.-O.; Feldmann, C. Nonagglomerated, Submicron α–Fe2O3 Particles: Preparation and Application. J. Mater. Res. 2000, 15, 2244–2248. [Google Scholar] [CrossRef]
- Feldmann, C. Preparation of Nanoscale Pigment Particles. Adv. Mater. 2001, 13, 1301–1303. [Google Scholar] [CrossRef]
- Bianco, A.; Gusmano, G.; Montanari, R.; Montesperelli, G.; Traversa, E. Preparation of NiCo Metal Powders by Co-Reduction of Ni(II) and Co(II) Hydroxides for Magnetoresistive Sensors. Mater. Lett. 1994, 19, 263–268. [Google Scholar] [CrossRef]
- Bianco, A.; Gusmano, G.; Montanari, R.; Montesperelli, G.; Traversa, E. Microstructural Characterisation of Ni, Co and Ni-Co Fine Powders for Physical Sensors. Thermochim. Acta 1995, 269–270, 117–132. [Google Scholar] [CrossRef]
- Kurihara, L.K.; Chow, G.M.; Schoen, P.E. Nanocrystalline Metallic Powders and Films Produced by the Polyol Method. Nanostruct. Mater. 1995, 5, 607–613. [Google Scholar] [CrossRef]
- Ammar, S.; Helfen, A.; Jouini, N.; Fiévet, F.; Rosenman, I.; Villain, F.; Molinié, P.; Danot, M. Magnetic Properties of Ultrafine Cobalt Ferrite Particles Synthesized by Hydrolysis in a Polyol MediumBasis of a Presentation given at Materials Discussion No. 3, 26–29 September, 2000, University of Cambridge, UK. J. Mater. Chem. 2001, 11, 186–192. [Google Scholar] [CrossRef]
- Yu, W.; Wang, Y.; Liu, H.; Zheng, W. Preparation and Characterization of Polymer-Protected PtCo Bimetallic Colloids and Their Catalytic Properties in the Selective Hydrogenation of Cinnamaldehyde. J. Mol. Catal. A Chem. 1996, 112, 105–113. [Google Scholar] [CrossRef]
- Kooli, F.; Rives, V.; Jones, W. Reduction of Ni2+−Al3+ and Cu2+−Al3+ Layered Double Hydroxides to Metallic Ni0 and Cu0 via Polyol Treatment. Chem. Mater. 1997, 9, 2231–2235. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Kitajima, K. Reduction of Interlayer Co2+ Ions in Fluorine Mica Using Diethylene Glycol. J. Mater. Sci. 1998, 33, 653–657. [Google Scholar] [CrossRef]
- Toneguzzo, P.; Viau, G.; Acher, O.; Guillet, F.; Bruneton, E.; Fievet-Vincent, F.; Fievet, F. CoNi and FeCoNi Fine Particles Prepared by the Polyol Process: Physico-Chemical Characterization and Dynamic Magnetic Properties. J. Mater. Sci. 2000, 35, 3767–3784. [Google Scholar] [CrossRef]
- Poul, L.; Jouini, N.; Fiévet, F. Layered Hydroxide Metal Acetates (Metal = Zinc, Cobalt, and Nickel): Elaboration via Hydrolysis in Polyol Medium and Comparative Study. Chem. Mater. 2000, 12, 3123–3132. [Google Scholar] [CrossRef]
- Wu, M.; He, H.; Zhao, Z.; Yao, X. Preparation of Magnetic Cobalt Fibres and Their Microwave Properties. J. Phys. D Appl. Phys. 2000, 33, 2927–2930. [Google Scholar] [CrossRef]
- Elumalai, P.; Vasan, H.N.; Verelst, M.; Lecante, P.; Carles, V.; Tailhades, P. Synthesis and Characterization of Sub-Micron Size Co–Ni Alloys Using Malonate as Precursor. Mater. Res. Bull. 2002, 37, 353–363. [Google Scholar] [CrossRef]
- Teranishi, T.; Miyake, M. Novel Synthesis of Monodispersed Pd/Ni Nanoparticles. Chem. Mater. 1999, 11, 3414–3416. [Google Scholar] [CrossRef]
- Jézéquel, D.; Guenot, J.; Jouini, N.; Fiévet, F. Submicrometer Zinc Oxide Particles: Elaboration in Polyol Medium and Morphological Characteristics. J. Mater. Res. 1995, 10, 77–83. [Google Scholar] [CrossRef]
- Cai, W.; Wan, J. Facile Synthesis of Superparamagnetic Magnetite Nanoparticles in Liquid Polyols. J. Colloid Interface Sci. 2007, 305, 366–370. [Google Scholar] [CrossRef]
- Sra, A.K.; Ewers, T.D.; Schaak, R.E. Direct Solution Synthesis of Intermetallic AuCu and AuCu3 Nanocrystals and Nanowire Networks. Chem. Mater. 2005, 17, 758–766. [Google Scholar] [CrossRef]
- Joseyphus, R.J.; Kodama, D.; Matsumoto, T.; Sato, Y.; Jeyadevan, B.; Tohji, K. Role of Polyol in the Synthesis of Fe Particles. J. Magn. Magn. Mater. 2007, 310, 2393–2395. [Google Scholar] [CrossRef]
- Pascal, C.; Pascal, J.L.; Favier, F.; Elidrissi Moubtassim, M.L.; Payen, C. Electrochemical Synthesis for the Control of γ-Fe2O3 Nanoparticle Size. Morphology, Microstructure, and Magnetic Behavior. Chem. Mater. 1999, 11, 141–147. [Google Scholar] [CrossRef]
- Khan, H.R.; Petrikowski, K. Anisotropic Structural and Magnetic Properties of Arrays of Fe26Ni74 Nanowires Electrodeposited in the Pores of Anodic Alumina. J. Magn. Magn. Mater. 2000, 215–216, 526–528. [Google Scholar] [CrossRef]
- Fürstner, A. (Ed.) Active Metals: Preparation, Characterization, Applications; VCH: Weinheim, Germany; New York, NY, USA, 1996; ISBN 978-3-527-29207-3. [Google Scholar]
- Samrot, A.V.; Ali, H.H.; Selvarani, J.; Faradjeva, E.; Raji, P.; Prakash, P. Adsorption Efficiency of Chemically Synthesized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) on Crystal Violet Dye. Curr. Res. Green Sustain. Chem. 2021, 4, 100066. [Google Scholar] [CrossRef]
- Li, L.; Duan, H.; Wang, X.; Luo, C. Fabrication of Novel Magnetic Nanocomposite with a Number of Adsorption Sites for the Removal of Dye. Int. J. Biol. Macromol. 2015, 78, 17–22. [Google Scholar] [CrossRef]
- Pandey, N.; Surana, S.; Shukla, S.K.; Singh, N.B. Methylene Blue Removal on Nano-Fe3O4/Poly(Vinyl Alcohol)/Polyacrylamide Hydrogel. Emerg. Mater. Res. 2017, 6, 305–313. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; He, Y.; Kong, D.; Klein, B.; Yin, S.; Zhao, H. Preparation of Magnetic Activated Carbon by Activation and Modification of Char Derived from Co-Pyrolysis of Lignite and Biomass and Its Adsorption of Heavy-Metal-Containing Wastewater. Minerals 2022, 12, 665. [Google Scholar] [CrossRef]
- Farhana, A.; Jenifer Selvarani, A.; Samrot, A.V.; Alsrhani, A.; Raji, P.; Sahithya, C.S.; Jane Cypriyana, P.J.; Senthilkumar, P.; Ling, M.P.; Yishak, S. Utilization of Superparamagnetic Iron Oxide Nanoparticles (SPIONs) Impregnated Activated Carbon for Removal of Hexavalent Chromium. J. Nanomater. 2022, 2022, 4326939. [Google Scholar] [CrossRef]
- Le, T.D.; Tran, L.T.; Dang, H.T.M.; Tran, T.T.H.; Tran, H.V. Graphene Oxide/Polyvinyl Alcohol/Fe3O4 Nanocomposite: An Efficient Adsorbent for Co(II) Ion Removal. J. Anal. Methods Chem. 2021, 2021, 6670913. [Google Scholar] [CrossRef]
- Llenas, M.; Sandoval, S.; Costa, P.M.; Oró-Solé, J.; Lope-Piedrafita, S.; Ballesteros, B.; Al-Jamal, K.T.; Tobias, G. Microwave-Assisted Synthesis of SPION-Reduced Graphene Oxide Hybrids for Magnetic Resonance Imaging (MRI). Nanomaterials 2019, 9, 1364. [Google Scholar] [CrossRef]
- Bertran, A.; Sandoval, S.; Oró-Solé, J.; Sánchez, À.; Tobias, G. Particle Size Determination from Magnetization Curves in Reduced Graphene Oxide Decorated with Monodispersed Superparamagnetic Iron Oxide Nanoparticles. J. Colloid Interface Sci. 2020, 566, 107–119. [Google Scholar] [CrossRef]
- Alcalá, M.D.; Real, C. Synthesis Based on the Wet Impregnation Method and Characterization of Iron and Iron Oxide-Silica Nanocomposites. Solid State Ion. 2006, 177, 955–960. [Google Scholar] [CrossRef]
- Gushikem, Y.; Rosatto, S.S. Metal Oxide Thin Films Grafted on Silica Gel Surfaces: Recent Advances on the Analytical Application of These Materials. J. Braz. Chem. Soc. 2001, 12, 695–705. [Google Scholar] [CrossRef][Green Version]
- Woo, K.; Hong, J.; Ahn, J.-P. Synthesis and Surface Modification of Hydrophobic Magnetite to Processible Magnetite@silica-Propylamine. J. Magn. Magn. Mater. 2005, 293, 177–181. [Google Scholar] [CrossRef]
- van Ewijk, G.A.; Vroege, G.J.; Philipse, A.P. Convenient Preparation Methods for Magnetic Colloids. J. Magn. Magn. Mater. 1999, 201, 31–33. [Google Scholar] [CrossRef]
- Ma, D.; Guan, J.; Dénommée, S.; Enright, G.; Veres, T.; Simard, B. Multifunctional Nano-Architecture for Biomedical Applications. Chem. Mater. 2006, 18, 1920–1927. [Google Scholar] [CrossRef]
- Lesnikovich, A.I.; Shunkevich, T.M.; Naumenko, V.N.; Vorobyova, S.A.; Baykov, M.V. Dispersity of Magnetite in Magnetic Liquids and the Interaction with a Surfactant. J. Magn. Magn. Mater. 1990, 85, 14–16. [Google Scholar] [CrossRef]
- Sun, Y.; Duan, L.; Guo, Z.; DuanMu, Y.; Ma, M.; Xu, L.; Zhang, Y.; Gu, N. An Improved Way to Prepare Superparamagnetic Magnetite-Silica Core-Shell Nanoparticles for Possible Biological Application. J. Magn. Magn. Mater. 2005, 285, 65–70. [Google Scholar] [CrossRef]
- Im, S.H.; Herricks, T.; Lee, Y.T.; Xia, Y. Synthesis and Characterization of Monodisperse Silica Colloids Loaded with Superparamagnetic Iron Oxide Nanoparticles. Chem. Phys. Lett. 2005, 401, 19–23. [Google Scholar] [CrossRef]
- Yang, H.-H.; Zhang, S.-Q.; Chen, X.-L.; Zhuang, Z.-X.; Xu, J.-G.; Wang, X.-R. Magnetite-Containing Spherical Silica Nanoparticles for Biocatalysis and Bioseparations. Anal. Chem. 2004, 76, 1316–1321. [Google Scholar] [CrossRef]
- Xie, L.; Jiang, R.; Zhu, F.; Liu, H.; Ouyang, G. Application of Functionalized Magnetic Nanoparticles in Sample Preparation. Anal. Bioanal. Chem. 2014, 406, 377–399. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, P. Adsorption of Methylene Blue from Aqueous Solutions by Modified Expanded Graphite Powder. Desalination 2009, 249, 331–336. [Google Scholar] [CrossRef]
- Chung, D.D.L. Review Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Castro, C.S.; Guerreiro, M.C.; Gonçalves, M.; Oliveira, L.C.A.; Anastácio, A.S. Activated Carbon/Iron Oxide Composites for the Removal of Atrazine from Aqueous Medium. J. Hazard. Mater. 2009, 164, 609–614. [Google Scholar] [CrossRef]
- Imamoglu, M.; Yıldız, H.; Altundag, H.; Turhan, Y. Efficient Removal of Cd(II) from Aqueous Solution by Dehydrated Hazelnut Husk Carbon. J. Dispers. Sci. Technol. 2015, 36, 284–290. [Google Scholar] [CrossRef]
