Application of the FMEA Tool in an Accredited Testing Laboratory in the Context of the ISO/IEC 17025:2017 Standard
Abstract
1. Introduction
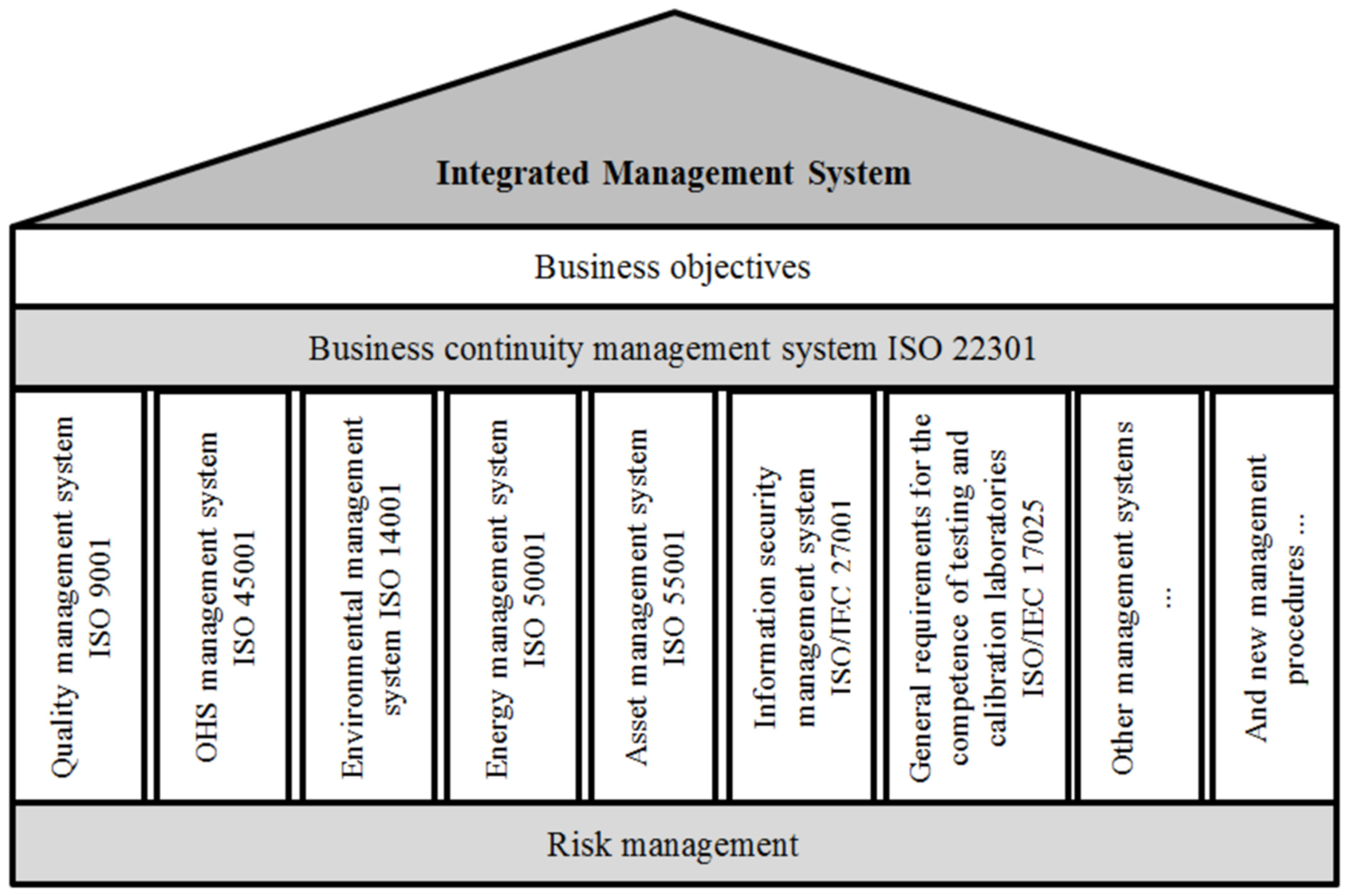
2. Methodology and Theoretical Requirements for Risk Management
