Preclinical Trial of Traditional Plant Remedies for the Treatment of Complications of Gestational Malaria
Abstract
1. Introduction
2. Methodology
2.1. Sample Collection and Preparation
2.2. Sample Size Determination
2.3. Animal Handling
2.4. Inoculation of Pregnant Rats
2.5. Grouping, Feeding, and Sample Collection
2.6. Measurement of Maternal Hemodynamics, Pregnancy Outcomes, and Sample Collection
2.7. Determination of Antimalarial Activity
2.8. Clinical Chemistry
2.9. Statistical Analysis
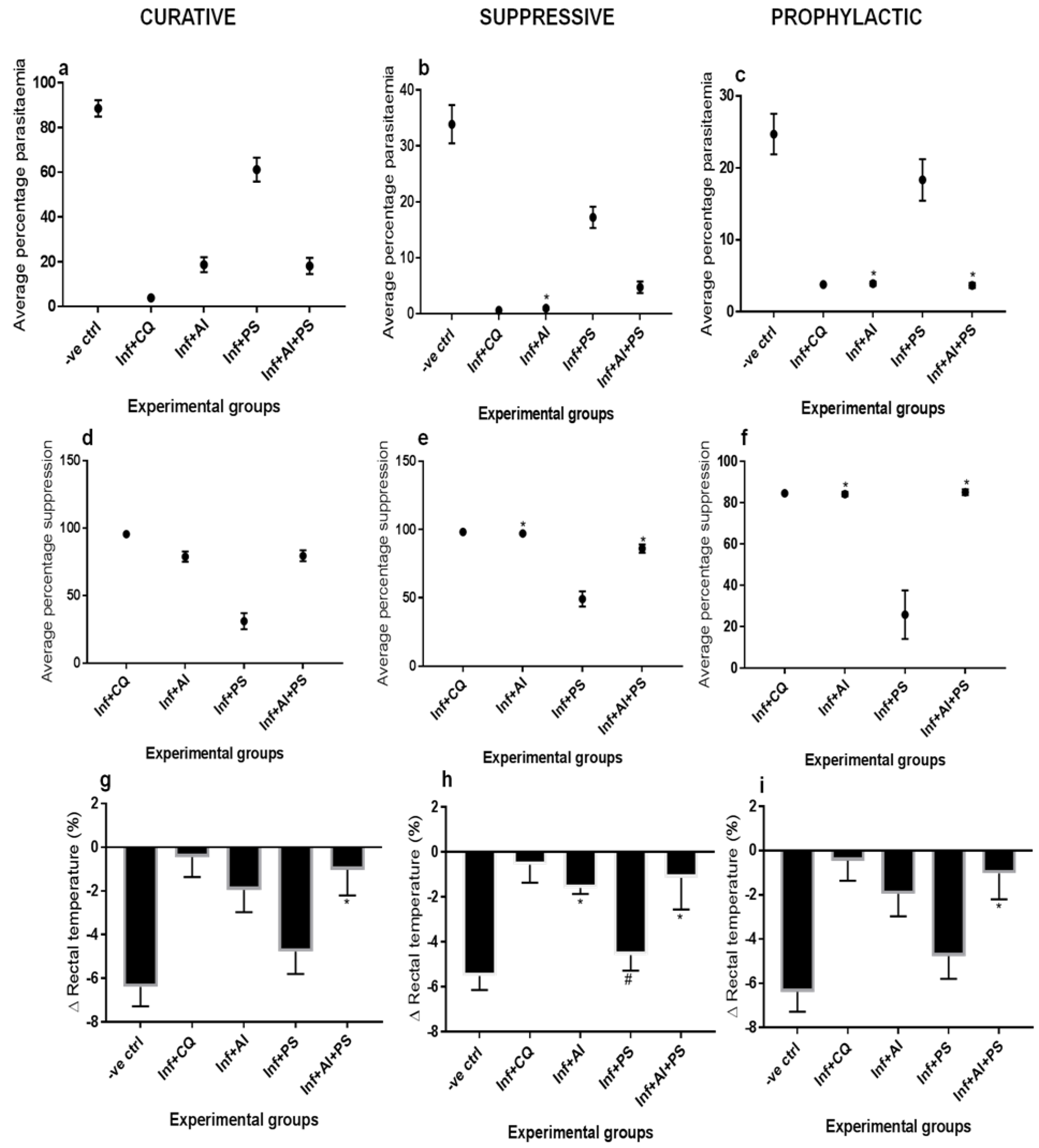
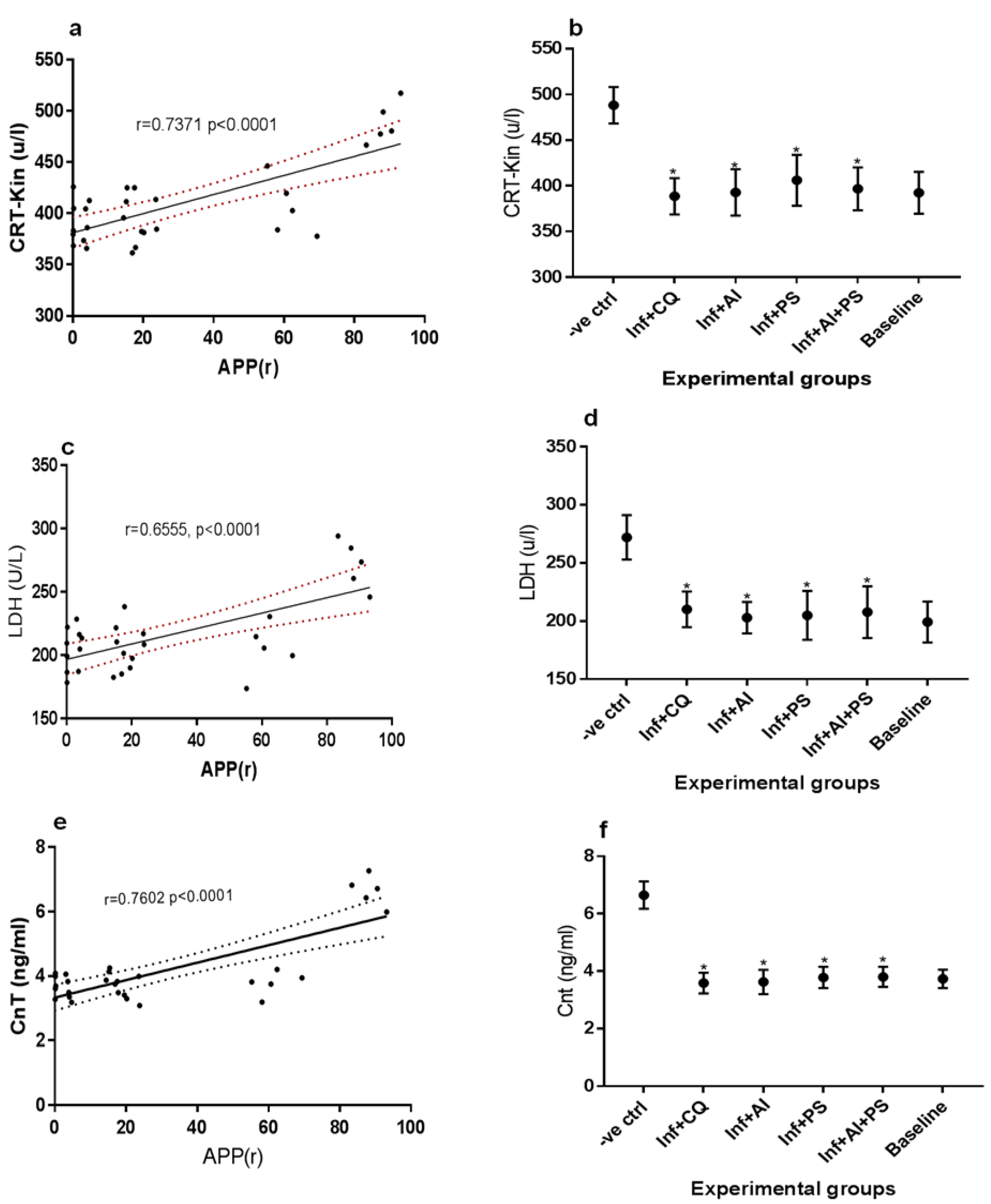
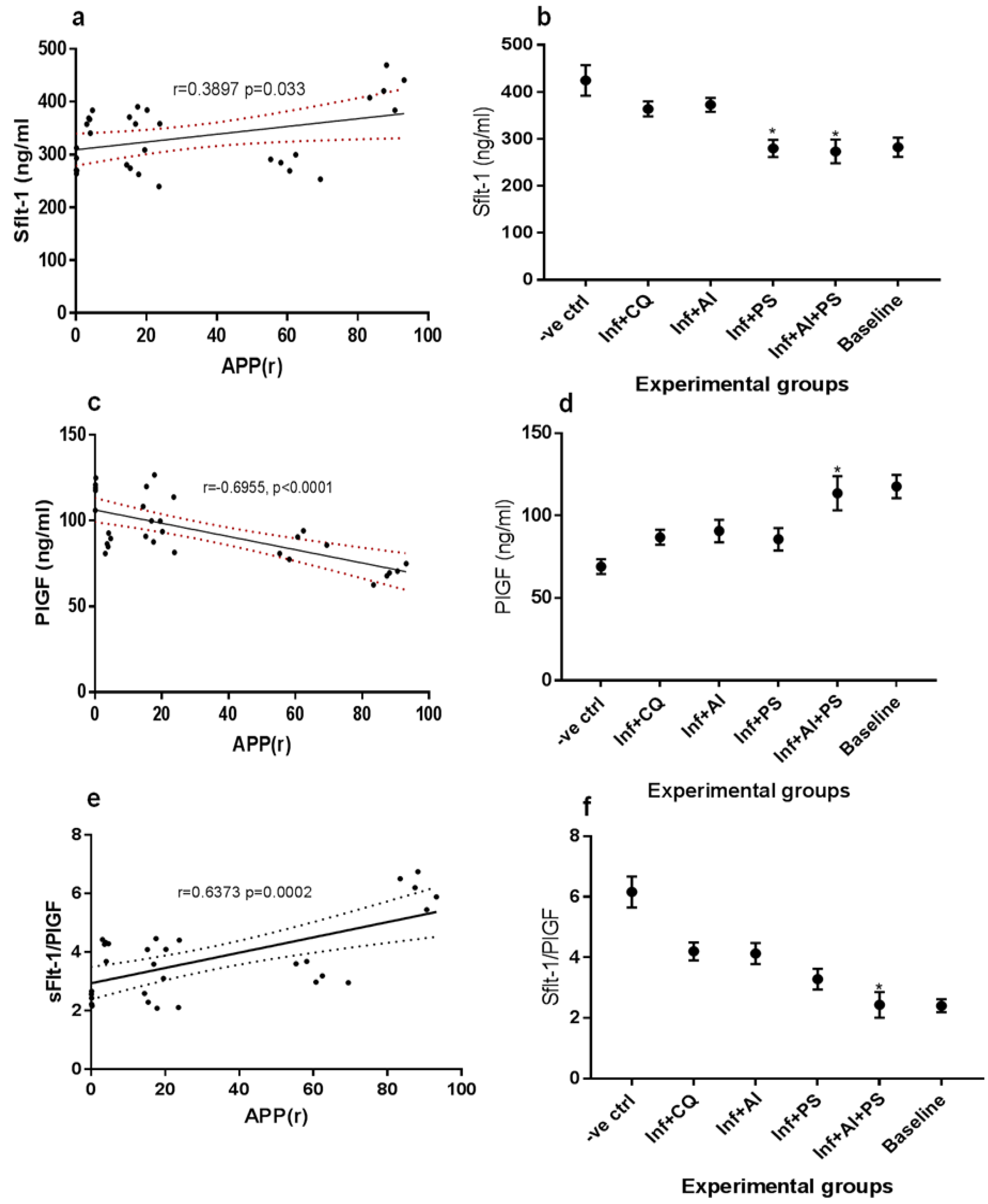
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boutayeb, A. The Burden of and Non-Communicable Diseases in Developing Countries. In