A Combination of GM-CSF and Released Factors from Gamma-Irradiated Tumor Cells Enhances the Differentiation of Macrophages from Bone Marrow Cells and Their Antigen-Presenting Function and Polarization to Type 1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gamma Ray Irradiation
2.2. In Vitro Culture of Mouse Bone Marrow Cells
2.3. Flow Cytometry
2.4. Real Time PCR Analysis
2.5. Statistical Analysis
3. Results
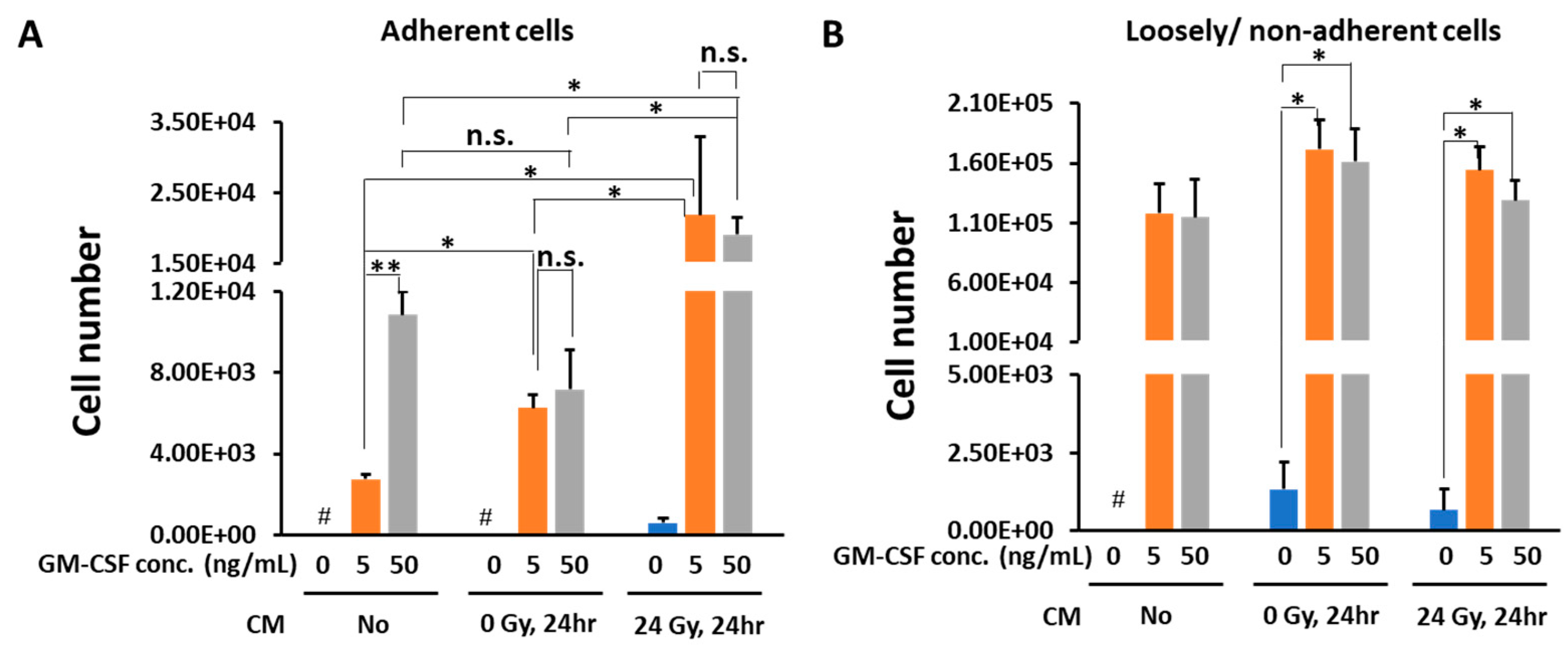
3.1. Conditioned Medium from Gamma-Irradiated B16 Cells Plus GM-CSF Enhanced the Differentiation of Macrophages from Bone Marrow Cells
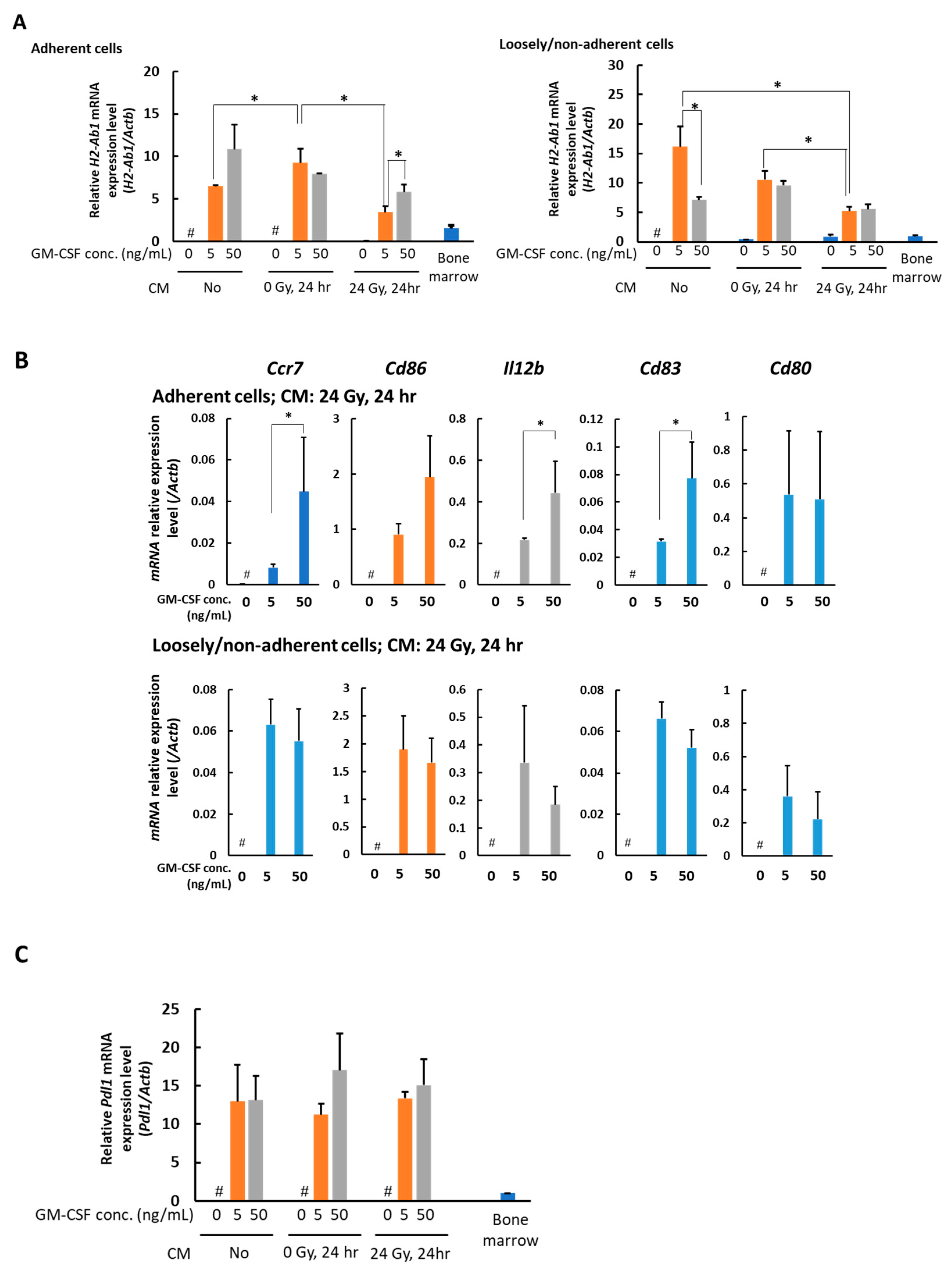
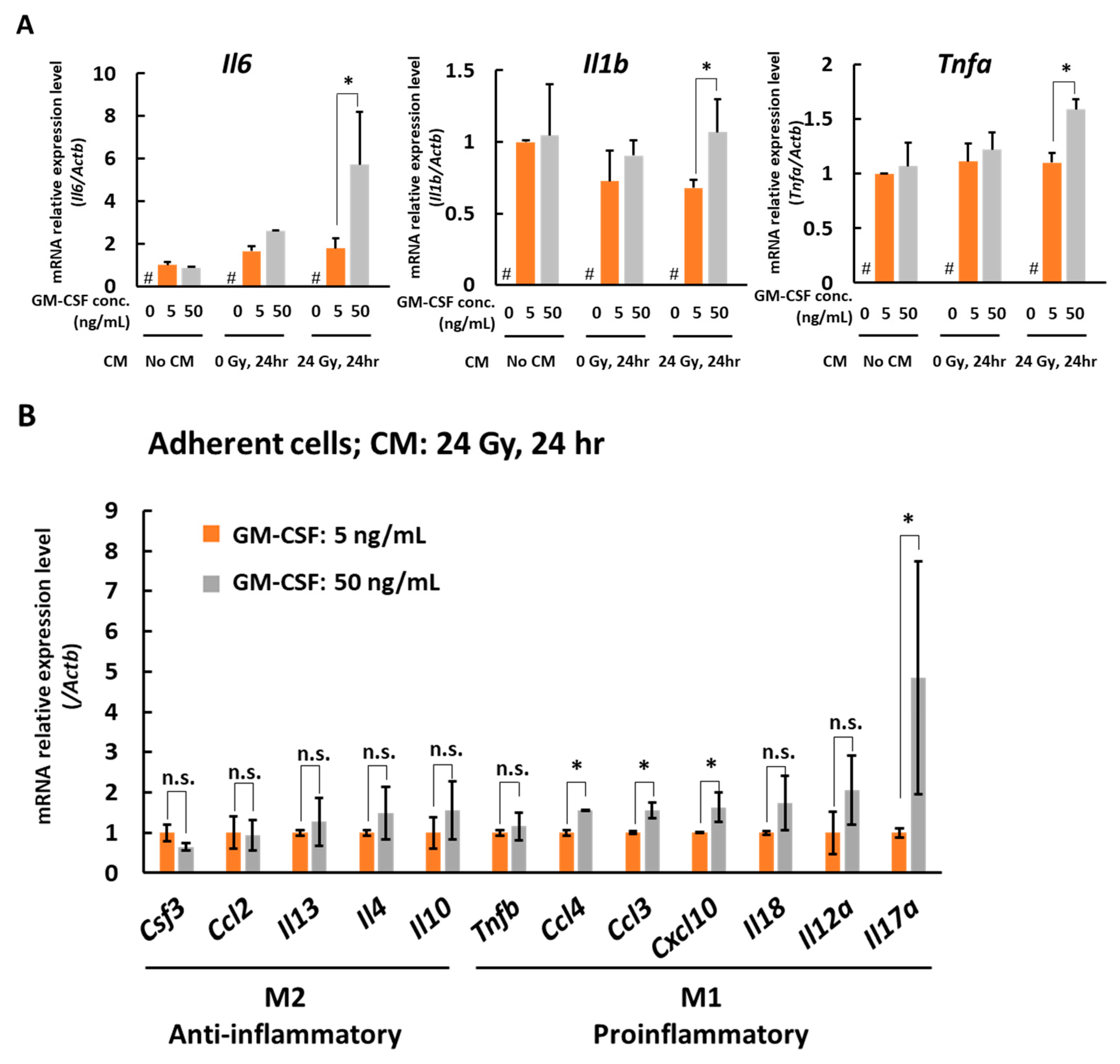
3.2. In the Presence of CM from 24 Gy Irradiated B16 Cells, the Antigen Presenting Function of Macrophages Increases Depending on GM-CSF Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Actb | actin beta |
| Ccr7 | C-C motif chemokine receptor 7 |
| Cd83 | CD83 molecule |
| Cd80 | CD80 molecule |
| Cd86 | CD86 molecule |
| H2-Ab1 | histocompatibility 2, class II antigen A, beta 1 |
| Il12b | interleukin 12B |
| Il6 | interleukin 6 |
| Il1b | interleukin 1 beta |
| Tnfa | tumor necrosis factor a |
| Csf3 | colony stimulating factor 3 |
| Ccl2 | C-C motif chemokine ligand 2 |
| Il13 | interleukin 13 |
| Il4 | interleukin 4 |
| Il10 | interleukin 10 |
| Tnfb | tumor necrosis factor b |
| Ccl4 | C-C motif chemokine ligand 4 |
| Ccl3 | C-C motif chemokine ligand 3 |
| Cxcl10 | C-X-C motif chemokine ligand 10 |
| Il18 | interleukin 18 |
| Il12a | interleukin 12A |
| Il17a | interleukin 17A |
References
- Becher, B.; Tugues, S.; Greter, M. GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity 2016, 45, 963–973. [Google Scholar] [CrossRef] [Green Version]
- Avalos, B.R. Molecular analysis of the granulocyte colony-stimulating factor receptor. Blood 1996, 88, 761–777. [Google Scholar] [CrossRef] [Green Version]
- Burgess, A.W.; Metcalf, D. The nature and action of granulocyte-macrophage colony stimulating factors. Blood 1980, 56, 947–958. [Google Scholar] [CrossRef]
