Efficiency and Prospects of the Use of Mechanochemical Treatment to Obtain Innovative Composite Systems
Abstract
:1. Introduction
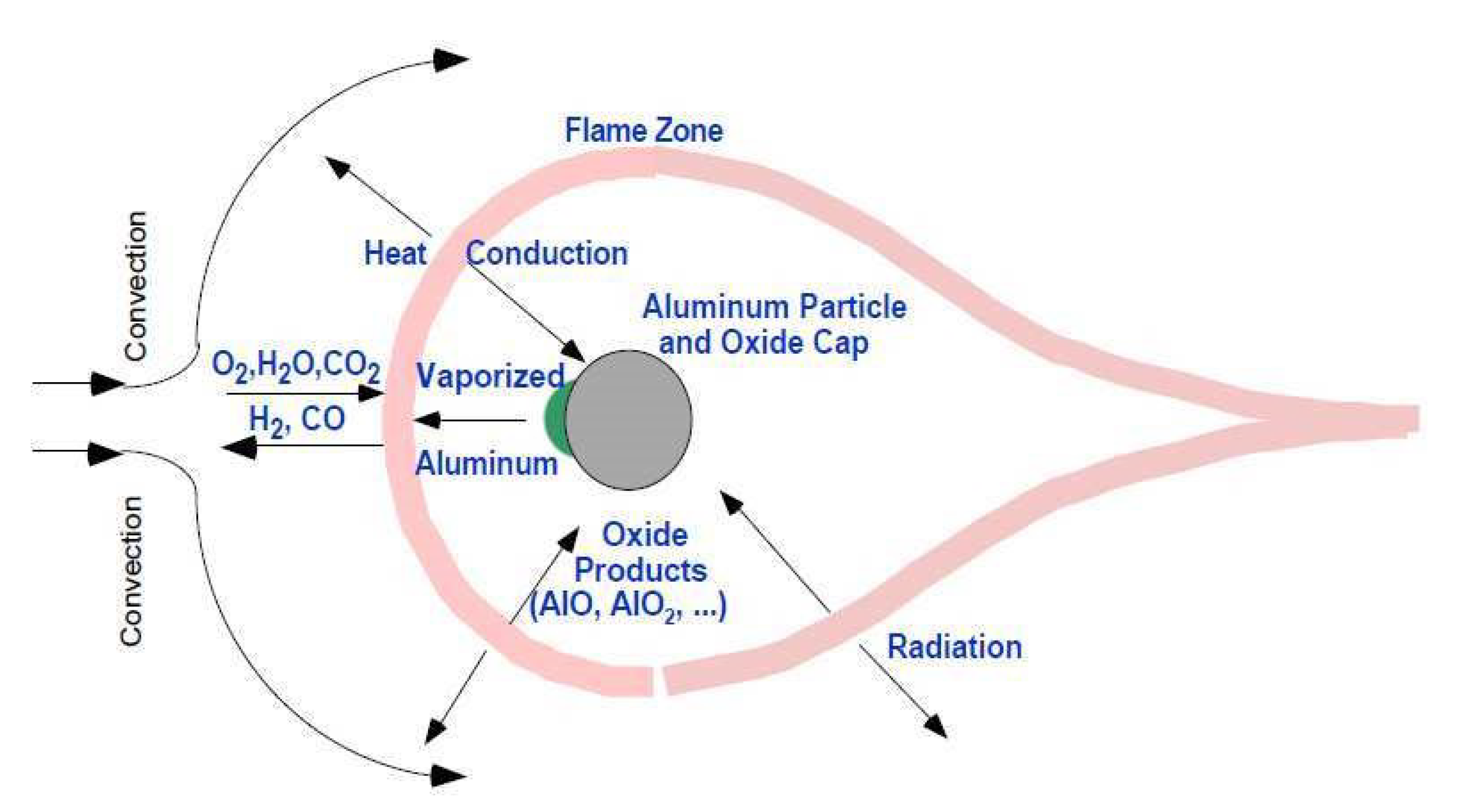
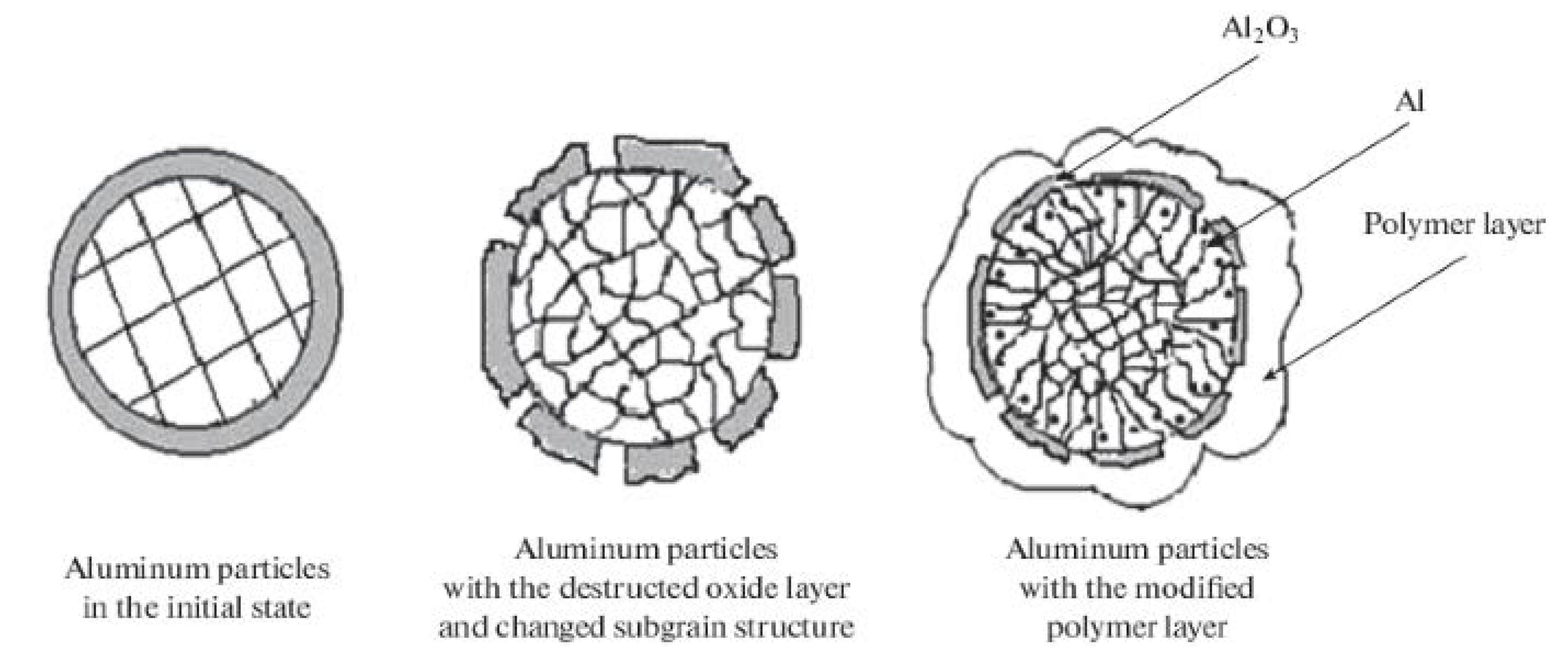
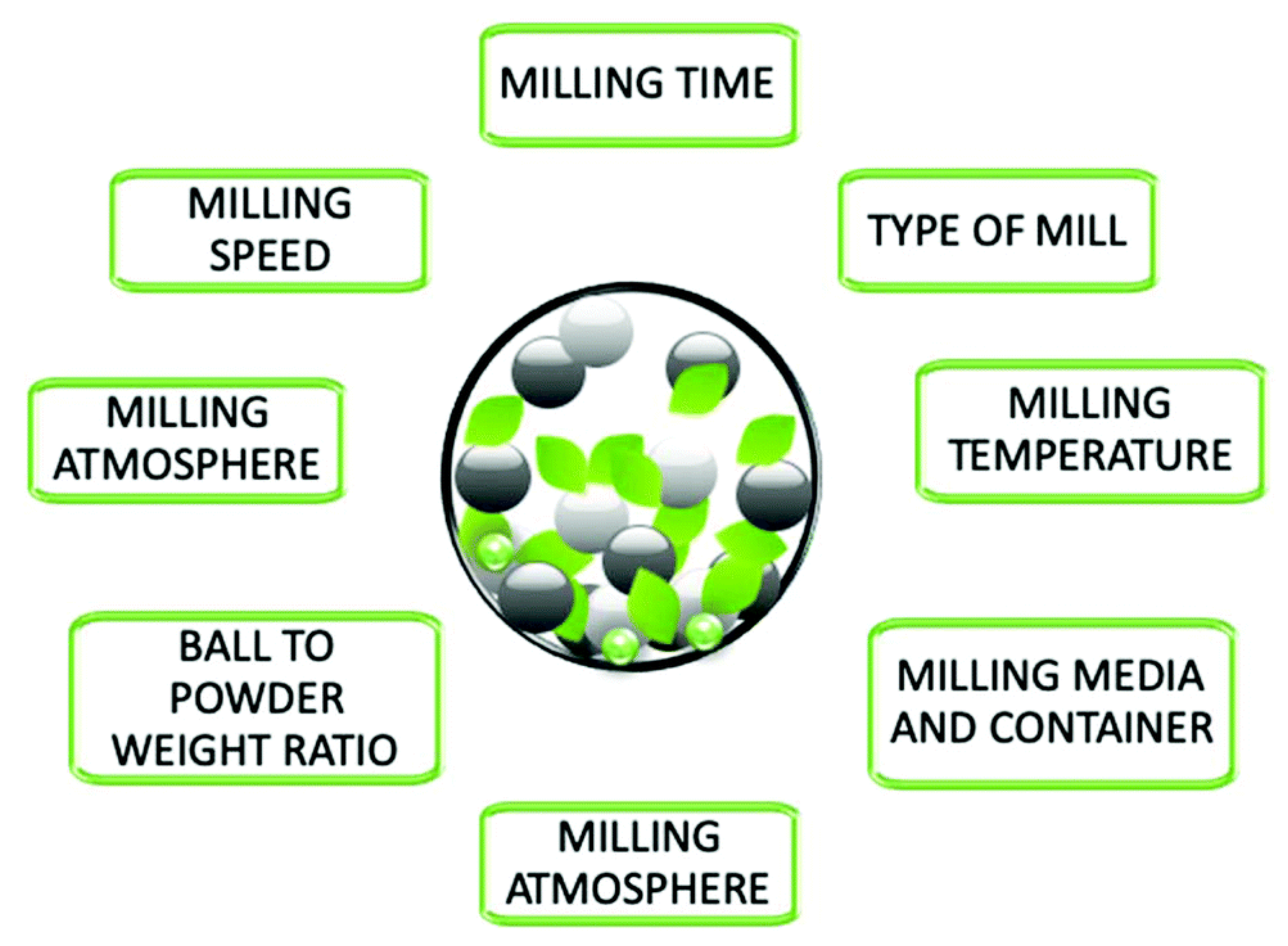
2. Features of the Structure and Properties of Energy-Intensive Metal Compositions Obtained by Mechanochemical Processing
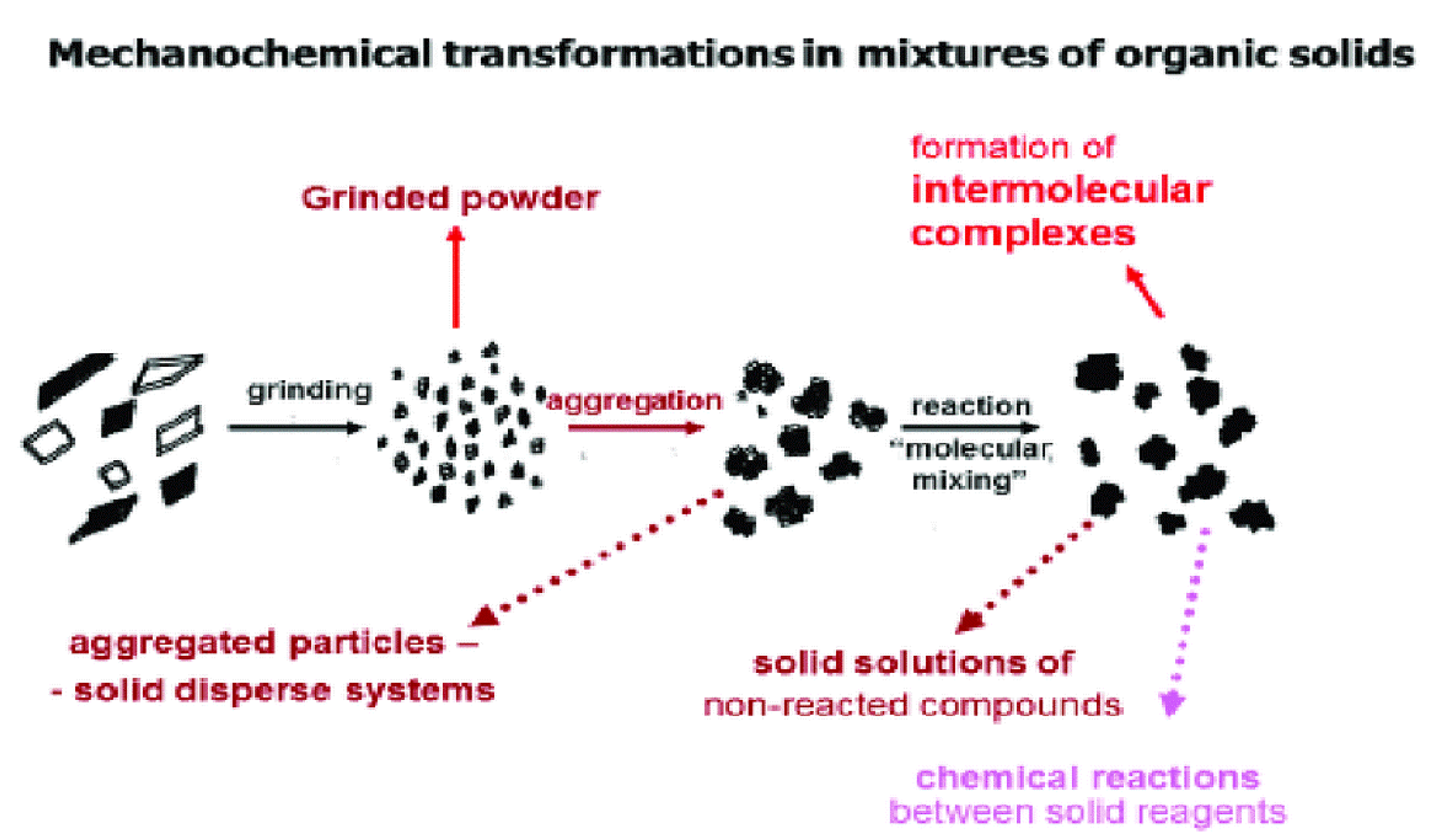
3. Prospects for the Use of Mechanochemical Processing for the Production of Physiologically Active Drugs from Plant Materials
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Avvakumo, E.G. (Ed.) Fundamental Foundations of Mechanical Activation, Mechanosynthesis and Mechanochemical Technologies. In Integration Projects of the Siberian Branch of the Russian Academy of Sciences Issue 19; Publishing House of the Siberian Branch of the Russian Academy of Sciences: Novosibirsk, Russia, 2009; 343p, Available online: http://www.prometeus.nsc.ru/contents/integrpr/019.ssi (accessed on 8 October 2021). (In Russian)
