Photocatalytic Hydrogen Production from Formic Acid Solution with Titanium Dioxide with the Aid of Simultaneous Rh Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Photocalytic Hydrogen Production
2.3. Characterization of Photocatalysts
3. Results and Discussion
3.1. Photocatalytic Hydrogen Production
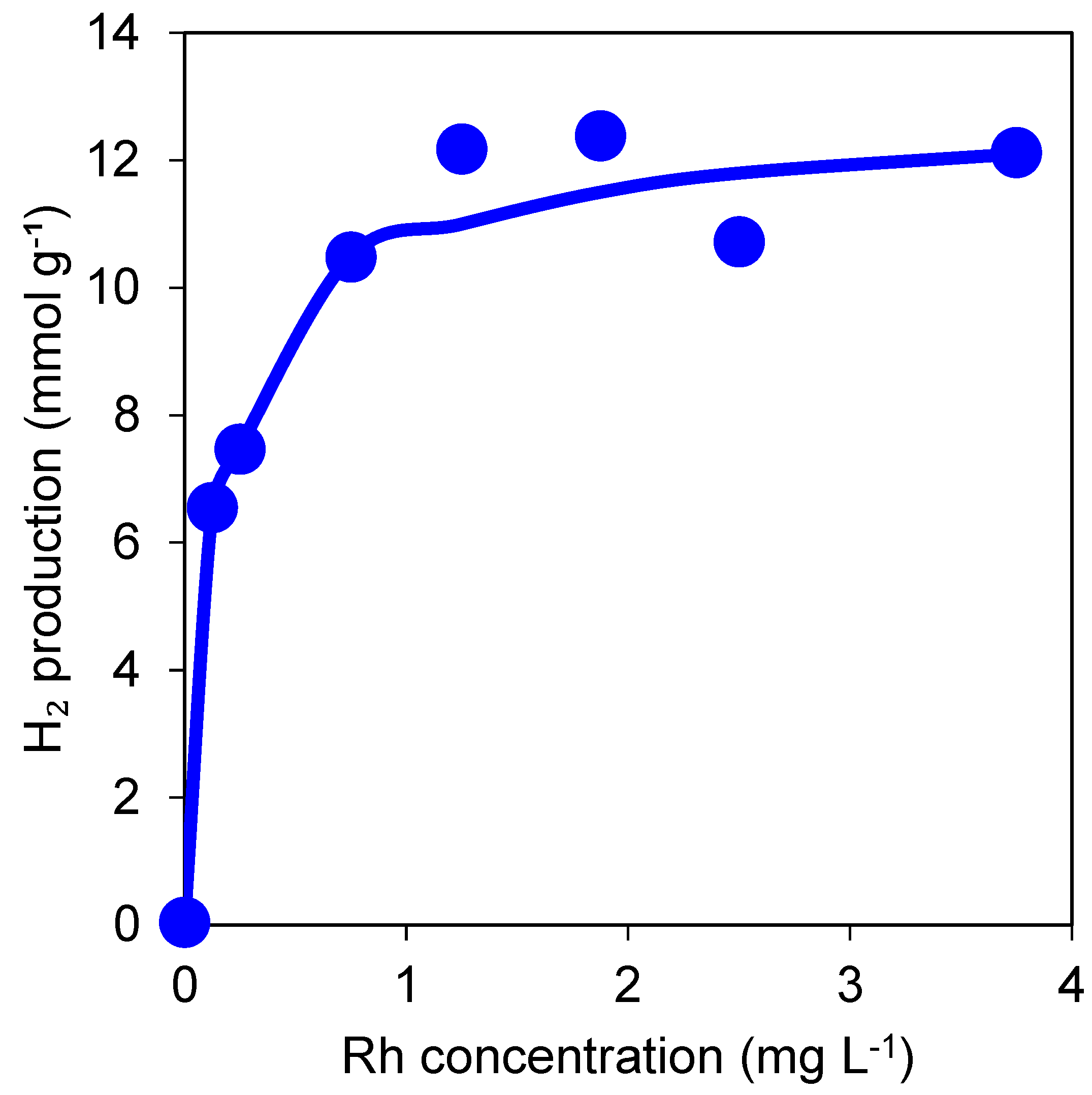
3.1.1. Effect of Rh Ion Concentration
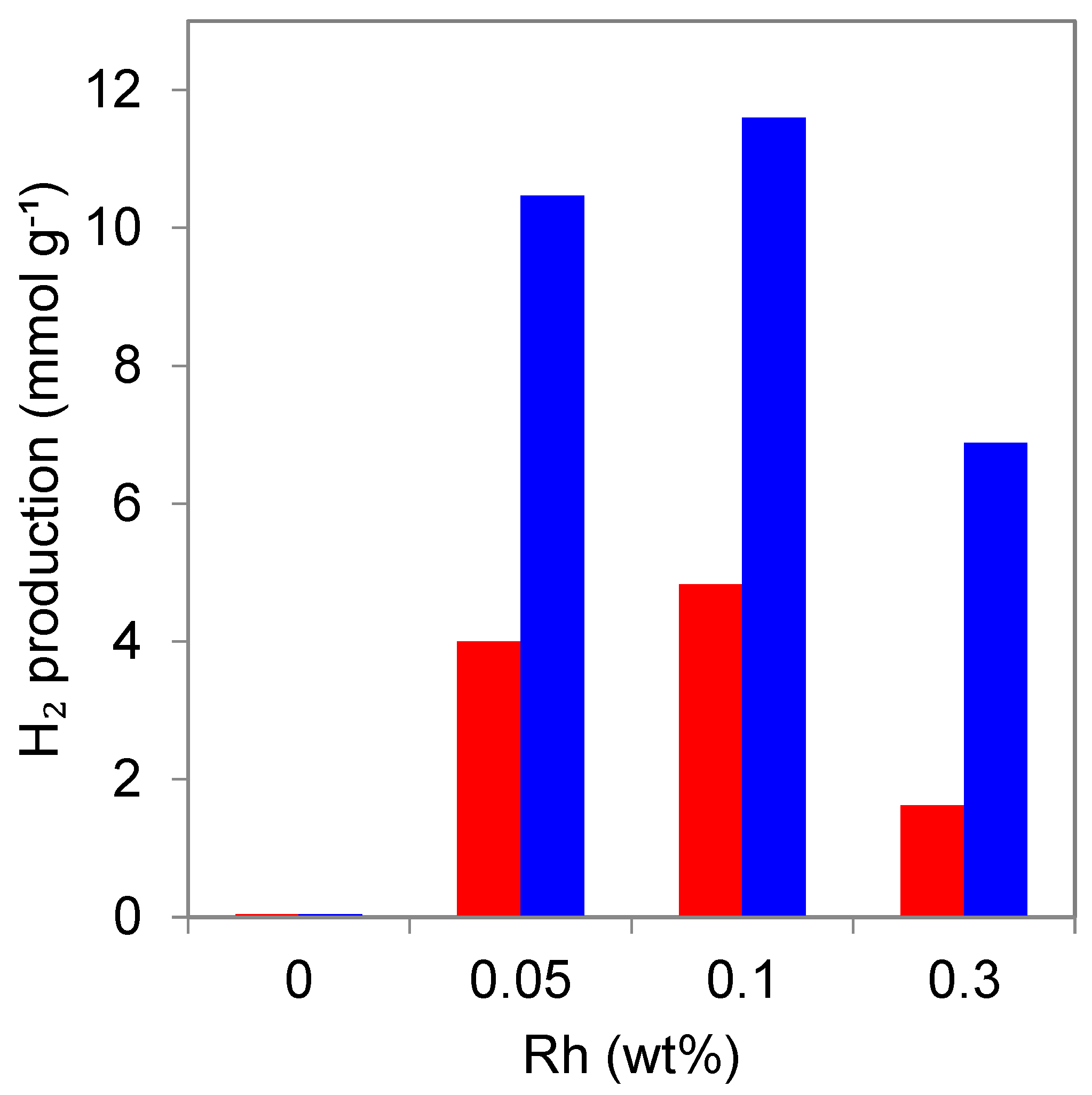
3.1.2. Effect of the Simultaneous Deposition of Rh in TiO2
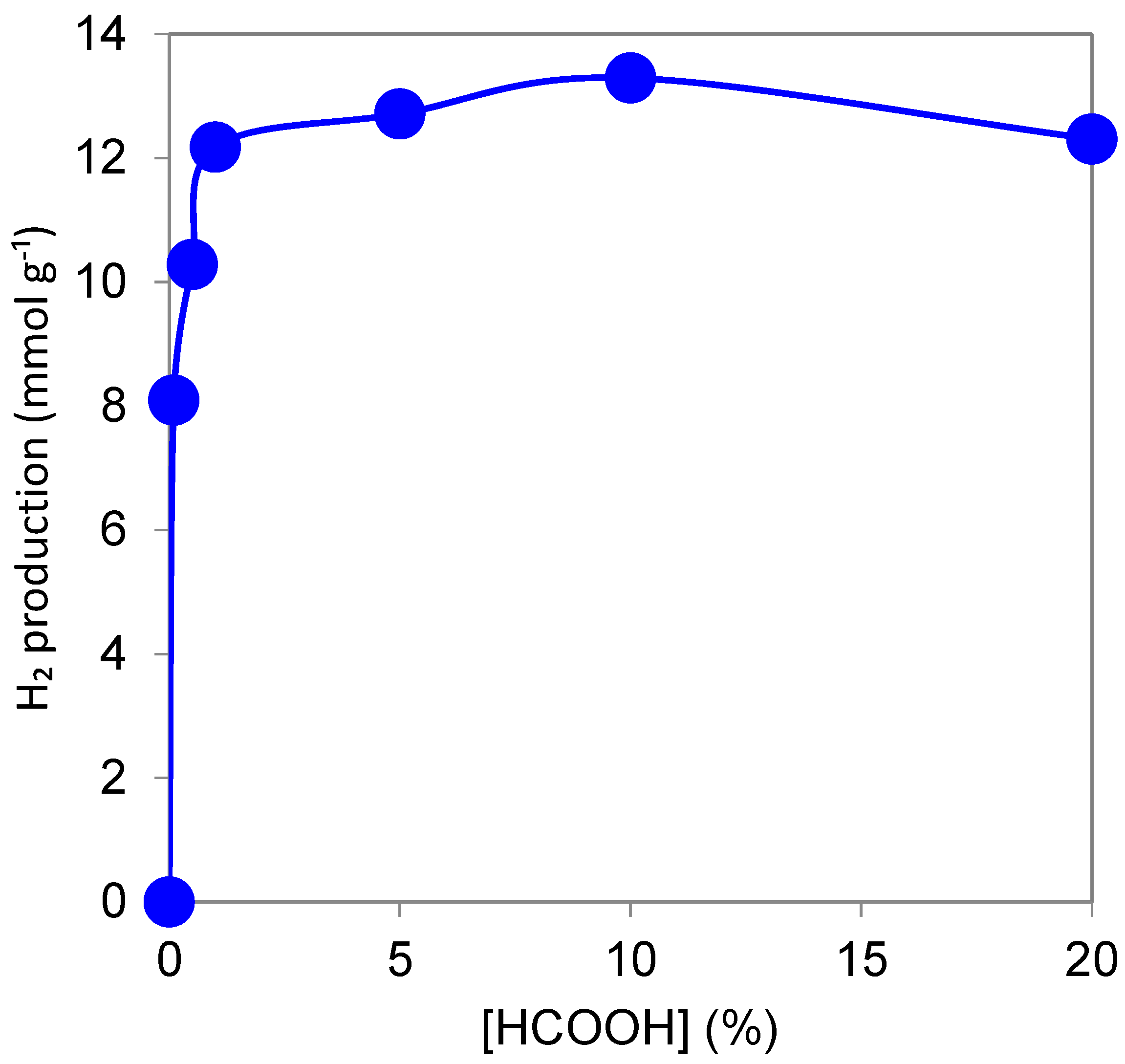
3.1.3. Effect of Formic Acid Concentration
3.1.4. Effect of the pH of the Reaction Solution
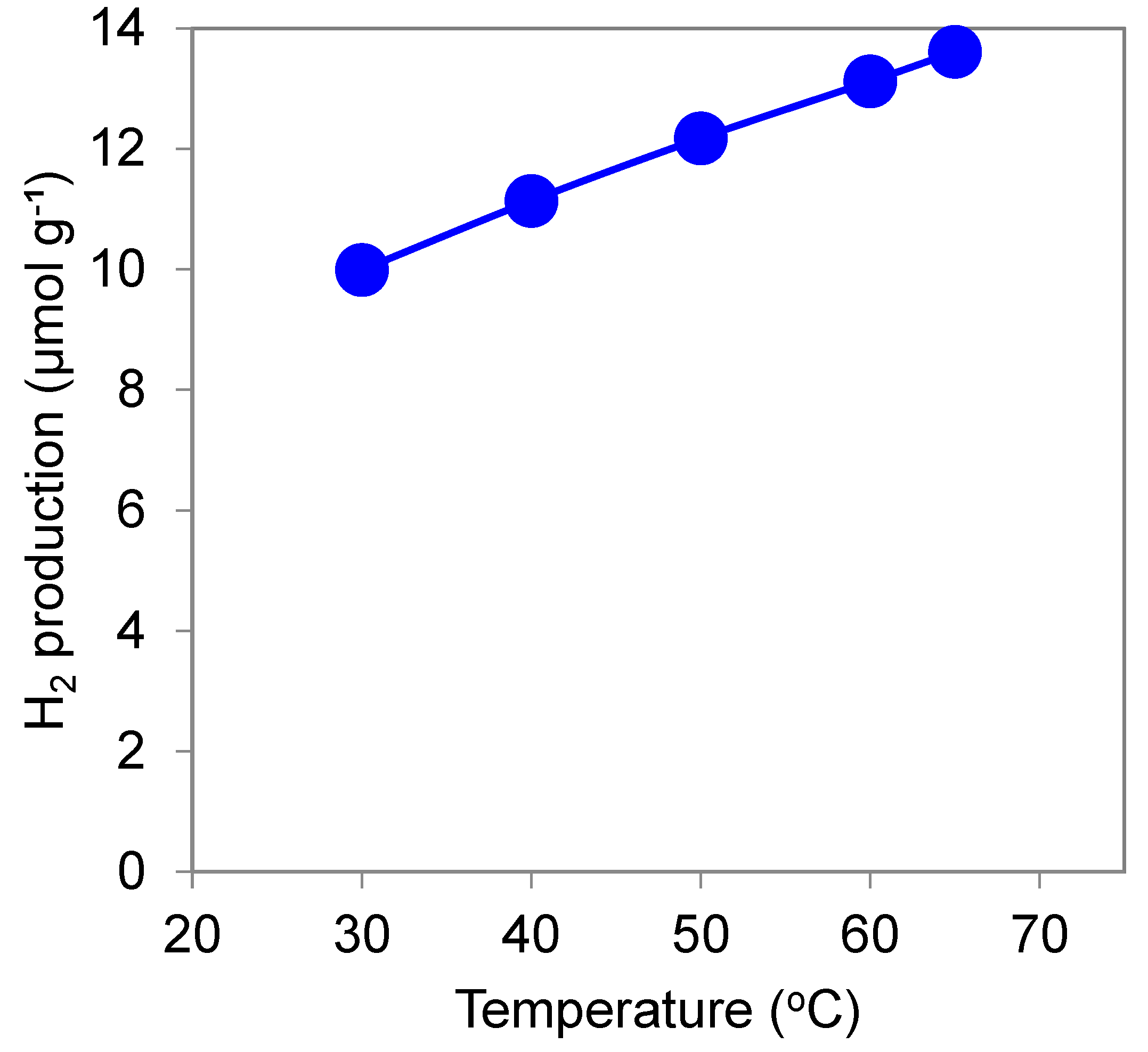
3.1.5. Effect of Temperature
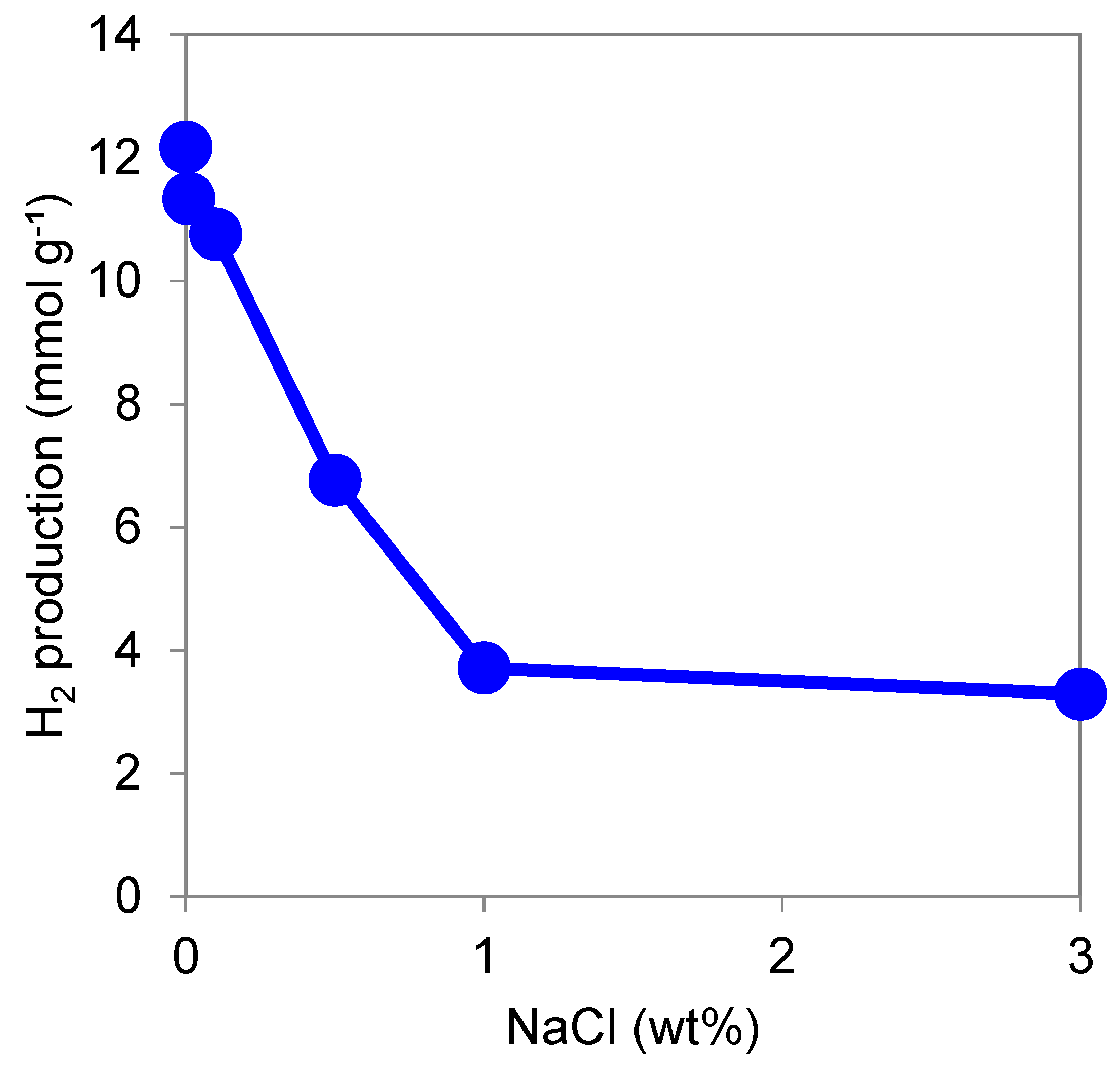
3.1.6. Effect of NaCl Concentration
3.1.7. Effect of Formate Type
3.2. Characterization of Photocatalysts
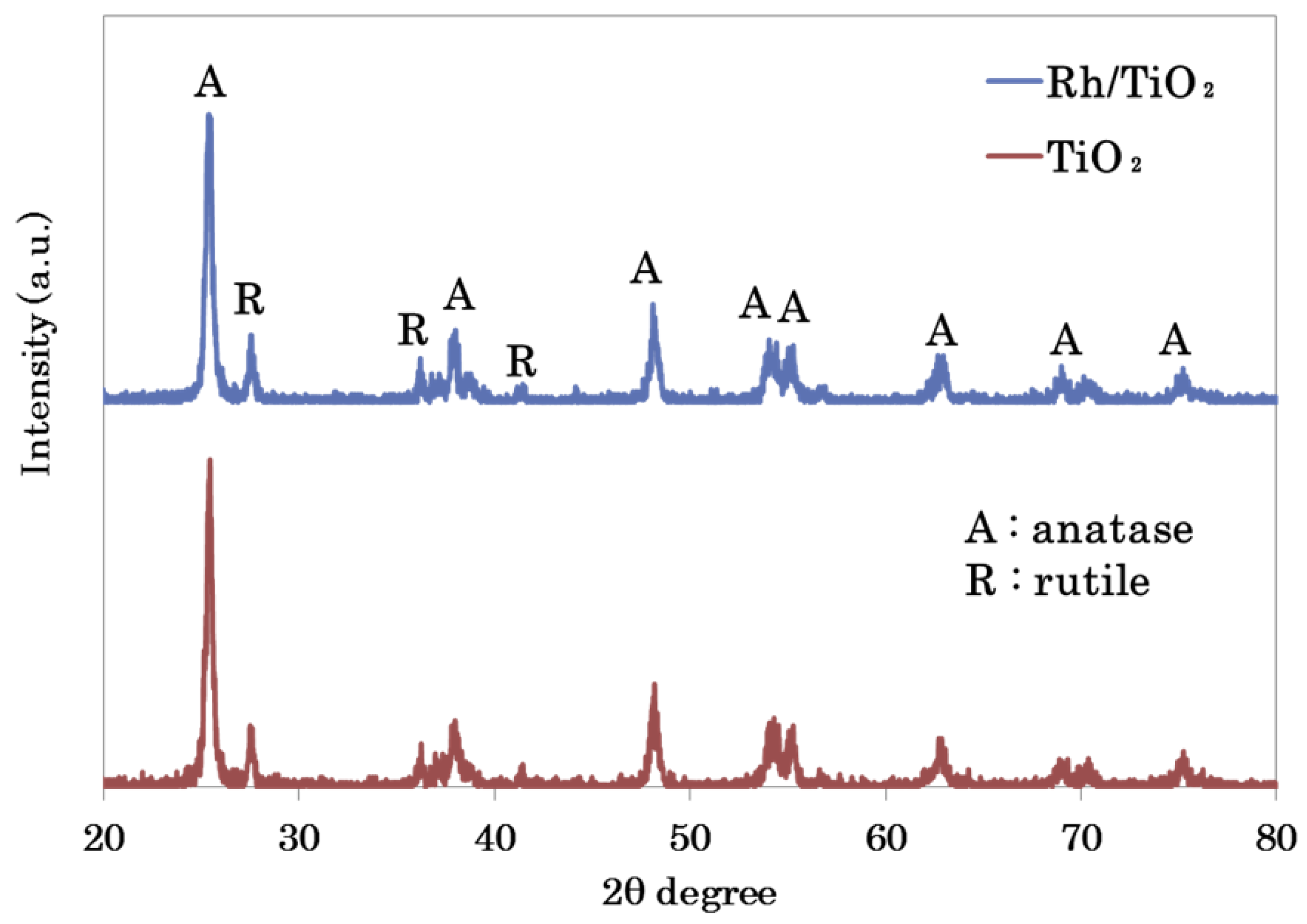
3.2.1. XRD Analysis
3.2.2. SEM Analysis
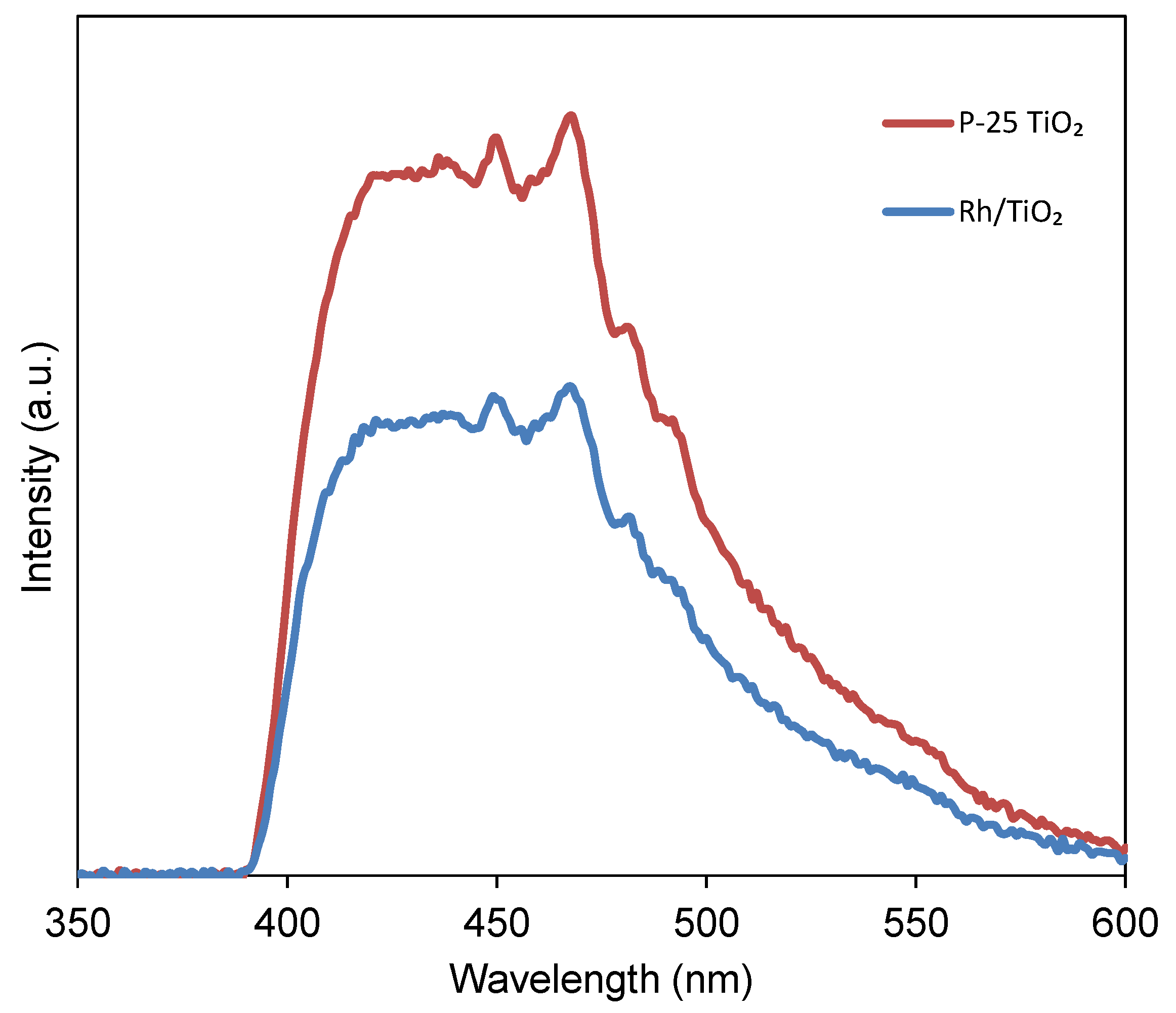
3.2.3. PL Analysis
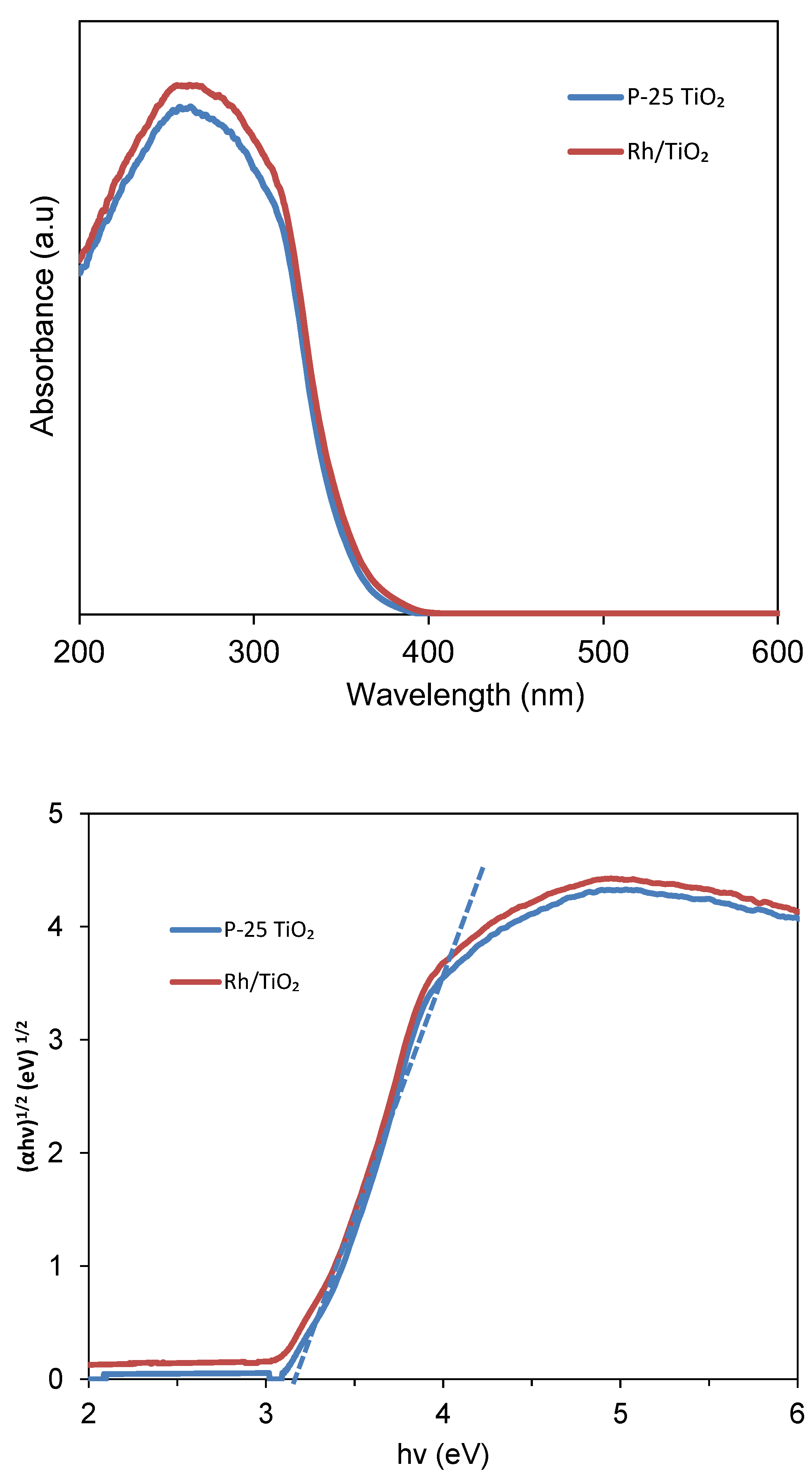
3.2.4. UV–Vis Diffuse Reflection Spectrum Analysis
3.2.5. BET Surface Area
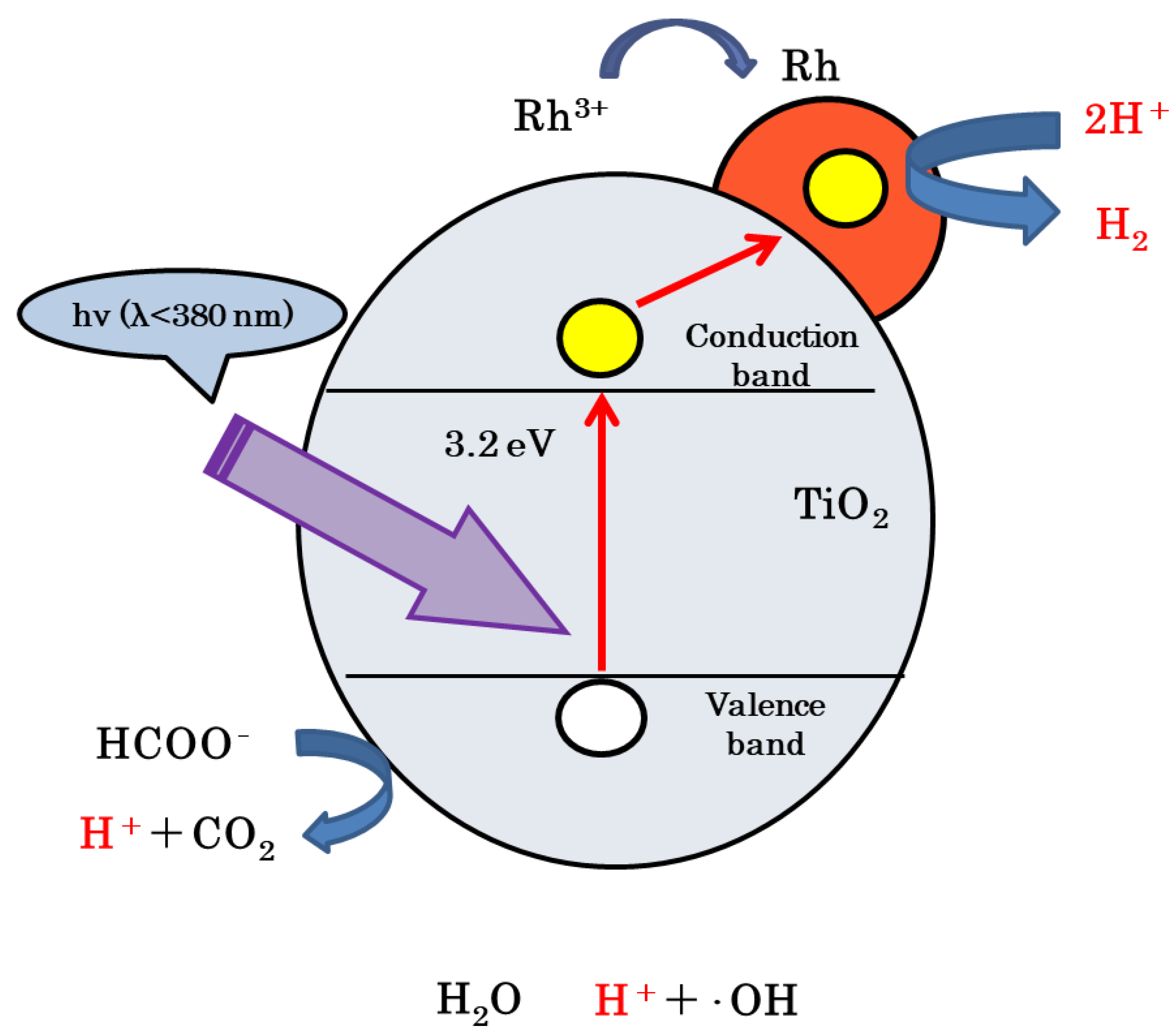
3.3. Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic hydrogen production using metal doped TiO2: A review of recent advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Fiorenza, R.; Sciré, S.; D’Urso, L.; Compagnini, G.; Bellardita, M.; Palmisano, L. Efficient H2 production by photocatalytic water splitting under UV or solar light over variously modified TiO2-based catalysts. Int. J. Hydrogen Energy 2019, 44, 14796–14807. [Google Scholar] [CrossRef]
