Nanocomposites of Barium Titanate Nanoparticles Embedded in Thermosetting Polymer Matrices (Novolac Resin/Unsaturated Polyesters/Epoxy Resin): A Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Polymer Synthesis
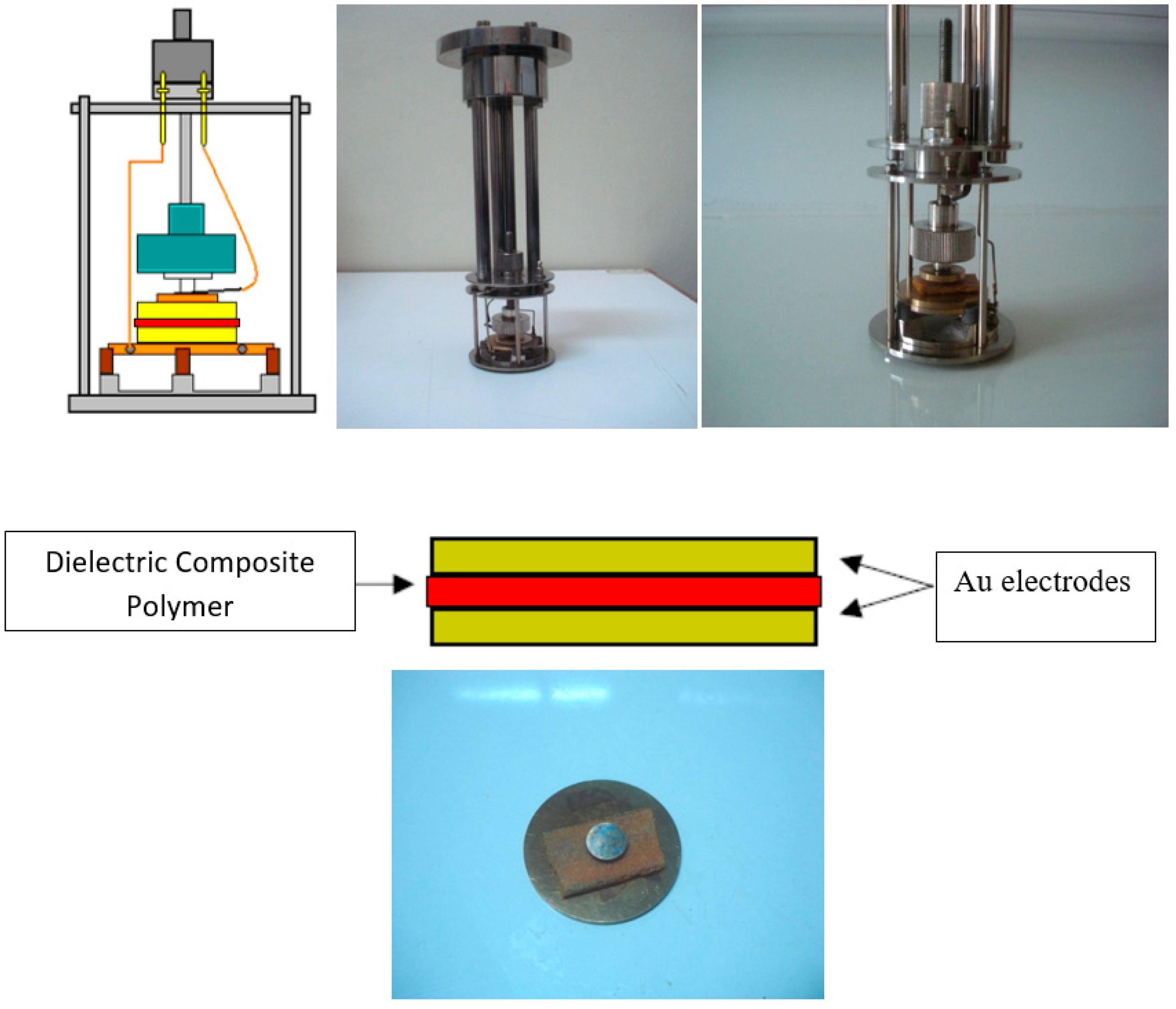
2.2. Composite Specimens Preparation—The Curing Process
2.3. Structural Characterization
2.4. Thermal Analysis
2.5. Mechanical Characterization
2.6. Dielectric Characterization
3. Results
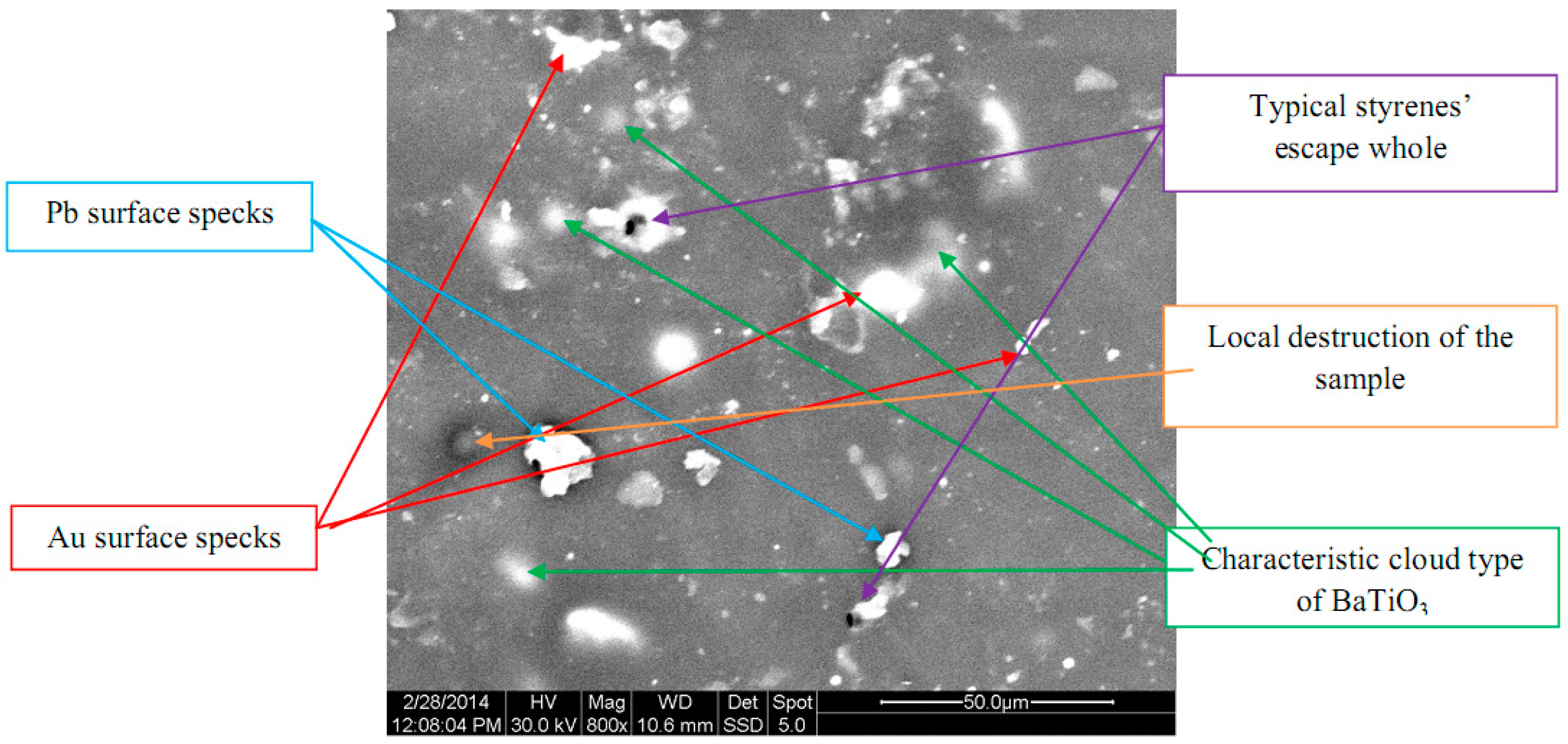
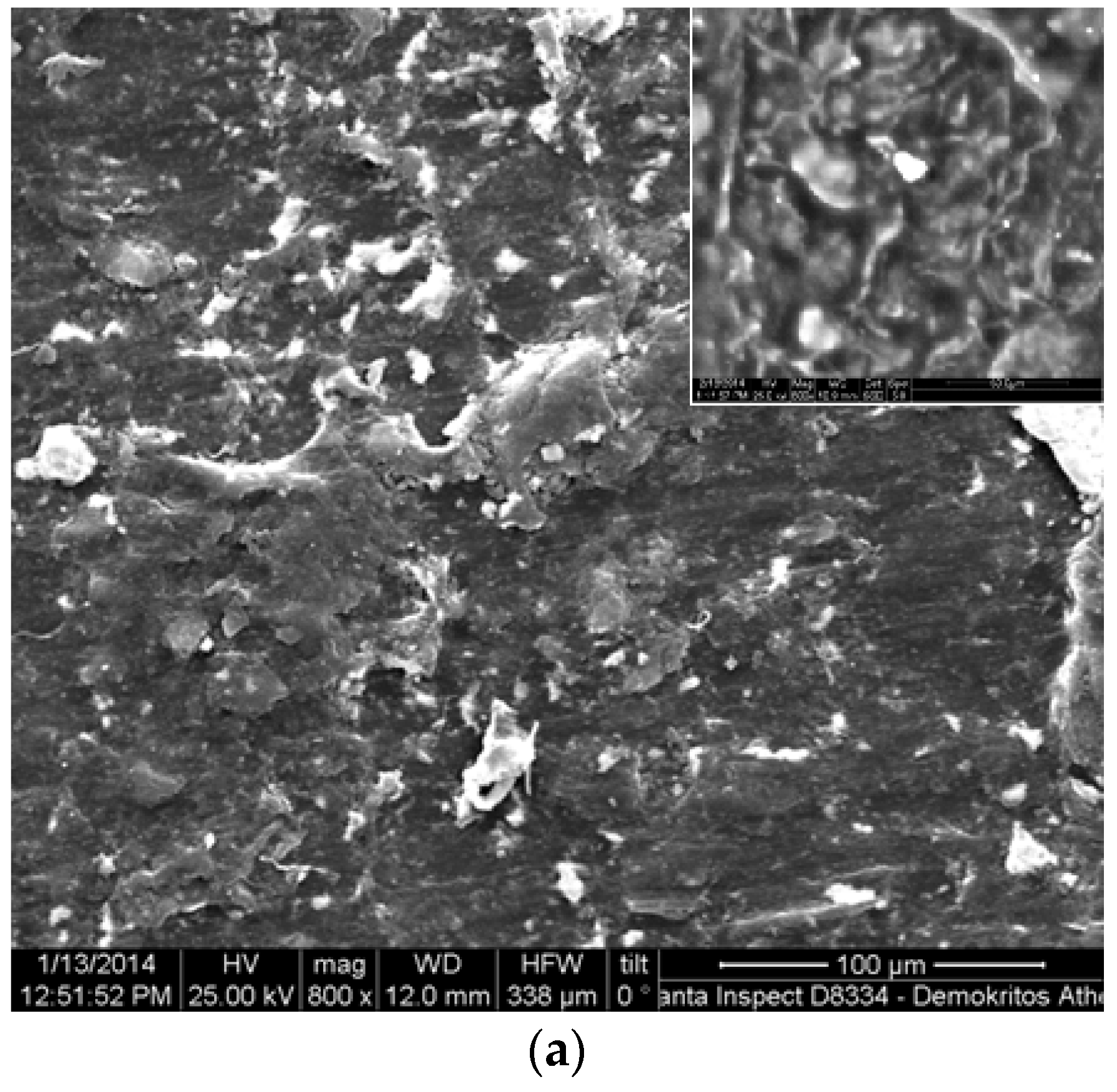
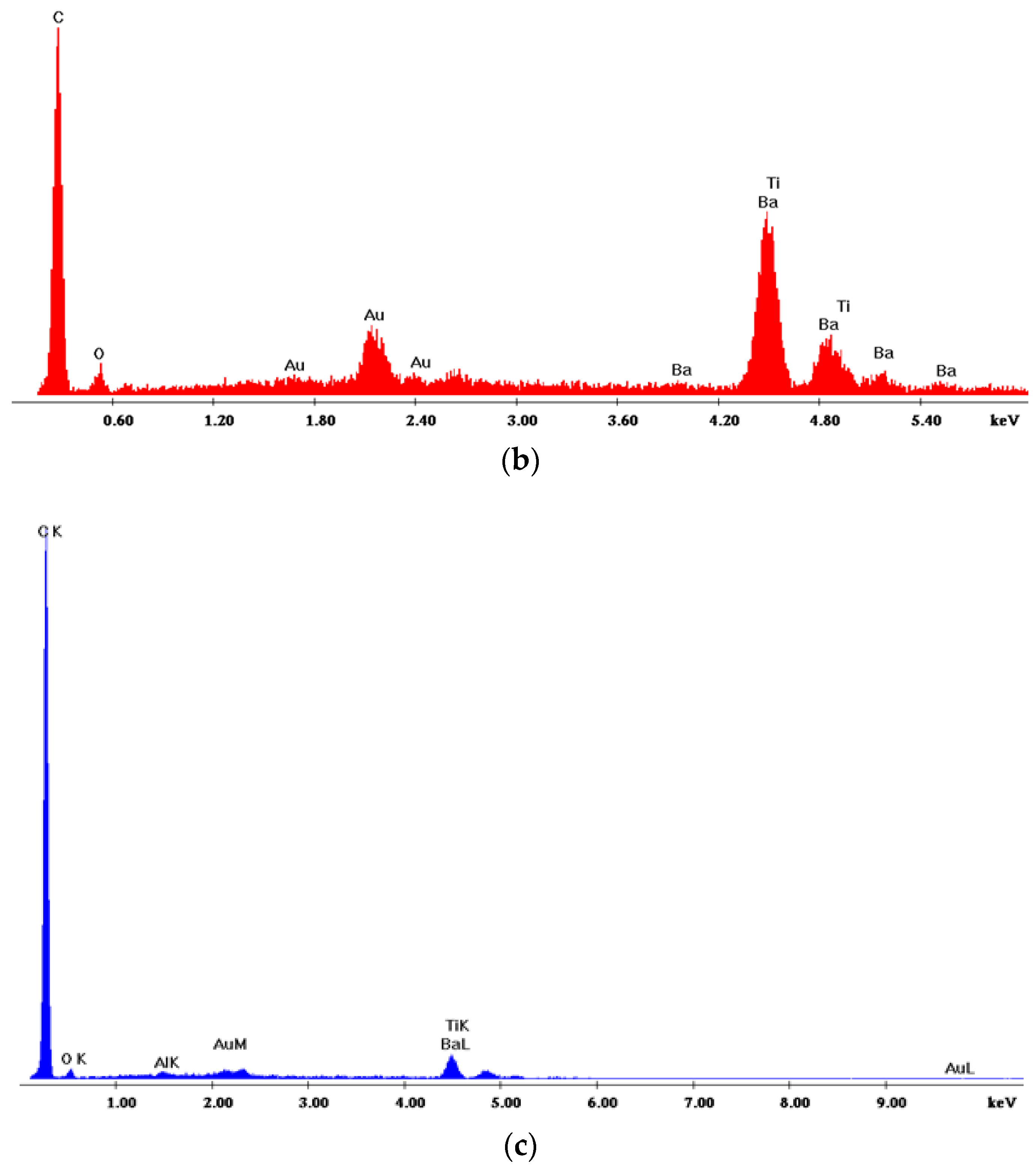
3.1. Scanning Electron Microscopy (SEM) and Energy Dispersive X-Ray Spectroscopy (EDAX) Characterization
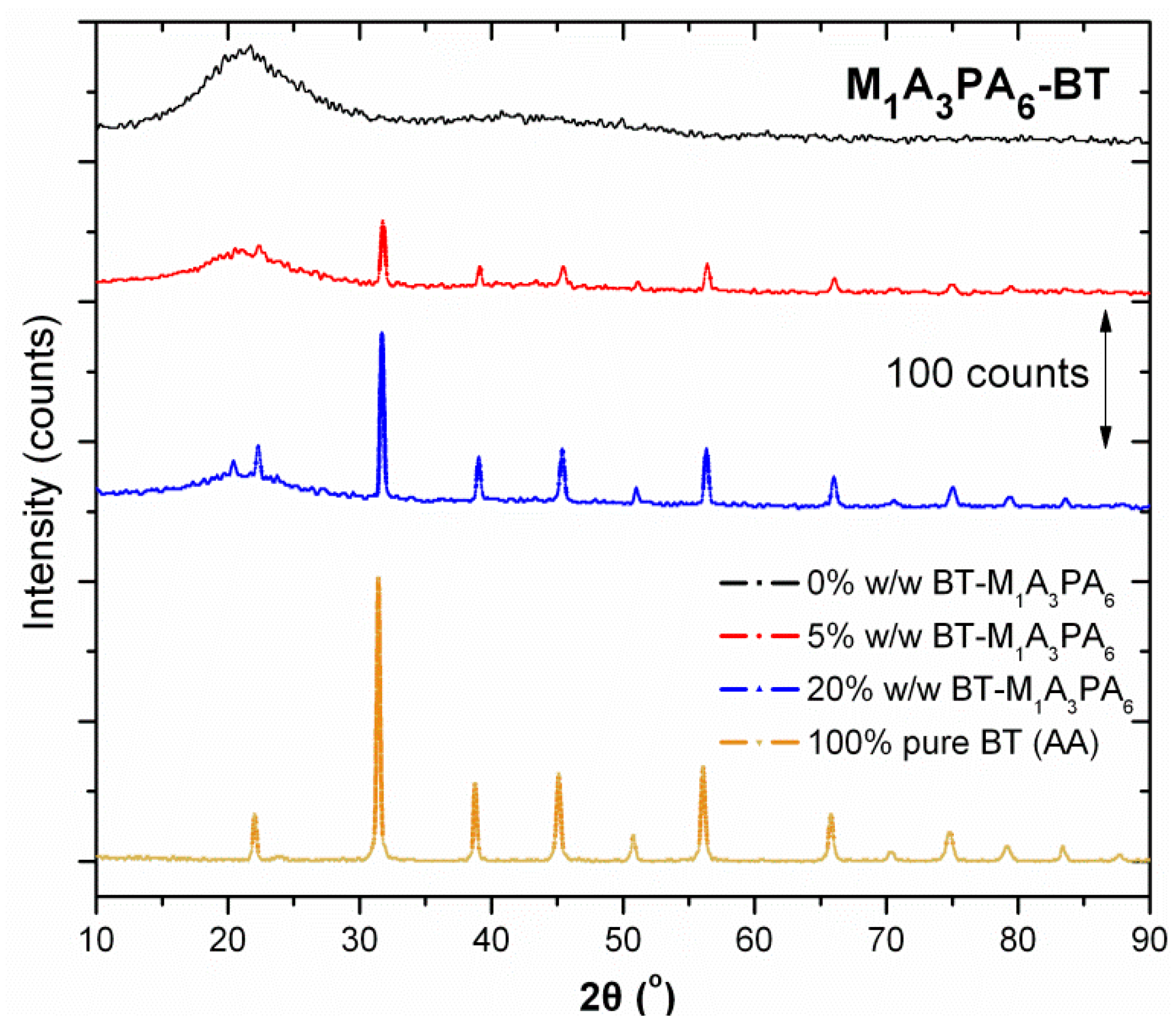
3.2. X-Ray Diffraction (XRD) Characterization
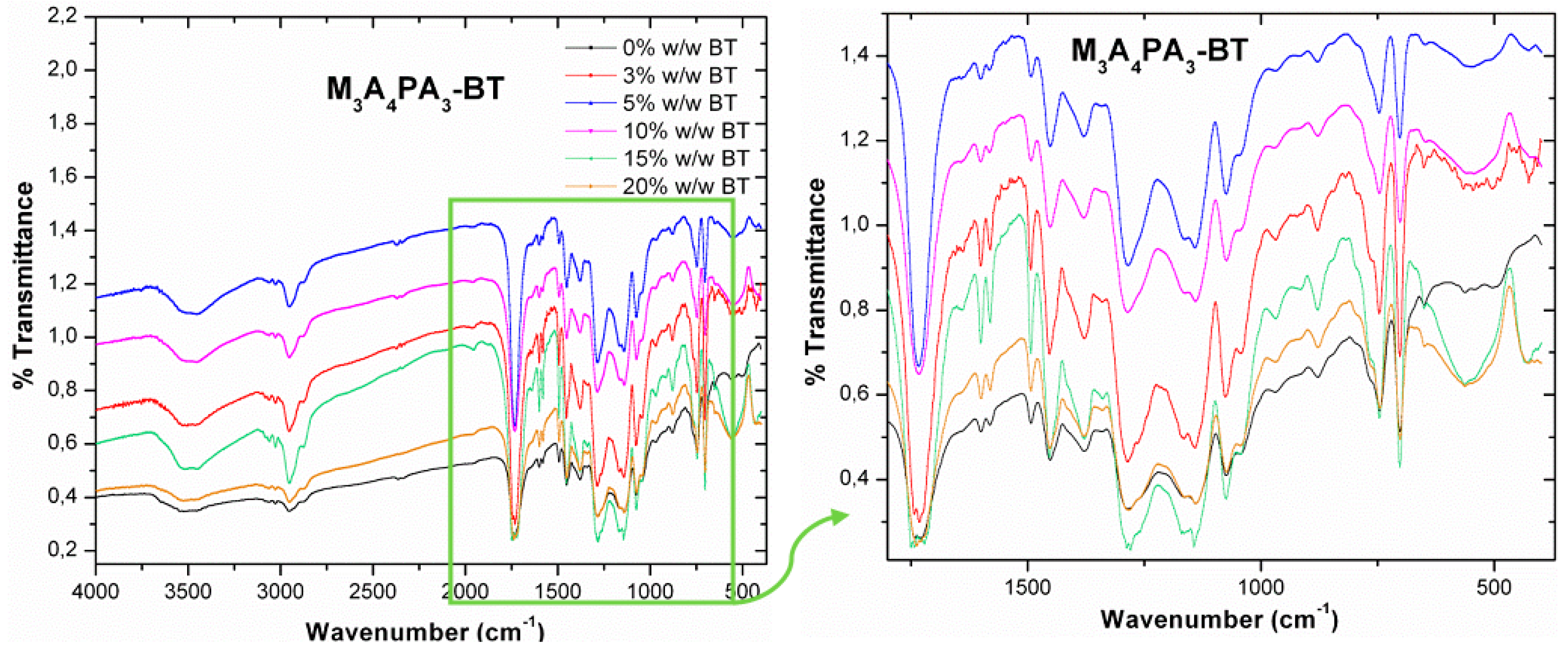
3.3. Infra Red Spectroscopy via Fourier Transformation (FT-IR) Characterization
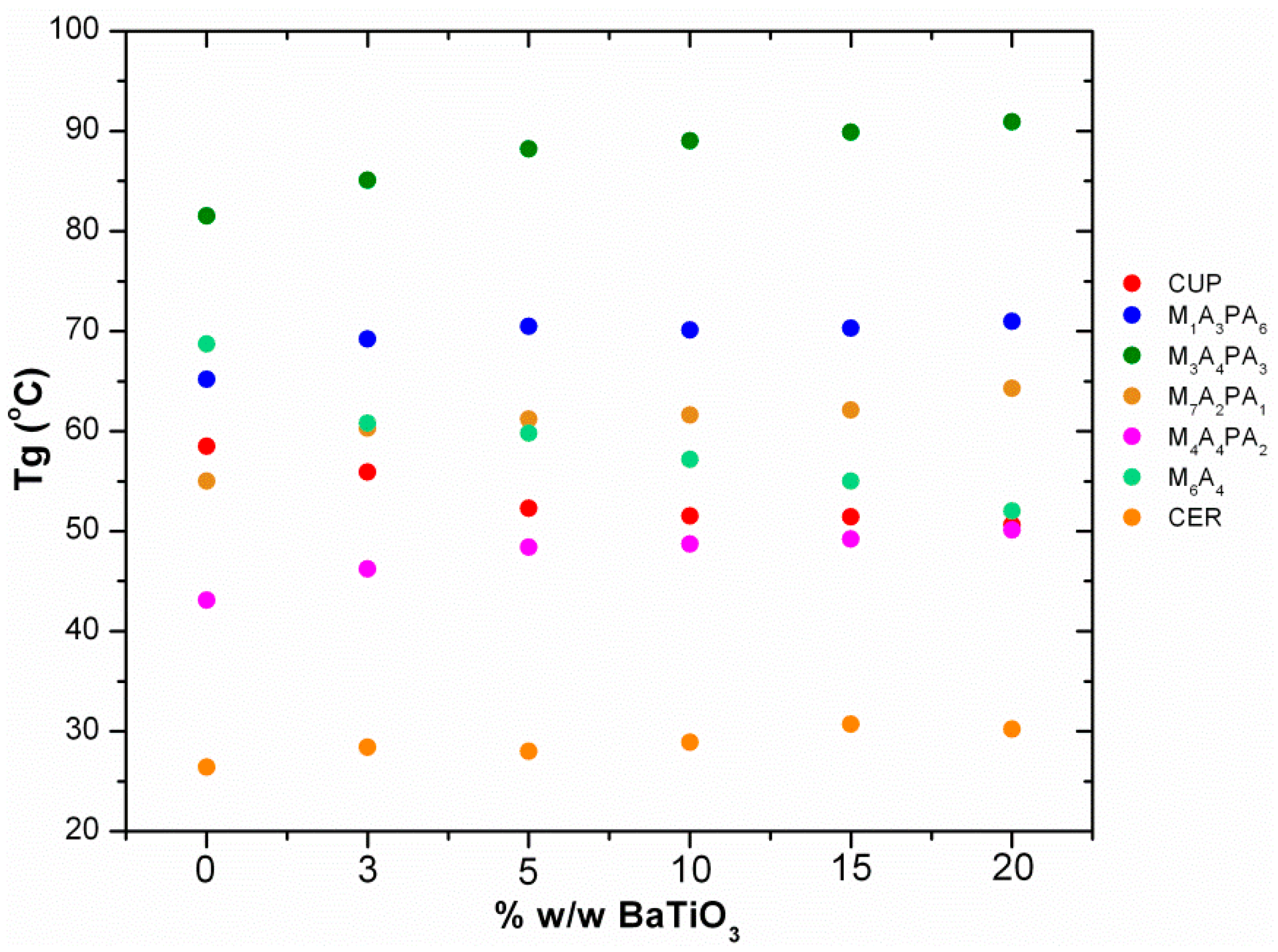
3.4. Thermal Characterization
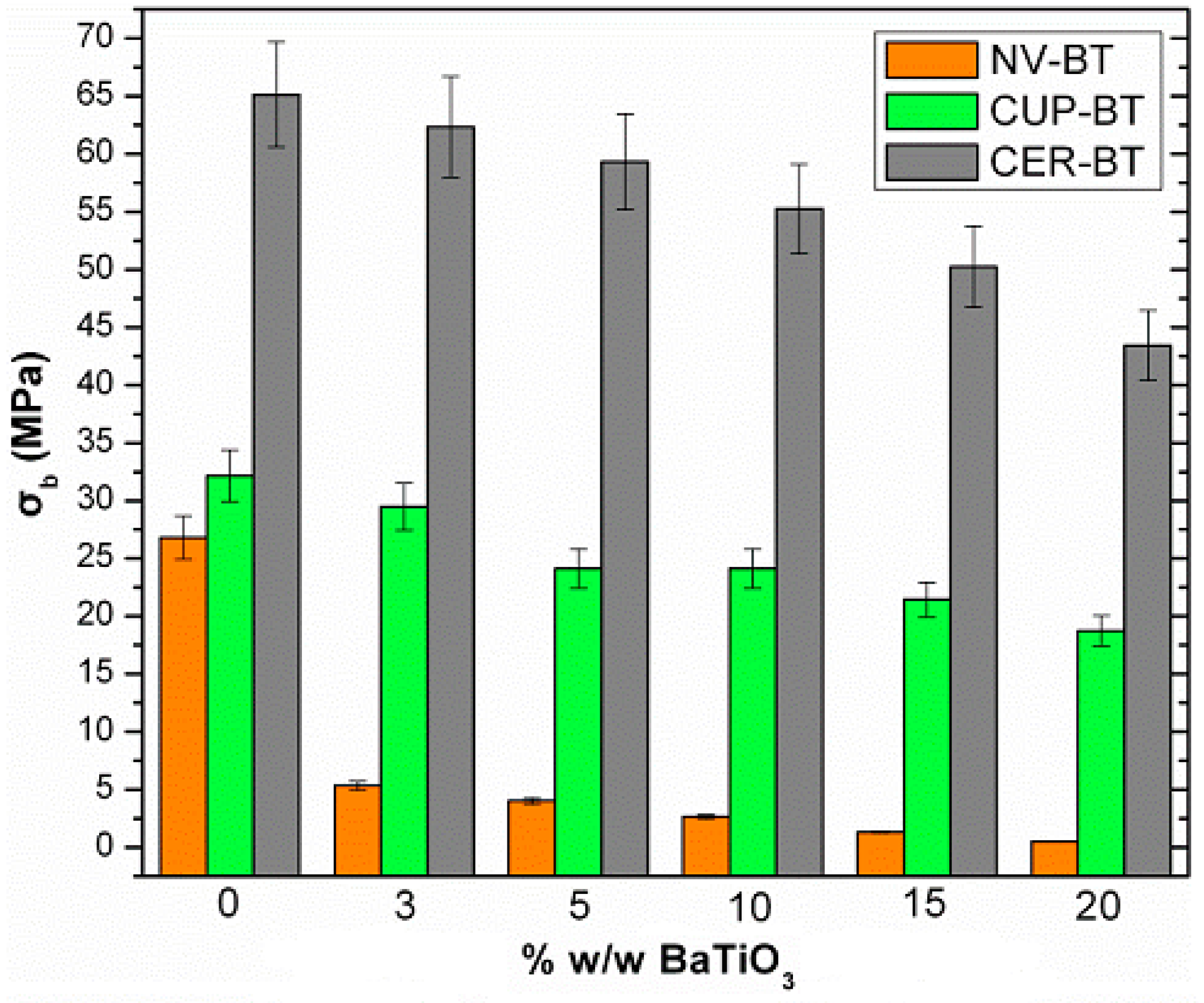
3.5. Mechanical Characterization
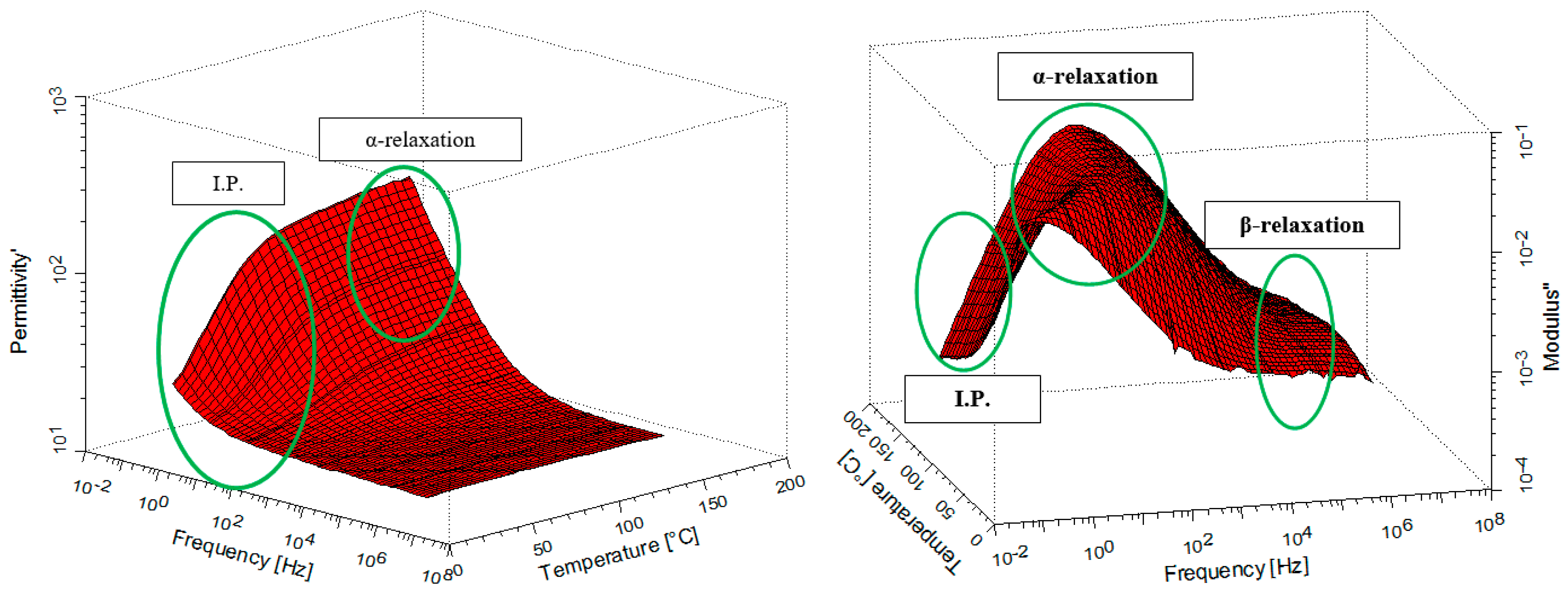
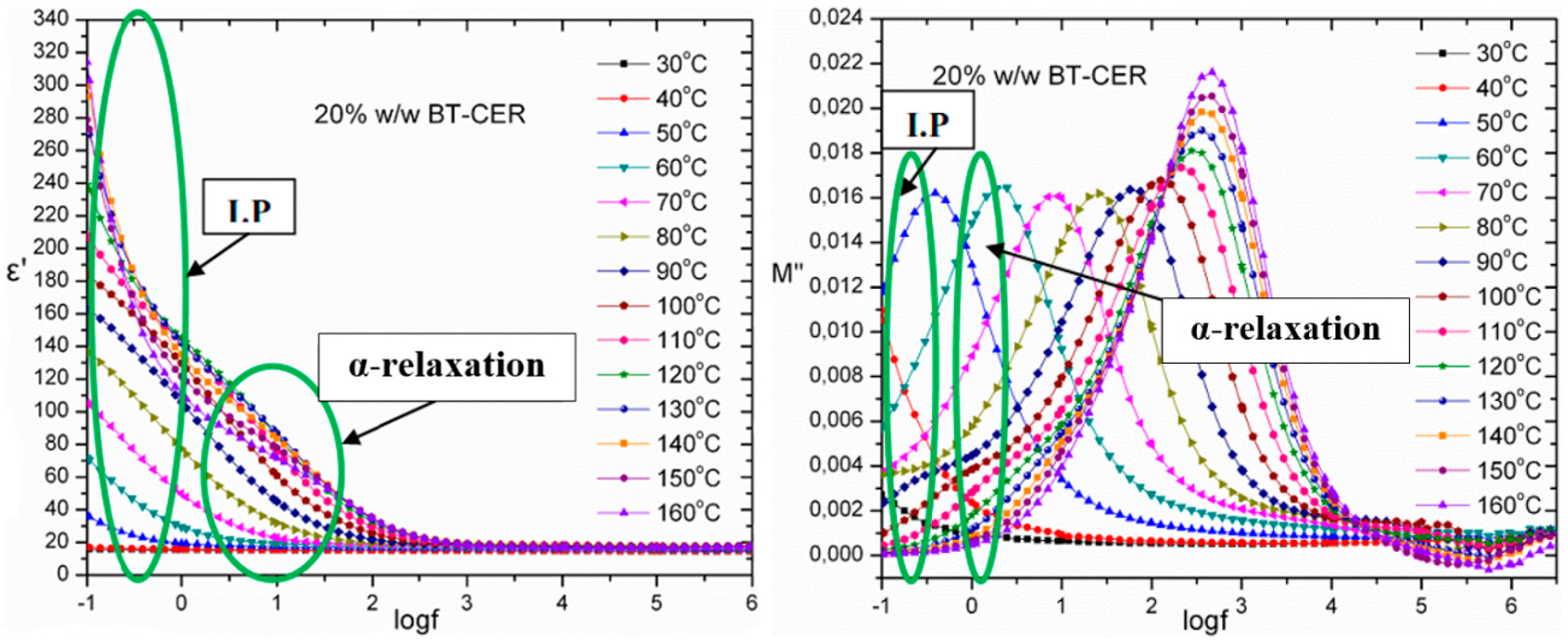
