DMSO Intercalation in Selected Kaolinites: Influence of the Crystallinity
Abstract
:1. Introduction
2. Materials and Methods
2.1. X-ray Diffraction
2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3. Thermal Analysis
2.4. Nitrogen Adsorption: BET Specific Surface Measurement
3. Results and Discussion
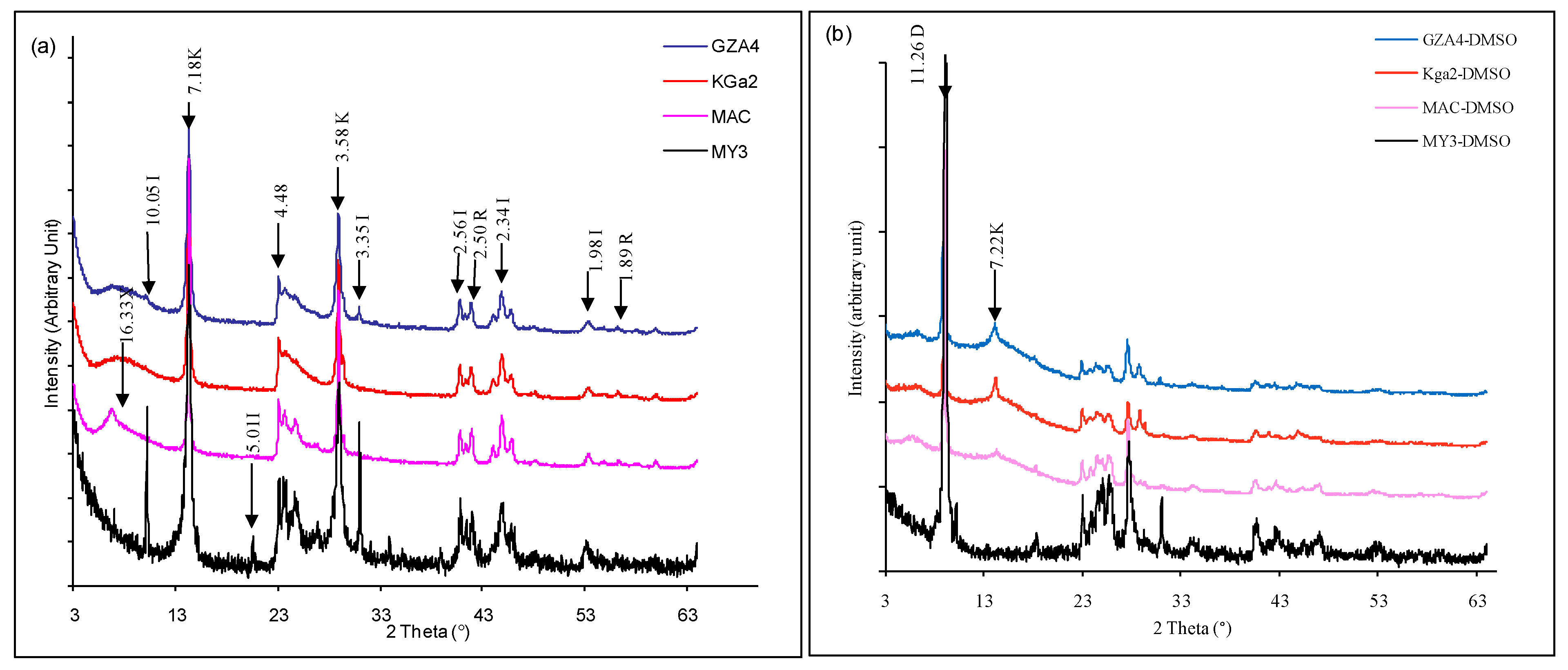
3.1. XRD: Crystalline Changes
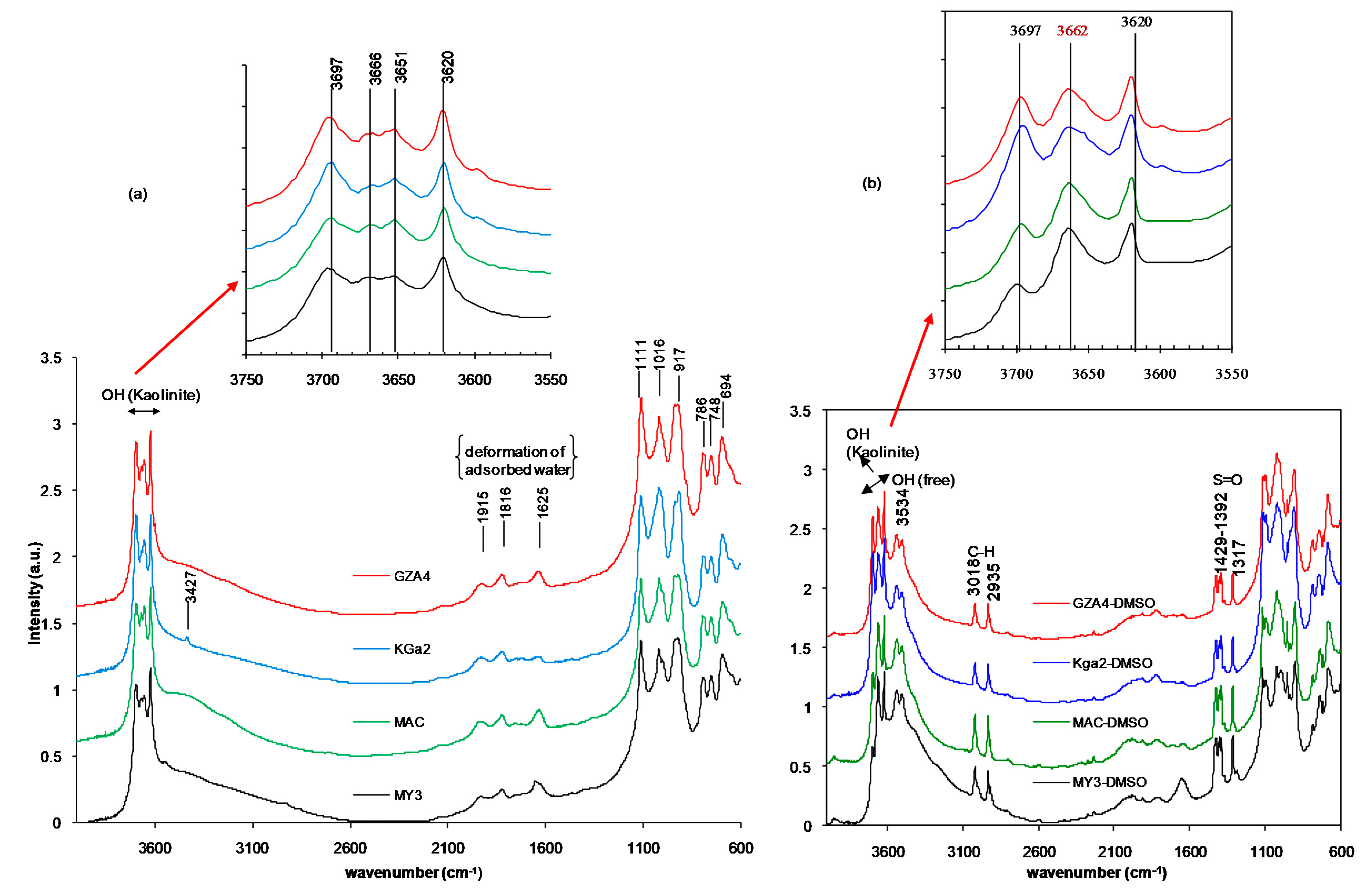
3.2. FTIR Analysis: DMSO-Clay Interactions
3.3. DSC-TGA: Interaction Energy and Quantitative Aspect
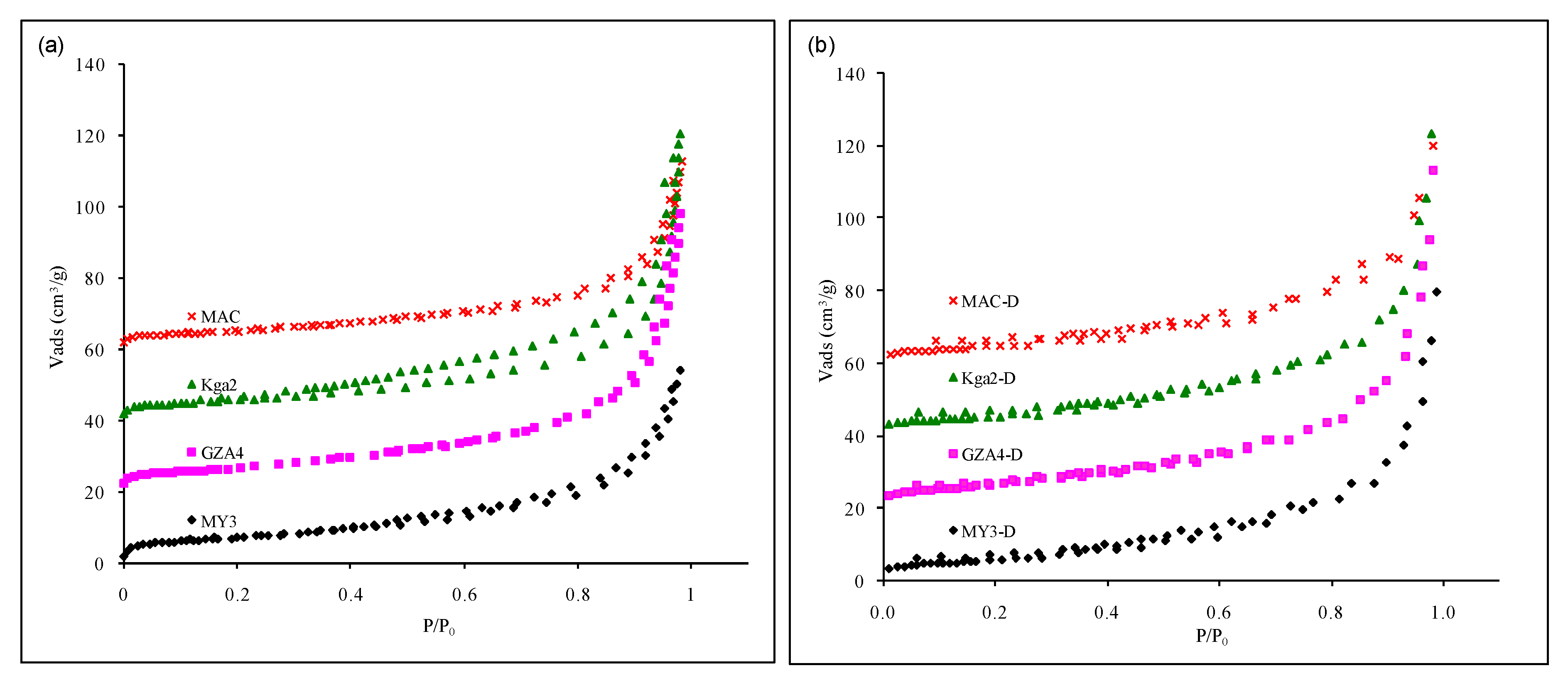
3.4. Nitrogen Adsorption and SSA: Textural Effects
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Mbey, J.A.; Hoppe, S.; Thomas, F. Cassava-starch kaolinite composite film. Effect of clay content and clay modification on film properties. Carbohydr. Polym. 2012, 88, 213–222. [Google Scholar] [CrossRef]
- Uysal Unalan, I.; Cerri, G.; Marcuzzo, E.; Cozzolino, C.A.; Farris, S. Nanocomposite films and coatings using inorganic nanobuilding blocks (NBB): Current applications and future opportunities in the food packaging sector. RSC Adv. 2014, 4, 29393–29428. [Google Scholar] [CrossRef] [Green Version]
- Olivato, J.B.; Marini, J.; Pollet, E.; Yamashita, F.; Grossmann, M.V.E.; Avérous, L. Elaboration, morphology and properties of starch/polyester nano-biocomposites based on sepiolite clay. Carbohydr. Polym. 2015, 118, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Gul, S.; Kausar, A.; Muhammad, B.; Jabeen, S. Research Progress on Properties and Applications of Polymer/Clay Nanocomposite. Polym. Plast. Technol. Eng. 2016, 55, 684–703. [Google Scholar] [CrossRef]
- La Mantia, F.P.; Mistretta, M.C.; Scaffarol, R.; Botta, L.; Ceraulo, M. Processing and characterization of highly oriented fibres of biodegradable nanocomposites. Compos. Part B Eng. 2015, 78, 1–7. [Google Scholar] [CrossRef]