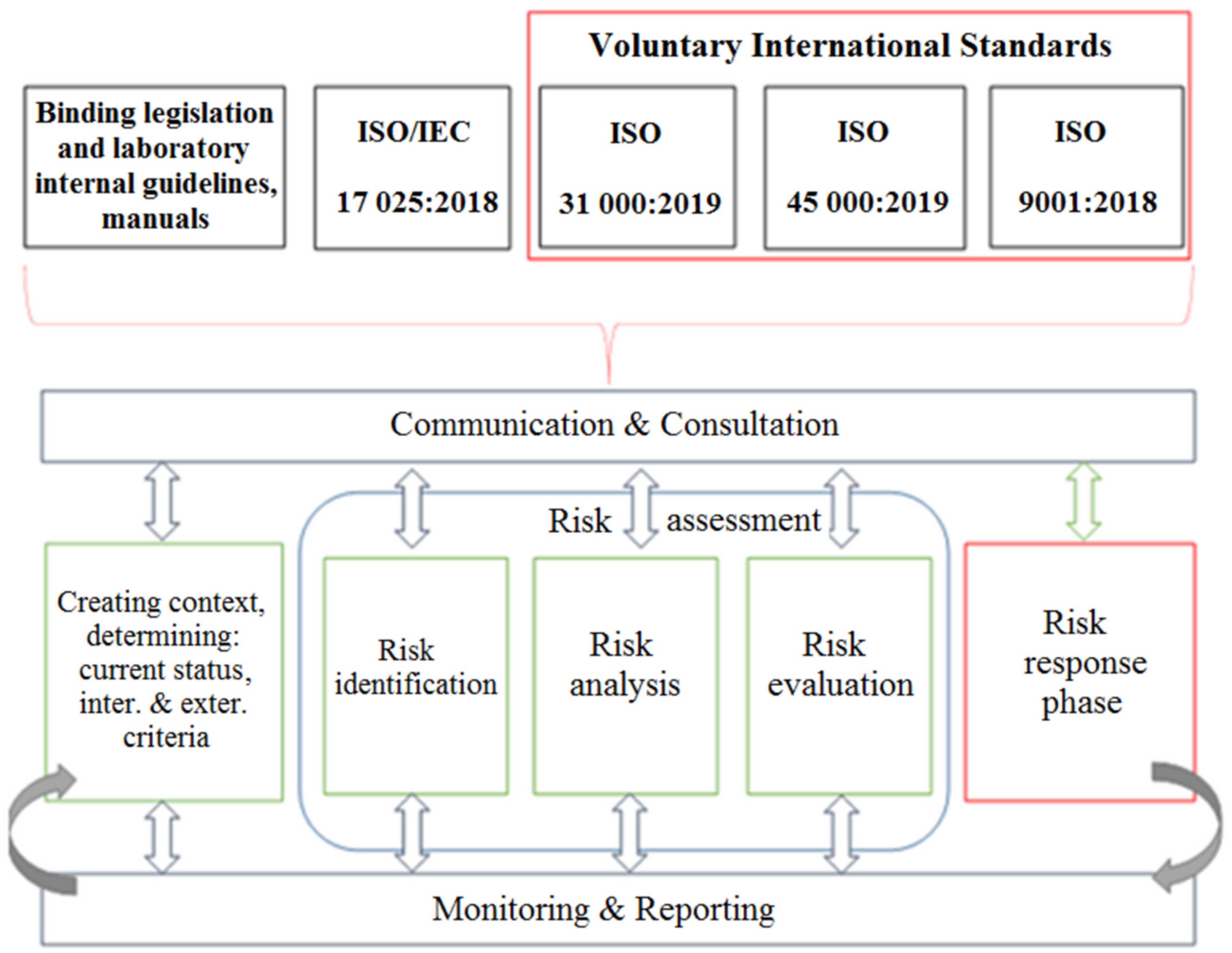
2.1. Methodology of Risk Management in Accredited Laboratories
2.2. International Standard ISO/IEC 17025:2018 and Theoretical Requirements for Risk Management of