1. Introduction
Fossil fuels are still the main sources of energy used worldwide, due to their high energy efficiency. However, in addition to the pollution caused by the gases released, fossil energy is finite, being currently depleted fast, boosting the search and the need to use renewable energies [
1]. Under this context, solid biomass shows great potential to become an energetic alternative to fossil energy sources. This renewable energy is abundant and has a virtually zero carbon dioxide (CO
2) balance since the CO
2 produced in power generation is equivalent to the CO
2 consumed during the growth of the plant from which the biomass was originated [
2].
Biomass is a source of energy that can potentially be obtained from forest-related industrial wastes, such as wood chips and sawdust, as well as from agricultural wastes, such as rice husk, straw, and sugarcane bagasse [
3,
4,
5,
6]. The amount of forest waste in Portugal is approximately 2.2 million tons per year. Portugal is the European country with the highest number of forest fires and the second with the most burned area. Most of the fires are caused by the use of fire to burn trash and waste. Thus, the use of forest biomass for energy production can minimize the damage to landowners, forest neighbors, and investors, as it provides a destination for waste, produces energy, and tends to reduce fires [
2,
7,
8].
Biomass gasification is the conversion of organic materials into an energetic gas, rich in hydrogen (H
2) and carbon monoxide (CO), through oxidation and reduction reactions at elevated temperatures. In the evaluation of gasification processes, estimating the composition of the fuel gas for different conditions is fundamental to identify the best operating conditions. In this way, gasification modeling and simulation provide an advance virtual analysis of the process performance, allowing for resource and time savings in pilot-scale process operations, as it predicts the behavior and analyzes the effects of different variables on the process [
9,
10,
11,
12,
13,
14].
Thus, the focus of this work is the modeling and simulation of biomass gasification processes using the UniSim Design chemical process software, applying the tools available in the simulator database in order to satisfactorily reproduce the operation behavior of a gasifier. The work is associated with a parallel project concerning the design, construction, and test of a pilot downdraft gasifier, aiming for the energetic valorization of residual biomass from fire-hazard areas. So, the developed model is focused specifically on the production of syngas from complex sources represented by mixtures of carbon rich organic wastes, which can show a variety of compositions. Hence, the chosen simulated gasifier has a downdraft design and it will be studied for the processing of forest and agricultural waste type biomasses. Optimization and sensitivity analysis will be performed to observe the influence of significant parameters affecting the process (e.g., equivalence ratio, steam to biomass ratio, and temperature) on the properties of the synthesis gas produced (e.g., composition and heating value).
This gas is a mixture composed mainly of carbon monoxide (CO), hydrogen (H
2), and methane (CH
4). The gas produced can be standardized in quality and is easier and more versatile to use than the original biomass, either for fueling gas engines and gas turbines or as a chemical raw material for liquid fuel production [
15]. Gasification adds value to low or negative value raw materials by converting them into fuels. This conversion process is more complex than simple combustion and is influenced by many factors, such as amount of oxidant, feedstock composition, gasifier temperature, and reactor geometry [
16,
17].
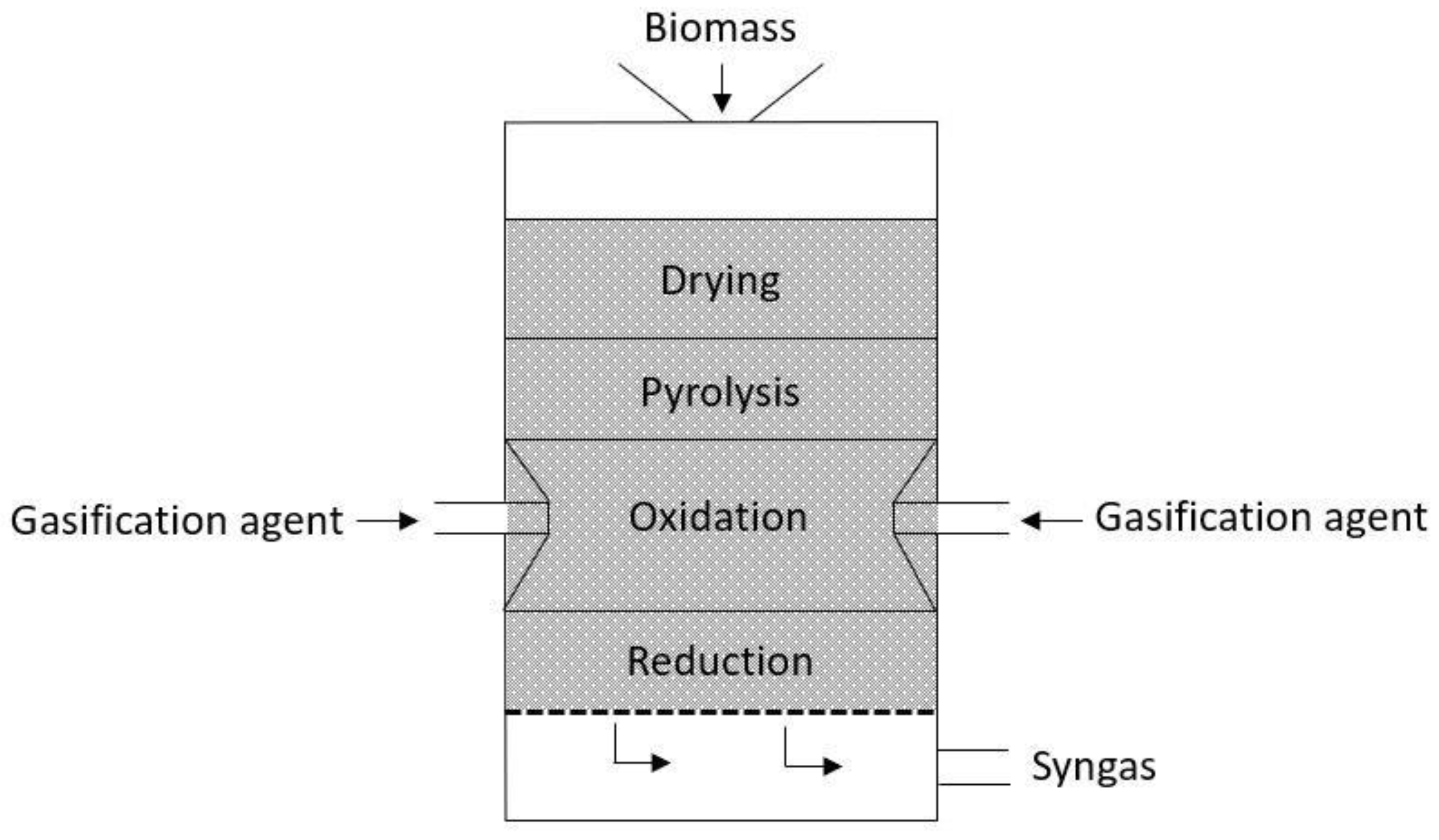
The downdraft gasifier design (see
Figure 1) is a reactor where the solid material is inserted in the top and the oxidizing gas enters the reactor laterally above the grid. The gasifying agent is introduced directly into the combustion zone, then flows into the reduction zone, and is extracted from the gasifier. The synthesis gas exits the gasifier after passing through the hot zone, allowing partial cracking of the tars formed during gasification, which provides a gas with low tar. However, the gases leave the gasifier at high temperatures (900–1000 °C), leading to low efficiencies, due to the high heat content carried by the hot gas. This reactor is suitable to convert biomass with high volatile content, but it is limited in scale. It also needs specific biomass conditions and is not suitable for various types of biomass [
9,
16].
In computer simulation, the processes and equipment operate following the sequence of input data, data processing, and return output data. Usually, these data are mass flows, temperatures, compositions, and pressures. For the construction, adaptation, or scaling of equipment, it is necessary to obtain well-dimensioned parameters. If these actions are done without prior study, the experimental data obtained may not be satisfactory and time and money have been spent on incorrect reactor sizing and operation [
20,
21].
Modeling and simulation of gasification systems help in predicting the outlet gas composition when operating conditions and scale size change. This assists in planning the construction or retrofitting of existing equipment. UniSim Design is a chemical process modeling software, with a similar design to that of Aspen Plus and Aspen Hysys. It is used in engineering to create dynamic and steady-state models for plant design, monitoring, troubleshooting, planning, and management. It is possible to build simulation processes in an integrated graphical environment including tools that allow the optimization of these processes.