- Fadil-Djenabou, S.; Ndjigui, P.-D.; Mbey, J.A. Morphological and physicochemical characterization of Ngaye alluvial clays (Northern Cameroon) and assessment of its suitability in ceramic production. J. Asian Ceram. Soc. 2015, 3, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Ekosse, G.E. Kaolin deposits and occurrences in Africa: Geology, mineralogy and utilization. Appl. Clay Sci. 2010, 50, 212–236. [Google Scholar] [CrossRef]
- Nkoumbou, C.; Njoya, A.; Grosbois, C.; Njopwouo, D.; Yvon, J.; Martin, F. Kaolin from Mayouom (Western Cameroon): Industrial suitability evaluation. Appl. Clay Sci. 2009, 43, 118–124. [Google Scholar] [CrossRef]
- de Macêdo Neto, J.C.; Botan, R.; Lona, L.M.F.; Net, J.E.; Pippo, W.A. Polystyrene/kaolinite nanocomposite synthesis and characterization via in situ emulsion polymerization. Polym. Bull. 2015, 72, 387–404. [Google Scholar] [CrossRef]
- Chen, B.; Evans, J.R.G. Thermoplastic starch–clay nanocomposites and their characteristics. Carbohydr. Polym. 2005, 61, 455–463. [Google Scholar] [CrossRef]
- Mbey, J.A.; Thomas, F.; Ngally Sabouang, C.J.; Liboum; Njopwouo, D. An insight on the weakening of the interlayer bonds in a cameroonian kaolinite through DMSO intercalation. Appl. Clay Sci. 2013, 83–84, 327–335. [Google Scholar] [CrossRef]
- Cabedo, L.; Giménez, E.; Lagaron, J.M.; Gavara, R.; Saura, J.J. Development of EVOH-kaolinite nanocomposites. Polymer 2004, 45, 5233–5238. [Google Scholar] [CrossRef]
- Giese, R.F. Kaolin Minerals: Structures and Stabilities. In Hydrous Phyllosilicates; Bailey, S.W., Ed.; Mineralogical Society of America: Chantilly, VA, USA, 1988; pp. 29–66. [Google Scholar]