- Mansurov, Z.A.; Mofa, N.N. Mechanochemical synthesis of composite materials. In Mekhanohimicheskij Sintez Kompozicionnyh Materialov; Almaty Kazakh University: Almaty, Kazakhstan, 2016; 376p, ISBN 978-601-04-1688-8. [Google Scholar]
- Lapshin, O.V.; Boldyreva, E.V.; Boldyrev, V.V. The role of mixing and dispersion in mechanochemical synthesis (review). J. Inorg. Chem. 2021, 66, 402–424. [Google Scholar] [CrossRef]
- Margetic, D.; Štrukil, V. Mechanochemical Organic Synthesis; Elsevier: Amsterdam, The Netherlands, 2016; 386p. [Google Scholar] [CrossRef]
- Ranu, B.; Stolle, A.; Cravotto, G. Ball milling towards green synthesis: Applications, projects, challenges. In Royal Society of Chemistry; Johnson Matthey: London, UK, 2014; p. 303. [Google Scholar] [CrossRef]
- Chukanov, N.V. IR Spectra of Minerals and Reference Samples Data. In Infrared Spectra of Mineral Species. Springer Geochemistry/Mineralogy; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Nikonenko, N.A.; Tretinnikov, O.N. Measurement of the thickness of thin polymer layers by infrared frustrated total internal reflection spectroscopy. J. Appl. Spectrosc. 2008, 75, 878–882. [Google Scholar] [CrossRef]