In the literature, there are studies of modeling and simulation of biomass gasification mainly using Aspen Plus and Aspen Hysys [
1,
2,
4,
18,
22,
23,
24,
25,
26,
27]. In this work, UniSim Design will be used as an alternative software for these applications. A small-scale gasifier will be modeled and simulated, in order to process several types of forest biomass and agricultural wastes, thus giving a purpose to this residue. The type of gasifier chosen is a downdraft due to the simple design, low cost, and quality of the outlet gas. Additionally, a non-stoichiometric equilibrium model is assumed considering that the reaction system is in its most stable state (lowest free energy). For this, the main hypothesis presumed is that the gasification reaction rates are fast enough and the residence time is long enough to reach equilibrium. This condition is met at gasification temperatures above 800 °C. Thus, the largest discrepancies between estimated and experimental values are found at low temperatures, where the CO and H
2 fractions are overestimated and the CO
2 and CH
4, tar, and residual carbon fractions are underestimated. This model is based on minimizing Gibbs free energy to determine the product composition obtained and the efficiency of the gasifier. The advantage of this method is that there is no need for the establishment of a specific set of reactions to solve the problem, requiring knowledge of only the approximate and ultimate analysis [
11].
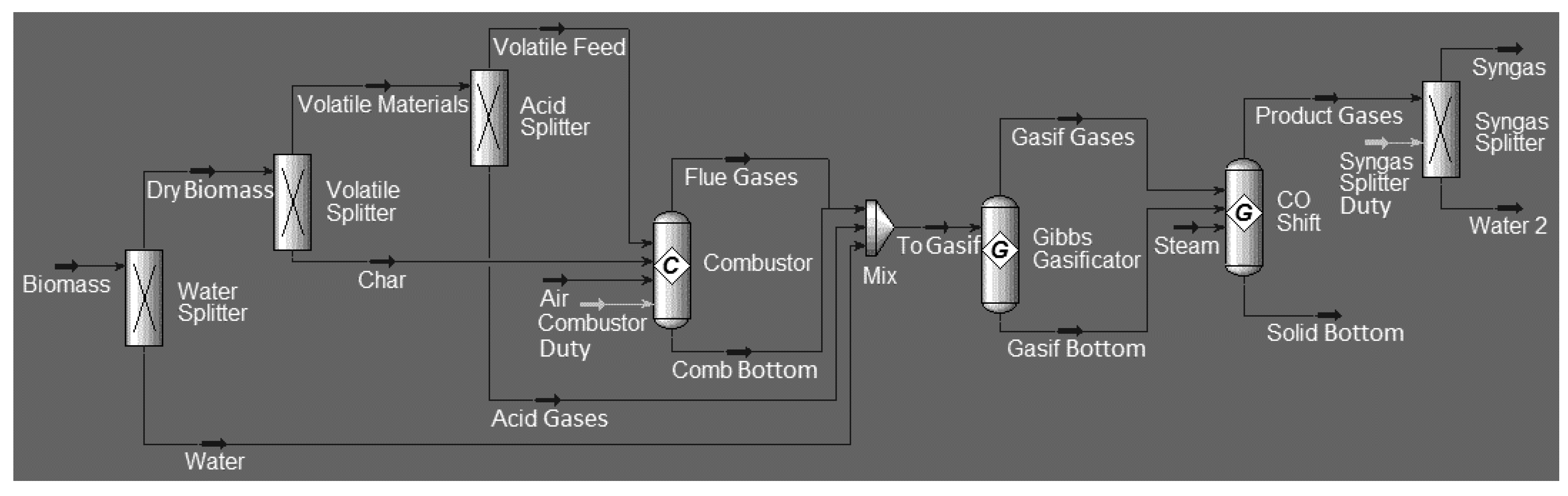