3.6. Broadband Dielectric Spectroscopy (BDS) Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Asimakopoulos, I.A.; Psarras, G.C.; Zoumpoulakis, L. Barium titanate/polyester resin nanocomposites: Development, structure-properties relationship and energy storage capability. Express Polym. Lett. 2014, 8, 692–707. [Google Scholar] [CrossRef]
- Asimakopoulos, I.; Zoumpoulakis, L.; Psarras, G.C. Development and Characterization of a Novolac Resin/BaTiO3 Nanoparticles Composite System. J. Appl. Polym. Sci. 2012, 125, 3737–3744. [Google Scholar] [CrossRef]
- Asimakopoulos, I.A. Development, Characterization and Properties of Polymer Matrix Dielectric Composite Materials. Ph.D. Thesis, National Technical University of Athens, Athens, Greece, 2015. [Google Scholar]
- Abraham, R.; Thomas, S.P.; Kuryan, S.; Isac, J.; Varughese, K.T.; Thomas, S. Mechanical properties of ceramic-polymer nanocomposites. Express Polym. Lett. 2009, 3, 177–189. [Google Scholar] [CrossRef]
- Smay, J.E.; Cerano, S.; Tuttle, B.A.; Lewis, J.A. Piezo electric properties of 3-X periodic Pb(ZrxTi1-x)O3-polymer composites. J. Appl. Phys. 2002, 92, 6119–6127. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Choudhary, R.N.P.; Samantaray, B.K. Studies of structural, thermal and electrical behavior of polymer nanocomposites electrolytes. Express Polym. Lett. 2008, 2, 630–638. [Google Scholar] [CrossRef]
- Simitzis, J. Correlation between the production parameters and the mechanical properties of novolac resins reinforced with carbon fibers. J. Angew. Makromol. Chem. 1989, 165, 21–34. [Google Scholar] [CrossRef]
- Braun, D.; Cherdron, H.; Kern, W. Praktikum der Makromolekularen Organischen Chemie; Huthig Verlag: Heidelberg, Germany, 1971. [Google Scholar]
- Simitzis, J.; Karagianis, K.; Zoumpoulakis, L. Curing of novolac—Lignocellulosic Composites. Polym. Int. 1995, 38, 183–189. [Google Scholar] [CrossRef]
- Simitzis, J.; Karagianis, K.; Zoumpoulakis, L. Influence of biomass on the curing of novolac-composites. Eur. Polym. J. 1996, 32, 857–863. [Google Scholar] [CrossRef]
- Asimakopoulos, I.; Psarras, G.C.; Zoumpoulakis, L. Development and Characterization of a Novolac resin/BaTiO3 nanoparticles composite system. In Proceedings of the 8th Hellenic Polymer Society Symposium (HPOL8), Hersonissos, Crete, Greece, 24–29 October 2010; p. 215. [Google Scholar]