- Cagnetta, G.; Huang, J.; Yu, G. A mini-review on mechanochemical treatment of contaminated soil: From laboratory to large-scale. Crit. Rev. Environm. Sci. Technol. 2018, 48, 723. [Google Scholar] [CrossRef]
- Bakkara, A.E.; Sadykov, B.S.; Sultanova, Z.L.; Khairullina, A.S.; Mofa, N.N.; Mansurov, Z.A. Energetic Compositions by Mechanochemical Treatment of Metal Powders: 3. Influence of Activated and Modified Aluminum Particles on Combustion of Thermite SiO2–Al Mixtures. Int. J. Self-Propagating High-Temp. Synth. 2021, 30, 165–169. [Google Scholar] [CrossRef]
- Popovich, A.A. Prospects for the Development of Mechanochemical Technologies in Solving Modern Problems of Materials Science Electronic Periodical Edition Bulletin of the Far Eastern State Technical University [Elektronnoe periodicheskoe izdanie «Vestnik Dal’nevostochnogo gosudarstvennogo tekhnicheskogo universiteta]. 2009, pp. 1–12. Available online: https://cyberleninka.ru/article/n/perspektivy-razvitiya-mehanohimicheskih-tehnologiy-pri-reshenii-sovremennyh-problem-materialovedeniya/viewer (accessed on 27 September 2009).
- Lyakhov, N.Z.; Talako, T.L.; Grigorieva, T.F. Influence of mechanical activation on the processes of phase and structure formation during self-propagating high-temperature synthesis [Vliyanie mekhanoaktivacii na processy fazo- i strukturoobrazovaniya pri samorasprostranyayushchemsya vysokotemperaturnom sinteze]. Novosib. Parallel 2008, 168. Available online: https://www.elibrary.ru/item.asp?id=19471273 (accessed on 27 November 2009). (In Russian).
- Chen, Z.; Lu, S.; Mao, Q.; Buekens, A.; Wang, Y.; Yan, J. Energy transfer and kinetics in mechanochemistry. Environ. Sci. Pollut. Res. 2017, 24, 24562–24571. [Google Scholar] [CrossRef]
- Mansurov, Z.A.; Mofa, N.N.; Shabanova, T.A.; Aknazarov, S.K.h. Synthesis and application of dispersed systems based on quartz with sacrificial nanostructural formations. Nanotechnics 2009, 17, 61–68. Available online: https://www.elibrary.ru/item.asp?id=11787653 (accessed on 2 March 2009). (In Russian).