Regardless of the software used for modeling and simulation of the gasification process, there is a pattern of steps that must be followed in order to successfully perform the simulation. These steps represent the four main zones present in a downdraft gasifier, namely: drying, pyrolysis, oxidation, and reduction zones. Therefore, the simulation consists of several unit operation blocks and, as in this work, the process is divided into three blocks: drying and decomposition, combustion, and gasification. In the drying and decomposition block, the moisture content is reduced, and the biomass is decomposed into volatile and char compounds. In the combustion and gasification block, the oxidation and reduction reactions will be modeled, minimizing Gibbs free energy. The approach will be non-stoichiometric, requiring the specification of the ultimate and approximate biomass composition and the reactions involved in the process.
Chemical engineering software provides databases for conventional fluids and solids properties. Therefore, when unconventional materials such as biomass are employed, a strategy should be used to inform the software about the composition of the material entering the process. The strategy that will be used in this work is to consider biomass as a generic char material. This allows, from the approximate and ultimate analysis of the material, the calculation of the properties of this unconventional material, hence, obtaining the feed stream characteristics.
The next step is the drying and decomposition block, where the goal is to reduce the moisture content of the biomass and the dried material will be converted into components such as carbon, hydrogen, oxygen, nitrogen, and ashes, specifying the distribution according to the ultimate and approximate analysis.
Finally, there is the combustion and gasification stage, where a Gibbs reactor will be used to minimize Gibbs free energy by calculating the gas composition reaching full chemical equilibrium. In this block, air will be introduced as a gasification agent. Upon exiting this reactor, the gas produced will pass a separating block that will remove water. Therefore, the gaseous output stream obtained from this separator contains the desired syngas at the end of the process.
4. Conclusions
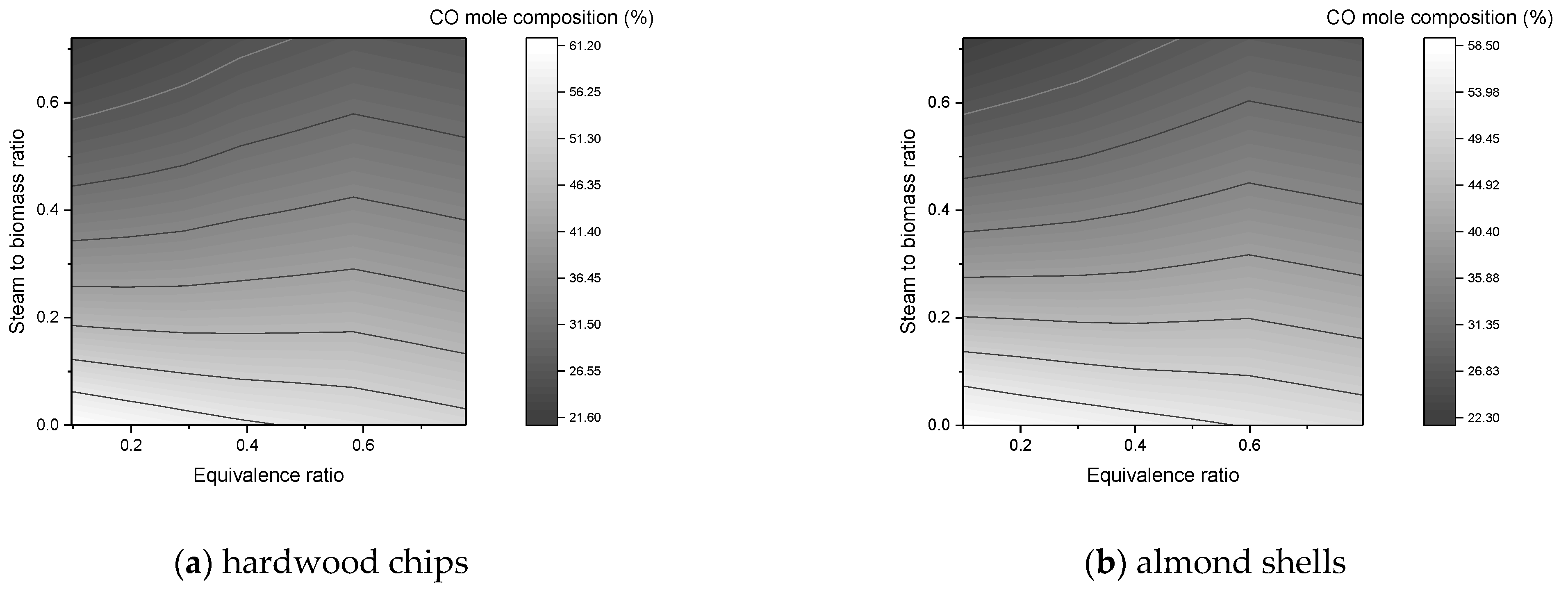
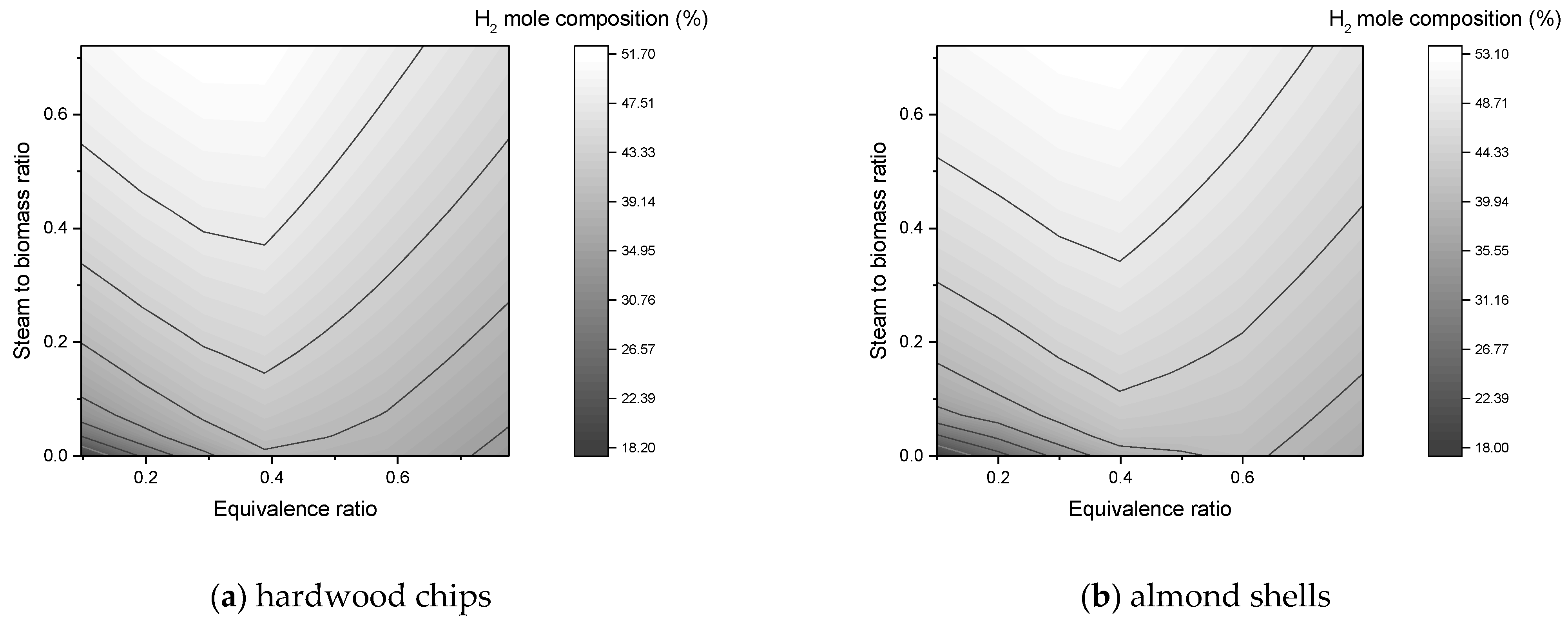
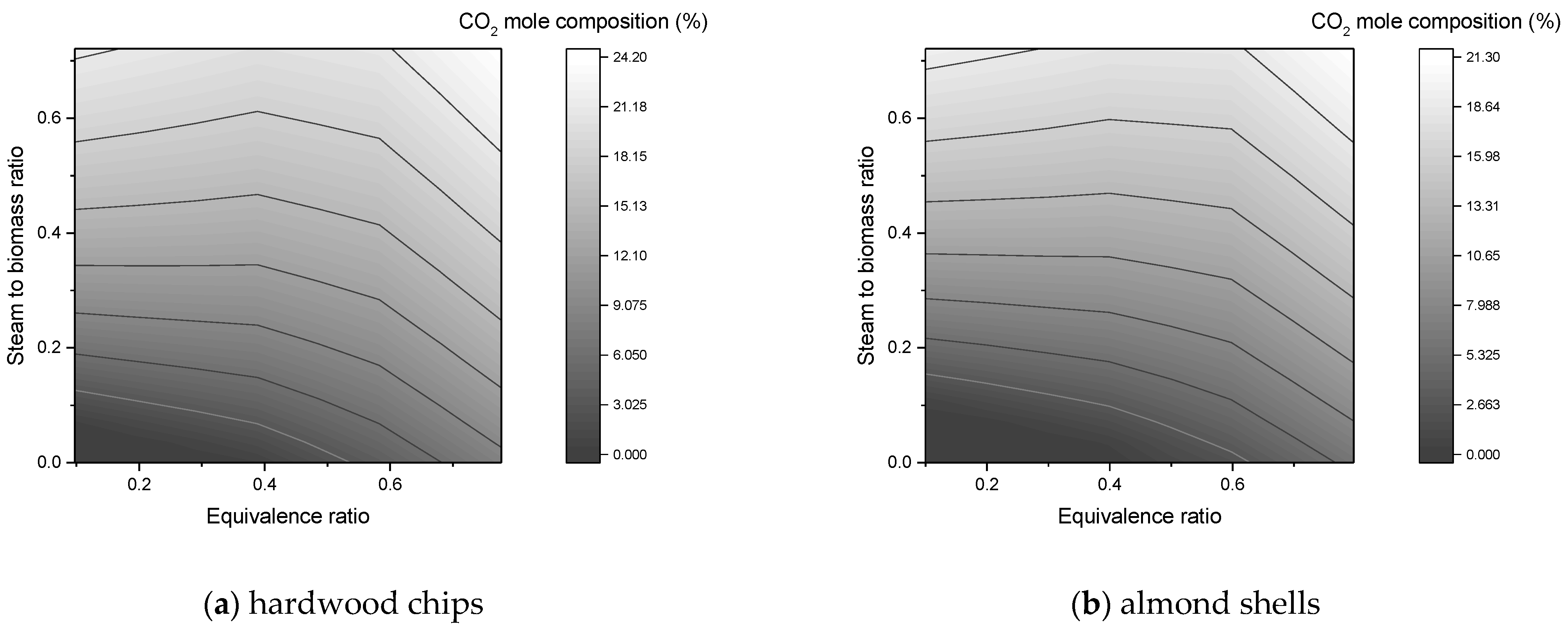
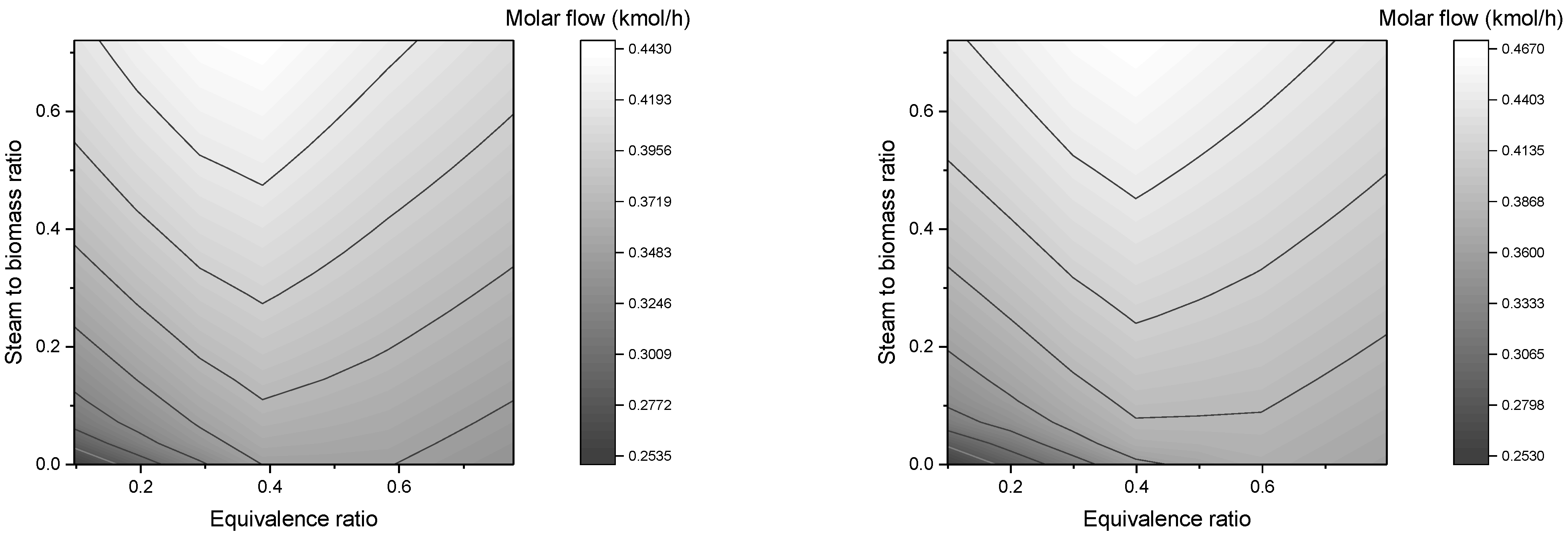
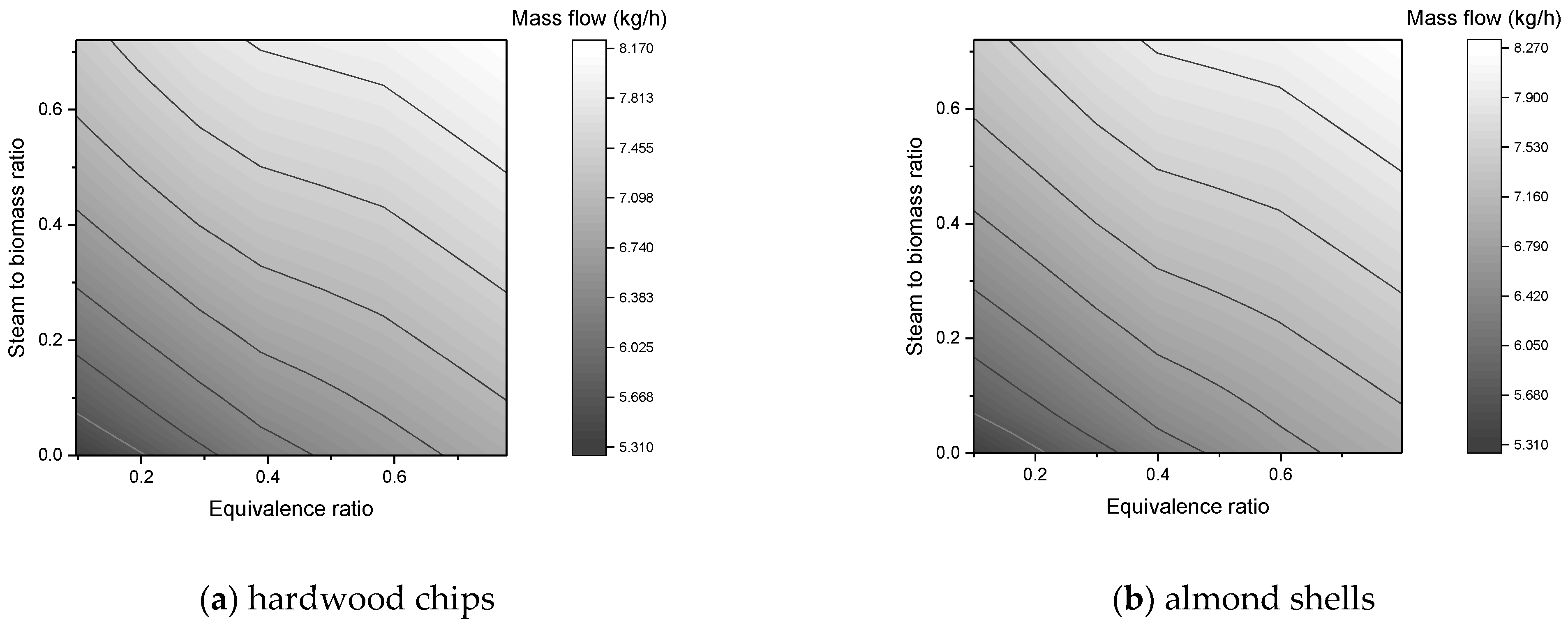
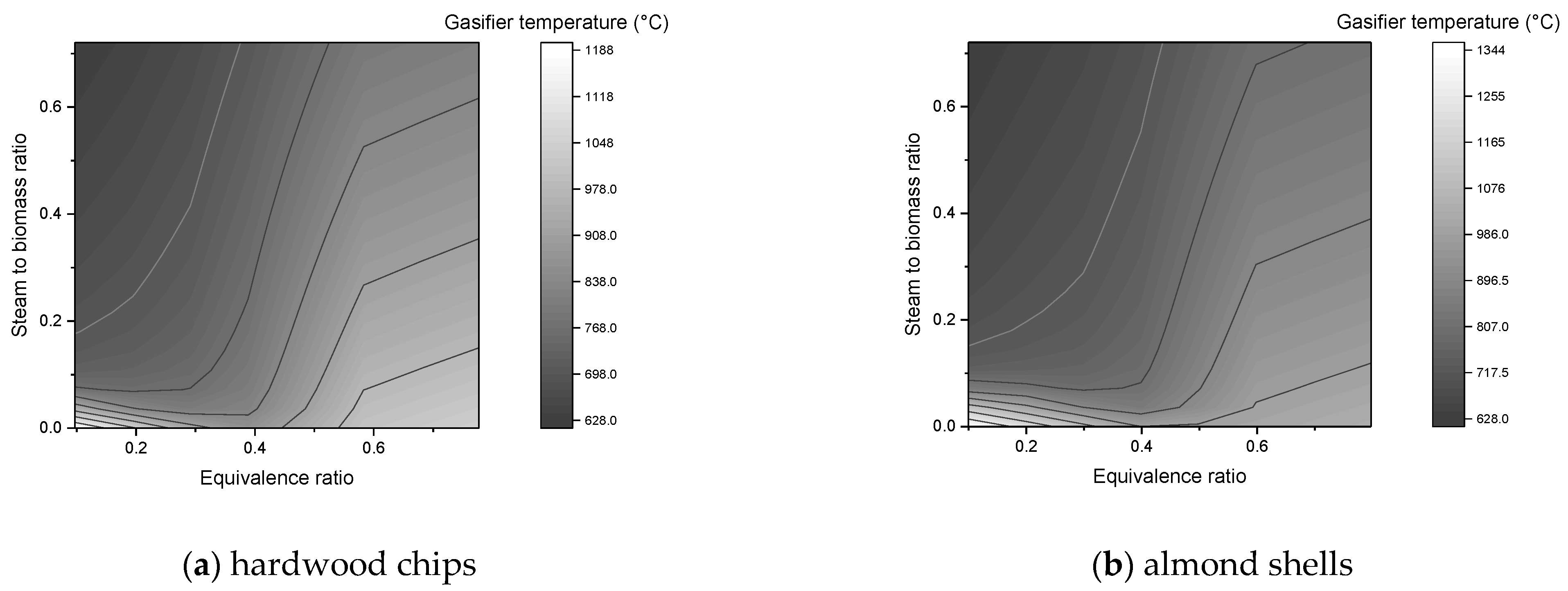
In this work, a downdraft gasifier was modeled and simulated for two residual biomasses (forest and agricultural) in order to predict the composition of the syngas produced. The reactors simulated gasification by minimizing the free energy of Gibbs. The main operating parameters were the equivalence ratio, steam to biomass ratio, and gasification temperature. In the simulations, a sensitivity analysis was carried out, where the effects of these parameters on the composition of syngas, flow of syngas, and heating values were studied.