- Aygun, A.; Yenisoy-Karakas, S.; Duman, I. Production of Granular Activated Carbon from Fruit Stones and Nutshells and Evaluation of Their Physical, Chemical and Adsorption Properties. Microporous Mesoporous Mater. 2003, 66, 189–195. [Google Scholar] [CrossRef]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of Graphene and Graphene Oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef]
- Bian, Y.; Bian, Z.-Y.; Zhang, J.-X.; Ding, A.-Z.; Liu, S.-L.; Wang, H. Effect of the Oxygen-Containing Functional Group of Graphene Oxide on the Aqueous Cadmium Ions Removal. Appl. Surf. Sci. 2015, 329, 269–275. [Google Scholar] [CrossRef]
- Gao, P.; Liu, M.; Tian, J.; Deng, F.; Wang, K.; Xu, D.; Liu, L.; Zhang, X.; Wei, Y. Improving the Drug Delivery Characteristics of Graphene Oxide Based Polymer Nanocomposites through the “One-Pot” Synthetic Approach of Single-Electron-Transfer Living Radical Polymerization. Appl. Surf. Sci. 2016, 378, 22–29. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The Reduction of Graphene Oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Wang, B.; Yao, J.; Park, J. Synthesis and Characterisation of Hydrophilic and Organophilic Graphene Nanosheets. Carbon 2009, 47, 1359–1364. [Google Scholar] [CrossRef]
- Ayazi, H.; Akhavan, O.; Raoufi, M.; Varshochian, R.; Hosseini Motlagh, N.S.; Atyabi, F. Graphene Aerogel Nanoparticles for In-Situ Loading/PH Sensitive Releasing Anticancer Drugs. Colloids Surf. B Biointerfaces 2020, 186, 110712. [Google Scholar] [CrossRef]
- Depan, D.; Shah, J.; Misra, R.D.K. Controlled Release of Drug from Folate-Decorated and Graphene Mediated Drug Delivery System: Synthesis, Loading Efficiency, and Drug Release Response. Mater. Sci. Eng. C 2011, 31, 1305–1312. [Google Scholar] [CrossRef]
- Ma, D.; Lin, J.; Chen, Y.; Xue, W.; Zhang, L.-M. In Situ Gelation and Sustained Release of an Antitumor Drug by Graphene Oxide Nanosheets. Carbon 2012, 50, 3001–3007. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion Behaviour of Graphene Oxide and Reduced Graphene Oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Toxicity of Graphene and Graphene Oxide Nanowalls Against Bacteria. ACS Nano 2010, 4, 5731–5736. [Google Scholar] [CrossRef] [PubMed]
- Akhavan, O.; Ghaderi, E.; Akhavan, A. Size-Dependent Genotoxicity of Graphene Nanoplatelets in Human Stem Cells. Biomaterials 2012, 33, 8017–8025. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, H.; Feng, J.; Zhang, J.; Deng, X.; Zhou, B.; Zhang, H.; Xue, D.; Li, F.; Mellors, N.J.; et al. One-Pot Polylol Synthesis of Graphene Decorated with Size- and Density-Tunable Fe3O4 Nanoparticles for Porcine Pancreatic Lipase Immobilization. Carbon 2013, 60, 488–497. [Google Scholar] [CrossRef]
- Baaziz, W.; Truong-Phuoc, L.; Duong-Viet, C.; Melinte, G.; Janowska, I.; Papaefthimiou, V.; Ersen, O.; Zafeiratos, S.; Begin, D.; Begin-Colin, S.; et al. Few Layer Graphene Decorated with Homogeneous Magnetic Fe3O4 Nanoparticles with Tunable Covering Densities. J. Mater. Chem. A 2014, 2, 2690. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.-J. Preparation and Application of Iron Oxide/Graphene Based Composites for Electrochemical Energy Storage and Energy Conversion Devices: Current Status and Perspective. Nano Energy 2015, 11, 277–293. [Google Scholar] [CrossRef]
- Xu, C.; Sun, S. New Forms of Superparamagnetic Nanoparticles for Biomedical Applications. Adv. Drug Deliv. Rev. 2013, 65, 732–743. [Google Scholar] [CrossRef]
- Mekonnen, T.W.; Birhan, Y.S.; Andrgie, A.T.; Hanurry, E.Y.; Darge, H.F.; Chou, H.-Y.; Lai, J.-Y.; Tsai, H.-C.; Yang, J.M.; Chang, Y.-H. Encapsulation of Gadolinium Ferrite Nanoparticle in Generation 4.5 Poly(Amidoamine) Dendrimer for Cancer Theranostics Applications Using Low Frequency Alternating Magnetic Field. Colloids Surf. B Biointerfaces 2019, 184, 110531. [Google Scholar] [CrossRef] [PubMed]
- Morawski, A.M.; Winter, P.M.; Crowder, K.C.; Caruthers, S.D.; Fuhrhop, R.W.; Scott, M.J.; Robertson, J.D.; Abendschein, D.R.; Lanza, G.M.; Wickline, S.A. Targeted Nanoparticles for Quantitative Imaging of Sparse Molecular Epitopes with MRI. Magn. Reson. Med. 2004, 51, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, P.G.; Popplewell, J.; Charles, S.W. A Method of Producing Ferrofluid with Gadolinium Particles. J. Phys. D Appl. Phys. 1970, 3, 1985–1986. [Google Scholar] [CrossRef]
- Xu, H.K.; Sorensen, C.M.; Klabunde, K.J.; Hadjipanayis, G.C. Aerosol Synthesis of Gadolinium Iron Garnet Particles. J. Mater. Res. 1992, 7, 712–716. [Google Scholar] [CrossRef]
- Arora, H.C.; Jensen, M.P.; Yuan, Y.; Wu, A.; Vogt, S.; Paunesku, T.; Woloschak, G.E. Nanocarriers Enhance Doxorubicin Uptake in Drug-Resistant Ovarian Cancer Cells. Cancer Res. 2012, 72, 769–778. [Google Scholar] [CrossRef]
- Lyon, J.L.; Fleming, D.A.; Stone, M.B.; Schiffer, P.; Williams, M.E. Synthesis of Fe Oxide Core/Au Shell Nanoparticles by Iterative Hydroxylamine Seeding. Nano Lett. 2004, 4, 719–723. [Google Scholar] [CrossRef]
- Lin, J.; Zhou, W.; Kumbhar, A.; Wiemann, J.; Fang, J.; Carpenter, E.E.; O’Connor, C.J. Gold-Coated Iron (Fe@Au) Nanoparticles: Synthesis, Characterization, and Magnetic Field-Induced Self-Assembly. J. Solid State Chem. 2001, 159, 26–31. [Google Scholar] [CrossRef]
- Kim, J.; Park, S.; Lee, J.E.; Jin, S.M.; Lee, J.H.; Lee, I.S.; Yang, I.; Kim, J.-S.; Kim, S.K.; Cho, M.-H.; et al. Designed Fabrication of Multifunctional Magnetic Gold Nanoshells and Their Application to Magnetic Resonance Imaging and Photothermal Therapy. Angew. Chem. 2006, 118, 7918–7922. [Google Scholar] [CrossRef]
- Du, J.; Jing, C. Preparation of Thiol Modified Fe3O4@Ag Magnetic SERS Probe for PAHs Detection and Identification. J. Phys. Chem. C 2011, 115, 17829–17835. [Google Scholar] [CrossRef]
- A DNA-Assembled Fe3O4@Ag Nanorod in Silica Matrix for Cholesterol Biosensing|SpringerLink. Available online: https://link.springer.com/article/10.1007/s11665-015-1532-z (accessed on 10 July 2023).
- Tamaura, Y.; Takahashi, K.; Kodera, Y.; Saito, Y.; Inada, Y. Chemical Modification of Lipase with Ferromagnetic Modifier ? A Ferromagnetic-Modified Lipase. Biotechnol. Lett. 1986, 8, 877–880. [Google Scholar] [CrossRef]
- Butterworth, M.D.; Illum, L.; Davis, S.S. Preparation of Ultrafine Silica- and PEG-Coated Magnetite Particles. Colloids Surf. A Physicochem. Eng. Asp. 2001, 179, 93–102. [Google Scholar] [CrossRef]
- Kohler, N.; Fryxell, G.E.; Zhang, M. A Bifunctional Poly(Ethylene Glycol) Silane Immobilized on Metallic Oxide-Based Nanoparticles for Conjugation with Cell Targeting Agents. J. Am. Chem. Soc. 2004, 126, 7206–7211. [Google Scholar] [CrossRef] [PubMed]
- Velusamy, P.; Chia-Hung, S.; Shritama, A.; Kumar, G.V.; Jeyanthi, V.; Pandian, K. Synthesis of Oleic Acid Coated Iron Oxide Nanoparticles and Its Role in Anti-Biofilm Activity against Clinical Isolates of Bacterial Pathogens. J. Taiwan Inst. Chem. Eng. 2016, 59, 450–456. [Google Scholar] [CrossRef]
- Chang, P.R.; Yu, J.; Ma, X.; Anderson, D.P. Polysaccharides as Stabilizers for the Synthesis of Magnetic Nanoparticles. Carbohydr. Polym. 2011, 83, 640–644. [Google Scholar] [CrossRef]
- Gaihre, B.; Khil, M.S.; Lee, D.R.; Kim, H.Y. Gelatin-Coated Magnetic Iron Oxide Nanoparticles as Carrier System: Drug Loading and in Vitro Drug Release Study. Int. J. Pharm. 2009, 365, 180–189. [Google Scholar] [CrossRef]
- Naha, P.C.; Liu, Y.; Hwang, G.; Huang, Y.; Gubara, S.; Jonnakuti, V.; Simon-Soro, A.; Kim, D.; Gao, L.; Koo, H.; et al. Dextran-Coated Iron Oxide Nanoparticles as Biomimetic Catalysts for Localized and PH-Activated Biofilm Disruption. ACS Nano 2019, 13, 4960–4971. [Google Scholar] [CrossRef]
- Tyukova, I.S.; Safronov, A.P.; Kotel’nikova, A.P.; Agalakova, D.Y. Electrostatic and Steric Mechanisms of Iron Oxide Nanoparticle Sol Stabilization by Chitosan. Polym. Sci. Ser. A 2014, 56, 498–504. [Google Scholar] [CrossRef]
- Khandhar, A.P.; Keselman, P.; Kemp, S.J.; Ferguson, R.M.; Goodwill, P.W.; Conolly, S.M.; Krishnan, K.M. Evaluation of PEG-Coated Iron Oxide Nanoparticles as Blood Pool Tracers for Preclinical Magnetic Particle Imaging. Nanoscale 2017, 9, 1299–1306. [Google Scholar] [CrossRef]
- Walker, M.; Will, I.; Pratt, A.; Chechik, V.; Genever, P.; Ungar, D. Magnetically Triggered Release of Entrapped Bioactive Proteins from Thermally Responsive Polymer-Coated Iron Oxide Nanoparticles for Stem-Cell Proliferation. ACS Appl. Nano Mater. 2020, 3, 5008–5013. [Google Scholar] [CrossRef]
- Arsianti, M.; Lim, M.; Marquis, C.P.; Amal, R. Assembly of Polyethylenimine-Based Magnetic Iron Oxide Vectors: Insights into Gene Delivery. Langmuir 2010, 26, 7314–7326. [Google Scholar] [CrossRef]
- Zhang, M.; O’Connor, C.J. Synthesis and Characterization of PMMA Coated Magnetite Nanocomposites by Emulsion Polymerization. MRS Online Proc. Libr. OPL 2007, 1032, 1442. [Google Scholar] [CrossRef]
- Padwal, P.; Bandyopadhyaya, R.; Mehra, S. Polyacrylic Acid-Coated Iron Oxide Nanoparticles for Targeting Drug Resistance in Mycobacteria. Langmuir 2014, 30, 15266–15276. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Singh, V.K.; Chauhan, S. A Review on Mechanical and Water Absorption Properties of Polyvinyl Alcohol Based Composites/Films. J. Mech. Behav. Mater. 2017, 26, 213–222. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Phung, T.K.; Bui, X.-T.; Doan, V.-D.; Tran, T.V.; Nguyen, D.V.; Lim, K.T.; Nguyen, T.D. Removal of Cationic Dye Using Polyvinyl Alcohol Membrane Functionalized by D-Glucose and Agar. J. Water Process Eng. 2021, 40, 101982. [Google Scholar] [CrossRef]