- Tahir, M.; Tasleem, S.; Tahir, B. Recent development in band engineering of binary semiconductor materials for solar driven photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020, 45, 15985–16038. [Google Scholar] [CrossRef]
- Trang, T.N.Q.; Nam, N.D.; Tu, L.T.N.; Quoc, H.P.; Van Man, T.; Ho, V.T.T.; Thu, V.T.H. In Situ Spatial Charge Separation of an Ir@TiO2 Multiphase Photosystem toward Highly Efficient Photocatalytic Performance of Hydrogen Production. J. Phys. Chem. C 2020, 124, 16961–16974. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Fu, X.; Wang, C.; Ni, M.; Leung, M.K.H.; Wang, X.; Fu, X. Hydrogen production over titania-based photocatalysts. ChemSusChem 2010, 3, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Do, H.H.; Nguyen, D.L.T.; Nguyen, X.C.; Le, T.-H.; Nguyen, T.P.; Trinh, Q.T.; Ahn, S.H.; Vo, D.V.N.; Kim, S.Y.; Van Le, Q. Recent progress in TiO2-based photocatalysts for hydrogen evolution reaction: A review. Arab. J. Chem. 2020, 13, 3653–3671. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Zhu, B.; Liu, M.; Zhang, L.; Yu, J.; Zhou, M. Direct Z-scheme ZnO/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Catal. B Environ. 2019, 243, 19–26. [Google Scholar] [CrossRef]
- Karthik, P.; Kumar, T.R.N.; Neppolian, B. Redox couple mediated charge carrier separation in g-C3N4/CuO photocatalyst for enhanced photocatalytic H2 production. Int. J. Hydrogen Energy 2020, 45, 7541–7551. [Google Scholar] [CrossRef]