- Makó, E.; Kristóf, J.; Horváth, E.; Vágvölgyi, V. Mechanochemical intercalation of low reactivity kaolinite. Appl. Clay Sci. 2013, 83–84, 24–31. [Google Scholar] [CrossRef]
- Makó, E.; Kovács, A.; Kristóf, T. Influencing parameters of direct homogenization intercalation of kaolinite with urea, dimethyl sulfoxide, formamide, and N-methylformamide. Appl. Clay Sci. 2019, 182, 105287. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristóf, J.; Paroz, G.N.; Kloprogge, J.T. Intercalation of kaolinite with acetamide. Phys. Chem.Miner. 1999, 26, 257–263. [Google Scholar] [CrossRef]
- Frost, R.L.; Makó, E.; Kristóf, J.; Horváth, E.; Cseh, T. The effect of machanochemical activation upon the intercalation of a high-defect kaolinite with formamide. J. Colloid Inter. Sci. 2003, 265, 386–395. [Google Scholar] [CrossRef]
- Frost, R.L.; Kristof, J.; Horvath, E. Vibrational spectroscopy of intercalated kaolinites. Part, I. Appl.Spectrosc. Rev. 2010, 45, 130–147. [Google Scholar] [CrossRef] [Green Version]
- Itagaki, T.; Komori, Y.; Sugahara, Y.; Kuroda, K. Synthesis of a kaolinite–poly(β-357 alanine) intercalation compound. J. Mater. Chem. 2001, 11, 3291–3295. [Google Scholar] [CrossRef]
- Olejnik, V.S.; Posner, A.M.; Quirk, J.P. The intercalation ofpolar organic compound into kaolinite. Clay Miner. 1970, 8, 421–434. [Google Scholar] [CrossRef]
- Olejnik, V.S.; Aylmore, L.A.G.; Posner, A.M.; Quirk, J.P. Infrared Spectra of Kaolin Mineral-Dimethyl Sulfoxide Complexes. J. Phys. Chem. 1968, 72, 241–249. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.; Cheng, H.; Gao, F.; Liu, C.; Teppen, B.J. Mechanism Responsible for Intercalation of Dimethyl Sulfoxide in Kaolinite: Molecular Dynamics Simulations. Appl. Clay Sci. 2018, 151, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Huang, S.; Wang, W. Intercalation of dimethyl sulphoxide in kaolinite: Molecular dynamics simulation study. Chem. Phys. Lett. 2005, 411, 233–237. [Google Scholar] [CrossRef]