- Jun, L.Y.; Mubarak, N.M.; Yon, L.S.; Bing, C.H.; Khalid, M.; Jagadish, P.; Abdullah, E.C. Immobilization of Peroxidase on Functionalized MWCNTs-Buckypaper/Polyvinyl Alcohol Nanocomposite Membrane. Sci. Rep. 2019, 9, 2215. [Google Scholar] [CrossRef] [PubMed]
- Merdas, S.; Al-Graiti, W.; Al-Ameer, A. Using PVA@WNS Composite as Adsorbent for Methylene Blue Dye from Aqueous Solutions. J. Med. Chem. Sci. 2022, 5, 1289–1298. [Google Scholar]
- Al Naim, A.F.; El-Shamy, A.G. A New Reusable Adsorbent of Polyvinyl Alcohol/Magnesium Peroxide (PVA/MgO2) for Highly Selective Adsorption and Dye Removal. Mater. Chem. Phys. 2021, 270, 124820. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Du, Q.; Wang, X.; Hu, S.; Chen, L.; Wang, Z.; Xia, Y.; Xia, L. Adsorption of Methylene Blue from Aqueous Solutions by Polyvinyl Alcohol/Graphene Oxide Composites. J. Nanosci. Nanotechnol. 2016, 16, 1775–1782. [Google Scholar] [CrossRef]
- Yokoi, H.; Kantoh, T. Thermal Decomposition of the Iron(III) Hydroxide and Magnetite Composites of Poly(Vinyl Alcohol). Preparation of Magnetite and Metallic Iron Particles. Bull. Chem. Soc. Jpn. 1993, 66, 1536–1541. [Google Scholar] [CrossRef]
- Sairam, M.; Naidu, B.V.K.; Nataraj, S.K.; Sreedhar, B.; Aminabhavi, T.M. Poly(Vinyl Alcohol)-Iron Oxide Nanocomposite Membranes for Pervaporation Dehydration of Isopropanol, 1,4-Dioxane and Tetrahydrofuran. J. Membr. Sci. 2006, 283, 65–73. [Google Scholar] [CrossRef]
- Schöpf, B.; Neuberger, T.; Schulze, K.; Petri, A.; Chastellain, M.; Hofmann, M.; Hofmann, H.; von Rechenberg, B. Methodology Description for Detection of Cellular Uptake of PVA Coated Superparamagnetic Iron Oxide Nanoparticles (SPION) in Synovial Cells of Sheep. J. Magn. Magn. Mater. 2005, 293, 411–418. [Google Scholar] [CrossRef]
- Doondani, P.; Jugade, R.; Gomase, V.; Shekhawat, A.; Bambal, A.; Pandey, S. Chitosan/Graphite/Polyvinyl Alcohol Magnetic Hydrogel Microspheres for Decontamination of Reactive Orange 16 Dye. Water 2022, 14, 3411. [Google Scholar] [CrossRef]
- Sekhavat Pour, Z.; Ghaemy, M. Removal of Dyes and Heavy Metal Ions from Water by Magnetic Hydrogel Beads Based on Poly(Vinyl Alcohol)/Carboxymethyl Starch-g-Poly(Vinyl Imidazole). RSC Adv. 2015, 5, 64106–64118. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Graphite Oxide/Chitosan Composite for Reactive Dye Removal. Chem. Eng. J. 2013, 217, 256–265. [Google Scholar] [CrossRef]
- Bryan, M.Y.K.; Chai, P.V.; Law, J.Y.; Mahmoudi, E. Graphene Oxide-Chitosan Composite Material as Adsorbent in Removing Methylene Blue Dye from Synthetic Wastewater. Mater. Today Proc. 2022, 64, 1587–1596. [Google Scholar] [CrossRef]
- Dissanayake, N.S.L.; Pathirana, M.A.; Wanasekara, N.D.; Nandasiri, G.K. Chitosan-Graphene Oxide Composite Membrane for Methylene Blue Removal. In Proceedings of the 2022 Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 27 July 2022; pp. 1–6. [Google Scholar]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, B.; Wu, Y.; Liu, Z.; Wang, J.; Xu, J.; Tong, Z.; Mu, X.; Liu, B. Fe3O4@PDA@PEI Core-Shell Microspheres as a Novel Magnetic Sorbent for the Rapid and Broad-Spectrum Separation of Bacteria in Liquid Phase. Materials 2022, 15, 2039. [Google Scholar] [CrossRef]
- Li, G.; Jiang, Y.; Huang, K.; Ding, P.; Chen, J. Preparation and Properties of Magnetic Fe3O4–Chitosan Nanoparticles. J. Alloys Compd. 2008, 466, 451–456. [Google Scholar] [CrossRef]
- Amini-Fazl, M.S.; Mohammadi, R.; Kheiri, K. 5-Fluorouracil Loaded Chitosan/Polyacrylic Acid/Fe3O4 Magnetic Nanocomposite Hydrogel as a Potential Anticancer Drug Delivery System. Int. J. Biol. Macromol. 2019, 132, 506–513. [Google Scholar] [CrossRef]
- Bixner, O.; Kurzhals, S.; Virk, M.; Reimhult, E. Triggered Release from Thermoresponsive Polymersomes with Superparamagnetic Membranes. Materials 2016, 9, 29. [Google Scholar] [CrossRef]
- Shin, J.R.; An, G.S.; Choi, S.-C. Influence of Carboxylic Modification Using Polyacrylic Acid on Characteristics of Fe3O4 Nanoparticles with Cluster Structure. Processes 2021, 9, 1795. [Google Scholar] [CrossRef]
- Sarkar, S.; Guibal, E.; Quignard, F.; SenGupta, A.K. Polymer-Supported Metals and Metal Oxide Nanoparticles: Synthesis, Characterization, and Applications. J. Nanopart. Res. 2012, 14, 715. [Google Scholar] [CrossRef]
- Paul, K.G.; Frigo, T.B.; Groman, J.Y.; Groman, E.V. Synthesis of Ultrasmall Superparamagnetic Iron Oxides Using Reduced Polysaccharides. Bioconjug. Chem. 2004, 15, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Zhang, Y.; Kehr, J.; Klason, T.; Bjelke, B.; Muhammed, M. Characterization and MRI Study of Surfactant-Coated Superparamagnetic Nanoparticles Administered into the Rat Brain. J. Magn. Magn. Mater. 2001, 225, 256–261. [Google Scholar] [CrossRef]
- Shultz, M.D.; Calvin, S.; Fatouros, P.P.; Morrison, S.A.; Carpenter, E.E. Enhanced Ferrite Nanoparticles as MRI Contrast Agents. J. Magn. Magn. Mater. 2007, 311, 464–468. [Google Scholar] [CrossRef]
- Cheng, J.; Zheng, Z.; Tang, W.; Shao, J.; Jiang, H.; Lin, H. A New Strategy for Stem Cells Therapy for Erectile Dysfunction: Adipose-Derived Stem Cells Transfect Neuregulin-1 Gene through Superparamagnetic Iron Oxide Nanoparticles. Investig. Clin. Urol. 2022, 63, 359–367. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, G.; Naushad, M.; Thakur, S. SPION/β-Cyclodextrin Core–Shell Nanostructures for Oil Spill Remediation and Organic Pollutant Removal from Waste Water. Chem. Eng. J. 2015, 280, 175–187. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Chauhan, C.; Okram, A.; Sharma, S.; Pathania, D.; Kalia, S. Pectin-Crosslinked-Guar Gum/SPION Nanocomposite Hydrogel for Adsorption of m-Cresol and o-Chlorophenol. Sustain. Chem. Pharm. 2017, 6, 96–106. [Google Scholar] [CrossRef]
- López, J.; González-Bahamón, L.F.; Prado, J.; Caicedo, J.C.; Zambrano, G.; Gómez, M.E.; Esteve, J.; Prieto, P. Study of Magnetic and Structural Properties of Ferrofluids Based on Cobalt–Zinc Ferrite Nanoparticles. J. Magn. Magn. Mater. 2012, 324, 394–402. [Google Scholar] [CrossRef]
- Vaidyanathan, G.; Sendhilnathan, S.; Arulmurugan, R. Structural and Magnetic Properties of Co1−xZnxFe2O4 Nanoparticles by Co-Precipitation Method. J. Magn. Magn. Mater. 2007, 313, 293–299. [Google Scholar] [CrossRef]
- Tajabadi, M.; Khosroshahi, M.E.; Bonakdar, S. An Efficient Method of SPION Synthesis Coated with Third Generation PAMAM Dendrimer. Colloids Surf. A Physicochem. Eng. Asp. 2013, 431, 18–26. [Google Scholar] [CrossRef]
- Hah, H.Y.; Gray, S.; Johnson, C.E.; Johnson, J.A.; Kolesnichenko, V.; Kucheryavy, P.; Goloverda, G. Mössbauer Spectroscopy of Superparamagnetic Fe3O4 Nanoparticles. J. Magn. Magn. Mater. 2021, 539, 168382. [Google Scholar] [CrossRef]
- Sodipo, B.K.; Aziz, A.A. A Sonochemical Approach to the Direct Surface Functionalization of Superparamagnetic Iron Oxide Nanoparticles with (3-Aminopropyl)Triethoxysilane. Beilstein J. Nanotechnol. 2014, 5, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhou, H.; Liu, G.; Qiao, J.; Wang, J.; Lu, H.; Yang, L.; Wu, Y. Methylene Blue Adsorption onto Swede Rape Straw (Brassica napus L.) Modified by Tartaric Acid: Equilibrium, Kinetic and Adsorption Mechanisms. Bioresour. Technol. 2012, 125, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Lazaridis, N.K.; Mitropoulos, A.C. Removal of Dyes from Aqueous Solutions with Untreated Coffee Residues as Potential Low-Cost Adsorbents: Equilibrium, Reuse and Thermodynamic Approach. Chem. Eng. J. 2012, 189, 148–159. [Google Scholar] [CrossRef]
- Fang, C.; Kievit, F.M.; Veiseh, O.; Stephen, Z.R.; Wang, T.; Lee, D.; Ellenbogen, R.G.; Zhang, M. Fabrication of Magnetic Nanoparticles with Controllable Drug Loading and Release through a Simple Assembly Approach. J. Control. Release 2012, 162, 233–241. [Google Scholar] [CrossRef]
- Munnier, E.; Cohen-Jonathan, S.; Linassier, C.; Douziech-Eyrolles, L.; Marchais, H.; Soucé, M.; Hervé, K.; Dubois, P.; Chourpa, I. Novel Method of Doxorubicin–SPION Reversible Association for Magnetic Drug Targeting. Int. J. Pharm. 2008, 363, 170–176. [Google Scholar] [CrossRef]
- Pang, K.; Sun, W.; Ye, F.; Yang, L.; Pu, M.; Yang, C.; Zhang, Q.; Niu, J. Sulfur-Modified Chitosan Derived N,S-Co-Doped Carbon as a Bifunctional Material for Adsorption and Catalytic Degradation Sulfamethoxazole by Persulfate. J. Hazard. Mater. 2022, 424, 127270. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Cytotoxicity Suppression and Cellular Uptake Enhancement of Surface Modified Magnetic Nanoparticles. Biomaterials 2005, 26, 1565–1573. [Google Scholar] [CrossRef]
- Majeed, M.I.; Lu, Q.; Yan, W.; Li, Z.; Hussain, I.; Tahir, M.N.; Tremel, W.; Tan, B. Highly Water-Soluble Magnetic Iron Oxide (Fe3O4) Nanoparticles for Drug Delivery: Enhanced in Vitro Therapeutic Efficacy of Doxorubicin and MION Conjugates. J. Mater. Chem. B 2013, 1, 2874. [Google Scholar] [CrossRef]
- Kayal, S.; Ramanujan, R.V. Doxorubicin Loaded PVA Coated Iron Oxide Nanoparticles for Targeted Drug Delivery. Mater. Sci. Eng. C 2010, 30, 484–490. [Google Scholar] [CrossRef]
- An, G.S.; Chae, D.H.; Hur, J.U.; Oh, A.H.; Choi, H.-H.; Choi, S.-C.; Oh, Y.-S.; Jung, Y.-G. Hollow-Structured Fe3O4@SiO2 Nanoparticles: Novel Synthesis and Enhanced Adsorbents for Purification of Plasmid DNA. Ceram. Int. 2018, 44, 18791–18795. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Lee, S.-H.; Xu, C.; Xie, J.; Lee, J.-H.; Wu, B.; Leen Koh, A.; Wang, X.; Sinclair, R.; Wang, S.X.; et al. Synthesis and Characterization of PVP-Coated Large Core Iron Oxide Nanoparticles as an MRI Contrast Agent. Nanotechnology 2008, 19, 165101. [Google Scholar] [CrossRef]