- Asimakopoulos, I.A.; Psarras, G.C.; Zoumpoulakis, L. Study on maleic acid/adipic acid/phthalic anhydride/ethylene glycol based unsaturated polyesters, with embedded ferroelectric ΒaTiO3 nano-particles, composite systems. In Proceedings of the 10th Hellenic Polymer Society Conference (10th HPSC) with International Participation, Patras, Greece, 4–6 December 2014; pp. 296–298. [Google Scholar]
- Simitzis, J.; Zoumpoulakis, L.; Soulis, S. Effect of composition and polyesterification catalysts on the optical properties of cured polyesters. Polym. Int. 2002, 51, 297–307. [Google Scholar] [CrossRef]
- Simitzis, J.; Zoumpoulakis, L.; Soulis, S. DSC curing study of catatytically synthesized maleic-acid-based unsaturated polyesters. Polym. Int. 2002, 51, 308–318. [Google Scholar] [CrossRef]
- Simitzis, J.; Zoumpoulakis, L.; Soulis, S. Review of the research results concerning the synthesis, curing, structure and properties of unsaturated polyesters. Curr. Trends Polym. Sci. 2003, 8, 107–125. [Google Scholar]
- Pomakis, I.; Simitzis, I. A new method to control the polyesterification process. Prospects of application in the production plants. Angew. Makromol. Chem. 1981, 99, 145–170. [Google Scholar] [CrossRef]
- Asimakopoulos, I.A.; Psarras, G.C.; Zoumpoulakis, L. Mechanical and Dielectric Properties of Barium Titanate/Polyester Nano-Composite Materials. In Proceedings of the 9th International Conference on Nanosciences & Nanotechnologies (NN12), Thessaloniki, Greece, 3–6 July 2012; p. 215. [Google Scholar]
- Asimakopoulos, I.A.; Psarras, G.C.; Zoumpoulakis, L. Mechanical and Dielectric Properties versus Structure: Study of epoxy resin /barium titanate nanocomposites. In Proceedings of the 30th Panhellenic Conference on Solid-State Physics and Materials Science (30th SSPMS), Heraklion, Crete, Greece, 21–24 September 2014; p. 187. [Google Scholar]
- Asimakopoulos, I.A.; Psarras, G.C.; Zoumpoulakis, L. Structure-Properties relationship and Energy Storage capability of Nano-Composite System: Polyester polymer matrix/barium titanate particles. In Proceedings of the 28th Panhellenic Conference on Solid State Physics and Materials Science (28th SSPMS), Patras, Greece, 23–26 September 2012; Available online: http://xxviii.physics.upatras.gr/program.html (accessed on 20 September 2012).