Risk Management in Accredited Laboratories
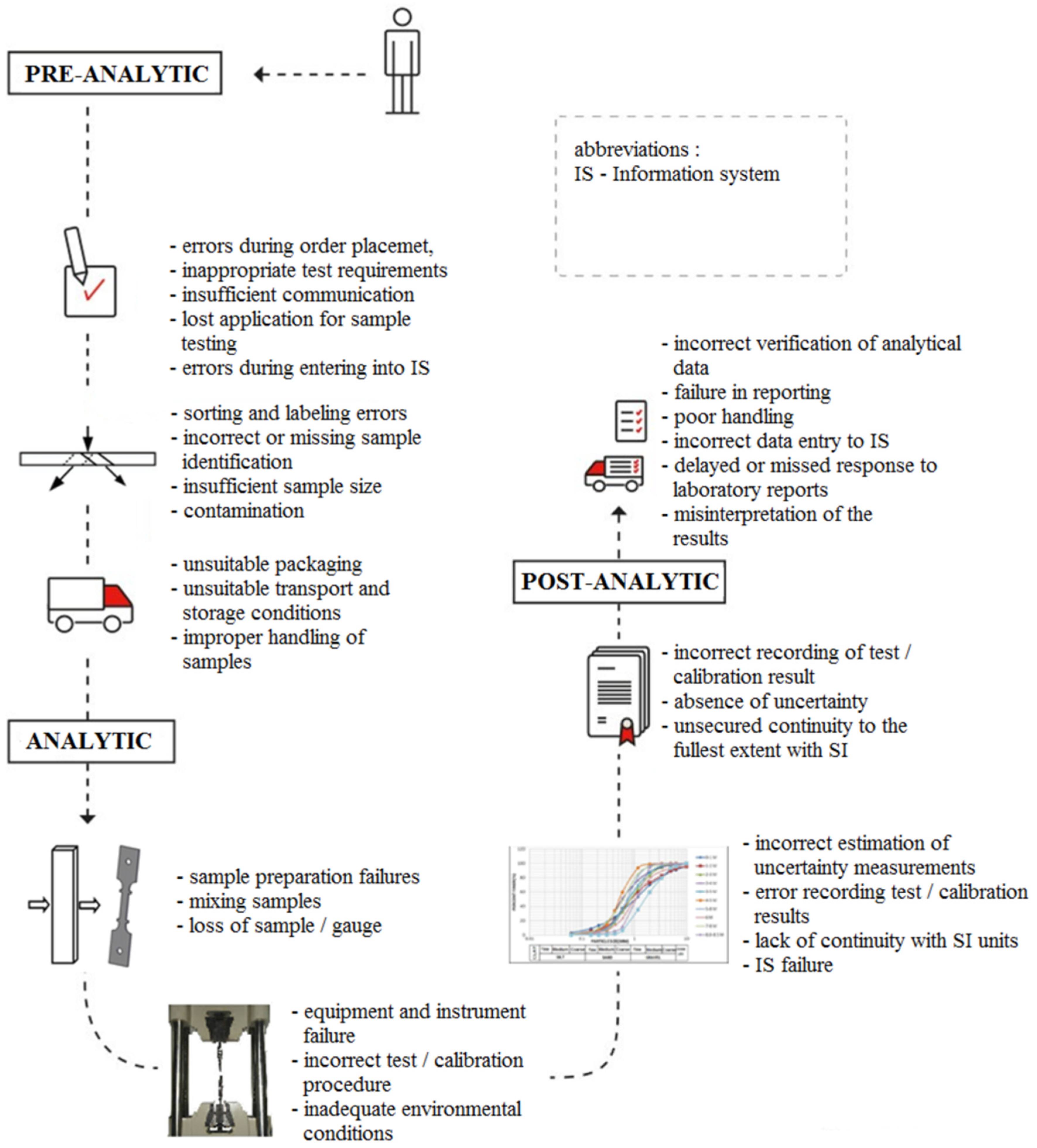
2.3. Failures in the Phases of the Laboratory Testing Process
3. Failure Mode and Effect Analysis (FMEA)
3.1. FMEA Method
- Verbal phase
- 2.