- Kopanja, L.; Kralj, S.; Zunic, D.; Loncar, B.; Tadic, M. Core–Shell Superparamagnetic Iron Oxide Nanoparticle (SPION) Clusters: TEM Micrograph Analysis, Particle Design and Shape Analysis. Ceram. Int. 2016, 42, 10976–10984. [Google Scholar] [CrossRef]
- Wanna, Y.; Sirapat Pratontep, S.; Pui-ngam, R.; Nukeaw, J.; Chindaduang, A.; Tumcharern, G. Preparation And Characterization of PEG Bis(Amine) Grafted PMMA/SPION Composite Nanoparticles. Adv. Mater. Lett. 2016, 7, 176–180. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Atarod, M.; Sajjadi, M.; Sajadi, S.M.; Issaabadi, Z. Plant-Mediated Green Synthesis of Nanostructures: Mechanisms, Characterization, and Applications. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 199–322. ISBN 978-0-12-813586-0. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Nishi, Y.; Inagaki, M. Chapter 11—Gas Adsorption/Desorption Isotherm for Pore Structure Characterization. In Materials Science and Engineering of Carbon; Inagaki, M., Kang, F., Eds.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 227–247. ISBN 978-0-12-805256-3. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Kumar, K.V.; Gadipelli, S.; Wood, B.; Ramisetty, K.A.; Stewart, A.A.; Howard, C.A.; Brett, D.J.L.; Rodriguez-Reinoso, F. Characterization of the Adsorption Site Energies and Heterogeneous Surfaces of Porous Materials. J. Mater. Chem. A 2019, 7, 10104–10137. [Google Scholar] [CrossRef]
- Yurdakal, S.; Garlisi, C.; Özcan, L.; Bellardita, M.; Palmisano, G. Chapter 4—(Photo)Catalyst Characterization Techniques: Adsorption Isotherms and BET, SEM, FTIR, UV–Vis, Photoluminescence, and Electrochemical Characterizations. In Heterogeneous Photocatalysis; Marcì, G., Palmisano, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 87–152. ISBN 978-0-444-64015-4. [Google Scholar]
- Levine, I.N. Physical Chemistry; Higher Education: New York, NY, USA, 2021. [Google Scholar]
- Das, A.; Guo, H. Raman Spectroscopy. In Reference Module in Earth Systems and Environmental Sciences; Elsevier: Amsterdam, The Netherlands, 2022; p. B9780128229743000000. ISBN 978-0-12-409548-9. [Google Scholar]
- de Jesús Ruíz-Baltazar, Á.; Reyes-López, S.Y.; de Lourdes Mondragón-Sánchez, M.; Robles-Cortés, A.I.; Pérez, R. Eco-Friendly Synthesis of Fe3O4 Nanoparticles: Evaluation of Their Catalytic Activity in Methylene Blue Degradation by Kinetic Adsorption Models. Results Phys. 2019, 12, 989–995. [Google Scholar] [CrossRef]
- Papadimitriou, S.; Bikiaris, D. Novel Self-Assembled Core–Shell Nanoparticles Based on Crystalline Amorphous Moieties of Aliphatic Copolyesters for Efficient Controlled Drug Release. J. Control. Release 2009, 138, 177–184. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and Mechanism of Removal of Methylene Blue by Adsorption on Various Carbons—A Comparative Study. Dye. Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Vimonses, V.; Lei, S.; Jin, B.; Chow, C.W.; Saint, C. Kinetic Study and Equilibrium Isotherm Analysis of Congo Red Adsorption by Clay Materials. Chem. Eng. J. 2009, 148, 354–364. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Aboluwoye, C.O.; Oladimeji, Y.B.; Ashogbon, A.O.; Otemuyiwa, I.O. Studies on Castor Seed Shell as a Sorbent in Basic Dye Contaminated Wastewater Remediation. Desalination 2008, 227, 190–203. [Google Scholar] [CrossRef]
- Nandi, B.K.; Goswami, A.; Purkait, M.K. Removal of Cationic Dyes from Aqueous Solutions by Kaolin: Kinetic and Equilibrium Studies. Appl. Clay Sci. 2009, 42, 583–590. [Google Scholar] [CrossRef]
- Özcan, A.S.; Erdem, B.; Özcan, A. Adsorption of Acid Blue 193 from Aqueous Solutions onto BTMA-Bentonite. Colloids Surf. A Physicochem. Eng. Asp. 2005, 266, 73–81. [Google Scholar] [CrossRef]
- Do, T.H.; Nguyen, V.T.; Dung, N.Q.; Chu, M.N.; Van Kiet, D.; Ngan, T.T.K.; Van Tan, L. Study on Methylene Blue Adsorption of Activated Carbon Made from Moringa Oleifera Leaf. Mater. Today Proc. 2021, 38, 3405–3413. [Google Scholar] [CrossRef]
- Cengiz, S.; Cavas, L. Removal of Methylene Blue by Invasive Marine Seaweed: Caulerpa Racemosa Var. Cylindracea. Bioresour. Technol. 2008, 99, 2357–2363. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, F.; Chen, M.; Xu, Z.; Zhu, Z. Adsorption Behavior of Methylene Blue on Carbon Nanotubes. Bioresour. Technol. 2010, 101, 3040–3046. [Google Scholar] [CrossRef]
- Sahu, S.; Pahi, S.; Tripathy, S.; Singh, S.K.; Behera, A.; Sahu, U.K.; Patel, R.K. Adsorption of Methylene Blue on Chemically Modified Lychee Seed Biochar: Dynamic, Equilibrium, and Thermodynamic Study. J. Mol. Liq. 2020, 315, 113743. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Adsorptive Removal of Organic Pollutant Methylene Blue Using Polysaccharide-Based Composite Hydrogels. Chemosphere 2022, 286, 131890. [Google Scholar] [CrossRef]
- Yukselen, Y.; Kaya, A. Suitability of the Methylene Blue Test for Surface Area, Cation Exchange Capacity and Swell Potential Determination of Clayey Soils. Eng. Geol. 2008, 102, 38–45. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Liao, F.; Wang, Y.; Li, M.; Meng, L.; Jiang, J. Removal of Methylene Blue from Aqueous Solution with Magnetite Loaded Multi-Wall Carbon Nanotube: Kinetic, Isotherm and Mechanism Analysis. J. Hazard. Mater. 2011, 198, 282–290. [Google Scholar] [CrossRef]
- Oboh, I.O.; Aluyor, E.O.; Audu, T.O.K. Second-Order Kinetic Model for the Adsorption of Divalent Metal Ions on Sida Acuta Leaves. Int. J. Phys. Sci. 2013, 8, 1722–1728. [Google Scholar]
- Sahoo, T.R.; Prelot, B. Adsorption Processes for the Removal of Contaminants from Wastewater. In Nanomaterials for the Detection and Removal of Wastewater Pollutants; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. ISBN 978-0-12-818489-9. [Google Scholar]
- Sharma, G.; Naushad, M.; Al-Muhtaseb, A.H.; Kumar, A.; Khan, M.R.; Kalia, S.; Shweta; Bala, M.; Sharma, A. Fabrication and Characterization of Chitosan-Crosslinked-Poly(Alginic Acid) Nanohydrogel for Adsorptive Removal of Cr(VI) Metal Ion from Aqueous Medium. Int. J. Biol. Macromol. 2017, 95, 484–493. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Devi, K.; Sharma, S.; Naushad, M.; Ghfar, A.A.; Ahamad, T.; Stadler, F.J. Guar Gum-Crosslinked-Soya Lecithin Nanohydrogel Sheets as Effective Adsorbent for the Removal of Thiophanate Methyl Fungicide. Int. J. Biol. Macromol. 2018, 114, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Kumar, A.; Naushad, M.; García-Peñas, A.; Al-Muhtaseb, A.H.; Ghfar, A.A.; Sharma, V.; Ahamad, T.; Stadler, F.J. Fabrication and Characterization of Gum Arabic-Cl-Poly(Acrylamide) Nanohydrogel for Effective Adsorption of Crystal Violet Dye. Carbohydr. Polym. 2018, 202, 444–453. [Google Scholar] [CrossRef]
- Ma, J.; Huang, D.; Zou, J.; Li, L.; Kong, Y.; Komarneni, S. Adsorption of Methylene Blue and Orange II Pollutants on Activated Carbon Prepared from Banana Peel. J. Porous Mater. 2015, 22, 301–311. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.-B.; Ding, L.; Luo, S.-L. 4—Application of Nanotechnology in the Removal of Heavy Metal From Water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; Micro and Nano Technologies; Luo, X., Deng, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. ISBN 978-0-12-814837-2. [Google Scholar]
- Bayazit, Ş.S. Magnetic Multi-Wall Carbon Nanotubes for Methyl Orange Removal from Aqueous Solutions: Equilibrium, Kinetic and Thermodynamic Studies. Sep. Sci. Technol. 2014, 49, 1389–1400. [Google Scholar] [CrossRef]
- Ghaedi, M.; Haghdoust, S.; Kokhdan, S.N.; Mihandoost, A.; Sahraie, R.; Daneshfar, A. Comparison of Activated Carbon, Multiwalled Carbon Nanotubes, and Cadmium Hydroxide Nanowire Loaded on Activated Carbon as Adsorbents for Kinetic and Equilibrium Study of Removal of Safranine O. Spectrosc. Lett. 2012, 45, 500–510. [Google Scholar] [CrossRef]
- Reed, B.E.; Matsumoto, M.R. Modeling Cadmium Adsorption by Activated Carbon Using the Langmuir and Freundlich Isotherm Expressions. Sep. Sci. Technol. 1993, 28, 2179–2195. [Google Scholar] [CrossRef]
- Sheha, R.R.; Metwally, E. Equilibrium Isotherm Modeling of Cesium Adsorption onto Magnetic Materials. J. Hazard. Mater. 2007, 143, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Chabani, M.; Amrane, A.; Bensmaili, A. Kinetic Modelling of the Adsorption of Nitrates by Ion Exchange Resin. Chem. Eng. J. 2006, 125, 111–117. [Google Scholar] [CrossRef]
- Özcan, A.; Özcan, A.S.; Tunali, S.; Akar, T.; Kiran, I. Determination of the Equilibrium, Kinetic and Thermodynamic Parameters of Adsorption of Copper(II) Ions onto Seeds of Capsicum Annuum. J. Hazard. Mater. 2005, 124, 200–208. [Google Scholar] [CrossRef]
- Helfferich, F.G. Ion Exchange; Courier Corporation: North Chelmsford, MA, USA, 1995; ISBN 978-0-486-68784-1. [Google Scholar]
- Onyango, M.S.; Kojima, Y.; Aoyi, O.; Bernardo, E.C.; Matsuda, H. Adsorption Equilibrium Modeling and Solution Chemistry Dependence of Fluoride Removal from Water by Trivalent-Cation-Exchanged Zeolite F-9. J. Colloid Interface Sci. 2004, 279, 341–350. [Google Scholar] [CrossRef]
- Üner, O.; Geçgel, Ü.; Bayrak, Y. Adsorption of Methylene Blue by an Efficient Activated Carbon Prepared from Citrullus Lanatus Rind: Kinetic, Isotherm, Thermodynamic, and Mechanism Analysis. Water Air Soil Pollut. 2016, 227, 247. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Teng, H. Langmuir and Dubinin-Radushkevich Analyses on Equilibrium Adsorption of Activated Carbon Fabrics in Aqueous Solutions. J. Chem. Technol. Biotechnol. 2000, 75, 1066–1072. [Google Scholar] [CrossRef]