- Bashkov, O.V.; Zaikov, V.I.; Khun, K.; Bashkov, I.O.; Romashko, R.V.; Bezruk, M.N.; Panin, S.V. Detecting acoustic-emission signals with fiber-optic interference transducers. Russ. J. Nondestruct. Test. 2017, 53, 415–421. [Google Scholar] [CrossRef]
- Miikkulainen, V.; Leskela, M.; Ritala, M.; Puurunen, R.L. Crystallinity of inorganic films grown by atomic layer deposition: Overview and general trends. J. Appl. Phys. 2013, 113, 021301–021401. [Google Scholar] [CrossRef]
- Reedy, B.S.B.; Rajasekhar, K.; Venu, M.; Dilip, J.J.S.; Das, S.; Das, K. Mechanical activation-assited solid-state combustion synthesis of in-situ aluminum matrix hybrid (al3Ni/al2o3) nanocomposites. J. Alloys Compd. 2008, 465, 97–105. [Google Scholar] [CrossRef]
- Reva, V.P.; Onishchenko, D.V. Mechanochemical grinding of metal using a destructible polymer. Probl. Mech. Eng. Reliab. Mach. 2013, 2, 77–83. Available online: https://www.elibrary.ru/item.asp?id=19548773 (accessed on 29 October 2012). (In Russian).
- Romanovsky, V.I.; Martsul, V.N. Influence of mechanochemical activation of waste ion exchangers on the dispersed composition and properties of the obtained products. Bull. Natl. Acad. Sci. Belarus. Ser. Chem. Sci. 2008, 2, 111–117. Available online: https://www.elibrary.ru/item.asp?id=32685974 (accessed on 5 June 2008). (In Russian).
- Kokhanovsky, V.A.; Petrov, Y.A. Friction and wear of fluoroplastic-containing composites (review). Bull. Don State Tech. Univ. 2009, 9, 30–36. Available online: https://cyberleninka.ru/article/n/trenie-i-iznashivanie-ftoroplastsoderzhaschih-kompozitov-obzor/viewer (accessed on 25 November 2009). (In Russian).
- Madyakin, F.P.; Tikhonova, N.A.; Madyakin, F.P. Components and combustion products of pyrotechnic compositions. In Polymers and Oligomers: Textbook. Allowance; Kazan Publishing House, State Technol Unta: Moscow, Russia, 2008; 492p, ISBN 978-5-7882-0540-3. (In Russian) [Google Scholar]
- Andreev, E.E.; Tikhonov, O.N. Crushing, Grinding and Preparation of Raw Materials for Enrichment; St. Petersburg Mining Institute: St. Petersburg, Russia, 2007; 439p, ISBN 978-5-94211-308-7. Available online: https://www.geokniga.org/bookfiles/geokniga-droblenie-izmelchenie-i-podgotovka-syrya-k-obogashcheniyu.pdf (accessed on 15 September 2022). (In Russian)
- Poluboyarov, V.A.; Andryushkova, O.V.; Pauli, I.A.; Korotaeva, Z.A. Influence of Mechanical Influences on Physical and Chemical Processes in Solids; Publishing House of NGU: Novosibirsk, Siberia, 2011; 604p, Available online: https://znanium.com/read?id=276232 (accessed on 12 November 2022). (In Russian)
- Alikin, V.N.; Vakhrushev, A.V.; Golubchikov, V.B.; Ermilov, A.S.; Lipanov, A.M.; Serebrennikov, S.Y. Solid Propellants for Jet Engines. Fuels, Charges, Engines; Mashinostroyeniye: Moscow, Russia, 2011; Volume IV, 380p, Available online: https://www.elibrary.ru/item.asp?id=43092389 (accessed on 29 June 2011). (In Russian)
- Ghule, V.D.; Sarangapani, R.; Jadhav, P.M.; Tewari, S.P. Quantum-chemical Investigation of Substituted s-Tetrazine Derivatives as Energetic Materials. Bull. Korean Chem. Soc. 2012, 33, 564–570. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Risha, G.A.; Yang, V.; Yetter, R.A. Combustion of bimodal nano/micron-sized aluminum particle dust in air. In Proceedings of the 31st International Symposium on Combustion, Heidelberg, Germany, 6–11 August 2006; The Combustion Institute, 2007. pp. 2001–2009. [Google Scholar] [CrossRef]
- Chintersingh, K.-L.; Schoenitz, M.; Dreizin, E.L. Oxidation kinetics and combustion of boron particles with modified surface. Combust. Flame 2016, 173, 288–295. [Google Scholar] [CrossRef]
- Young, G.; Sullivan, K.; Zachariah, M.R.; Yu, K. Combustion characteristics of boron nanoparticles. Combust. Flame 2009, 156, 322–333. [Google Scholar] [CrossRef]
- Fedorov, A.V.; Shulgin, A.V. Complex modeling of melting of aluminum nanoparticles. Phys. Combust. Explos. 2013, 49, 442–449. [Google Scholar] [CrossRef]
- Puri, P.; Yang, V. Effect of particle size on melting of aluminum at nano scales. J. Phys. Chem. C. 2007, 111, 11776–11783. [Google Scholar] [CrossRef]
- Beckstead, M.W. A Summary of Aluminum Combustion. Internal Aerodynamics in Solid Rocket Propulsion; Rhode-Saint-Genèse: Brabant, Belgium, 2002; pp. 1–46. Available online: https://apps.dtic.mil/sti/pdfs/ADA425147.pdf (accessed on 31 May 2002).