- Lawson, D.H.; Lee, S.; Zhao, F.; Tarhini, A.A.; Margolin, K.; Ernstoff, M.S.; Atkins, M.B.; Cohen, G.I.; Whiteside, T.L.; Butterfield, L.H.; et al. Randomized, Placebo-Controlled, Phase III Trial of Yeast-Derived Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Versus Peptide Vaccination Versus GM-CSF Plus Peptide Vaccination Versus Placebo in Patients With No Evidence of Disease After Complete Surgical Resection of Locally Advanced and/or Stage IV Melanoma: A Trial of the Eastern Cooperative Oncology Group-American College of Radiology Imaging Network Cancer Research Group (E4697). J. Clin. Oncol. 2015, 33, 4066–4076. [Google Scholar]
- Simons, J.W.; Jaffee, E.M.; Weber, C.E.; Levitsky, H.I.; Nelson, W.G.; Carducci, M.A.; Lazenby, A.J.; Cohen, L.K.; Finn, C.C.; Clift, S.M.; et al. Bioactivity of autologous irradiated renal cell carcinoma vaccines generated by ex vivo granulocyte-macrophage colony-stimulating factor gene transfer. Cancer Res. 1997, 57, 1537–1546. [Google Scholar]
- Golden, E.B.; Chhabra, A.; Chachoua, A.; Adams, S.; Donach, M.; Fenton-Kerimian, M.; Friedman, K.; Ponzo, F.; Babb, J.; Goldberg, J.; et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: A proof-of-principle trial. Lancet Oncol. 2015, 16, 795–803. [Google Scholar] [CrossRef]
- Grass, G.D.; Krishna, N.; Kim, S. The immune mechanisms of abscopal effect in radiation therapy. Curr. Probl. Cancer 2016, 40, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Gargett, T.; Christo, S.N.; Hercus, T.R.; Abbas, M.; Singhal, N.; Lopez, A.F.; Brown, M.P. GM-CSF signalling blockade and chemotherapeutic agents act in concert to inhibit the function of myeloid-derived suppressor cells in vitro. Clin. Transl. Immunol. 2016, 5, e119. [Google Scholar] [CrossRef] [PubMed]