- Gardolinski, J.E.; Carrera, L.C.M.; Wypych, F. Layered polymer-kaolinite nanocomposites. J. Mater. Sci. 2000, 35, 3113–3119. [Google Scholar] [CrossRef]
- Hinckley, D.N. Variability in “crystallinity” Values among the Kaolin Deposits of the Coastal Plain of Georgia and South Carolina. Clays Clay Miner. 1962, 11, 229–235. [Google Scholar] [CrossRef]
- Mbey, J.A.; Thomas, F.; Razafitianamaharavo, A.; Caillet, C.; Villieras, F. A comparative study of some kaolinites surface properties. Appl. Clay Sci. 2019, 172, 135–145. [Google Scholar] [CrossRef]
- Cases, J.M.; Lietard, O.; Yvon, J.; Delon, J.F. Etude des propriétés cristallochimiques, morphologiques, superficielles des kaolinites désordonnées. Bull. Mineral. 1982, 105, 439–455. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Wu, Z.; Zheng, Q.; Cheng, H. Thermal behavior analysis of kaolinite–dimethylsulfoxide intercalation complex. J. Therm. Anal. Calorim. 2012, 110, 1167–1172. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquérol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sayed Hassan, M.; Villiéras, F.; Razafitianamaharavo, A.; Michot, L.J. Role of exchangeable cations on geometrical and energetic surface heterogeneity of kaolinites. Langmuir 2005, 21, 12283–12289. [Google Scholar] [CrossRef]
- Delineau, T. Les Argiles Kaoliniques Du Bassin de Charentes (France): Analyses Typologiques, Cristallochimique, Speciation du Fer et Applications. Ph.D. Thesis, Université de Lorraine, INPL-Nancy, France, 1994; 627p. [Google Scholar]


| Samples | MY3 | MY3-D | MAC | MAC-D | KGa2 | Kga2-D | GZA4 | GZA4-D |