- Asimakopoulos, I.A.; Psarras, G.C.; Zoumpoulakis, L. Composite Materials Comparative Study: Consisting of three kinds of Thermosetting Polymer Matrices (phenol-formaldehyde, 8 kinds of different composition unsaturated polyesters and epoxy) and Ferroelectric Ceramic Nano-Particles of Barium Titanate. In Proceedings of the 10th Chemical Engineering Panhellenic Conference, Patras, Greece, 4–6 June 2015; p. 49. [Google Scholar]
- Orthamann, M. Die Prūfung thermoplastischer Kunstoffe; Carl Hanser Verlag: München, Germany, 1971. [Google Scholar]
- Bϋrger, A. Kohlenstoffasen-Verstärker Polymere und deren Ehernischer, Abbaw bis zu Kohlenstoff-Verbund-Werkstoffen (in German). Ph.D. Thesis, Karlsruhe Institute of Technology, Karlsruhe, Germany, 1973. [Google Scholar]
- Skoog, D.A.; Holler, F.J.; Nieman, T.A. Principles in Instrumental Analysis; Kostarakis Publ.: Athens, Greece, 2005. [Google Scholar]
- Valavanides, T. Basic Principles in Molecular Spectroscopy and Applications in Organic Chemistrie; Sichrona Themata Publ.: Athens, Greece, 2008. [Google Scholar]
- Mc Murry, J. Organic Chemistry; Crete University Publ.: Herakleion, Greek, 2007. [Google Scholar]
- Atta, A.M.; Nassar, I.F.; Bedawy, H.M. Unsaturated polyester resins based on rosin maleic anhydride adduct as corrosion protections of steel. React. Funct. Polym. 2007, 67, 617–626. [Google Scholar] [CrossRef]
- Abdallh, M.; Bakir, E.; Yousif, E. Study the electrical conductivity of crosslinked polyester doped with different metal salts. J. Saudi Chem. Soc. 2014, 18, 387–391. [Google Scholar] [CrossRef]
- Cho, L.L. Identification of textile fiber by Raman microspectroscopy. Forensic Sci. J. 2007, 6, 55–62. [Google Scholar]
- Oswal, S.L.; Pandya, A.K. Synthesis and Characterization of Linear Aromatic Polyester-amides from Diacid Chlorides and Aminophenols. Iran. Polym. J. 2004, 13, 205–212. [Google Scholar]
- Psarras, G.C.; Manolakaki, E.; Tsangaris, G.M. Dielectric Dispersion and ac Conductivity in—Iron Particles Loaded—Polymer Composites. J. Compo. A 2003, 34, 1187–1198. [Google Scholar] [CrossRef]
- Psarras, G.C.; Manolakaki, E.; Tsangaris, G.M. Electrical Relaxations in Polymeric Particulate Composites of Epoxy Resin and Metal Particles. J. Compos. A 2002, 33, 375–384. [Google Scholar] [CrossRef]
- Tsangaris, G.M.; Psarras, G.C.; Kouloumbi, N. Electric modulus and interfacial polarization in composite polymeric systems. J. Mater. Sci. 1998, 33, 2027–2037. [Google Scholar] [CrossRef]
- Dang, Z.M.; Yuan, J.K.; Zha, J.W.; Zhou, T.; Li, S.T.; Hu, G.H. Fundamentals, processes and applications of high-permittivity polymer-matrix composites. Prog. Mater. Sci. 2012, 57, 660–723. [Google Scholar] [CrossRef]


| Unsaturated Polyester Code Name | % mol Diacid/Total mol | % mol Diole/Total mol | ||
|---|---|---|---|---|
| Maleic Acid (M) | Adipic Acid (A) | Phthalic Anydride (PA) | Ethylene Glycol (EG) | |
| M1A3PA6 | 10 | 30 | 60 | 110 |
| M3A4PA3 | 30 | 40 | 30 | 110 |
| M4A4PA2 | 40 | 40 | 20 | 110 |
| M7A2PA1 | 70 | 20 | 10 | 110 |
| M6A4 | 60 | 40 | 0 | 110 |
| Unsaturated Polyester Code Name | A.N. (mg KOH/gr Polyester) |
|---|---|
| M1A3PA6 | 34 |
| M3A4PA3 | 37 |
| M4A4PA2 | 41 |
| M7A2PA1 | 47 |
| M6A4 | 43 |
| Unsaturated Polyester Code Name | Ea | ko (kg∙mol−2∙min−1) | R2 | |
|---|---|---|---|---|
| (kcal/mol) | (kJ/mol) | |||
| M1A3PA6 | 32.19 | 134.69 | 6.53 × 1013 | 0.957 |
| M3A4PA3 | 19.87 | 83.14 | 3.60 × 107 | 0.871 |
| M4A4PA2 | 14.45 | 60.44 | 2.27 × 104 | 0.738 |
| M7A2PA1 | 17.60 | 73.66 | 2.19 × 106 | 0.779 |
| M6A4 | 26.27 | 110.09 | 2.92 × 1010 | 0.986 |
| Specimen Type | Chemical Element % w/w | ||||
|---|---|---|---|---|---|
| C (Κ) | O (Κ) | Ba (L) | Ti (Κ) | Total | |
| 20% w/w NV-BT | 86.73 | 5.44 | 6.35 | 1.49 | 100.00 |
| 20% w/w M7A2PA1-BT | 43.27 | 16.79 | 33.42 | 6,52 | 100.00 |
| 20% w/w CER-BT | 65.57 | 5.00 | 23.67 | 5.76 | 100.00 |
| Composite Polymer Matrix | 2θ (°) |
|---|---|
| M1A3PA6 | 21.20 |
| M3A4PA3 | 21.37 |
| M4A4PA2 | 21.50 |
| M6A4 | 21.40 |
| M7A2PA1 | 21.49 |
| CUP | 19.72 |
| NV | 18.38 |
| CER | 17.67 |
| Characteristic Chemical Group | Wavelength from Literature (cm−1) | Peak Appearance Wavelength (cm−1) |
|---|---|---|
| Cured Novolac Resin (NV) (Phenol-Phormaldehyde resin) | ||
| -OH stretch vibration | 3435–3383 | 3367 w/br |
| CH, >CH2, -CH3 stretch vibration, aliphatic parts | 2960–2850 | 2954 w/sh, 2923 m/sh, 2856 m/br |
| C=C stretch vibration within the aromatic ring | 1635–1630 | 1637 s/br |
| C=C stretch vibration within the aromatic ring | 1615–1600 | 1610 s/br |
| C=C stretch vibration within the aromatic ring | 1510–1504 | 1509 w/sh, 1498 w/sh |
| CH, >CH2, -CH3 bending vibration aliphatic parts (asymmetric bending) | 1470–1457 | 1473 w/sh, 1457 m/sh |