The model is able to predict the gasifier’s performance and is qualified to analyze the behavior of the independent parameters in the gasification results. The following are the main results achieved with the simulation:
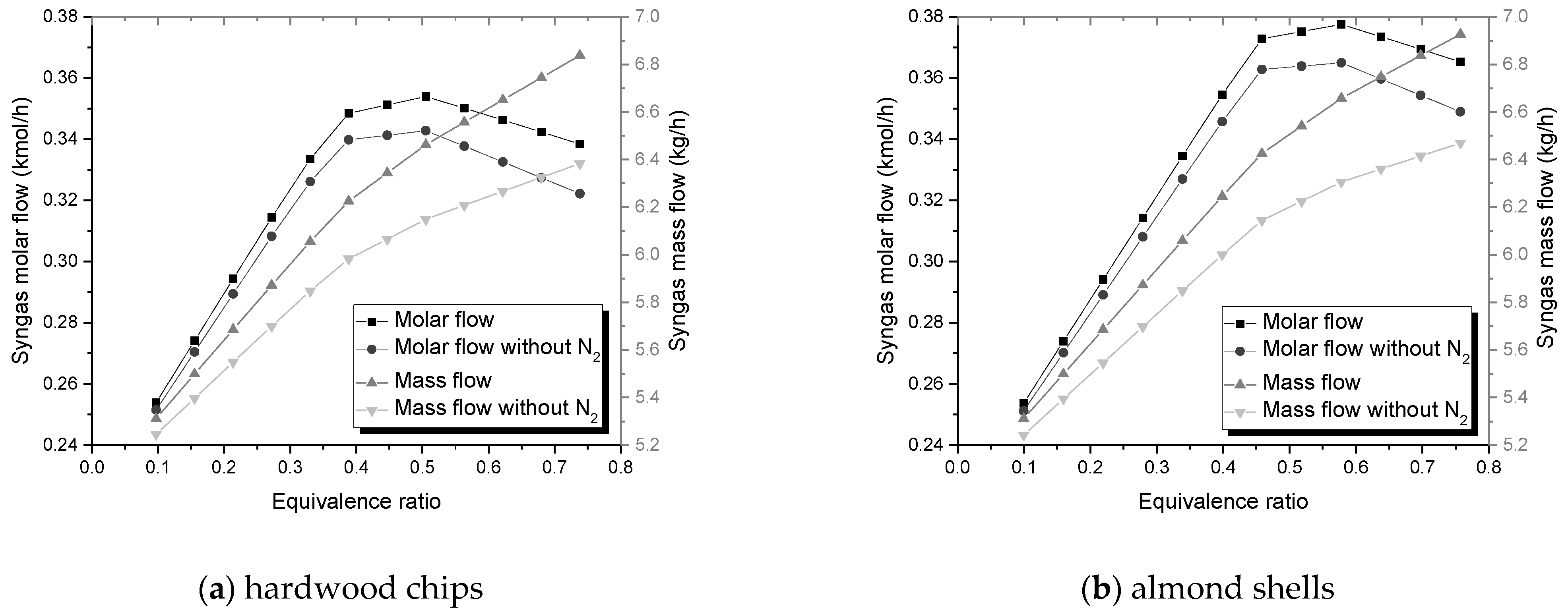
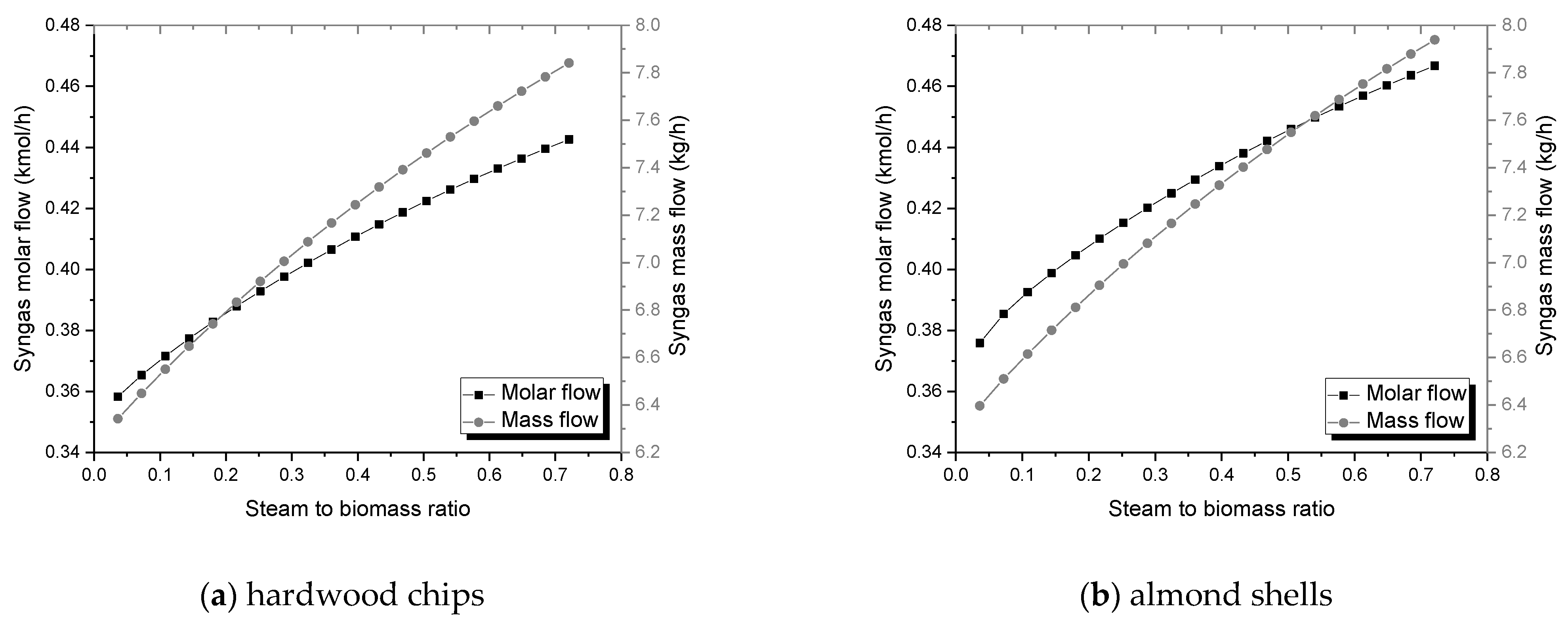
The mass flow of syngas is favored by the increase of ER and SBR;
The molar flow upsurges with higher SBR, however, it reaches a maximum value with rising ER;
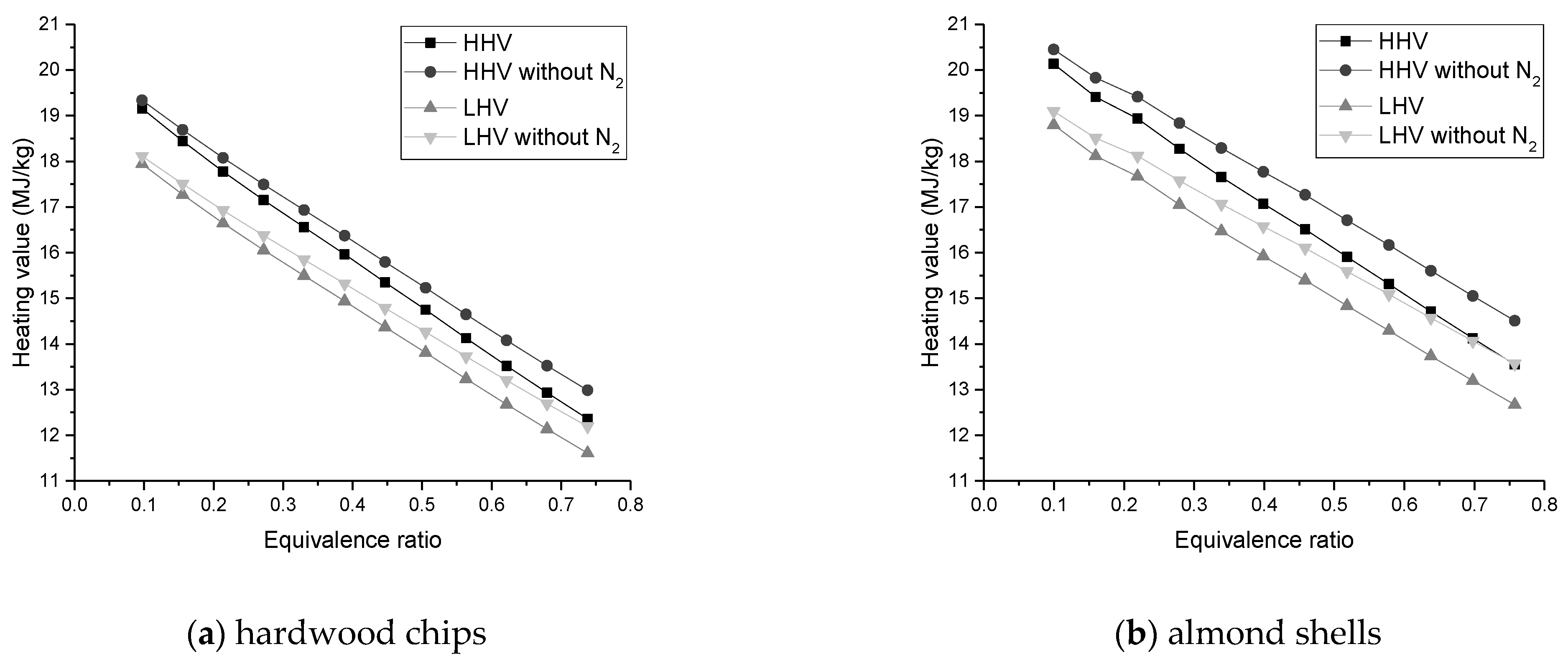
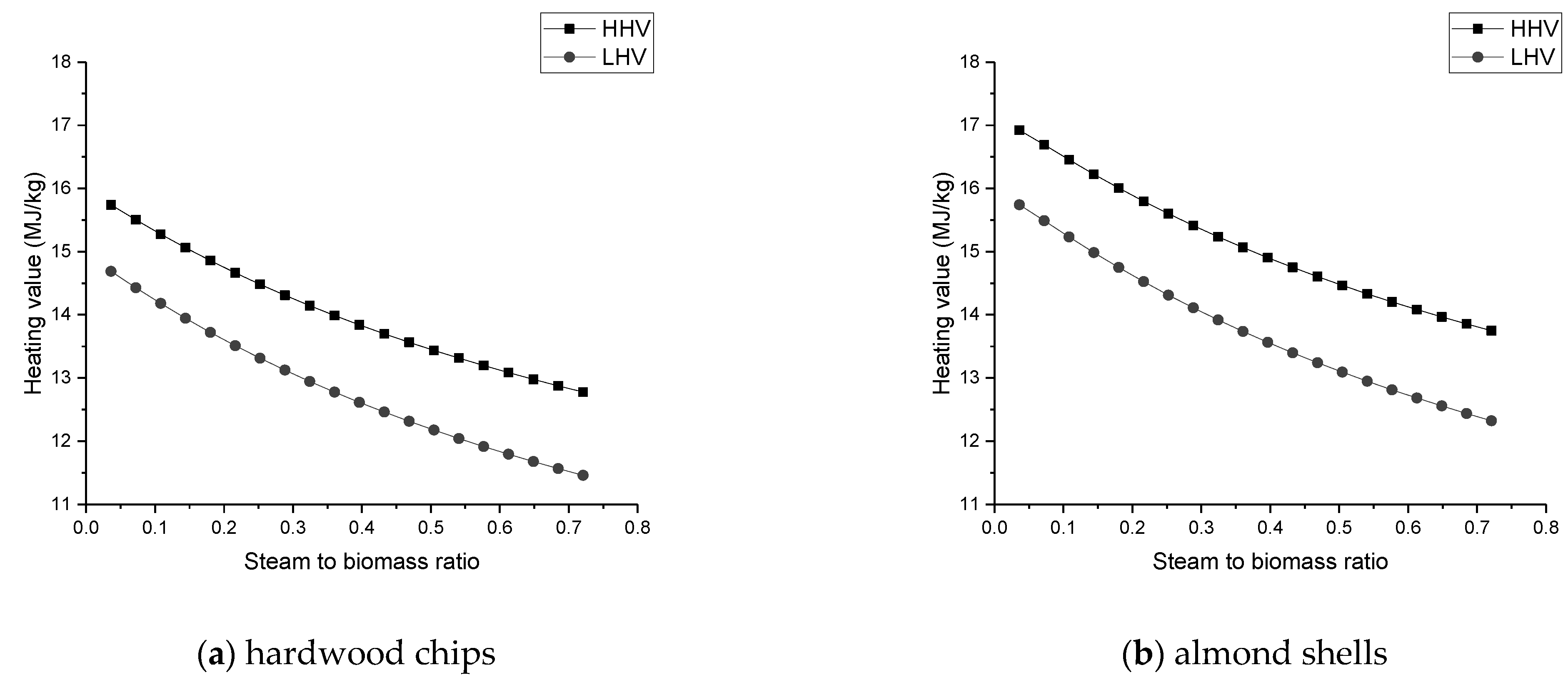