- Wang, Q.; Edalati, K.; Koganemaru, Y.; Nakamura, S.; Watanabe, M.; Ishihara, T.; Horita, Z. Photocatalytic hydrogen generation on low-bandgap black zirconia (ZrO2) produced by high-pressure torsion. J. Mater. Chem. A 2020, 8, 3643–3650. [Google Scholar] [CrossRef]
- Preethi, V.; Kanmani, S. Photocatalytic hydrogen production using Fe2O3-based core shell nano particles with ZnS and CdS. Int. J. Hydrogen Energy 2014, 39, 1613–1622. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Zhu, Y.; Li, Z.; Vajtai, R.; Ci, L.; Ajayan, P.M. Nanostructured VO2 photocatalysts for hydrogen production. ACS Nano 2008, 2, 1492–1496. [Google Scholar] [CrossRef]
- Ye, L.; Wen, Z. ZnIn2S4 nanosheets decorating WO3 nanorods core-shell hybrids for boosting visible-light photocatalysis hydrogen generation. Int. J. Hydrogen Energy 2019, 44, 3751–3759. [Google Scholar] [CrossRef]
- Wang, P.; Li, H.; Sheng, Y.; Chen, F. Inhibited photocorrosion and improved photocatalytic H2-evolution activity of CdS photocatalyst by molybdate ions. Appl. Surf. Sci. 2019, 463, 27–33. [Google Scholar] [CrossRef]
- Mei, F.; Zhang, J.; Dai, K.; Zhu, G.; Liang, C. A Z-scheme Bi 2 MoO6/CdSe-diethylenetriamine heterojunction for enhancing photocatalytic hydrogen production activity under visible light. Dalt. Trans. 2019, 48, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.Y.; Wang, Y.Y.; Tong, X.L.; Jin, G.Q.; Guo, X.Y. Photocatalytic hydrogen production over modified SiC nanowires under visible light irradiation. Int. J. Hydrogen Energy 2012, 37, 15038–15044. [Google Scholar] [CrossRef]