| -ΟΗ bending vibration | 1438–1415 | 1438 w/sh |
| CH, >CH2, -CH3 bending vibration aliphatic parts (symmetric bending) | 1378–1370 | 1371 m/sh |
| -ΟΗ bending vibration | 1330–1320 | 1340 w/br |
| CH, bending Hexa (HMTA) | 1244–1240 | 1238 s/sh |
| stretch vibration within the aromatic ring | 1210–1100 | 1213 w/br, 1141 w/br |
| Aromatic ring asymmetric rings | 1100–1109 | 1100 w/sh |
| Aromatic ring asymmetric rings | 1097–1050 | 1072 w/br, 1047 w/br |
| Hexa (HMTA) | 1007–1000 | 1008 s/sh |
| C-H Deformation vibration 1 or 2 neighbour Η of benzene ring (para-substituents) | 814–810 | 813 m/sh |
| C-H Deformation vibration 3 or 4 neighbour Η of benzene ring (meta- or ortho-substituents) | 778–750 | 757 m/br |
| C-H Deformation vibration 5 neighbour Η of benzene ring | 672–666 | 673 m/sh |
| Characteristic Chemical Group | Wavelength from Literature (cm−1) | Peak Appearance Wavelength (cm−1) | |||||
|---|---|---|---|---|---|---|---|
| Composite’s Cured Unsaturated Polyester Polymer Matrix | |||||||
| M1A3PA6 | M3A4PA3 | M4A4PA2 | Μ6A4 | M7A2PA1 | CUP | ||
| -OH stretch vibration | 3600–3200 | 3512 m/br 3452 m/br | 3523 m/br 3452 m/br | 3446 s/br | 3540 w/br 3459 w/br | 3456 s/br | 3534 w/br 3443 w/br |
| C-H stretch vibration, styrene’s aromatic ring | 3024 | 3061 w/sh 3026 w/sh | 3064 w/sh 3028 w/sh | 3064 w/sh 3030 w/sh | 3061w/sh 3029 w/sh | 3062 w/sh 3026 w/sh | 3062 w/sh 3028 w/sh |
| >CH2, -CH3 stretch vibration, aliphatic parts | 2980–2950 | 2953 m/sh | 2954 m/sh | 2956 m/sh | 2958 m/sh | 2951 m/sh | 2933 m/sh |
| >CH2 vibration stretch, circular and linear parts | 2890–2850 | 2885 w/sh | 2875 w/sh | 2881 w/sh | 2878 w/sh | 2883 w/sh | 28857 w/sh |
| CO2 | 2360 | 2345 w/sh | 2362 w/sh 2339 w/br | 2364 m/sh 2337 w/sh | 2351 w/sh | 2343 w/sh | 2346 w/sh |
| >C=O Ester bond stretch vibration | 1736–1726 | 1734 s/sh | 1728 s/sh | 1734 s/sh | 1736 s/sh | 1734 s/sh | 1731 s/sh |
| C=C stretch vibration within the aromatic ring | 1600, 1580, 1500 | 1598 m/sh, 1579 m/sh, 1492 m/sh | 1598 w/sh, 1579 w/sh, 1492 w/sh | 1602 w/br, 1583 w/sh, 1492 w/sh | 1603 w/sh, -, 1495 w/sh | -, -, 1490 w/sh | 1600 m/sh, 1581 m/sh, 1494 m/sh |
| C-C stretch vibration within the aromatic ring | 1480–1400 | 1450 m/sh | 1452 s/sh | 1452 m/sh | 1456 m/sh | 1450 m/sh | 1453 s/sh |
| CH3 Symmetric bending vibration | 1390–1370 | 1373 m/sh | 1379 m/sh | 1386 m/sh | 1393 m/sh | 1394 m/sh | 1381 w/sh |
| Csp3-O (ester bond) | 1330–1200 | 1284 s/sh | 1284 s/sh | 1286 s/sh | 1250 w/br | 1282 s/sh | 1283 s/sh |
| Csp3-O (alcohol bond) | 1200–1100 | 1166 w/sh, 1136 m/sh | 1163 w/sh, 1139 m/sh | 1155 m/br | 1162 m/sh | 1161 m/sh | 1122 m/sh |
| C-H «in plane» vibrations | 1080–1036 | 1072 m/sh | 1074 m/sh | 1074 m/sh | 1065 m/br | 1074 m/sh | 1068 m/sh |
| C=C unsaturated polyester’s double bond | 982 | 964 w/sh | 970 w/br | - | 978 w/sh | 981 w/br | 990 w/br |
| C=C Styrene’s double bond | 912 | 904 w/sh | - | 925 w/br | _ | - | 913 w/br |
| C-H Deformation vibration «out of plane» 1 or 2 neighbour Η of benzene ring | 876–815 | 877 w/sh | 877 w/sh | 879 w/sh | 877 w/sh 814 w/br | 879 w/sh | 847 m/br |
| C-H Deformation vibration «out of plane» 3 or 4 neighbour Η of benzene ring | 750 | 744 s/sh | 746 s/sh | 748 s/sh | 764 m/sh | 754 s/sh | 745 s/sh |
| C-H Deformation vibration «out of plane» 5 neighbour Η of benzene ring | 700, 666 | 700 s/sh, 650 w/sh | 702 s/sh | 702 s/sh, 650 w/br | 703 s/sh | 702 s/sh | 700 s/sh 649 w/br |
| Characteristic Chemical Group | Wavelength from Literature (cm−1) | Peak Appearance Wavelength (cm−1) |
|---|---|---|
| Cured Epoxy Resin (CER) | ||
| -OH stretch vibration | 4000–3400 | 3416 s/br |
| C-H Aromatic ring stretch vibration, | 3024 | 3034 w/sh |
| >CH2, -CH3 stretch vibration, aliphatic parts | 3100–2800 (usually between 2960–2850) | 2923 s/sh, 2854 w/sh |
| CO2 | 2360 | 2360 m/sh |
| Csp3-O alcohol BPA stretch vibration | 1753–1743 | 1750 w/br |
| Aromatic ring (benzene’s backbone vibration, BPA molecule) | 1610–1605 | 1608 s/sh |
| Isophorono-Diamine (IPD) | 1580 | 1582 w/sh |
| C=C vibration stretch within the aromatic ring | 1510–1505 | 1509 s/sh |
| >CH2, -CH3 aliphatic parts bending vibration («in plane» deformation vibration) | 1450 ± 20 | 1456 m/sh |
| -ΟΗ bending vibration | 1410–1260 | 1384 w/sh, 1362 w/sh, 1297 w/sh |
| Csp3-O stretch vibration, (aromatic ether) | 1330–1200 | 1247 s/sh |
| stretch vibration, aromatic ring | 1180 | 1181 s/sh |
| Csp3-OΗ Alcohol stretch vibration, | 1150–1050 | 1109 m/sh, 1083 w/sh |
| aromatic C trans formations of ether bond (C-O-C) stretch vibration | 1039–1034 | 1037 s/sh |