- Numerical phase
- SEV = Severity (1 = Least Severe, 10 = Most Severe),
- Occur = Probability of Occurrence (1 = Least Likely, 10 = Most Likely),
- Det = Probability of Detection (1 = Most Likely, 10 = Least Likely).
- Step I—Preparation
- Step II—Analysis
- Step III—Risk Minimization
3.2. Practical Application of FMEA
- The impact on performing laboratory,
- The probability of risk occurs in the laboratory,
- The possibility of risk detection in the laboratory.
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Croxatto, A.; Greub, G. Project Management: Importance for Diagnostic Laboratories. Clin. Microbiol. Infect. 2017, 23, 434–440. [Google Scholar] [CrossRef]
- Wong, W.W.-S. Risk Management in Clinical Laboratories. J. Hong Kong Inst. Med. Lab. Sci. 2018, 15, 21. [Google Scholar]
- Frikha, G.; Lamine, E.; Kamissoko, D.; Benaben, F.; Pingaud, H. Toward a Modeling Tool for Business Continuity Management. IFAC-PapersOnLine 2021, 54, 1156–1161. [Google Scholar] [CrossRef]
- Tranchard, S. ISO/IEC 17025 Moves to Final Stage of Revision. 2017. Available online: https://www.iso.org/news/ref2212.html (accessed on 7 April 2022).
- Slovak National Accreditation Service SNAS. Akreditačný Informačný Systém. 2022. Available online: https://ais.snas.sk/ais/#!WebReports (accessed on 7 April 2022).
- ISO/IEC 17025; General Requirements for the Competence of Testing and Calibration Laboratories. International Organization for Standardization: Geneva, Switzerland, 2017.
- Njoroge, S.W.; Nichols, J.H. Risk Management in the Clinical Laboratory. Ann. Lab. Med. 2014, 34, 274–278. [Google Scholar] [CrossRef]
- Monteiro Bastos da Silva, J.; Chaker, J.; Martail, A.; Costa Moreira, J.; David, A.; Le Bot, B. Improving Exposure Assessment Using Non-Targeted and Suspect Screening: The ISO/IEC 17025: 2017 Quality Standard as a Guideline. JoX 2021, 11, 1–15. [Google Scholar] [CrossRef]
- Verma, K.L. Quality management—ISO/IEC 17025:2017 (General requirements for the competence of testing and calibration laboratories): Nurturing Confidence. In Proceedings of the Conference Quality Management: ISO/IEC 17025:2017 Forensic, FSL Delhi, Online, June 2020. [Google Scholar]
- Riglietti, F.G. Business Continuity Management as a Key Enabler of Supply Chain Resilience: A Conceptual Paper. IFAC-PapersOnLine 2022, 55, 2197–2202. [Google Scholar] [CrossRef]
- Tranchard, S. New Edition of ISO/IEC 17025 Just Published. 2017. Available online: https://www.iso.org/news/ref2250.html (accessed on 7 April 2022).