- Erlich, Z.; Shlomovitz, I.; Edry-Botzer, L.; Cohen, H.; Frank, D.; Wang, H.; Lew, A.M.; Lawlor, K.E.; Zhan, Y.; Vince, J.E.; et al. Macrophages, rather than DCs, are responsible for inflammasome activity in the GM-CSF BMDC model. Nat. Immunol. 2019, 20, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Umansky, V.; Blattner, C.; Gebhardt, C.; Utikal, J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Hutchison, S.; Sahay, B.; de Mello, S.C.; Sayour, E.; Lejeune, A.; Szivek, A.; Livaccari, A.; Fox-Alvarez, S.; Salute, M.; Powers, L.; et al. Characterization of myeloid-derived suppressor cells and cytokines GM-CSF, IL-10 and MCP-1 in dogs with malignant melanoma receiving a GD3-based immunotherapy. Veter Immunol. Immunopathol. 2019, 216, 109912. [Google Scholar] [CrossRef]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef]
- Umansky, V.; Sevko, A.; Gebhardt, C.; Utikal, J. Myeloid-derived suppressor cells in malignant melanoma. J. Dtsch. Dermatol. Ges. 2014, 12, 1021–1027. [Google Scholar] [CrossRef]
- Unanue, E.R. Antigen-presenting function of the macrophage. Annu. Rev. Immunol. 1984, 2, 395–428. [Google Scholar] [CrossRef]
- Rimaniol, A.-C.; Gras, G.; Verdier, F.; Capel, F.; Grigoriev, V.B.; Porcheray, F.; Sauzeat, E.; Fournier, J.-G.; Clayette, P.; Siegrist, C.-A.; et al. Aluminum hydroxide adjuvant induces macrophage differentiation towards a specialized antigen-presenting cell type. Vaccine 2004, 22, 3127–3135. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S. The macrophage: Past, present and future. Eur. J. Immunol. 2007, 37, S9–S17. [Google Scholar] [CrossRef] [PubMed]
- Azizi, E.; Carr, A.J.; Plitas, G.; Cornish, A.E.; Konopacki, C.; Prabhakaran, S.; Nainys, J.; Wu, K.; Kiseliovas, V.; Setty, M.; et al. Single-Cell Map of Diverse Immune Phenotypes in the Breast Tumor Microenvironment. Cell 2018, 174, 1293–1308.e36. [Google Scholar] [CrossRef] [Green Version]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef] [PubMed]
- Unanue, E.R.; Turk, V.; Neefjes, J. Variations in MHC Class II Antigen Processing and Presentation in Health and Disease. Annu. Rev. Immunol. 2016, 34, 265–297. [Google Scholar] [CrossRef]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Schweitzer, A.N.; Borriello, F.; Wong, R.C.; Abbas, A.K.; Sharpe, A.H. Role of costimulators in T cell differentiation: Studies using antigen-presenting cells lacking expression of CD80 or CD86. J. Immunol. 1997, 158, 2713–2722. [Google Scholar]