- Nizovskij, A.I.; Novikov, A.A.; Kirillov, V.A.; Bukhtijarov, V.I. Method of Activating Aluminium and Apparatus for Realising Said. Method. Patent No. RU 2 414 424, 20 March 2011. [Google Scholar]
- Maggi, F.; Bandera, A.; Galfetti, L.; De Luca, L.T.; Jackson, T.L. Efficient solid rocket propulsion for access to space. Acta Astronaut. 2010, 66, 1563–1573. [Google Scholar] [CrossRef]
- Lerner, M.I.; Glazkova, E.A.; Vorozhtsov, A.B.; Rodkevich, N.G.; Volkov, S.A.; Ivanov, A.N. Passivation of aluminum nanopowders for use in energetic materials. Russ. J. Phys. Chem. 2015, 9, 56–61. [Google Scholar] [CrossRef]
- Mofa, N.N.; Sadykov, B.S.; Bakkara, A.E.; Mansurov, Z.A. Obtaining by mechanochemical processing of metal powders for energy-intensive combustible compositions. 2. Structure and reactivity of mechanically activated mixtures of Al–modifier–SiO2. Izvestiya vuzov. Powder Metall. Funct. Coat. 2019, 3, 13–25. (In Russian) [Google Scholar] [CrossRef]
- Massalimov, I.A. Fluctuation mechanism of chemical bonds breaking in metals under intensive influences. Bash. Chem. J. 2007, 14, 127–131. Available online: https://cyberleninka.ru/article/n/fluktuatsionnyy-mehanizm-razryva-himicheskih-svyazey-metallov-pri-intensivnyh-vozdeystviyah/viewer (accessed on 19 June 2007). (In Russian).
- Streletskii, A.N.; Kolbanev, I.V.; Vorob’eva, G.A.; Leonov, A.V.; Borunova, A.B.; Dubinsky, A.A.; Appl, J. Mechanochemistry of BI2O3. 1. Defect structure and reactivity of mechanically activated BI2O3. Colloid J. 2019, 81, 617–624. (In Russian) [Google Scholar] [CrossRef]
- Grigoryeva, T.F.; Barinova, A.P.; Lyakhov, N.Z. Mechanochemical synthesis in metallic systems. Novosibirsk 2008, 309. Available online: https://books.google.kz/books/about/%D0%9C%D0%B5%D1%85%D0%B0%D0%BD%D0%BE%D1%85%D0%B8%D0%BC%D0%B8%D1%87%D0%B5%D1%81%D0%BA%D0%B8%D0%B9_%D1%81%D0%B8%D0%BD.html?id=kW8tcgAACAAJ&redir_esc=y. (accessed on 27 November 2008). (In Russian).
- Sabourin, J.L.; Yetter, R.; Asay, B.; Lloyd, J.M.; Sanders, V.; Risha, G.; Son, S.F. Effect of nano-aluminum and furmed silica particles on deflagration and detonation of nitromethane. Propell. Explos. Pyrotech. 2009, 34, 385–393. [Google Scholar] [CrossRef]
- Galfetti, L.; DeLuca, L.T.; Severini, F.; Colombo, G.; Meda, L.; Marra, G. Pre and post-burning analysis of nano-aluminized solid rocket propellants. Aerosp. Sci. Technol. 2007, 11, 26–32. [Google Scholar] [CrossRef]
- Starik, A.M.; Saveliev, A.M.; Titova, N.S. Features of ignition and combustion of composite fuels containing aluminum nanoparticles (review). Phys. Combust. Explos. 2015, 51, 64–91. Available online: https://www.sibran.ru/upload/iblock/418/4186dc047c035762cb63e0df74dbab69.pdf (accessed on 24 April 2015). (In Russian). [CrossRef]
- Streletskii, A.N.; Kolbanev, I.V.; Troshin, K.Y.; Borisov, A.A.; Leonov, A.V.; Mudretsova, S.N.; Artemov, V.V.; Yu, A. Structure and reactivity of mechanically activated Mg(Al)/MoO3 nanocomposites. Chem. Phys. 2016, 35, 79–91. (In Russian) [Google Scholar] [CrossRef]
- Mofa, N.N.; Sadykov, B.S.; Bakkara, A.E.; Mansurov, Z.A. Features of combustion of energy condensed systems with mechanoactivated metallized composites. In Proceedings of the 7th International Conference SPACE’ 2015 “SPACE CHALLENGE XXI CENTURY”, Sevastopol, Crimea. 2015, pp. 61–63. (In Russian). [CrossRef]
- Khrapovsky, V.E.; Khudaverdiev, V.G. Occurrence and development of convective combustion in highly porous charges of ammonium perchlorate and its mixture with aluminum. Chem. Phys. 2010, 29, 39–48. Available online: https://www.elibrary.ru/item.asp?id=13044805 (accessed on 30 January 2010). (In Russian).
- Khrapovsky, V.E.; Khudaverdiev, V.G.; Sulimov, A.A. On convective combustion of mixtures of ammonium perchlorate with aluminum. Phys. Combust. 2011, 4, 119–126. Available online: https://www.sibran.ru/upload/iblock/acd/acd55bf5b4ec3adbc7023754e49b06c5.pdf (accessed on 20 August 2011). (In Russian).