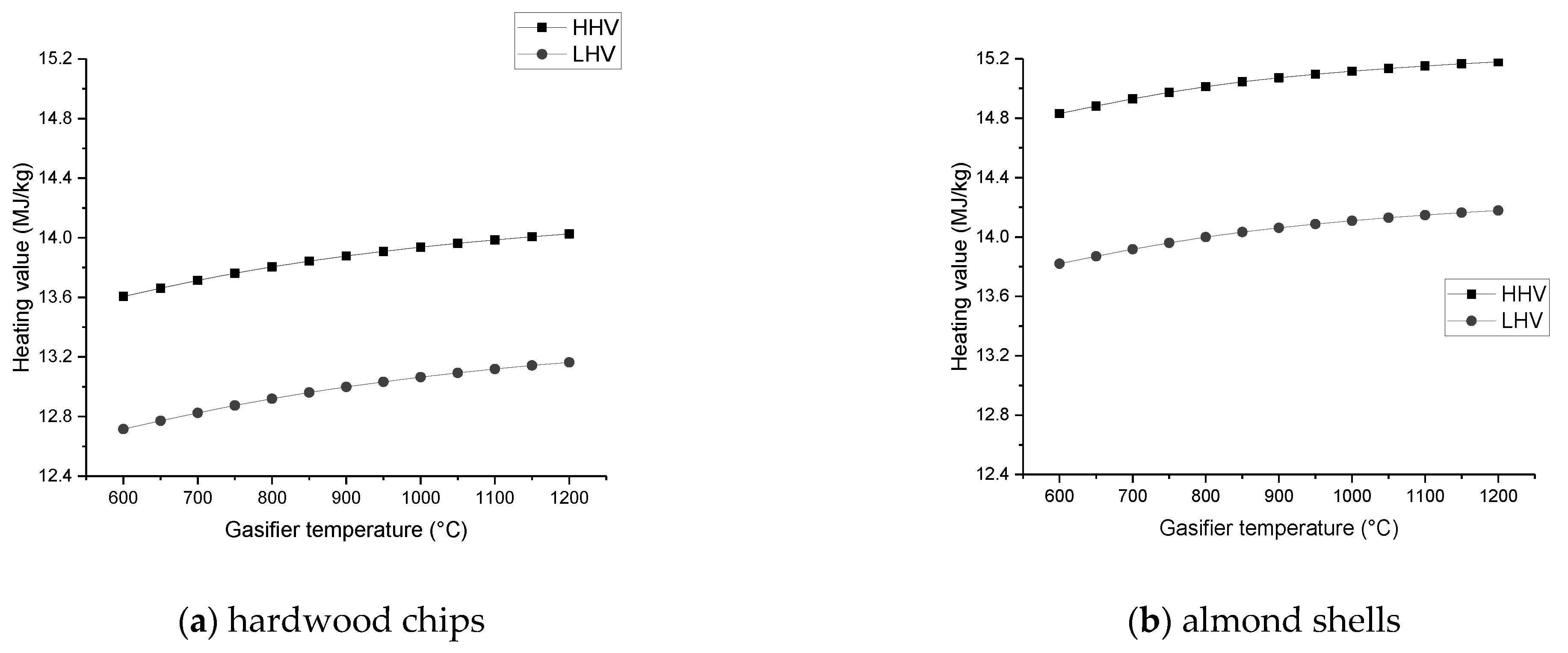
The heating value of the syngas declines with increasing ER and SBR, but increases with the growth of gasification temperature;
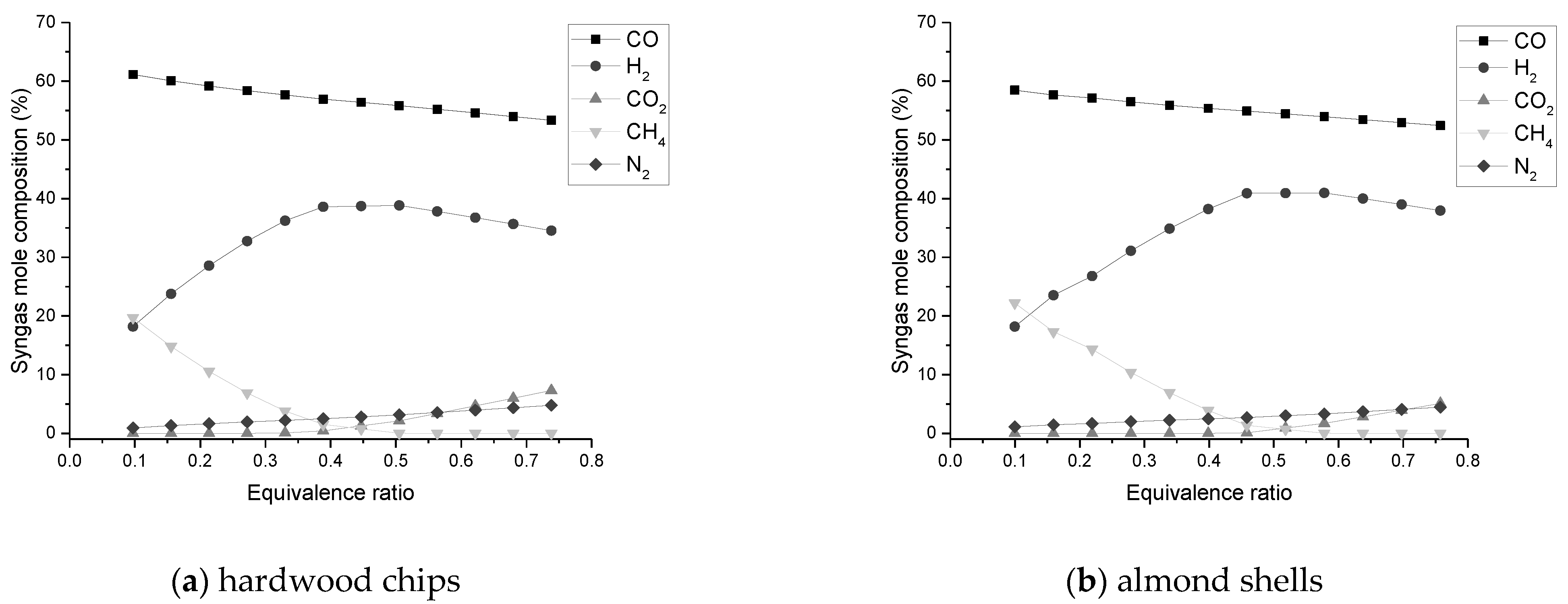
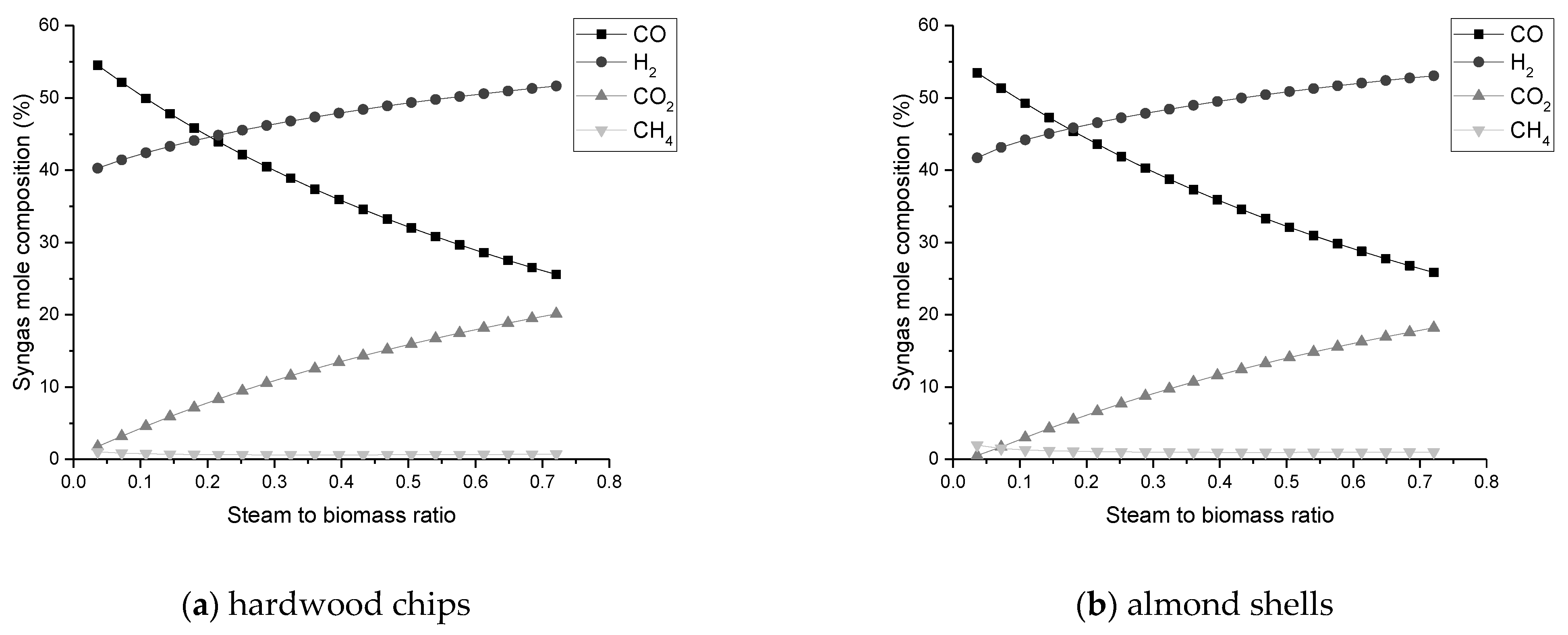
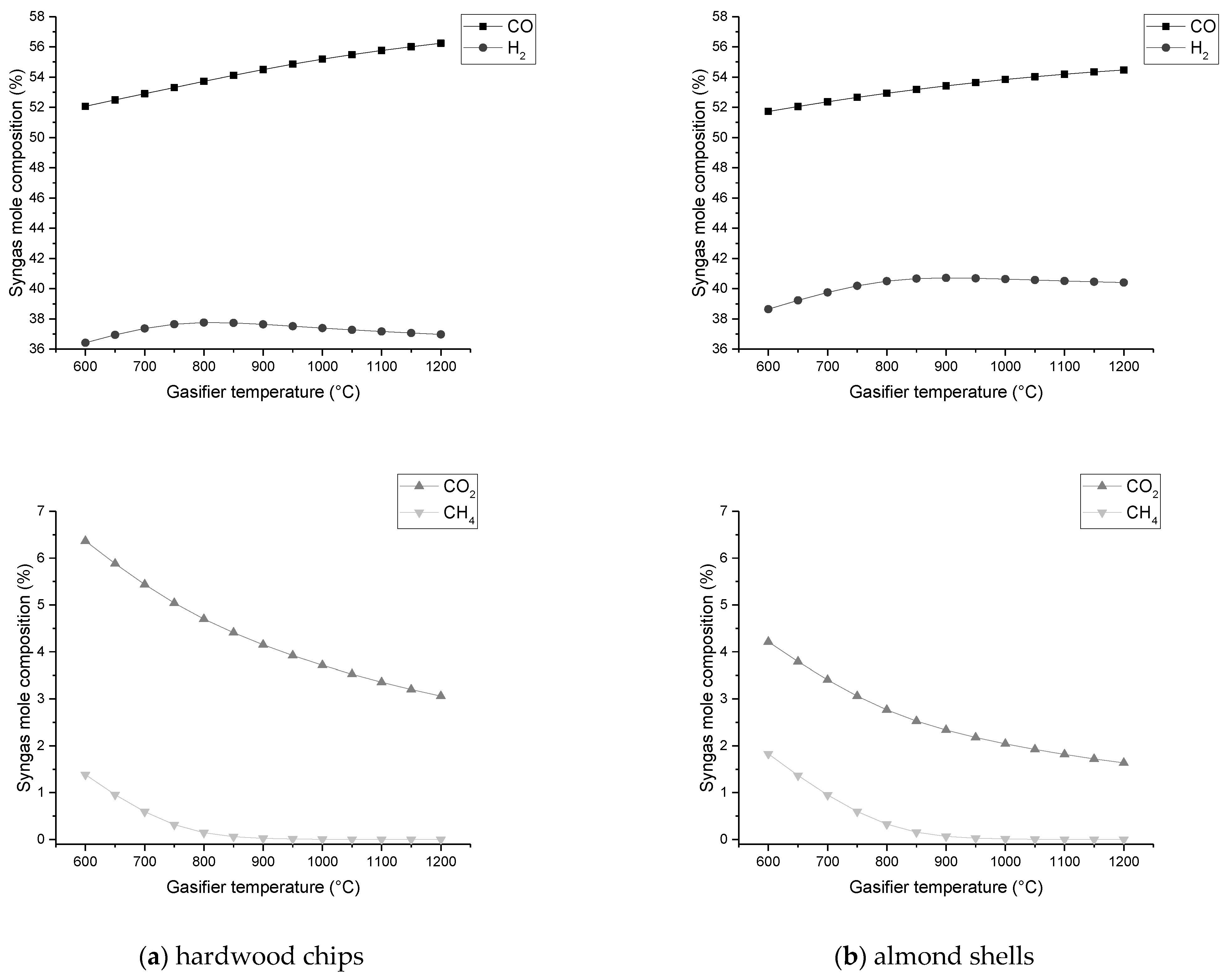
The composition of H2 and CO2 improves with SBR increase, while CO declines continuously;
An SBR value up to 0.2 is an acceptable value to promote the production of H2 without so much formation of CO2 in the syngas;