- Abdi, K. IL-12: The role of p40 versus p75. Scand. J. Immunol. 2002, 56, 1–11. [Google Scholar] [CrossRef]
- Andersen, M.N.; Al-Karradi, S.N.H.; Kragstrup, T.W.; Hokland, M. Elimination of erroneous results in flow cytometry caused by antibody binding to Fc receptors on human monocytes and macrophages. Cytom. Part A 2016, 89, 1001–1009. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Nieto, C.; Domínguez-Soto, Á.; Barroso, R.; Sánchez-Mateos, P.; Puig-Kroger, A.; López-Bravo, M.; Joven, J.; Ardavín, C.; Fernandez, J.L.R.; et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: Identification of CCL2/CCR2-dependent gene expression profile. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Q.; Kong, Y.; Zhao, H.-Y.; Zhang, Y.-Y.; Han, T.-T.; Wang, Y.; Xu, L.-P.; Zhang, X.-H.; Huang, X.-J. G-CSF-induced macrophage polarization and mobilization may prevent acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2019, 54, 1419–1433. [Google Scholar] [CrossRef] [PubMed]
- Orihuela, R.; McPherson, C.A.; Harry, G.J. Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 2016, 173, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.-H. The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J. Leukoc. Biol. 2014, 97, 61–69. [Google Scholar] [CrossRef]
- Climaco-Arvizu, S.; Domínguez-Acosta, O.; Cabañas-Cortés, M.A.; Rodríguez-Sosa, M.; Gonzalez, F.J.; Vega, L.; Elizondo, G. Aryl hydrocarbon receptor influences nitric oxide and arginine production and alters M1/M2 macrophage polarization. Life Sci. 2016, 155, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liao, M.-Y.; Gao, X.-L.; Zhong, Q.; Tang, T.-T.; Yu, X.; Liao, Y.-H.; Cheng, X. IL-17A induces pro-inflammatory cytokines production in macrophages via MAPKinases, NF-κB and AP-1. Cell. Physiol. Biochem. 