- Sadykov, B.S. Mechanochemical synthesis of energy-intensive powders based on aluminum: Thesis for the degree of Doctor of Philosophy. Ph.D. Thesis, 6D073400–Chemical Technology of Explosives and Pyrotechnics, Almaty, Kazakhstan, 2017; p. 120. Available online: https://www.kaznu.kz/content/files/pages/folder17928/%D0%94%D0%B8%D1%81%D1%81%D0%B5%D1%80%D1%82%D0%B0%D1%86%D0%B8%D1%8F%20%D0%A1%D0%B0%D0%B4%D1%8B%D0%BA%D0%BE%D0%B2%20%D0%91%D0%A1.pdf (accessed on 24 November 2017). (In Russian).
- Gogulya, M.F.; Brazhnikov, M.A.; Makhov, M.N.; Dolgoborodov AYu Lyubimov, A.V.; Sokolova, I.L. Influence of aluminum on the propelling ability of mixed compositions based on regular explosives. Chem. Phys. 2012, 31, 33–66. Available online: https://naukarus.com/vliyanie-alyuminiya-na-metatelnuyu-sposobnost-smesevyh-sostavov-na-osnove-shtatnyh-vzryvchatyh-veschestv (accessed on 29 November 2012). (In Russian).
- Meda, L.; Marra GGalfetti, L.; Severini, F.; DeLuca, L.T. Nano-aluminum as energetic material for rocket propellants. Mater. Sci. Eng. C 2007, 27, 1393–1396. [Google Scholar] [CrossRef]
- Mironov, V.V.; Volkov, N.N.; Volkova, L.I.; Tsatsuev, S.M.; Berdov, R.D. Investigation of non-uniform mixture formation in liquid-propellant rocket engines based on the results of firing tests. Izv. Ross. Akad. Nauk. Energy 2015, 4, 143–151. Available online: https://elibrary.ru/item.asp?id=23944701 (accessed on 25 December 2015). (In Russian).
- Kubota, N. Propellants and explosives; Wiley: Hoboken, NJ, USA, 2007; 509p, Available online: https://www.studmed.ru/kubota-n-tverdye-raketnye-topliva-i-vzryvchatye-veschestva_2452b3bbb2f.html (accessed on 15 July 2007). (In Russian)
- Baláž, P. Mechanochemistry in Minerals Engineering. In Mechanochemistry in Nanoscience and Minerals Engineering; Springer: Berlin/Heidelberg, Germany, 2008; p. 257. [Google Scholar] [CrossRef]
- Mikhailenko, M.A.; Shakhtshneider, T.P.; Drebushchak, V.A.; Skvortsova, G.P.; Boldyrev, V.V. Influence of mechanical treatment on the properties of betulin, betulin diacetate, and their mixture with water-soluble polymers. Chem. Nat. Compd. 2011, 47, 229–233. [Google Scholar] [CrossRef]
- Bychkov, A.L.; Lomovskii, O.I. Mechanical treatment effect on the cellulolytic specimen activity. Russ. J. Appl. Chem. 2010, 83, 1106. [Google Scholar] [CrossRef]
- Shapolova, E.G.; Lomovsky, O.I. Mechanochemical solubilization of silicon dioxide with polyphenol compounds of plant origin. Russ. J. Bioorganic Chem. 2013, 39, 765–770. [Google Scholar] [CrossRef]
- Bazarnova, N.G.; Markin, V.I.; Katrakov, I.B.; Kolosov, P.V.; Cheprasova, M.Y.; Kalyuta, E.V. Methods of obtaining lignin-carbohydrate compounds from chemically modified plant raw materials. Russ. J. Gen. Chem. 2015, 82, 947–954. [Google Scholar] [CrossRef]
- Zhilyakova, E.T.; Novikova MYu Fadeeva, D.A.; Novikov, O.O.; Popov, N.N. Preparation of Submicro- and/or Nanostructured Taurine Substance and Experimental Confirmation of Changes in its Structure. Belgorod State University. Series Medicine. Pharmacy 2011, 22, 104–113. Available online: http://dspace.bsu.edu.ru/handle/123456789/14963 (accessed on 23 December 2011). (In Russian).
- Zhilyakova, E.T.; Novikova MYu Popov, N.N.; Pridachina, D.V.; Bondarev, A.V. Solid-phase mechanochemical processing—a promising method for modifying starches for the pharmaceutical industry. Mod. Probl. Sci. Educ. 2012, 6, 745–753. Available online: http://science-education.ru/ru/article/view?id=7805 (accessed on 19 December 2012). (In Russian).
- Dushkin, A.V.; Suntsova, L.P.; Khalikov, S.S. Mechanochemical technology for increasing the solubility of medicinal substances. Basic research. Pharm. Sci. 2013, 1, 448–457. Available online: https://fundamental-research.ru/ru/article/view?id=30969 (accessed on 29 December 2012). (In Russian).
- Politov, A.; Golyazimova, O. Increasing the energy yield of mechanochemical transformations: Selected case studies. Faraday Discuss. 2014, 170, 345–356. [Google Scholar] [CrossRef]
- Zhilyakova, E.T.; Popov, N.N.; Tsvetkova, Z.E.; Agarina, A.V. Study of the effect of mechanochemical treatment on the physicochemical parameters of high-molecular compounds used in the technology of drugs to prolong the therapeutic effect. Young Sci. 2015, 24, 263–266. Available online: https://moluch.ru/archive/104/24658/ (accessed on 18 December 2015). (In Russian).