The equivalence ratio is a key parameter in the process as it favors the production of H2. A low amount causes the absence of gasification and a high amount causes formation of CO2, decrease of CO, and the presence of N2 in the synthesis gas;
An ER value between 0.3 and 0.5 is within the favorable range to maximize the amount of CO and H2 in the process;
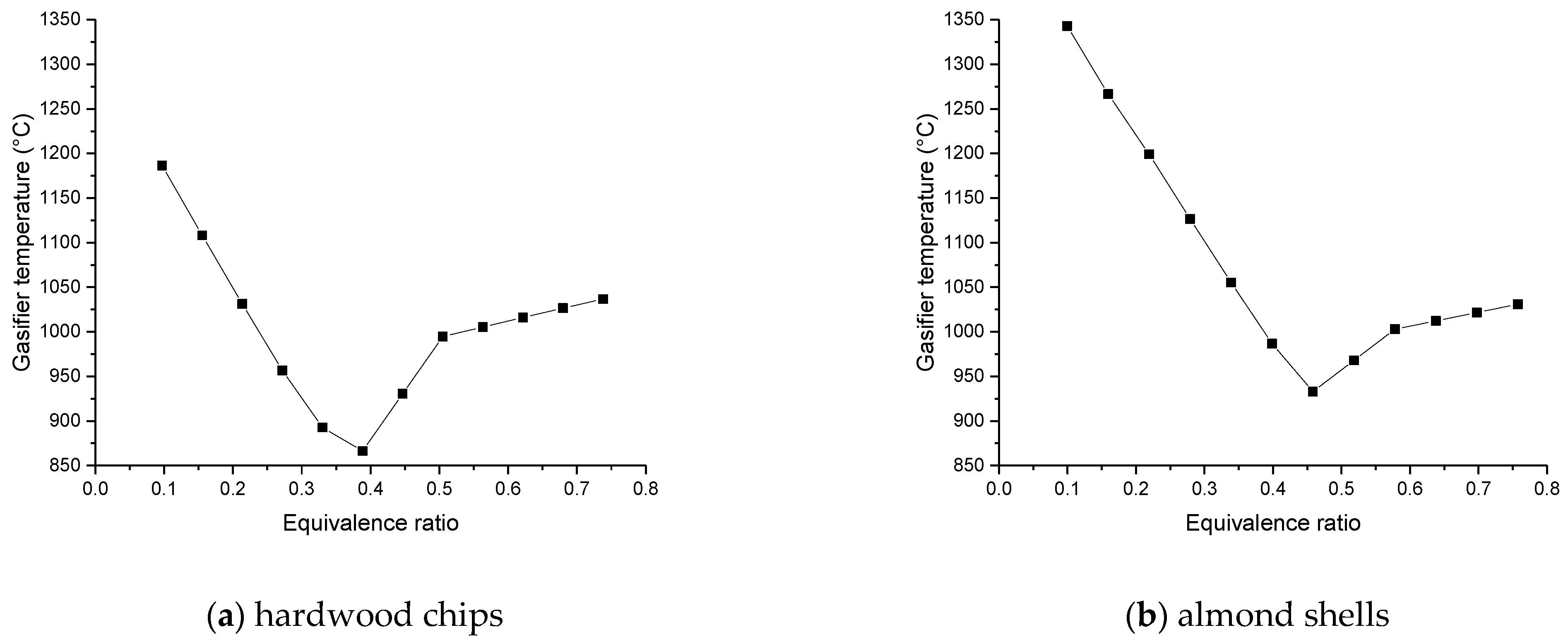
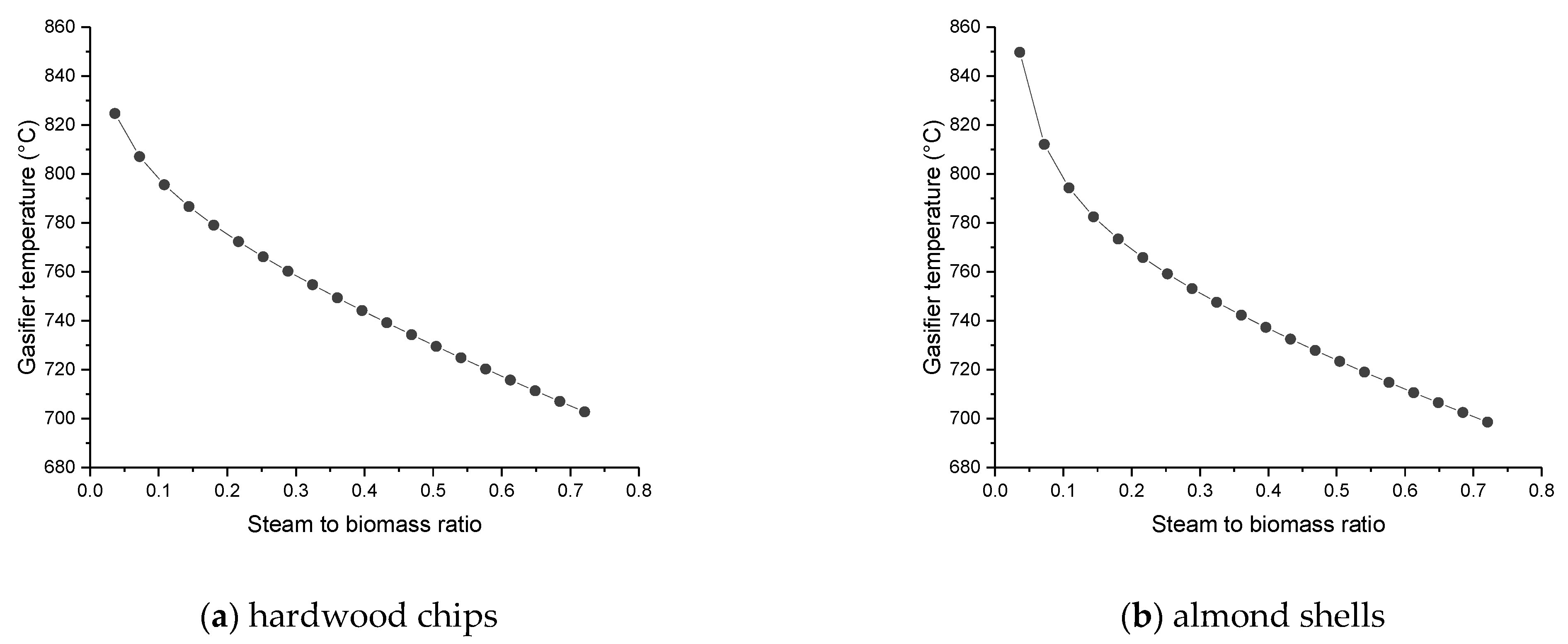
The favorable temperature of the gasifier must be between 850 and 950 °C, controlling the feeds of air and steam to obtain these values.
Additionally, it is concluded that in the specific conditions where the gasification temperature is specified and changed for a fixed air intake, the content of CO tends to rise with increasing temperature, but the content of H2 reaches a maximum for an optimal temperature value, while CO2 and CH4 tend to decrease continuously.
In summary, with a temperature between 850 and 950 °C, SBR values up to 0.2 and ER values between 0.3 and 0.5, the best operating conditions are obtained to maximize the composition of the syngas rich in CO and H2.