2013, 32, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; E Ruby, C.; Hughes, T.; Slingluff, C.L. Current status of granulocyte–macrophage colony-stimulating factor in the immunotherapy of melanoma. J. Immunother. Cancer 2014, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, J.M.; Lee, S.; Moschos, S.J.; Albertini, M.R.; Michalak, J.C.; Sander, C.; Whiteside, T.; Butterfield, L.H.; Weiner, L. Immunogenicity and antitumor effects of vaccination with peptide vaccine+/− granulocyte-monocyte colony-stimulating factor and/or IFN-α2b in advanced metastatic melanoma: Eastern Cooperative Oncology Group Phase II Trial E1696. Clin. Cancer Res. 2009, 15, 1443–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajewski, T.F.; Schreiber, H.; Fu, Y.-X. Innate and adaptive immune cells in the tumor microenvironment. Nat. Immunol. 2013, 14, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krysko, D.; Garg, A.D.; Kaczmarek, A.; Krysko, O.; Agostinis, P.; Vandenabeele, P. Immunogenic cell death and DAMPs in cancer therapy. Nat. Rev. Cancer 2012, 12, 860–875. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward | Reverse |
|---|---|---|
| Actb | GCCAACCGTGAAAAGATGACC | GCGTGAGGGAGAGCATAGC |
| Ccr7 | TCATTGCCGTGGTGGTAGTCTTCA | ATGTTGAGCTGCTTGCTGGTTTCG |
| Cd83 | GTGGCACTGAGAGTGTGGAG | TTGGATCGTCAGGGAATAGG |
| Cd80 | CTGGGAAAAACCCCCAGAAG | TGACAACGATGACGACGACTG |
| Cd86 | ATCAAGGACATGGGCTCGTA | GAAGTTGGCGATCACTGACA |
| H2-Ab1 | AGCCCCATCACTGTGGAGT | GATGCCGCTCAACATCTTGC |
| Il12b | ATGGAGTCATAGGCTCTGGAAA | CCGGAGTAATTTGGTGCTTCAC |
| Il6 | CTGCAAGAGACTTCCATCCAG | AGTGGTATAGACAGGTCTGTTGG |
| Il1b | TGAAATGCCACCTTTTGACAG | CCACAGCCACAATGAGTGATAC |
| Tnfa | GGCAGGTCTACTTTGGAGTCAT | CAGAGTAAAGGGGTCAGAGTGG |
| Csf3 | GCCACCTACAAGCTGTGTCACC | GCTGGCTTAGGCACTGTGTCTG |
| Ccl2 | ATTGGGATCATCTTGCTGGT | CCTGCTGTTCACAGTTGCC |
| Il13 | CCTGGCTCTTGCTTGCCTT | GGTCTTGTGTGATGTTGCTCA |
| Il4 | GGTCTCAACCCCCAGCTAGT | GCCGATGATCTCTCTCAAGTGAT |
| Il10 | TAACTGCACCCACTTCCCAG | AGGCTTGGCAACCCAAGTAA |