- Amin, N.K. Removal of Direct Blue-106 Dye from Aqueous Solution Using New Activated Carbons Developed from Pomegranate Peel: Adsorption Equilibrium and Kinetics. J. Hazard. Mater. 2009, 165, 52–62. [Google Scholar] [CrossRef]
- Ghaedi, M.; Sadeghian, B.; Pebdani, A.A.; Sahraei, R.; Daneshfar, A.; Duran, C. Kinetics, Thermodynamics and Equilibrium Evaluation of Direct Yellow 12 Removal by Adsorption onto Silver Nanoparticles Loaded Activated Carbon. Chem. Eng. J. 2012, 187, 133–141. [Google Scholar] [CrossRef]
- Başar, C.A. Applicability of the Various Adsorption Models of Three Dyes Adsorption onto Activated Carbon Prepared Waste Apricot. J. Hazard. Mater. 2006, 135, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Ertugay, M.; Certel, M.; Gurses, A. Moisture Adsorption Isotherms of Tarhana at 25 °C and 35 °C and the Investigation of Fitness of Various Isotherm Equations to Moisture Sorption Data of Tarhana. J. Sci. Food Agric. 2000, 80, 2001–2004. [Google Scholar] [CrossRef]
- El Qada, E.N.; Allen, S.J.; Walker, G.M. Adsorption of Methylene Blue onto Activated Carbon Produced from Steam Activated Bituminous Coal: A Study of Equilibrium Adsorption Isotherm. Chem. Eng. J. 2006, 124, 103–110. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Jawad, A.H.; Surip, S.N. Upgrading Low Rank Coal into Mesoporous Activated Carbon via Microwave Process for Methylene Blue Dye Adsorption: Box Behnken Design and Mechanism Study. Diam. Relat. Mater. 2022, 127, 109199. [Google Scholar] [CrossRef]
- Jawad, A.H.; Ismail, K.; Ishak, M.A.M.; Wilson, L.D. Conversion of Malaysian Low-Rank Coal to Mesoporous Activated Carbon: Structure Characterization and Adsorption Properties. Chin. J. Chem. Eng. 2019, 27, 1716–1727. [Google Scholar] [CrossRef]
- Jawad, A.H.; Mohd Firdaus Hum, N.N.; Abdulhameed, A.S.; Mohd Ishak, M.A. Mesoporous Activated Carbon from Grass Waste via H3PO4—Activation for Methylene Blue Dye Removal: Modelling, Optimisation, and Mechanism Study. Int. J. Environ. Anal. Chem. 2020, 102, 6061–6077. [Google Scholar] [CrossRef]
- Tan, K.L.; Hameed, B.H. Insight into the Adsorption Kinetics Models for the Removal of Contaminants from Aqueous Solutions. J. Taiwan Inst. Chem. Eng. 2017, 74, 25–48. [Google Scholar] [CrossRef]
- Srivastava, S.; Tyagi, R.; Pant, N. Adsorption of Heavy Metal Ions on Carbonaceous Material Developed from the Waste Slurry Generated in Local Fertilizer Plants. Water Res. 1989, 23, 1161–1165. [Google Scholar] [CrossRef]
- Demirbas, E.; Kobya, M.; Senturk, E.; Ozkan, T. Adsorption Kinetics for the Removal of Chromium (VI) from Aqueous Solutions on the Activated Carbons Prepared from Agricultural Wastes. Water SA 2004, 30, 533–539. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich Equation to the Kinetics of Phosphate Release and Sorption in Soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The Exchange Adsorption of Ions from Aqueous Solutions by Organic Zeolites. II. Kinetics. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef]
- Hameed, B.H.; El-Khaiary, M.I. Malachite Green Adsorption by Rattan Sawdust: Isotherm, Kinetic and Mechanism Modeling. J. Hazard. Mater. 2008, 159, 574–579. [Google Scholar] [CrossRef]
- Seki, Y.; Yurdakoç, K. Adsorption of Promethazine Hydrochloride with KSF Montmorillonite. Adsorption 2006, 12, 89–100. [Google Scholar] [CrossRef]
- Singh, D. Studies of the Adsorption Thermodynamics of Oxamyl on Fly Ash. Adsorpt. Sci. Technol. 2000, 18, 741–748. [Google Scholar] [CrossRef]
- Özcan, A.; Öncü, E.M.; Özcan, A.S. Kinetics, Isotherm and Thermodynamic Studies of Adsorption of Acid Blue 193 from Aqueous Solutions onto Natural Sepiolite. Colloids Surf. A Physicochem. Eng. Asp. 2006, 277, 90–97. [Google Scholar] [CrossRef]
- Bhattacharyya, K.G.; Sharma, A. Kinetics and Thermodynamics of Methylene Blue Adsorption on Neem (Azadirachta Indica) Leaf Powder. Dye. Pigment. 2005, 65, 51–59. [Google Scholar] [CrossRef]
- Sharma, P.; Kaur, R.; Baskar, C.; Chung, W.-J. Removal of Methylene Blue from Aqueous Waste Using Rice Husk and Rice Husk Ash. Desalination 2010, 259, 249–257. [Google Scholar] [CrossRef]
- Karaer, H.; Kaya, İ. Synthesis, Characterization of Magnetic Chitosan/Active Charcoal Composite and Using at the Adsorption of Methylene Blue and Reactive Blue4. Microporous Mesoporous Mater. 2016, 232, 26–38. [Google Scholar] [CrossRef]
- Srivastava, V.; Weng, C.H.; Singh, V.K.; Sharma, Y.C. Adsorption of Nickel Ions from Aqueous Solutions by Nano Alumina: Kinetic, Mass Transfer, and Equilibrium Studies. J. Chem. Eng. Data 2011, 56, 1414–1422. [Google Scholar] [CrossRef]
- Hasan, M.; Ahmad, A.L.; Hameed, B.H. Adsorption of Reactive Dye onto Cross-Linked Chitosan/Oil Palm Ash Composite Beads. Chem. Eng. J. 2008, 136, 164–172. [Google Scholar] [CrossRef]
- Nnadi, C.; Nwodo, N.; Obonga, W.; Uzor, P. In Vitro Adsorption Kinetic Model of Phenobarbital onto Powdered Seeds of Garcinia Kola. Afr. J. Pharm. Pharmacol. 2014, 8, 1079–1085. [Google Scholar]
- Lazaridis, N.K.; Asouhidou, D.D. Kinetics of Sorptive Removal of Chromium(VI) from Aqueous Solutions by Calcined Mg–Al–CO3 Hydrotalcite. Water Res. 2003, 37, 2875–2882. [Google Scholar] [CrossRef] [PubMed]
- Özçelik, G.; Kurtulbaş Şahin, E.; Şahin, S. Effect of Ionic Strength on Methylene Blue Sorption onto Macroporous Resins: A Comprehensive Study. J. Dispers. Sci. Technol. 2022, 43, 716–725. [Google Scholar] [CrossRef]
- Ghasemi, J.; Asadpour, S. Thermodynamics’ Study of the Adsorption Process of Methylene Blue on Activated Carbon at Different Ionic Strengths. J. Chem. Thermodyn. 2007, 39, 967–971. [Google Scholar] [CrossRef]
- Doğan, M.; Özdemir, Y.; Alkan, M. Adsorption Kinetics and Mechanism of Cationic Methyl Violet and Methylene Blue Dyes onto Sepiolite. Dye Pigment. 2007, 75, 701–713. [Google Scholar] [CrossRef]
- Imamura, K.; Ikeda, E.; Nagayasu, T.; Sakiyama, T.; Nakanishi, K. Adsorption Behavior of Methylene Blue and Its Congeners on a Stainless Steel Surface. J. Colloid Interface Sci. 2002, 245, 50–57. [Google Scholar] [CrossRef]
- Yazdani, O.; Irandoust, M.; Ghasemi, J.B.; Hooshmand, S. Thermodynamic Study of the Dimerization Equilibrium of Methylene Blue, Methylene Green and Thiazole Orange at Various Surfactant Concentrations and Different Ionic Strengths and in Mixed Solvents by Spectral Titration and Chemometric Analysis. Dye. Pigment. 2012, 92, 1031–1041. [Google Scholar] [CrossRef]
- Yang, S.-T.; Chen, S.; Chang, Y.; Cao, A.; Liu, Y.; Wang, H. Removal of Methylene Blue from Aqueous Solution by Graphene Oxide. J. Colloid Interface Sci. 2011, 359, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Mahmoodi, N.M.; Hayati, B.; Arami, M.; Lan, C. Adsorption of Textile Dyes on Pine Cone from Colored Wastewater: Kinetic, Equilibrium and Thermodynamic Studies. Desalination 2011, 268, 117–125. [Google Scholar] [CrossRef]
- Ghaedi, M.; Hassanzadeh, A.; Kokhdan, S.N. Multiwalled Carbon Nanotubes as Adsorbents for the Kinetic and Equilibrium Study of the Removal of Alizarin Red S and Morin. J. Chem. Eng. Data 2011, 56, 2511–2520. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and Anionic Dye Adsorption by Agricultural Solid Wastes: A Comprehensive Review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Saputra, E.; Saputra, R.; Nugraha, M.W.; Irianty, R.S.; Utama, P.S. Removal of Methylene Blue from Aqueous Solution Using Spent Bleaching Earth. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012008. [Google Scholar] [CrossRef]
- Kallel, F.; Chaari, F.; Bouaziz, F.; Bettaieb, F.; Ghorbel, R.; Chaabouni, S.E. Sorption and Desorption Characteristics for the Removal of a Toxic Dye, Methylene Blue from Aqueous Solution by a Low Cost Agricultural by-Product. J. Mol. Liq. 2016, 219, 279–288. [Google Scholar] [CrossRef]
- Noori, M.; Tahmasebpoor, M.; Foroutan, R. Enhanced Adsorption Capacity of Low-Cost Magnetic Clinoptilolite Powders/Beads for the Effective Removal of Methylene Blue: Adsorption and Desorption Studies. Mater. Chem. Phys. 2022, 278, 125655. [Google Scholar] [CrossRef]
- Dawood, S.; Sen, T.K. Removal of Anionic Dye Congo Red from Aqueous Solution by Raw Pine and Acid-Treated Pine Cone Powder as Adsorbent: Equilibrium, Thermodynamic, Kinetics, Mechanism and Process Design. Water Res. 2012, 46, 1933–1946. [Google Scholar] [CrossRef]
- Shahryari, Z.; Goharrizi, A.S.; Azadi, M. Experimental Study of Methylene Blue Adsorption from Aqueous Solutions onto Carbon Nano Tubes. Int. J. Water Resour. Environ. Eng. 2010, 2, 16–28. [Google Scholar]
- Mahmoud, D.K.; Salleh, M.A.M.; Karim, W.A.W.A.; Idris, A.; Abidin, Z.Z. Batch Adsorption of Basic Dye Using Acid Treated Kenaf Fibre Char: Equilibrium, Kinetic and Thermodynamic Studies. Chem. Eng. J. 2012, 181, 449–457. [Google Scholar] [CrossRef]
- Reddy, M.S.; Sivaramakrishna, L.; Reddy, A.V. The Use of an Agricultural Waste Material, Jujuba Seeds for the Removal of Anionic Dye (Congo Red) from Aqueous Medium. J. Hazard. Mater. 2012, 203, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, Y.; Xing, J.; Meng, A. Fast Removal of Methylene Blue by Fe3O4 Magnetic Nanoparticles and Their Cycling Property. J. Nanosci. Nanotechnol. 2019, 19, 2116–2123. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.V.; Rama Devi, D.; Mohan Botsa, S.; Basavaiah, K. Facile Green Synthesis of Fe3O4 Nanoparticles Using Aqueous Leaf Extract of Zanthoxylum Armatum DC. for Efficient Adsorption of Methylene Blue. J. Asian Ceram. Soc. 2018, 6, 145–155. [Google Scholar] [CrossRef]