|---|---|---|---|---|---|---|---|---|
| d001 (Å) | 7.20 | 11.26 | 7.20 | 11.35 | 7.20 | 11.35 | 7.21 | 11.48 |
| D (Å) | 169 | 292 | 439 | 518 | 339 | 434 | 262 | 373 |
| SSA (m2/g) BET | 25.9 | 20.3 | 18.7 | 18.0 | 21.0 | 19.4 | 24.9 | 25 |
| External SSA (m2/g) t-plot | 25.7 | 20.4 | 17.4 | 16.0 | 20.6 | 18.1 | 22.9 | 22.7 |
| Microporous SSA (m2/g) t-plot | 0.3 | 0 | 1.0 | 0 | 0 | 0.6 | 1.1 | 0 |
| Hinckley Index (HI) | 1.05 | / | 0.86 | / | 0.44 | / | 0.57 | / |
| p2 | 1.04 | 1.13 | 1.23 | 1.13 | ||||
| Number of Layer/Crystallite | 23 | 26 | 61 | 46 | 47 | 38 | 36 | 33 |
| Intercalation ratio (I.R) (%) | / | 96 | / | 88 | / | 71 | / | 73 |
Publisher’sNote: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbey, J.-A.; Siéwé, J.M.; Ngally Sabouang, C.J.; Razafitianamaharavo, A.; Kong, S.; Thomas, F. DMSO Intercalation in Selected Kaolinites: Influence of the Crystallinity. ChemEngineering 2020, 4, 66. https://doi.org/10.3390/chemengineering4040066
Mbey J-A, Siéwé JM, Ngally Sabouang CJ, Razafitianamaharavo A, Kong S, Thomas F. DMSO Intercalation in Selected Kaolinites: Influence of the Crystallinity. ChemEngineering. 2020; 4(4):66. https://doi.org/10.3390/chemengineering4040066
Chicago/Turabian StyleMbey, Jean-Aimé, Jean Mermoz Siéwé, Cyrill Joël Ngally Sabouang, Angelina Razafitianamaharavo, Sakeo Kong, and Fabien Thomas. 2020. "DMSO Intercalation in Selected Kaolinites: Influence of the Crystallinity" ChemEngineering 4, no. 4: 66. https://doi.org/10.3390/chemengineering4040066
APA StyleMbey, J.-A., Siéwé, J. M., Ngally Sabouang, C. J., Razafitianamaharavo, A., Kong, S., & Thomas, F. (2020). DMSO Intercalation in Selected Kaolinites: Influence of the Crystallinity. ChemEngineering, 4(4), 66. https://doi.org/10.3390/chemengineering4040066