- Zhou, P.; Lv, F.; Li, N.; Zhang, Y.; Mu, Z.; Tang, Y.; Lai, J.; Chao, Y.; Luo, M.; Lin, F.; et al. Strengthening reactive metal-support interaction to stabilize high-density Pt single atoms on electron-deficient g-C3N4 for boosting photocatalytic H2 production. Nano Energy 2019, 56, 127–137. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Sakthivel, S.; Shankar, M.V.; Palanichamy, M.; Arabindoo, B.; Bahnemann, D.W.; Murugesan, V. Enhancement of photocatalytic activity by metal deposition: Characterisation and photonic efficiency of Pt, Au and Pd deposited on TiO2 catalyst. Water Res. 2004, 38, 3001–3008. [Google Scholar] [CrossRef]
- Gomathisankar, P.; Yamamoto, D.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photocatalytic hydrogen production with aid of simultaneous metal deposition using titanium dioxide from aqueous glucose solution. Int. J. Hydrogen Energy 2013, 38, 5517–5524. [Google Scholar] [CrossRef]
- Gomathisankar, P.; Kawamura, T.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photocatalytic hydrogen production from aqueous methanol solution using titanium dioxide with the aid of simultaneous metal deposition. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 110–116. [Google Scholar] [CrossRef]
- Tseng, I.H.; Wu, J.C.S.; Chou, H.Y. Effects of sol-gel procedures on the photocatalysis of Cu/TiO2 in CO2 photoreduction. J. Catal. 2004, 221, 432–440. [Google Scholar] [CrossRef]
- Liu, S.X.; Qu, Z.P.; Han, X.W.; Sun, C.L. A mechanism for enhanced photocatalytic activity of silver-loaded titanium dioxide. Catal. Today 2004, 93–95, 877–884. [Google Scholar] [CrossRef]
- Clarizia, L.; Spasiano, D.; Di Somma, I.; Marotta, R.; Andreozzi, R.; Dionysiou, D.D. Copper modified-TiO2 catalysts for hydrogen generation through photoreforming of organics. A short review. Int. J. Hydrogen Energy 2014, 39, 16812–16831. [Google Scholar] [CrossRef]
- Montini, T.; Gombac, V.; Sordelli, L.; Delgado, J.J.; Chen, X.; Adami, G.; Fornasiero, P. Nanostructured Cu/TiO2 Photocatalysts for H2 Production from Ethanol and Glycerol Aqueous Solutions. ChemCatChem 2011, 3, 574–577. [Google Scholar] [CrossRef]
- Navlani-García, M.; Salinas-Torres, D.; Mori, K.; Kuwahara, Y.; Yamashita, H. Photocatalytic Approaches for Hydrogen Production via Formic Acid Decomposition; Springer International Publishing: Cham, Switzerland, 2019; Volume 377, ISBN 0123456789. [Google Scholar]
- Ravi, P.; Rao, V.N.; Shankar, M.V.; Sathish, M. CuO@NiO core-shell nanoparticles decorated anatase TiO2 nanospheres for enhanced photocatalytic hydrogen production. Int. J. Hydrogen Energy 2020, 45, 7517–7529. [Google Scholar] [CrossRef]