- Mohammadpour, A.; Karami, N.; Zabihi, R.; Fazeliyan, E.; Abbasi, A.; Karimi, S.; Barbosa de Farias, M.; Adeodato Vieira, M.G.; Shahsavani, E.; Mousavi Khaneghah, A. Green Synthesis, Characterization, and Application of Fe3O4 Nanoparticles for Methylene Blue Removal: RSM Optimization, Kinetic, Isothermal Studies, and Molecular Simulation. Environ. Res. 2023, 225, 115507. [Google Scholar] [CrossRef] [PubMed]
- Xiang, H.; Ren, G.; Zhong, Y.; Xu, D.; Zhang, Z.; Wang, X.; Yang, X. Fe3O4@C Nanoparticles Synthesized by In Situ Solid-Phase Method for Removal of Methylene Blue. Nanomaterials 2021, 11, 330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, L.Y.; Zhao, X.J.; Zhou, Z. Citrus Pectin Derived Ultrasmall Fe3O4@C Nanoparticles as a High-Performance Adsorbent toward Removal of Methylene Blue. J. Mol. Liq. 2016, 222, 995–1002. [Google Scholar] [CrossRef]
- Tripathy, S.; Sahu, S.; Patel, R.K.; Panda, R.B.; Kar, P.K. Novel Fe3O4-Modified Biochar Derived from Citrus Bergamia Peel: A Green Synthesis Approach for Adsorptive Removal of Methylene Blue. ChemistrySelect 2022, 7, e202103595. [Google Scholar] [CrossRef]
- Habila, M.A.; Moshab, M.S.; El-Toni, A.M.; ALOthman, Z.A.; Badjah Hadj Ahmed, A.Y. Thermal Fabrication of Magnetic Fe3O4 (Nanoparticle)@Carbon Sheets from Waste Resources for the Adsorption of Dyes: Kinetic, Equilibrium, and UV–Visible Spectroscopy Investigations. Nanomaterials 2023, 13, 1266. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, S.; Liu, S.; Ma, L.P.; Sun, H.; Wang, S. Synthesis, Characterization, and Adsorption Properties of Magnetic Fe3O4@graphene Nanocomposite. Chem. Eng. J. 2012, 184, 326–332. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Eldesoky, G.E.; Al-Saeedi, S.I.; Nafady, A.; Al-Kadhi, N.S.; Al-Muhtaseb, A.H.; Khan, A.A.; Khan, A. Effective and Fast Adsorptive Removal of Toxic Cationic Dye (MB) from Aqueous Medium Using Amino-Functionalized Magnetic Multiwall Carbon Nanotubes. J. Mol. Liq. 2019, 282, 154–161. [Google Scholar] [CrossRef]
- Wu, K.-H.; Huang, W.-C.; Hung, W.-C.; Tsai, C.-W. Modified Expanded Graphite/Fe3O4 Composite as an Adsorbent of Methylene Blue: Adsorption Kinetics and Isotherms. Mater. Sci. Eng. B 2021, 266, 115068. [Google Scholar] [CrossRef]
- Tishbi, P.; Mosayebi, M.; Salehi, Z.; Fatemi, S.; Faegh, E. Synthesizing Magnetic Graphene Oxide Nanomaterial (GO-Fe3O4) and Kinetic Modelling of Methylene Blue Adsorption from Water. Can. J. Chem. Eng. 2022, 100, 3321–3334. [Google Scholar] [CrossRef]
- Li, M.; Dong, C.; Guo, C.; Yu, L. Magnetic Activated Biochar Fe3O4-MOS Made from Moringa Seed Shells for the Adsorption of Methylene Blue. Processes 2022, 10, 2720. [Google Scholar] [CrossRef]
- Xie, J.; Lin, R.; Liang, Z.; Zhao, Z.; Yang, C.; Cui, F. Effect of Cations on the Enhanced Adsorption of Cationic Dye in Fe3O4-Loaded Biochar and Mechanism. J. Environ. Chem. Eng. 2021, 9, 105744. [Google Scholar] [CrossRef]
- Hingrajiya, R.D.; Patel, M.P. Fe3O4 Modified Chitosan Based Co-Polymeric Magnetic Composite Hydrogel: Synthesis, Characterization and Evaluation for the Removal of Methylene Blue from Aqueous Solutions. Int. J. Biol. Macromol. 2023, 244, 125251. [Google Scholar] [CrossRef]
- Niu, Y.; Han, X.; Song, J.; Huang, L. Removal of Methylene Blue and Lead(ii) via PVA/SA Double-Cross-Linked Network Gel Beads Loaded with Fe3O4 @KHA Nanoparticles. New J. Chem. 2021, 45, 5605–5620. [Google Scholar] [CrossRef]
- Abutaleb, A.; Imran, M.; Zouli, N.; Khan, A.H.; Hussain, S.; Ali, M.A.; Bakather, O.; Gondal, M.A.; Khan, N.A.; Panchal, H.; et al. Fe3O4-Multiwalled Carbon Nanotubes-Bentonite as Adsorbent for Removal of Methylene Blue from Aqueous Solutions. Chemosphere 2023, 316, 137824. [Google Scholar] [CrossRef]
- Chang, J.; Ma, J.; Ma, Q.; Zhang, D.; Qiao, N.; Hu, M.; Ma, H. Adsorption of Methylene Blue onto Fe3O4/Activated Montmorillonite Nanocomposite. Appl. Clay Sci. 2016, 119, 132–140. [Google Scholar] [CrossRef]
- Barakat, M.A.; Kumar, R.; Halawani, R.F.; Al-Mur, B.A.; Seliem, M.K. Fe3O4 Nanoparticles Loaded Bentonite/Sawdust Interface for the Removal of Methylene Blue: Insights into Adsorption Performance and Mechanism via Experiments and Theoretical Calculations. Water 2022, 14, 3491. [Google Scholar] [CrossRef]
- Maimaiti, T.; Hu, R.; Yuan, H.; Liang, C.; Liu, F.; Li, Q.; Lan, S.; Yu, B.; Yang, S.-T. Magnetic Fe3O4/TiO2/Graphene Sponge for the Adsorption of Methylene Blue in Aqueous Solution. Diam. Relat. Mater. 2022, 123, 108811. [Google Scholar] [CrossRef]
- Kazemi, J.; Javanbakht, V. Alginate Beads Impregnated with Magnetic Chitosan@Zeolite Nanocomposite for Cationic Methylene Blue Dye Removal from Aqueous Solution. Int. J. Biol. Macromol. 2020, 154, 1426–1437. [Google Scholar] [CrossRef]
- Rahmi; Ishmaturrahmi; Mustafa, I. Methylene Blue Removal from Water Using H2SO4 Crosslinked Magnetic Chitosan Nanocomposite Beads. Microchem. J. 2019, 144, 397–402. [Google Scholar] [CrossRef]
- Faaizatunnisa, N.; Ediati, R.; Fansuri, H.; Juwono, H.; Suprapto, S.; Hidayat, A.R.P.; Zulfa, L.L. Facile Green Synthesis of Core–Shell Magnetic MOF Composites (Fe3O4@SiO2@HKUST-1) for Enhanced Adsorption Capacity of Methylene Blue. Nano Struct. Nano Objects 2023, 34, 100968. [Google Scholar] [CrossRef]
- Wo, R.; Li, Q.-L.; Zhu, C.; Zhang, Y.; Qiao, G.; Lei, K.; Du, P.; Jiang, W. Preparation and Characterization of Functionalized Metal–Organic Frameworks with Core/Shell Magnetic Particles (Fe3O4@SiO2@MOFs) for Removal of Congo Red and Methylene Blue from Water Solution. J. Chem. Eng. Data 2019, 64, 2455–2463. [Google Scholar] [CrossRef]
- Aslam, S.; Zeng, J.; Subhan, F.; Li, M.; Lyu, F.; Li, Y.; Yan, Z. In Situ One-Step Synthesis of Fe3O4@MIL-100(Fe) Core-Shells for Adsorption of Methylene Blue from Water. J. Colloid Interface Sci. 2017, 505, 186–195. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Abdi, J.; Oveisi, M.; Alinia Asli, M.; Vossoughi, M. Metal-Organic Framework (MIL-100(Fe)): Synthesis, Detailed Photocatalytic Dye Degradation Ability in Colored Textile Wastewater and Recycling. Mater. Res. Bull. 2018, 100, 357–366. [Google Scholar] [CrossRef]
- Wang, R.; Ge, C.; Xing, T.; Zhang, Y.; Zhang, Y.; Zhang, X. Facile Synthesis of Magnetic Hybrid Metal–Organic Frameworks with High Adsorption Capacity for Methylene Blue. Appl. Organomet. Chem. 2017, 31, e3798. [Google Scholar] [CrossRef]
- Xu, Y.; Jin, J.; Li, X.; Song, C.; Meng, H.; Zhang, X. Adsorption Behavior of Methylene Blue on Fe3O4-Embedded Hybrid Magnetic Metal–Organic Framework. Desalination Water Treat. 2016, 57, 25216–25225. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.L.; Gao, M.; Hong, W.; Liu, G.Z.; Fan, L.; Hu, B.; Xia, Q.H.; Liu, L.; Song, G.W.; et al. The Adsorption on Magnetic Hybrid Fe3O4/HKUST-1/GO of Methylene Blue from Water Solution. J. Mater. Chem. A 2014, 2, 1795–1801. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, L.; Bao, C.; Ma, J.; Liu, M.; Wang, F. Magnetic Responsive Metal–Organic Frameworks Nanosphere with Core–Shell Structure for Highly Efficient Removal of Methylene Blue. Chem. Eng. J. 2016, 283, 1127–1136. [Google Scholar] [CrossRef]
- Rosa, E.V.; Fascineli, M.L.; da Silva, I.C.R.; Rodrigues, M.O.; Chaker, J.A.; Grisolia, C.K.; Moya, S.E.; Campos, A.F.C.; Sousa, M.H. Carbon Nitride Nanosheets Magnetically Decorated with Fe3O4 Nanoparticles by Homogeneous Precipitation: Adsorption-Photocatalytic Performance and Acute Toxicity Assessment. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100549. [Google Scholar] [CrossRef]
- Soni, S.; Bajpai, P.K.; Mittal, J.; Arora, C. Utilisation of Cobalt Doped Iron Based MOF for Enhanced Removal and Recovery of Methylene Blue Dye from Waste Water. J. Mol. Liq. 2020, 314, 113642. [Google Scholar] [CrossRef]
- Ali, H.; Ismail, A.M. Fabrication of Magnetic Fe3O4/Polypyrrole/Carbon Black Nanocomposite for Effective Uptake of Congo Red and Methylene Blue Dye: Adsorption Investigation and Mechanism. J. Polym. Environ. 2023, 31, 976–998. [Google Scholar] [CrossRef]
- ZabihiSahebi, A.; Koushkbaghi, S.; Pishnamazi, M.; Askari, A.; Khosravi, R.; Irani, M. Synthesis of Cellulose Acetate/Chitosan/SWCNT/Fe3O4/TiO2 Composite Nanofibers for the Removal of Cr(VI), As(V), Methylene Blue and Congo Red from Aqueous Solutions. Int. J. Biol. Macromol. 2019, 140, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wen, F.; Chen, J.; Chen, W.; Huang, Y.; Wang, B. Mussel-Inspired Synthesis of Magnetic Carboxymethyl Chitosan Aerogel for Removal Cationic and Anionic Dyes from Aqueous Solution. Polymer 2021, 213, 123316. [Google Scholar] [CrossRef]
- Munagapati, V.S.; Wen, H.-Y.; Gollakota, A.R.K.; Wen, J.-C.; Lin, K.-Y.A.; Shu, C.-M.; Yarramuthi, V.; Basivi, P.K.; Reddy, G.M.; Zyryanov, G.V. Magnetic Fe3O4 Nanoparticles Loaded Guava Leaves Powder Impregnated into Calcium Alginate Hydrogel Beads (Fe3O4-GLP@CAB) for Efficient Removal of Methylene Blue Dye from Aqueous Environment: Synthesis, Characterization, and Its Adsorption Performance. Int. J. Biol. Macromol. 2023, 246, 125675. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Zhang, H.; Hao, C.; Zhao, P. Efficient Removal of Cationic and Anionic Dyes by Surfactant Modified Fe3O4 Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2022, 633, 127680. [Google Scholar] [CrossRef]
- Bai, L.; Li, Z.; Zhang, Y.; Wang, T.; Lu, R.; Zhou, W.; Gao, H.; Zhang, S. Synthesis of Water-Dispersible Graphene-Modified Magnetic Polypyrrole Nanocomposite and Its Ability to Efficiently Adsorb Methylene Blue from Aqueous Solution. Chem. Eng. J. 2015, 279, 757–766. [Google Scholar] [CrossRef]