| Tnfb | TCACCTCAGACAGGACCCAT | AGCAGTGGCTGGCTTTTAGA |
| Ccl4 | ATGAAGCTCTGCGTGTCTGC | CTGCCGGGAGGTGTAAGAGA |
| Ccl3 | ACCATGACACTCTGCAACCAAGTC | GCGTGGAATCTTCCGGCTGTAG |
| Cxcl10 | AGAGACATCCCGAGCCAACC | AGTCCCACTCAGACCCAGCAG |
| Il18 | ATGCTTTCTGGACTCCTGCC | ATTGTTCCTGGGCCAAGAGG |
| Il12a | CCATCAACGCAGCACTTCAG | TCACCCTGTTGATGGTCACG |
| Il17a | TCTCTGATGCTGTTGCTGCT | CGTGGAACGGTTGAGGTAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Imamichi, S.; Tong, Y.; Sasaki, Y.; Onodera, T.; Nakamura, S.; Igaki, H.; Itami, J.; Masutani, M. A Combination of GM-CSF and Released Factors from Gamma-Irradiated Tumor Cells Enhances the Differentiation of Macrophages from Bone Marrow Cells and Their Antigen-Presenting Function and Polarization to Type 1. Medicines 2021, 8, 35. https://doi.org/10.3390/medicines8070035
Chen L, Imamichi S, Tong Y, Sasaki Y, Onodera T, Nakamura S, Igaki H, Itami J, Masutani M. A Combination of GM-CSF and Released Factors from Gamma-Irradiated Tumor Cells Enhances the Differentiation of Macrophages from Bone Marrow Cells and Their Antigen-Presenting Function and Polarization to Type 1. Medicines. 2021; 8(7):35. https://doi.org/10.3390/medicines8070035
Chicago/Turabian StyleChen, Lichao, Shoji Imamichi, Ying Tong, Yuka Sasaki, Takae Onodera, Satoshi Nakamura, Hiroshi Igaki, Jun Itami, and Mitsuko Masutani. 2021. "A Combination of GM-CSF and Released Factors from Gamma-Irradiated Tumor Cells Enhances the Differentiation of Macrophages from Bone Marrow Cells and Their Antigen-Presenting Function and Polarization to Type 1" Medicines 8, no. 7: 35. https://doi.org/10.3390/medicines8070035
APA StyleChen, L., Imamichi, S., Tong, Y., Sasaki, Y., Onodera, T., Nakamura, S., Igaki, H., Itami, J., & Masutani, M. (2021). A Combination of GM-CSF and Released Factors from Gamma-Irradiated Tumor Cells Enhances the Differentiation of Macrophages from Bone Marrow Cells and Their Antigen-Presenting Function and Polarization to Type 1. Medicines, 8(7), 35. https://doi.org/10.3390/medicines8070035