- Nada, A.A.; Barakat, M.H.; Hamed, H.A.; Mohamed, N.R.; Veziroglu, T.N. Studies on the photocatalytic hydrogen production using suspended modified TiO2 photocatalysts. Int. J. Hydrogen Energy 2005, 30, 687–691. [Google Scholar] [CrossRef]
- Kim, G.; Choi, H.J.; Kim, H.-I.; Kim, J.; Monllor-Satoca, D.; Kim, M.; Park, H. Temperature-boosted photocatalytic H2 production and charge transfer kinetics on TiO2 under UV and visible light. Photochem. Photobiol. Sci. 2016, 15, 1247–1253. [Google Scholar] [CrossRef]
- Gao, M.; Connor, P.K.N.; Ho, G.W. Plasmonic photothermic directed broadband sunlight harnessing for seawater catalysis and desalination. Energy Environ. Sci. 2016, 9, 3151–3160. [Google Scholar] [CrossRef] [Green Version]
- Jiang, K.; Xu, K.; Zou, S.; Cai, W. B-Doped Pd Catalyst: Boosting Room-Temperature Hydrogen Production from Formic Acid—Formate Solutions. J. Am. Chem. Soc. 2014, 136, 4861–4864. [Google Scholar] [CrossRef]
- Swapna, M.V.; Haridas, K.R. An easier method of preparation of mesoporous anatase TiO2 nanoparticles via ultrasonic irradiation. J. Exp. Nanosci. 2016, 11, 540–549. [Google Scholar] [CrossRef]
- Camposeco, R.; Hinojosa-Reyes, M.; Zanella, R. Highly efficient photocatalytic hydrogen evolution by using Rh as co-catalyst in the Cu/TiO2 system. Int. J. Hydrogen Energy 2021, 46, 26074–26086. [Google Scholar] [CrossRef]
- Duan, S.; Zhang, S.; Chang, S.; Meng, S.; Fan, Y.; Zheng, X.; Chen, S. Efficient photocatalytic hydrogen production from formic acid on inexpensive and stable phosphide/Zn3In2S6 composite photocatalysts under mild conditions. Int. J. Hydrogen Energy 2019, 44, 21803–21820. [Google Scholar] [CrossRef]