- Gong, J.; Yao, K.; Liu, J.; Wen, X.; Chen, X.; Jiang, Z.; Mijowska, E.; Tang, T. Catalytic Conversion of Linear Low Density Polyethylene into Carbon Nanomaterials under the Combined Catalysis of Ni2O3 and Poly(Vinyl Chloride). Chem. Eng. J. 2013, 215–216, 339–347. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Chen, Z. Removal of Methylene Blue from Aqueous Solution by a Solvothermal-Synthesized Graphene/Magnetite Composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef]
- Zhang, P.; Xiang, M.; Liu, H.; Yang, C.; Deng, S. Novel Two-Dimensional Magnetic Titanium Carbide for Methylene Blue Removal over a Wide PH Range: Insight into Removal Performance and Mechanism. ACS Appl. Mater. Interfaces 2019, 11, 24027–24036. [Google Scholar] [CrossRef] [PubMed]
- Tara, N.; Siddiqui, S.I.; Nirala, R.K.; Abdulla, N.K.; Chaudhry, S.A. Synthesis of Antibacterial, Antioxidant and Magnetic Nigella Sativa-Graphene Oxide Based Nanocomposite BC-GO@Fe3O4 for Water Treatment. Colloid Interface Sci. Commun. 2020, 37, 100281. [Google Scholar] [CrossRef]
- Anushree, C.; Philip, J. Efficient Removal of Methylene Blue Dye Using Cellulose Capped Fe3O4 Nanofluids Prepared Using Oxidation-Precipitation Method. Colloids Surf. A Physicochem. Eng. Asp. 2019, 567, 193–204. [Google Scholar] [CrossRef]
- Tran, H.V.; Bui, L.T.; Dinh, T.T.; Le, D.H.; Huynh, C.D.; Trinh, A.X. Graphene Oxide/Fe3O4/Chitosan Nanocomposite: A Recoverable and Recyclable Adsorbent for Organic Dyes Removal. Application to Methylene Blue. Mater. Res. Express 2017, 4, 035701. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, P.; Guo, R.; Wang, Y.; Zhan, H.; Yuan, Y. Synthesis of Rectorite/Fe3O4/ZnO Composites and Their Application for the Removal of Methylene Blue Dye. Catalysts 2018, 8, 107. [Google Scholar] [CrossRef]
- Chishti, A.N.; Guo, F.; Aftab, A.; Ma, Z.; Liu, Y.; Chen, M.; Gautam, J.; Chen, C.; Ni, L.; Diao, G. Synthesis of Silver Doped Fe3O4/C Nanoparticles and Its Catalytic Activities for the Degradation and Reduction of Methylene Blue and 4-Nitrophenol. Appl. Surf. Sci. 2021, 546, 149070. [Google Scholar] [CrossRef]
- Alinezhad, H.; Zabihi, M.; Kahfroushan, D. Design and Fabrication the Novel Polymeric Magnetic Boehmite Nanocomposite (Boehmite@ Fe3O4@PLA@SiO2) for the Remarkable Competitive Adsorption of Methylene Blue and Mercury Ions. J. Phys. Chem. Solids 2020, 144, 109515. [Google Scholar] [CrossRef]
- Saiphaneendra, B.; Saxena, T.; Singh, S.A.; Madras, G.; Srivastava, C. Synergistic Effect of Co-Existence of Hematite (α-Fe2O3) and Magnetite (Fe3O4) Nanoparticles on Graphene Sheet for Dye Adsorption. J. Environ. Chem. Eng. 2017, 5, 26–37. [Google Scholar] [CrossRef]
- Chen, J.; Chen, H. Removal of Anionic Dyes from an Aqueous Solution by a Magnetic Cationic Adsorbent Modified with DMDAAC. New J. Chem. 2018, 42, 7262–7271. [Google Scholar] [CrossRef]
- de Araújo Padilha, C.E.; da Costa Nogueira, C.; de Santana Souza, D.F.; de Oliveira, J.A.; dos Santos, E.S. Organosolv Lignin/Fe3O4 Nanoparticles Applied as a β-Glucosidase Immobilization Support and Adsorbent for Textile Dye Removal. Ind. Crops Prod. 2020, 146, 112167. [Google Scholar] [CrossRef]
- Yao, L.; Yang, J.; Zhang, P.; Deng, L. In Situ Surface Decoration of Fe3C/Fe3O4/C Nanosheets: Towards Bi-Functional Activated Carbons with Supercapacitance and Efficient Dye Adsorption. Bioresour. Technol. 2018, 256, 208–215. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Wu, Y.; Lu, G.; Cao, Z.; Cheng, J.; Wang, K.; Yang, H.; Xia, Y.; Wen, X.; et al. Facile Preparation of Polyacrylamide/Chitosan/Fe3O4 Composite Hydrogels for Effective Removal of Methylene Blue from Aqueous Solution. Carbohydr. Polym. 2020, 234, 115882. [Google Scholar] [CrossRef]
- Subhan, F.; Aslam, S.; Yan, Z.; Khan, M.; Etim, U.J.; Naeem, M. Effective Adsorptive Performance of Fe3O4@SiO2 Core Shell Spheres for Methylene Blue: Kinetics, Isotherm and Mechanism. J. Porous Mater. 2019, 26, 1465–1474. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, X.; Meng, X.; Wang, Z. The Preparation of Core–Shell Fe3O4@SiO2 Magnetic Nanoparticles with Different Surface Carboxyl Densities and Their Application in the Removal of Methylene Blue. Inorg. Chem. Commun. 2022, 139, 109381. [Google Scholar] [CrossRef]
- Qi, L.; Jiaqi, Z.; Yimin, D.; Danyang, L.; Shengyun, W.; Ling, C. Facile Synthesis of 5-Aminoisophthalic Acid Functionalized Magnetic Nanoparticle for the Removal of Methylene Blue. J. Mater. Sci. Mater. Electron. 2020, 31, 457–468. [Google Scholar] [CrossRef]
- Jiaqi, Z.; Yimin, D.; Danyang, L.; Shengyun, W.; Liling, Z.; Yi, Z. Synthesis of Carboxyl-Functionalized Magnetic Nanoparticle for the Removal of Methylene Blue. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 58–66. [Google Scholar] [CrossRef]
- Tan, X.; Lu, L.; Wang, L.; Zhang, J. Facile Synthesis of Bimodal Mesoporous Fe3O4@SiO2 Composite for Efficient Removal of Methylene Blue. Eur. J. Inorg. Chem. 2015, 2015, 2928–2933. [Google Scholar] [CrossRef]
- Zheng, J.; Cheng, C.; Fang, W.-J.; Chen, C.; Yan, R.-W.; Huai, H.-X.; Wang, C.-C. Surfactant-Free Synthesis of a Fe3O4@ZIF-8 Core–Shell Heterostructure for Adsorption of Methylene Blue. CrystEngComm 2014, 16, 3960–3964. [Google Scholar] [CrossRef]
- Kraithep, C.; Sajomsang, W.; Minami, H.; Busabok, C.; Tangboriboonrat, P.; Chaiyasat, P.; Chaiyasat, A. Fabrication of Porous Polymer Particles Containing BiVO4 and Fe3O4 Nanoparticles Using Block Copolymer as Porogen for Effective Dye Removal. Surf. Interfaces 2023, 37, 102738. [Google Scholar] [CrossRef]
- Srisawang, N.; Chaiyasat, A.; Ngernchuklin, P.; Chaiyasat, P. Novel Reusable PH-Responsive Photocatalyst Polymeric Microcapsules for Dye Treatment. Int. J. Energy Res. 2021, 45, 7535–7548. [Google Scholar] [CrossRef]
- Benhalima, T.; Ferfera-Harrar, H.; Saha, N.; Saha, P. Fe3O4 Imbuing Carboxymethyl Cellulose/Dextran Sulfate Nanocomposite Hydrogel Beads: An Effective Adsorbent for Methylene Blue Dye Pollutant. J. Macromol. Sci. Part A 2023, 60, 442–461. [Google Scholar] [CrossRef]
- Yusuf, M.S.; Rahmasari, R. Synthesis Processing Condition Optimization of Citrate Stabilized Superparamagnetic Iron Oxide Nanoparticles Using Direct Co-Precipitation Method. Biomed. Pharmacol. J. 2021, 14, 1533–1542. [Google Scholar] [CrossRef]
- Verma, M.; Dwivedi, P.K.; Saxena, N.S. Hollow Silica Nanoparticles Synthesized from Core-Shell Nanoparticles as Highly Efficient Adsorbent for Methylene Blue and Its Invitro Release: Mechanism and Kinetics Study. Colloids Surf. A Physicochem. Eng. Asp. 2020, 587, 124333. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical Models of Drug Release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. ISBN 978-0-08-100092-2. [Google Scholar]
- Ribeiro, S.C.; de Lima, H.H.C.; Kupfer, V.L.; da Silva, C.T.P.; Veregue, F.R.; Radovanovic, E.; Guilherme, M.R.; Rinaldi, A.W. Synthesis of a Superabsorbent Hybrid Hydrogel with Excellent Mechanical Properties: Water Transport and Methylene Blue Absorption Profiles. J. Mol. Liq. 2019, 294, 111553. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, W.; Sun, H.; Li, X.; Du, Q.; Wei, C.; Ge, X.; Li, C. The Transition from Locally Excited States to Twisted Intramolecular Charge Transfer States for Fluorescence Methylene Blue Labeled in Biodegradable Silica Particles. J. Mol. Liq. 2019, 291, 111312. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A Simple Equation for the Description of Solute Release. III. Coupling of Diffusion and Relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Chahal, C.; van den Akker, B.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and Particle Associations in Wastewater. Adv. Appl. Microbiol. 2016, 97, 63–119. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in Vitro Evaluating Antimicrobial Activity: A Review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]

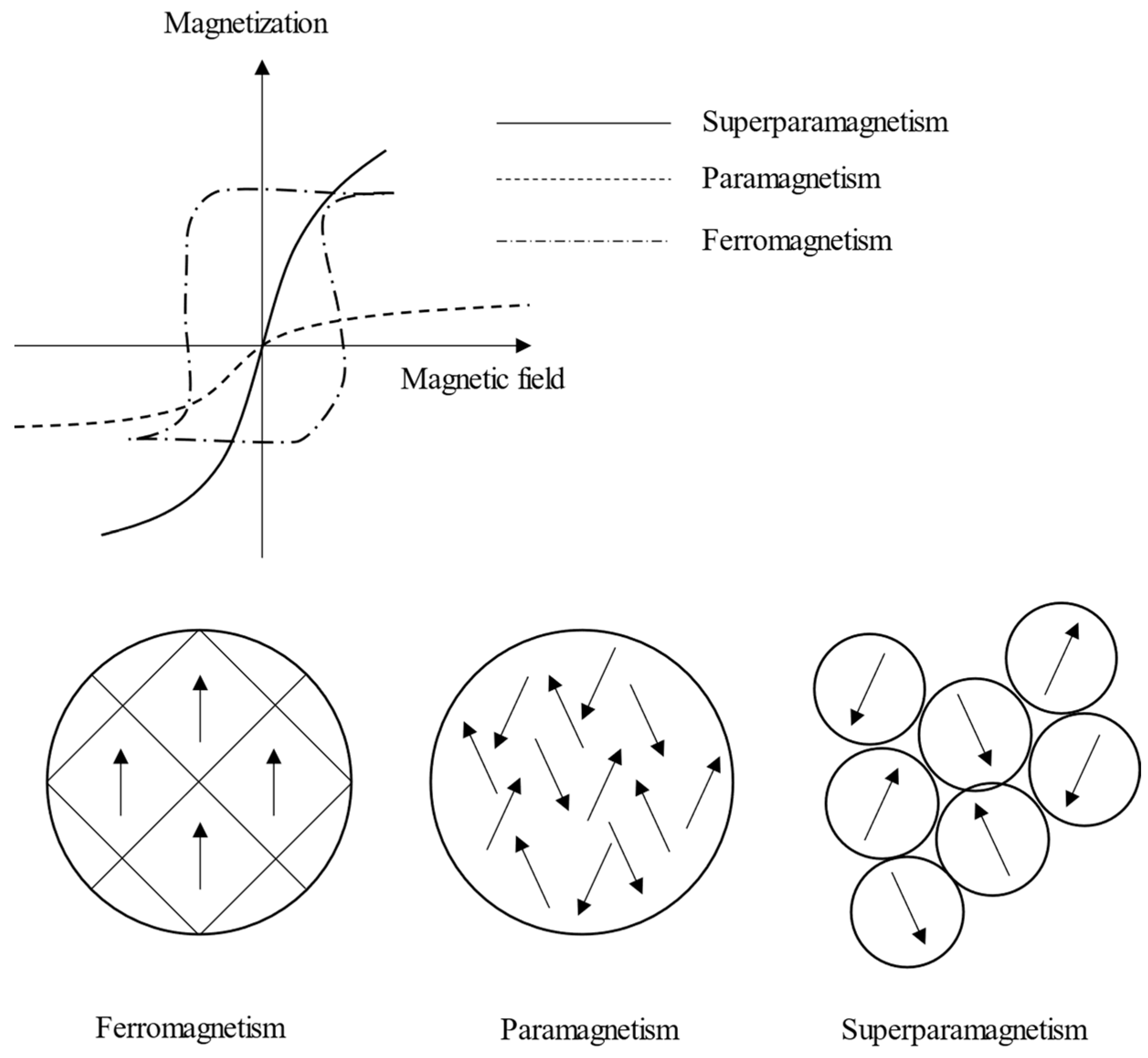
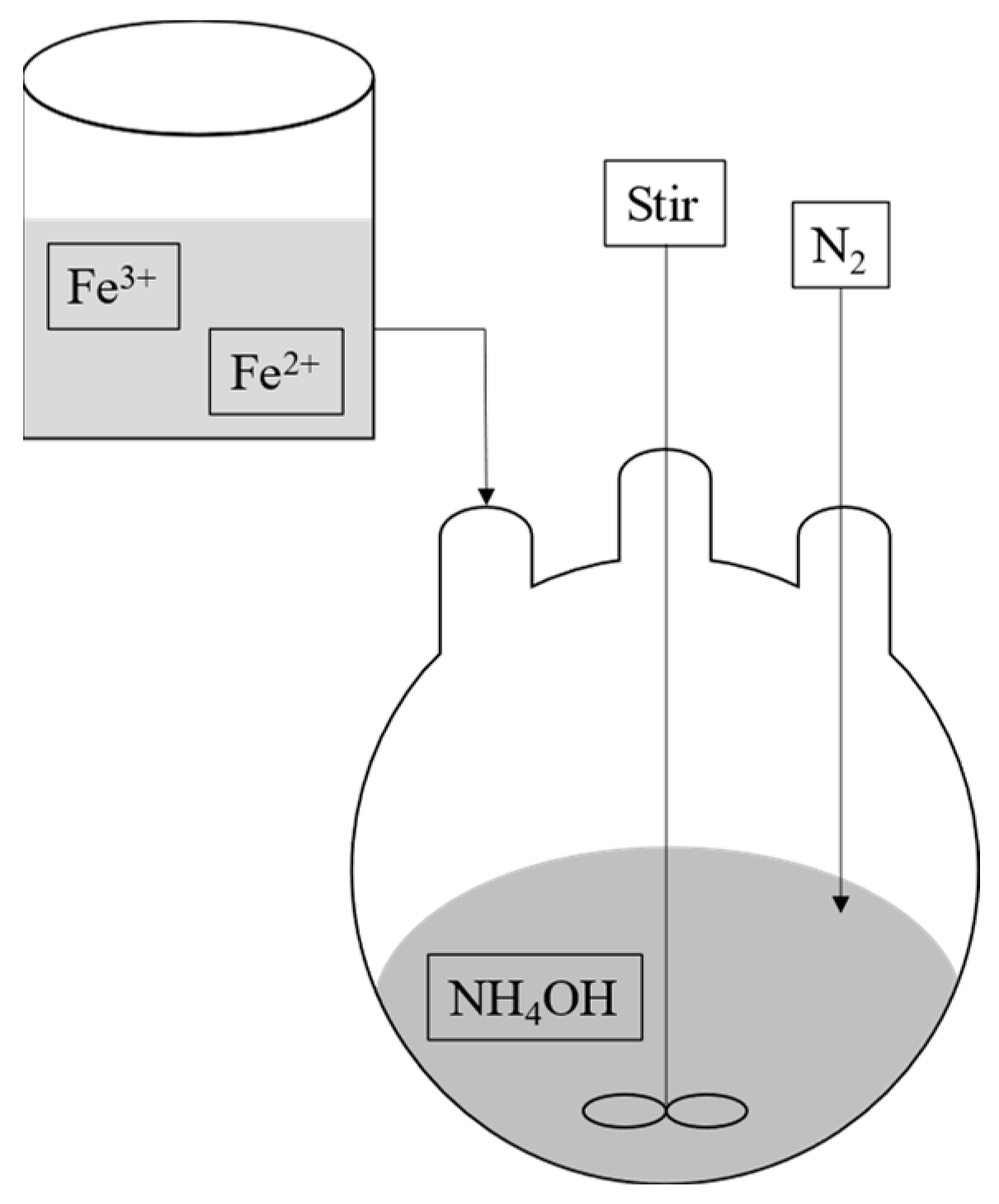
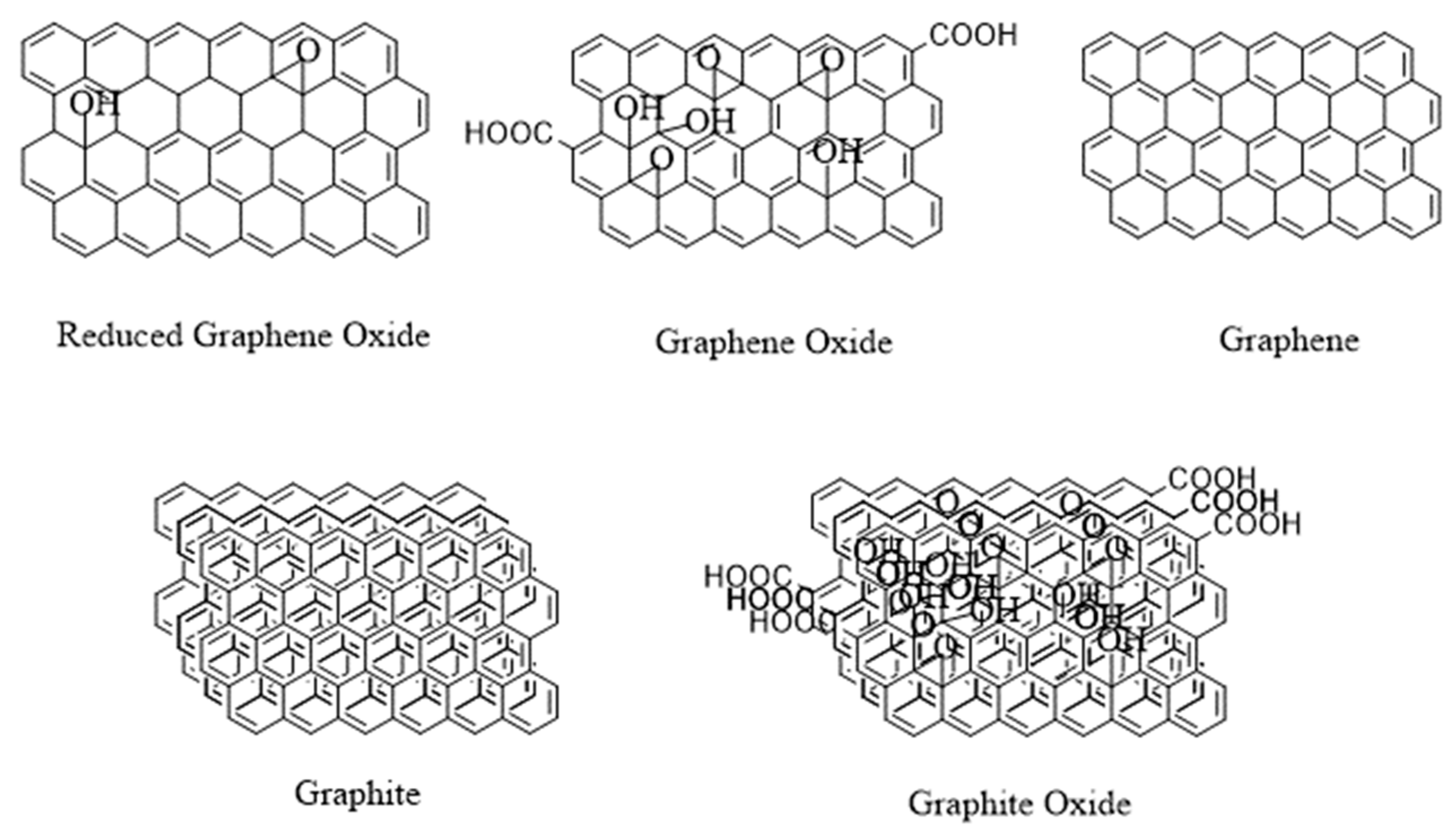
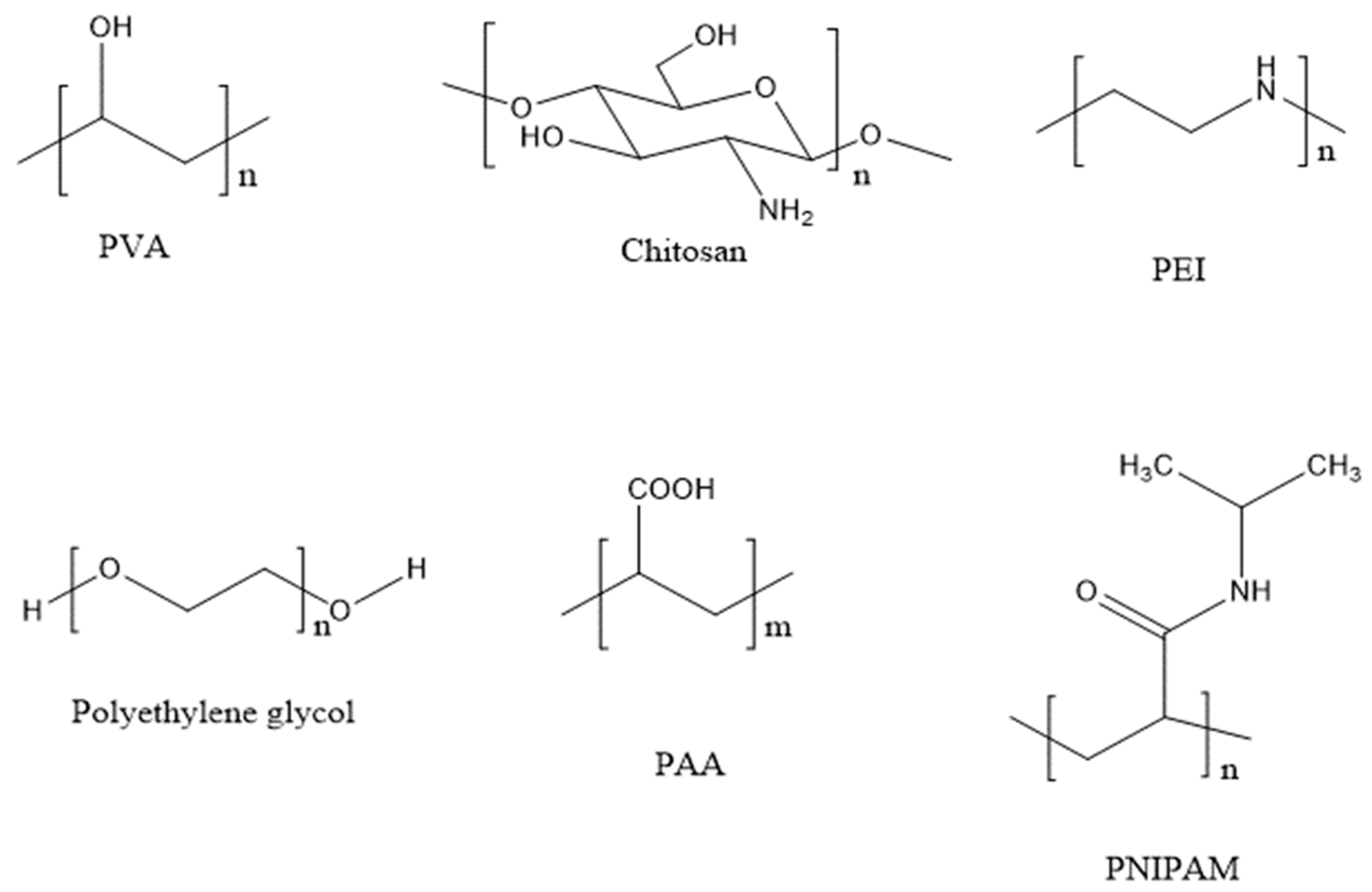



| Methods | Advantages | Disadvantages | Factors | Ref |
|---|---|---|---|---|
| Co-precipitation | Facile Rapid High yield Cheap | Weak size control Aggregation Oxidation | Iron salt precursors (Fe3+:Fe2+ = 2:1 mol/mol) Base (ammonia, CH3NH2, and NaOH) Optional additional cations (Na+, K+, Li+, NH4+, N(CH3)4+, CH3NH3+) pH = 9–14 | [33,34,40,42,43,44,45] |
| Hydrothermal and high-temperature decomposition | Small size distribution High yield Controllable size and shape | High temperature High pressure Long reaction time | Hydrolysis ferrous salts Oxidation of metal hydroxides Pressure > 2000 psi Temperature > 200 °C | [33,43,46,47,48,49,50] |
| Sol–gel | Controllable kinetics Controllable growth reactions | Expensive Long reaction time | Iron salt precursors Solvents Temperature pH Agitation | [38,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95] |
| Aerosol/vapor phase | High yield Non-aggregation | High temperature | Ferric salts Reducing agent | [33] |
| Electrochemical | Controllable size | Reproducibility | Iron salt precursors | [38,96,97,98] |
| Microemulsion | Controllable size Homogeneous | Low yield Long reaction time Substantial number of solvents | Iron salt precursors |
| Models | Equations | Plot | Equation |
|---|---|---|---|
| Kinetics | |||
| Pseudo-first order | |||