- Popov, G.; Lyon, B.K.; Hollcroft, B. Risk Assessment: A Practical Guide to Assessing Operational Risks; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 978-1-119-22091-6. [Google Scholar]
- Tun, T. Biomedical Laboratory: Its Safety and Risk Management. Biomed. Sci. Lett. 2017, 23, 155–160. [Google Scholar] [CrossRef]
- Mascia, A.; Cirafici, A.M.; Bongiovanni, A.; Colotti, G.; Lacerra, G.; Di Carlo, M.; Digilio, F.A.; Liguori, G.L.; Lanati, A.; Kisslinger, A. A Failure Mode and Effect Analysis (FMEA)-Based Approach for Risk Assessment of Scientific Processes in Non-Regulated Research Laboratories. Accred. Qual. Assur. 2020, 25, 311–321. [Google Scholar] [CrossRef]
- Pačaiová, H.; Nagyová, A.; Turisová, R.; Hijj, J.; Vilinský, T.; Firmentová, K. Principles of Management and Position of Maintenance in the I4.0 Environment. Acta Mech. Slov. 2021, 25, 14–19. [Google Scholar] [CrossRef]
- Morton, C.; Bowman, G. New Requirements for Labs—Laboratories and Accreditation Bodies Tackle Revised ISO Standards. 2018. Available online: https://synergist.aiha.org/201808-new-requirements-for-labs (accessed on 8 April 2022).
- Van Hoof, V.; Bench, S.; Soto, A.B.; Luppa, P.P.; Malpass, A.; Schilling, U.M.; Rooney, K.D.; Stretton, A.; Tintu, A.N. Failure Mode and Effects Analysis (FMEA) at the Preanalytical Phase for POCT Blood Gas Analysis: Proposal for a Shared Proactive Risk Analysis Model. Clin. Chem. Lab. Med. (CCLM) 2022, 60, 1186–1201. [Google Scholar] [CrossRef]
- Neogi, S.; Mehndiratta, M.; Gupta, S.; Puri, D. Pre-Analytical Phase in Clinical Chemistry Laboratory. J. Clin. Sci. Res. 2016, 5, 171. [Google Scholar] [CrossRef]
- Kalra, J. Medical Errors: Impact on Clinical Laboratories and Other Critical Areas. Clin. Biochem. 2004, 37, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Lima-Oliveira, G.; Volanski, W.; Lippi, G.; Picheth, G.; Guidi, G.C. Pre-Analytical Phase Management: A Review of the Procedures from Patient Preparation to Laboratory Analysis. Scand. J. Clin. Lab. Investig. 2017, 77, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Carraro, P.; Plebani, M. Errors in a Stat Laboratory: Types and Frequencies 10 Years Later. Clin. Chem. 2007, 53, 1338–1342. [Google Scholar] [CrossRef]
- Plebani, M.; Ceriotti, F.; Messeri, G.; Ottomano, C.; Pansini, N.; Bonini, P. Laboratory Network of Excellence: Enhancing Patient Safety and Service Effectiveness. Clin. Chem. Lab. Med. (CCLM) 2006, 44, 150–160. [Google Scholar] [CrossRef]
- Da Rin, G. Pre-Analytical Workstations: A Tool for Reducing Laboratory Errors. Clin. Chim. Acta 2009, 404, 68–74. [Google Scholar] [CrossRef]
- Plebani, M. Errors in Clinical Laboratories or Errors in Laboratory Medicine? Clin. Chem. Lab. Med. (CCLM) 2006, 44. [Google Scholar] [CrossRef]
- Heldman, K. Project Manager’s Spotlight on Risk Management; Harbor Light Press: San Francisco, CA, USA, 2005; ISBN 978-0-7821-4411-6. [Google Scholar]
- Lee, Y.-C.; Chu, W.-H.; Chen, Q.; Tsai, S.-B.; Wang, J.; Dong, W. Integrating Decision-Making Trial and Evaluation Laboratory Model and Failure Mode and Effect Analysis to Determine the Priority in Solving Production Problems. Adv. Mech. Eng. 2016, 8, 168781401664101. [Google Scholar] [CrossRef]
- Vasilnakova, A. Risk Management in Accredited Testing Laboratories. In DAAAM Proceedings; Katalinic, B., Ed.; DAAAM International Vienna: Vienna, Austria, 2018; Volume 1, pp. 1071–1075. ISBN 978-3-902734-20-4. [Google Scholar]
- Markulik, S.; Nagyova, A.; Turisova, R.; Villinsky, T. Improving Quality in the Process of Hot Rolling of Steel Sheets. Appl. Sci. 2021, 11, 5451. [Google Scholar] [CrossRef]