| C-O-C Epoxy group stretch vibration (oxirane bond) | 930–914 | 957 w/br, 934 w/br |
| C-H Deformation vibration «out of plane» individual Η within aromatic ring | 876 | 874 w/br |
| C-H Aromatic ring bending (out of plane) | 830–829 | 828 s/sh |
| C-H Deformation vibration «out of plane» 3 or 4 neighbour Η of aromatic ring | 750 | 749 m/br, 734 m/br |
| C-H Deformation vibration «out of plane» | 675 | 698 m/sh |
| C-H Deformation vibration «out of plane» 5 neighbour Η of aromatic ring | 666, 640 | 668 w/sh |
| Characteristic Chemical Group | Wavelength from Literature (cm−1) | Peak Appearance Wavelength (cm−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Composite Category | |||||||||
| NV-BT | M1A3PA6-BT | M3A4PA3-BT | M4A4PA2-BT | M6A4-BT | M7A2PA1-BT | CUP-BT | CER-BT | ||
| Ba-O, Ti-O stretch vibration | 450–405 m/sh (region ii) | 422 m/br (3%) 428 m/br (5%) 420 s/br (10%) 431 s/br (15%) 426 s/br (20%) | 400 s/br (3%–20%) | 426 m/br (3%, 5%) 424 s/br (10%) 430 s/sh (15%) 426 s/sh (20%) | very weak (3%) 443 w/br (5%) 428 s/br (10%) 432 s/br (15%) 432 s/sh (20%) | 432 s/br (3%, 5%) 428 s/br (10–20%) | 447 m/sh (3%) 439 w/br (5%) 439 m/br (10%) 405 s/br (15%) 430 s/br (20%) | 458 m/br (3%, 5%) 431 s/br (10%–20%) | 425 s/br (3%) 433 s/br (5%) 429 s/br (10%) 432 s/br (15%) 416 s/br (20%) |
| Ti-O stretch vibration | 700–530 s/sh (region i) | 698 w/br (3%) 700 s/br (5%–15%) 698 s/br (20%) | 540 s/br (3%–20%) | 545 m/br (3%) 547 m/br (5%) 543 m/br (10%) 565 s/br (15%) 563 s/br (20%) | very weak (3%) 563 s/br (5%) 559 s/br (10%) 553 s/br (15%) 561 s/br (20%) | 504 s/sh (3%, 5%) 504 s/br (10–20%) | 514 s/br (3%) 518 s/br (5%) 540 s/br (10%) 547 s/br (15%) 545 s/br (20%) | 541 s/br (3%–20%) | 558 s/br (3%) 561 s/br (5%) 560 s/br (10%) 559 s/br (15%, 20%) |
| Ba-O, Ti-O stretch vibration | 860–852 w/sh (region iv) | Overlap with NV peak: 874 w/br | Overlap with M1A3PA6 peak: 877 w/sh | Overlap with M3A4PA3 peak: 877 w/sh | Overlap with M4A4PA2 peak: 879 w/sh | Overlap with M6A4 peak: 877 w/sh | Overlap with M7A2PA1 peak: 879 w/sh | Overlap with CUP peak: 847 m/br | Overlap with CER peak: 874 w/br |
| C-O stretch vibration | 1444–1440 m/sh (region iii) | _ | Overlap with M1A3PA6 peak: 1450 m/sh | Overlap with M3A4PA3 peak: 1452 s/sh | Overlap with M4A4PA2 peak: 1452 m/sh | Overlap with M6A4 peak: 1456 m/sh | Overlap with M7A2PA1 peak: 1450 m/sh | Overlap with CUP peak: 1453 s/sh | Overlap with CER peak: 1456 m/sh |
| -OH stretch vibration | 3433–3389 m/br (region v) | Overlap with NV peak: 3416 s/br | Overlap with M1A3PA6 peak: 3452 m/br | Overlap with M3A4PA3 peak: 3452 m/br | Overlap with M4A4PA2 peak: 3446 s/br | Overlap with M6A4 peak: 3459 w/br | Overlap with M7A2PA1 peak: 3456 s/br | Overlap with CUP peak: 3443 w/br | Overlap with CER peak: 3416 s/br |
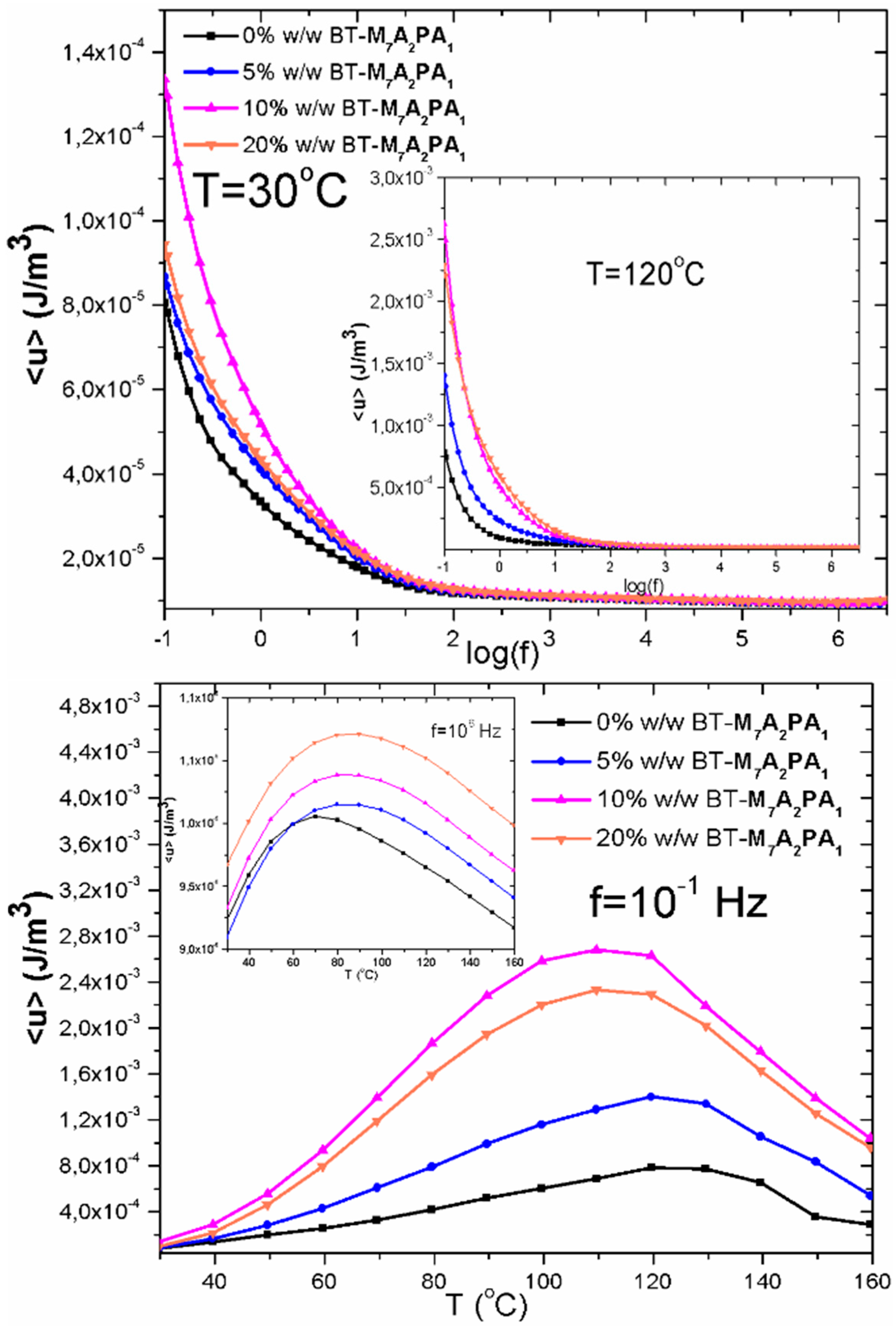
| Type of Composite | <u> vs logf Graphs, at 30 °C | <u> vs Τemperature Graphs, at 10−1 Hz | ||
|---|---|---|---|---|
| % w/w BaTiO3 | <u>max (J/m3) | % w/w BaTiO3 | <u>max (J/m3) | |
| NV-BT | 10 | 1.15 × 10−5 | 10 | 7.40 × 10−5 |
| CUP-BT | 20 | 4 70 × 10−6 | 5 | 5.25 × 10−4 |
| M1A3PA6-BT | 20 | 3.50 × 10−5 | 20 | 3.60 × 10−4 |
| M3A4PA3-BT | 20 | 2.30 × 10−5 | 20 | 2.80 × 10−4 |
| M4A4PA2-BT | 10 | 3.62 × 10−5 | 10 | 2.00 × 10−4 |
| M7A2PA1-BT | 10 | 1.35 × 10−4 | 10 | 2.80∙10−3 |
| M6A4-BT | 5 | 2.30 × 10−5 | 10 | 2.23 × 10−3 |
| CER-BT | 20 | 7.75 × 10−6 | 20 | 1.50 × 10−4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asimakopoulos, I.A.; Psarras, G.C.; Zoumpoulakis, L. Nanocomposites of Barium Titanate Nanoparticles Embedded in Thermosetting Polymer Matrices (Novolac Resin/Unsaturated Polyesters/Epoxy Resin): A Comparative Study. ChemEngineering 2019, 3, 12. https://doi.org/10.3390/chemengineering3010012
Asimakopoulos IA, Psarras GC, Zoumpoulakis L. Nanocomposites of Barium Titanate Nanoparticles Embedded in Thermosetting Polymer Matrices (Novolac Resin/Unsaturated Polyesters/Epoxy Resin): A Comparative Study. ChemEngineering. 2019; 3(1):12. https://doi.org/10.3390/chemengineering3010012
Chicago/Turabian StyleAsimakopoulos, Ioannis A., Georgios C. Psarras, and Loukas Zoumpoulakis. 2019. "Nanocomposites of Barium Titanate Nanoparticles Embedded in Thermosetting Polymer Matrices (Novolac Resin/Unsaturated Polyesters/Epoxy Resin): A Comparative Study" ChemEngineering 3, no. 1: 12. https://doi.org/10.3390/chemengineering3010012
APA StyleAsimakopoulos, I. A., Psarras, G. C., & Zoumpoulakis, L. (2019). Nanocomposites of Barium Titanate Nanoparticles Embedded in Thermosetting Polymer Matrices (Novolac Resin/Unsaturated Polyesters/Epoxy Resin): A Comparative Study. ChemEngineering, 3(1), 12. https://doi.org/10.3390/chemengineering3010012