- Wang, Q.; An, N.; Bai, Y.; Hang, H.; Li, J.; Lu, X.; Liu, Y.; Wang, F.; Li, Z.; Lei, Z. High photocatalytic hydrogen production from methanol aqueous solution using the photocatalysts CuS/TiO2. Int. J. Hydrogen Energy 2013, 38, 10739–10745. [Google Scholar] [CrossRef]
- Chen, W.-T.; Chan, A.; Sun-Waterhouse, D.; Moriga, T.; Idriss, H.; Waterhouse, G.I.N. Ni/TiO2: A promising low-cost photocatalytic system for solar H2 production from ethanol-water mixtures. J. Catal. 2015, 326, 43–53. [Google Scholar] [CrossRef]
- Tahir, M. La-modified TiO2/carbon nanotubes assembly nanocomposite for efficient photocatalytic hydrogen evolution from glycerol-water mixture. Int. J. Hydrogen Energy 2019, 44, 3711–3725. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, X.; Jia, Y.; Chen, X.; Han, H.; Li, C. Titanium dioxide-based nanomaterials for photocatalytic fuel generations. Chem. Rev. 2014, 114, 9987–10043. [Google Scholar] [CrossRef]
- Halasi, G.; Schubert, G.; Solymosi, F. Photodecomposition of formic acid on N-doped and metal-promoted TiO2 production of CO-free H2. J. Phys. Chem. C 2012, 116, 15396–15405. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Melvin, A.A.; Matthews, T.; Dash, S.; Tyagi, A.K. TiO2 modification by gold (Au) for photocatalytic hydrogen (H2) production. Renew. Sustain. Energy Rev. 2016, 58, 1366–1375. [Google Scholar] [CrossRef]