- Ullah, E.; Baig, M.M.; GholamHosseini, H.; Lu, J. Failure Mode and Effect Analysis (FMEA) to Identify and Mitigate Failures in a Hospital Rapid Response System (RRS). Heliyon 2022, 8, e08944. [Google Scholar] [CrossRef]
- Claxton, K.; Campbell-Allen, N.M. Failure Modes Effects Analysis (FMEA) for Review of a Diagnostic Genetic Laboratory Process. Int. J. Qual. Reliab. Manag. 2017, 34, 265–277. [Google Scholar] [CrossRef]
- Shin, J.; Lee, S.; Yoon, B. Identification and Prioritisation of Risk Factors in R&D Projects Based on an R&D Process Model. Sustainability 2018, 10, 972. [Google Scholar] [CrossRef]
- Vulanovic, S.; Delic, M.; Kamberovic, B.; Beker, I.; Lalic, B. Integrated Management Systems Based on Risk Assessment: Methodology Development and Case Studies. Adv. Prod. Eng. Manag. 2020, 15, 93–106. [Google Scholar] [CrossRef]
- Stamatis, D.H. Introduction to Risk and Failures: Tools and Methodologies; CRC Press: Boca Raton, FL, USA, 2014; ISBN 978-1-4822-3480-0. [Google Scholar]
- Bognár, F.; Benedek, P. Case Study on a Potential Application of Failure Mode and Effects Analysis in Assessing Compliance Risks. Risks 2021, 9, 164. [Google Scholar] [CrossRef]
- Sigma Zone. Quantum XL Software for Microsoft® Excel. Available online: https://sigmazone.com/quantumxl/ (accessed on 7 June 2021).
- Qin, J.; Xi, Y.; Pedrycz, W. Failure Mode and Effects Analysis (FMEA) for Risk Assessment Based on Interval Type-2 Fuzzy Evidential Reasoning Method. Appl. Soft Comput. 2020, 89, 106134. [Google Scholar] [CrossRef]
- Feng, X.; Qian, Y.; Li, Z.; Wang, L.; Wu, M. Functional Model-Driven FMEA Method and Its System Implementation. In Proceedings of the 2018 12th International Conference on Reliability, Maintainability, and Safety (ICRMS), Shanghai, China, 17–19 October 2018; IEEE: Shanghai, China, 2018; pp. 345–350. [Google Scholar]
- de Andrade, J.M.M.; Leite, A.F.C.S.d.M.; Canciglieri, M.B.; Szejka, A.L.; Loures, E.d.F.R.; Canciglieri Junior, O. A Multi-Criteria Approach for FMEA in Product Development in Industry 4.0. In Advances in Transdisciplinary Engineering; Pokojski, J., Gil, M., Newnes, L., Stjepandić, J., Wognum, N., Eds.; IOS Press: Amsterdam, The Netherlands, 2020; ISBN 978-1-64368-110-8. [Google Scholar]
- Chang, T.-W.; Lo, H.-W.; Chen, K.-Y.; Liou, J. A Novel FMEA Model Based on Rough BWM and Rough TOPSIS-AL for Risk Assessment. Mathematics 2019, 7, 874. [Google Scholar] [CrossRef]
- Jin, G.; Meng, Q.; Feng, W. Optimization of Logistics System with Fuzzy FMEA-AHP Methodology. Processes 2022, 10, 1973. [Google Scholar] [CrossRef]
- Bognár, F.; Benedek, P. A Novel Risk Assessment Methodology—A Case Study of the PRISM Methodology in a Compliance Management Sensitive Sector. Acta Polytech. Hung. 2021, 18, 89–108. [Google Scholar] [CrossRef]
| ISO/IEC 17025: 2017 | Risk, Requirements |
|---|---|
| 4.1.4; 4.1.5 | Risk to impartiality |
| 7.8.6 | Level of risk associated with a decision rule used to make a statement of conformity to a specification (such as false accept and false reject and statistical assumptions) |
| 7.10 | Action taken for nonconforming work based upon risk level established by the laboratory |
| 8.5 | Actions to address risks and opportunities |
| 8.7 | Updated risks and opportunities when corrective action is taken |
| 8.9 | Management review includes results of risk identification |
| Examples of Particular Risks of ISO/IEC 17025:2017 | |