| Pseudo-first order [225,226] | Determining the initial steps of the adsorption process. Relationship between changes in concentration and time. The rate depends on the adsorbate concentration [227]. | ||
| Nonlinear | (7) | ||
| Linear | (7a) | ||
| Pseudo-second order | |||
| Pseudo-second order [225,226] | The adsorption rate depends on the adsorption capacity [227]. MB adsorbed on the adsorbents via the chemisorption process (electrons transferring) [170,228,229,230,231]. | ||
| Nonlinear | (8) | ||
| Linear Type I | = 1/slope = slope2/intercept h = 1/intercept | (8a) | |
| Linear Type II | = 1/slope = intercept2/slope h = 1/slope | (8b) | |
| Linear Type III | = intercept = −1/intercept × slope h = −intercept/slope | (8c) | |
| Linear Type IV | = −intercept/slope = slope2/intercept h = intercept | (8d) | |
| (9) | |||
| Isotherm | |||
| Langmuir [232,233] | Assumptions: the adsorption and desorption rates are equal at equilibrium when is in direct proportion to the rate of desorption from the surface [233] | (10) | |
| (11) | |||
| Nonlinear | Assumptions: when a single molecule occupies a single surface site, there is no lateral interaction between adjacent adsorbed molecules [233]. | (12) | |
| Linear | vs. = slope = intercept = slope/intercept | (13) | |
| If , then the adsorption was favorable [225,234] | (14) | ||
| Freundlich [233] | , favorable adsorption [233]. and > 1, favorable physical process [234,235] < 1: bond energies increase with surface density [236]. | ||
| Nonlinear | (15) | ||
| Linear | (16) | ||
| BET [233] | |||
| Nonlinear | (17) | ||
| Linear | vs. = slope = intercept | (18) | |
| Dubinin-Radushkevich (D-R) [233] | E < 0, the sorption process is exothermic [237]. 8 < E < 16 kJ/mol: ion exchange [238,239,240,241]. E < 8 kJ/mol: physisorption [238,239,240,241]. KDR < 1: surface heterogeneity increases due to the interaction between adsorbents and MB [242]. | ||
| vs. = −slope = intercept average free energy of adsorption, E (kJ mol−1) [233,243] | (19) | ||
| Temkin and Pyzhev [244,245] | decreases when increases in temperature: exothermic [244] | ||
| Nonlinear | (20) | ||
| Linear | vs. = the slope = intercept/slope | (21) | |
| (22) | |||
| Harkins-Jura [244,246] | vs. = −slope = intercept/slope | (23) | |
| Halsey and Henderson [246,247] | n decreases when increases in temperature: endothermic [246,247] | ||
| Halsey [246] | vs. n = −1/slope = intercept/slope | (24) | |
| Henderson [246] | vs. n = slope = intercept | (25) | |
| Redlich–Peterson [248] | For simplicity, | ||
| Nonlinear | (26) | ||
| Linear | (27) | ||
| Diffusion | |||
| Intraparticle diffusion [225,249] | vs. t1/2 ki = slope I = intercept If I = 0: the adsorption process is the intraparticle diffusion. If I > 0: the film diffusion and intraparticle diffusion occurred at the same time [250,251,252] If I < 0: combined impacts of surface response control and film diffusion processes [253,254,255] | (28) | |
| Simplified Elovich model [234,256] | Boundary conditions:
| vs. = slope | (29) |
| Boyd’s model [234,257] | Plot: Bt vs. t Linear: the controlling step is pore-diffusion [234,258] Nonlinear or linear, not passing through the origin: film diffusion or chemical reaction [234]. | ||
| (30) | |||
| Applied when f > 0.85 | (31) | ||
| Applied when f < 0.85 | (32) | ||
| Thermodynamics [233,234,244] | |||
| vs. (Extrapolating it to zero) K0 = when = 0 vs. 1/T = slope = intercept | (33) | ||
| : not spontaneous [234]. : spontaneous. : the randomness decreasing on the surface. : the randomness increasing on the surface [234]. : exothermic : endothermic [234], monolayer adsorption [259]. Small : weak forces of attraction, weak electrostatic interactions, and the existence of loose bonding between adsorbents and MB [260,261,262]. : dominated by physisorption [221,263] : dominated by van der Waals forces [264]. | (34) | ||
| Activation energy | |||
| Arrhenius [221,265,266] | ln Kads vs. 1/T (K−1) −EA/R = slope EA < 40 kJ/mol: chemisorption [267] 25 < EA < 30 kJ/mol: diffusion-controlled [268] EA > 40kJ/mol: physisorption [265,266]. | (35) | |
| Fittings parameters | |||
| Chi-square | Small : calculated values are similar to experimental data [244]. Large : calculated values are different from experimental data [244] | (36) | |
| Adsorbent | Adsorption Capacity (mg/g) | Isotherm, Kinetics, Thermodynamics | Ref. |
|---|---|---|---|
| SPION | 45.43 | Langmuir, PSO | [285] |
| SPION (Zanthoxylum armatum DC. via green route method) | 7.26 | Langmuir, PSO | [286] |
| SPION (P. factra extract via green route method) | 26.81 | Freundlich, PSO | [287] |
| SPION@C using FeSO4, FeS2, PVP K30 as raw materials | 17.26 | Redlich–Peterson, PSO | [288] |
| SPION@C using FeCl3·6H2O, citrus pectin as raw materials | 141.3 | Freundlich, PSO | [289] |
| SPION@C using citrus bergamia as raw materials | 31 | PSO, intraparticle diffusion, spontaneous, endothermic | [290] |
| SPION@Carbon sheets | 95 | Freundlich, PSO | [291] |
| SPION@Graphene | 45.27 | Langmuir, PSO | [292] |
| SPION@NH2-MWCTNs | 178.5 | Langmuir, PSO, spontaneous, exothermic | [293] |
| SPION/EG | 76.2 | Redlich–Peterson, PSO | [294] |
| SPION/GO | 280.26 | Langmuir, PFO, spontaneous, endothermic | [295] |
| SPION/MWCNT | 48.06 | Langmuir, PSO, film diffusion, intraparticle diffusion | [225] |
| SPION/moringa seed shell biochar | 219.60 | Freundlich, PSO, Elovich, spontaneous, endothermic, chemisorption | [296] |
| SPION/pyrolyzed sorghum straw | 136.53 | Langmuir, PSO, intraparticle diffusion | [297] |
| SPION/CS/p(Aam/NVIm) hydrogels | 860 | Langmuir, PSO | [298] |
| PVA/SA/SPION@KHA gel beads | 781.92 | Langmuir, PSO, spontaneous, endothermic | [299] |
| SPION-MWCNT-Bentonite | 48.2 | Redlich–Peterson, PFO, physisorption, non-spontaneous, endothermic | [300] |
| SPION/AMMT | 106.38 | Langmuir, PSO | [301] |
| SPION/Bentonite/Sawdust | 144.2 | Freundlich, PSO | [302] |
| SPION/TiO2-graphene sponge | 224 | Temkin, PSO, spontaneous, endothermic | [303] |
| Alg/Clin/SPION | 12.48 | Langmuir, PSO, spontaneous, exothermic | [280] |
| Clin/SPION | 45.66 | Langmuir, PSO, spontaneous, exothermic | [280] |
| Alg beads impregnated with SPION/CS@Zeolite | 6.14 | Freundlich, PSO, spontaneous, exothermic | [304] |
| H2SO4 crosslinked SPION/CS | 20.408 | Langmuir | [305] |
| SPION@SiO2@HKUST-1 | 434.78 | Langmuir, PSO | [306] |
| SPION@SiO2@Zn–TDPAT | 20.83 | Langmuir, PSO, spontaneous, endothermic | [307] |
| SPION@MIL-100(Fe) | 221 | Langmuir, PSO, spontaneous, exothermic | [308,309] |
| SPION-COOH/HKUST-1 | 118.6 | Langmuir, PSO, spontaneous, endothermic | [310] |
| SPION/PVP embedded HKUST-1 | 2.96 | Langmuir, PSO, spontaneous, endothermic | [311] |
| SPION/HKUST-1/GO | 150 | Langmuir, PFO | [312] |
| SPION@PAA/MIL-100(Fe) | 34.53 | Langmuir, PSO, spontaneous, endothermic | [313] |
| SPION/g-C3N4 | 20.5 | PSO | [314] |
| Co doped Fe-BDC MOF | 23.92 | Langmuir, PSO, spontaneous, endothermic | [315] |
| SPION/PPy/C | 90.9 | Langmuir, PSO | [316] |
| CA/CS/SWCNT/SPION/TiO2 | 14.3 | Redlich–Peterson, PSO | [317] |
| SPION@PDA/CMC | 217.43 | Langmuir, PSO, spontaneous, endothermic | [318] |
| SPION-GLP@CAB | 70.43 | Langmuir, PFO, spontaneous, exothermic | [319] |
| SDS@SPION | 62.43 | Langmuir, PSO, spontaneous, endothermic | [320] |
| SPION@PPy/RGO | 270.3 | Langmuir, PSO, spontaneous, endothermic | [321] |
| SPION/Ni/C | 175.2 | [322] | |
| SPION/GNS | 35.42 | Langmuir, PSO, spontaneous, endothermic | [323] |
| Ti3C2@SPION | 11.68 | Langmuir, non-spontaneous, exothermic | [324] |
| BC-GO@SPION | 9.87 | Freundlich, PSO, spontaneous, endothermic | [325] |
| Cellulose/SPION | 19.49 | Dubini-Radushkevich | [326] |
| GO/SPION/CS | 30.01 | Langmuir | [327] |
| Rectorite/SPION/ZnO | 35.1 | Langmuir | [328] |
| SPION@C/Ag | 40.16 | Langmuir, PSO, spontaneous, endothermic | [329] |
| Boehmite@SPION@PLA@SiO2 | 70.03 | Langmuir, PSO | [330] |
| RGO-Fe2O3-SPION | 72.8 | Langmuir, PSO | [331] |
| SPION@SiO2–VTEOS–DMDAAC | 109.89 | Freundlich, PSO | [332] |
| Lignin/SPION | 203.66 | Langmuir | [333] |
| Fe3C/SPION/C nanosheets | 918 | Langmuir, PSO, Elovich, spontaneous, endothermic | [334] |
| paAm/CS/SPION | 1603 | Langmuir, PFO | [335] |
| SPION@SiO2 | 123 | Freundlich, PFO | [336] |
| Multi-carboxyl functionalized SPION@SiO2 | 34.75 | Langmuir, PSO | [337] |
| SPION@SiO2-APTA | 46.24 | Freundlich, PSO | [338] |
| SPION@SiO2-EDA-COOH | 43.15 | Freundlich, PSO | [339] |
| Mesoporous SPION@SiO2 | 33.12 | [340] | |
| SPION@ZIF-8 | 20.2 | [341] | |
| HPPs-BiVO4/SPION | 33.6 | [342] | |
| P(MMA-AA-DVB)/BiVO4/ SPION microcapsules | 5 | [343] | |
| m-SPION0.3-C/D0.5 hydrogel | 529 | [344] |
| Model | Linear | Nonlinear |
|---|---|---|
| Zeroth order | ||
| Higuchi | ||
| Korsmeyer–Peppas |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doan, L. Modifying Superparamagnetic Iron Oxide Nanoparticles as Methylene Blue Adsorbents: A Review. ChemEngineering 2023, 7, 77. https://doi.org/10.3390/chemengineering7050077
Doan L. Modifying Superparamagnetic Iron Oxide Nanoparticles as Methylene Blue Adsorbents: A Review. ChemEngineering. 2023; 7(5):77. https://doi.org/10.3390/chemengineering7050077
Chicago/Turabian StyleDoan, Linh. 2023. "Modifying Superparamagnetic Iron Oxide Nanoparticles as Methylene Blue Adsorbents: A Review" ChemEngineering 7, no. 5: 77. https://doi.org/10.3390/chemengineering7050077
APA StyleDoan, L. (2023). Modifying Superparamagnetic Iron Oxide Nanoparticles as Methylene Blue Adsorbents: A Review. ChemEngineering, 7(5), 77. https://doi.org/10.3390/chemengineering7050077