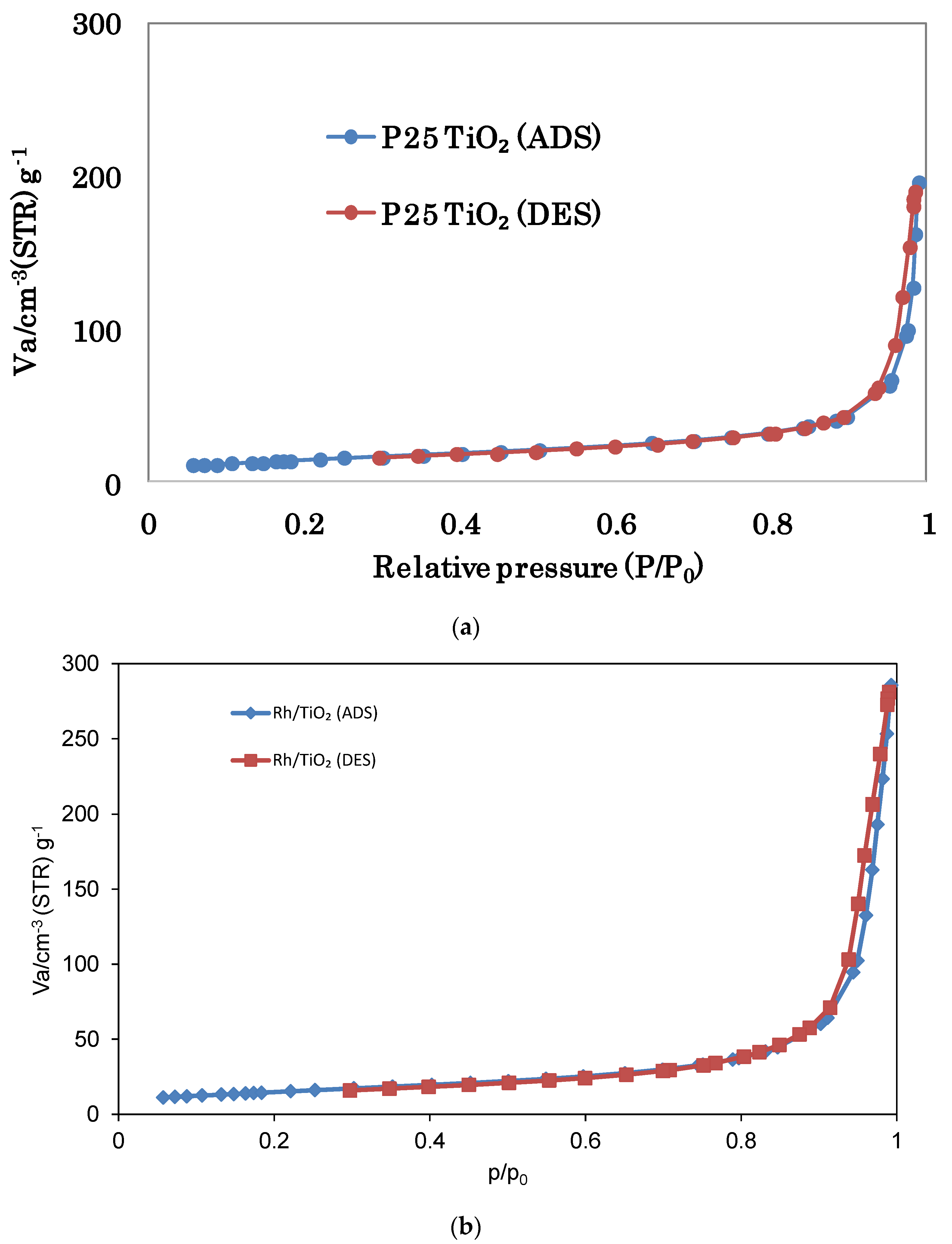
| Photocatalyst | BET Surface Area [m2 g−1] | Total Pore Volume [cm3 g−1] | Average Pore Diameter [nm] |
|---|---|---|---|
| P-25 TiO2 | 53 | 0.296 | 22.3 |
| Rh/TiO2 | 54 | 0.417 | 31.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suhag, M.H.; Tateishi, I.; Furukawa, M.; Katsumata, H.; Khatun, A.; Kaneco, S. Photocatalytic Hydrogen Production from Formic Acid Solution with Titanium Dioxide with the Aid of Simultaneous Rh Deposition. ChemEngineering 2022, 6, 43. https://doi.org/10.3390/chemengineering6030043
Suhag MH, Tateishi I, Furukawa M, Katsumata H, Khatun A, Kaneco S. Photocatalytic Hydrogen Production from Formic Acid Solution with Titanium Dioxide with the Aid of Simultaneous Rh Deposition. ChemEngineering. 2022; 6(3):43. https://doi.org/10.3390/chemengineering6030043
Chicago/Turabian StyleSuhag, Mahmudul Hassan, Ikki Tateishi, Mai Furukawa, Hideyuki Katsumata, Aklima Khatun, and Satoshi Kaneco. 2022. "Photocatalytic Hydrogen Production from Formic Acid Solution with Titanium Dioxide with the Aid of Simultaneous Rh Deposition" ChemEngineering 6, no. 3: 43. https://doi.org/10.3390/chemengineering6030043
APA StyleSuhag, M. H., Tateishi, I., Furukawa, M., Katsumata, H., Khatun, A., & Kaneco, S. (2022). Photocatalytic Hydrogen Production from Formic Acid Solution with Titanium Dioxide with the Aid of Simultaneous Rh Deposition. ChemEngineering, 6(3), 43. https://doi.org/10.3390/chemengineering6030043