| Confidence between employer and laboratory personnel; relations between personnel involved in testing and calibration process; results falsification; unsatisfactory technical conditions of equipment; incorrect calibration of machines and equipment; unsatisfactory climatic conditions in the laboratory during testing; incorrectly selected methods; protocol error; non-updating risk register and protocol; incorrect procedures of testing and calibration; inappropriate shipping and storage conditions; sample damage; no internal audit; poor communication with customers; insufficient staff training; no goals achievement; information system failure; bad working machines; non-compliance with laws and standards; faulty equipment for measuring climate changes in the laboratory chamber. | |
| Value | Severity of Effect | Likelihood of Detection | Probability of Occurrence | |
|---|---|---|---|---|
| Safety/Regulatory/Legal Zone | 10 | May result in safety issue or regulatory violation without warning | Absolutely uncertain that failure will be detected | 1 in 2 |
| 9 | May result in safety issue or regulatory violation with warning | Very remote chance that failure will be detected | 1 in 10 | |
| Warranty/ Field Failure Zone | 8 | Primary function is lost or seriously degraded | Remote chance that failure will be detected | 1 in 50 |
| 7 | Primary function is reduced, and customer is impacted | Very low chance that failure will be detected | 1 in 250 | |
| 6 | Secondary function is lost or seriously degraded | Low chance that failure will be detected | 1 in 1000 | |
| 5 | Secondary function is reduced, and customer is impacted | Moderate chance that failure will be detected | 1 in 5000 | |
| 4 | Loss of function or appearance such that most customers would return product or stop using service | Moderately high chance that failure will be detected | 1 in 10,000 | |
| 3 | Loss of function or appearance that is noticed by customers but would not result in a return or loss of service | High chance that failure will be detected | 1 in 50,000 | |
| 2 | Loss of function or appearance that is unlikely to be noticed by customers and would not result in a return or loss of service | Very high chance that failure will be detected | 1 in 250,000 | |
| 1 | Little to no impact | Almost certainty that failure will be detected | 1 in 1 million |
| Areas | |
|---|---|
| 1. | Economic circumstances in the laboratory |
| 2. | Legislative changes |
| 3. | Changes in technical standards |
| 4. | Personal security |
| 5. | Technical support |
| 6. | Spatial security, working conditions, information systems |
| 7. | Management activities and management interventions |
| 8. | Process risks |
| Evaluation | Measure | RPN |
|---|---|---|
| High risk | Necessary intervention in the process is required | >150 |
| Moderate risk | Process control is required | 121–150 |
| Low risk | No special measures required | <121 |
| Areas | Risk | Potential Effect of Failure | SEV | Current Process Controls | |||||
|---|---|---|---|---|---|---|---|---|---|
| Potential Cause | Occur | Prevention | Detection | Det | RPN | ||||
| 2. | Suspension or revocation of accreditation | Sales recession, customer loss, restriction in operation | 9 | Not meeting conditions of impartiality (4.1) | 3 | Multiple inspections, quality manual, regulations in employment contracts | Quality manager, double check by two correctors | 4 | 108 |
| Result interpretations according to own purpose | 3 | 3 | 135 | ||||||
| 4. | Confidential break of laboratory test data (4.2) | Accidental or intentional transmission of data (to the customer or to the third person) | 10 | Attitude of employees to partial and final results | 2 | Training of personal data protection, retraining of employees | - | 7 | 140 |