- Bychkov, A.L.; Ryabchikova, E.I.; Korolev, K.G.; Lomovsky, O.I. Ultrastructural changes of cell walls under intense mechanical treatment of selective plant raw material. Biomass Bioenergy 2012, 47, 260–267. [Google Scholar] [CrossRef]
- Lomovsky, O.I.; Lomovskiy, I.O.; Orlov, D.V. Mechanochemical solid acid/base reactions for obtaining biologically active preparations and extracting plant materials. Green Chem. Lett. Rev. 2017, 10, 171–185. [Google Scholar] [CrossRef] [Green Version]
- Lomovsky, I.O. Mechanochemical Reactions of Phenolic Compounds of Plant Origin and Their Technological Application. Candidate’s Dissertations Chem. Sciences, 02.00.21. Novosibirsk. 2012. Available online: https://www.dissercat.com/content/mekhanokhimicheskie-reaktsii-fenolnykh-soedinenii-rastitelnogo-proiskhozhdeniya-i-ikh-tekhno (accessed on 11 April 2013). (In Russian).
- Varlamova, A.I.; Arkhipov, I.A.; Abramov, V.E.; Arisov, M.V.; Khalikov, S.S.; Dushkin, A.V. Pharmacological activity and targeted delivery of supramolecular fenbendazole obtained by mechanochemical technology with various components. Russ. Parasitol. J. 2021, 15, 64–71. (In Russian) [Google Scholar] [CrossRef]
- Solares-Briones, M.; Coyote-Dotor, G.; Páez-Franco, J.C.; Zermeño-Ortega, M.R.; de la OContreras, C.M.; Canseco-González, D.; Avila-Sorrosa, A.; Morales-Morales, D.; Germán-Acacio, J.M.; Mechanochemistry, A. Green Approach in the Preparation of Pharmaceutical Cocrystals. Pharmaceutics 2021, 13, 790. [Google Scholar] [CrossRef]
- Bychkov, A.L.; Lomovsky, O.I. Modern achievements in mechano-fermentative processing of vegetable raw materials. Chem. Veg. Raw Mater. 2017, 2, 35–47. (In Russian) [Google Scholar] [CrossRef] [Green Version]
- Chemat, F.; Vian, M.A.; Fabiano-Tixier, A.-S.; Nutrizio, M.; Jambrak, A.R.; Munekata, P.E.S.; Lorenzo, J.M.; Barbara, F.J.; Binello, A.; Cravotto, G. A review of sustainable and intensified techniques for extraction of food and natural products. Green Chem. 2020, 22, 2325–2353. [Google Scholar] [CrossRef]
- Golyazimova, O.V.; Politov, A.A.; Lomovsky, O.I. Mechanical activation of enzymatic hydrolysis of lignocellulose. Chem. Veg. Raw Mater. 2009, 2, 59–63. Available online: https://cyberleninka.ru/article/n/mehanicheskaya-aktivatsiya-fermentativnogo-gidroliza-lignotsellyulozy/viewer (accessed on 12 February 2009). (In Russian).
- Golyazimova, O.V.; Politov, A.A.; Lomovsky, O.I. Increasing the efficiency of grinding lignocellulosic plant raw materials using chemical processing. Chem. Veg. Raw Mater. 2009, 2, 53–57. Available online: https://static.freereferats.ru/_avtoreferats/01004636800.pdf (accessed on 20 January 2010). (In Russian).




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakkara, A.; Sadykov, B.; Zhapekova, A.; Oserov, T.; Batkal, A.; Khairullina, A.; Mofa, N. Efficiency and Prospects of the Use of Mechanochemical Treatment to Obtain Innovative Composite Systems. ChemEngineering 2022, 6, 90. https://doi.org/10.3390/chemengineering6060090
Bakkara A, Sadykov B, Zhapekova A, Oserov T, Batkal A, Khairullina A, Mofa N. Efficiency and Prospects of the Use of Mechanochemical Treatment to Obtain Innovative Composite Systems. ChemEngineering. 2022; 6(6):90. https://doi.org/10.3390/chemengineering6060090
Chicago/Turabian StyleBakkara, Ayagoz, Bakhtiyar Sadykov, Anar Zhapekova, Timur Oserov, Aisulu Batkal, Ainur Khairullina, and Nina Mofa. 2022. "Efficiency and Prospects of the Use of Mechanochemical Treatment to Obtain Innovative Composite Systems" ChemEngineering 6, no. 6: 90. https://doi.org/10.3390/chemengineering6060090
APA StyleBakkara, A., Sadykov, B., Zhapekova, A., Oserov, T., Batkal, A., Khairullina, A., & Mofa, N. (2022). Efficiency and Prospects of the Use of Mechanochemical Treatment to Obtain Innovative Composite Systems. ChemEngineering, 6(6), 90. https://doi.org/10.3390/chemengineering6060090