| 5. | External influences—energy sources, weather, etc. (6.3) | Inability to perform the measurement | 7 | Supply voltage fluctuations, influence of different electromagnetic fields | 4 | Verification of power supply, external power supply, location of the measuring vehicle of the frequency fields | Verification of the measure before measurement | 1 | 28 |
| Bad weather—storm, rain | 6 | - | Responsible person | 6 | 252 | ||||
| 7. | Failure to update the risk list (8.5) | Errors in laboratory processes | 7 | Person responsible for risk management in the laboratory | 5 | - | - | 7 | 245 |
| Areas | Risk | Potential Effect of Failure | RPN | Recommended Actions | Action Results | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Recommended Actions | Responsible | Target Completion Date | Actions Taken | SEV | Occur | Det | RPN | ||||
| 2. | Suspension or revocation of accreditation | Sales recession, customer loss, restriction in operation | 108 | Multiple checks, trainings, knowledge of procedures | Quality manager | April 2022 | Established training system | 9 | 2 | 4 | 72 |
| 135 | Increased control level | 3 | 3 | 81 | |||||||
| 4. | Confidential break of laboratory test data (4.2) | Accidental or intentional transmission of data (to the customer | 140 | Make a list of employees who have the right to see the test results | Executive manager | March 2022 | List of responsible employees | 10 | 1 | 5 | 50 |
| 5. | External influences—energy sources, weather, etc. (6.3) | Inability to perform the measurement | 28 | Improving communication with the customer | Measuring technician | March 2022 | Established communication manual | 7 | 3 | 1 | 21 |
| 252 | Weather monitoring | Measuring technician | March 2022 | Established weather monitoring system | 5 | 5 | 175 | ||||
| 7. | Failure to update the risk list (8.5) | Errors in laboratory processes | 245 | Identify the person responsible for updates and implement the updated procedure | Quality manager | February 2022 | Established procedure for updating risks | 7 | 3 | 6 | 126 |
| Areas | Before Recommended Actions | After Recommended Actions | ||||
|---|---|---|---|---|---|---|
| High Risk | Moderate Risk | Low Risk | High Risk | Moderate Risk | Low Risk | |
| 1. | - | - | 7 | - | - | 7 |
| 2. | 1 | 1 | 4 | - | 1 | 5 |
| 3. | 1 | 2 | 2 | - | 1 | 4 |
| 4. | 7 | - | 3 | 2 | 2 | 6 |
| 5. | 2 | - | 24 | 1 | 1 | 24 |
| 6. | 2 | - | 3 | - | - | 5 |
| 7. | 1 | - | 3 | - | 1 | 3 |
| 8. | 2 | 2 | 16 | - | 1 | 19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaško, P.; Šolc, M.; Petrík, J.; Girmanová, L.; Blašková, A. Application of the FMEA Tool in an Accredited Testing Laboratory in the Context of the ISO/IEC 17025:2017 Standard. Standards 2023, 3, 57-69. https://doi.org/10.3390/standards3010006
Blaško P, Šolc M, Petrík J, Girmanová L, Blašková A. Application of the FMEA Tool in an Accredited Testing Laboratory in the Context of the ISO/IEC 17025:2017 Standard. Standards. 2023; 3(1):57-69. https://doi.org/10.3390/standards3010006
Chicago/Turabian StyleBlaško, Peter, Marek Šolc, Jozef Petrík, Lenka Girmanová, and Andrea Blašková. 2023. "Application of the FMEA Tool in an Accredited Testing Laboratory in the Context of the ISO/IEC 17025:2017 Standard" Standards 3, no. 1: 57-69. https://doi.org/10.3390/standards3010006
APA StyleBlaško, P., Šolc, M., Petrík, J., Girmanová, L., & Blašková, A. (2023). Application of the FMEA Tool in an Accredited Testing Laboratory in the Context of the ISO/IEC 17025:2017 Standard. Standards, 3(1), 57-69. https://doi.org/10.3390/standards3010006