Handbook of Disease Burdens and Quality of Life Measures; Springer: New York, NY, USA, 2010; pp. 531–546. [Google Scholar]
- Gajida, A.U.; Iliyasu, Z.; Zoakah, A.I. Malaria among antenatal clients attending primary health care facilities in Kano State, Nigeria. Ann. Afr. Med. 2010, 9, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Onwujekwe, O.; Uguru, N.; Etiaba, E.; Chikezie, I.; Uzochukwu, B.; Adjagba, A. The Economic Burden of Malaria on Households and the Health System in Enugu State Southeast Nigeria. PLoS ONE 2013, 8, e78362. [Google Scholar]
- Bhalla, D.; Cleenewerck, L.; Okorafor Kalu, S.; Abubakar Gulma, K. Malaria Prevention Measures among Pregnant Women: A Population-Based Survey in Nnewi, Nigeria. Sci. World J. 2019, 2019, 6402947. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The World Malaria Report 2019 at a Glance; Regional and Global Trends in Burden of Malaria Cases and Deaths. 2019. Available online: https://www.who.int/news-room/feature-stories/detail/world-malaria-report-2019 (accessed on 30 July 2021).
- Okafor, I.P.; Ezekude, C.; Oluwole, E.O.; Onigbogi, O.O. Malaria in pregnancy: A community-based study on the knowledge, perception, and prevention among Nigerian women. J. Family Med. Prim. Care 2019, 8, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Sam-Wobo, S.O.; Akinboroye, T.; Anosike, J.C.; Adewale, B. Knowledge and practices on malaria treatment measures among pregnant women in Abeokuta, Nigeria. Tanzan. J. Health Res. 2008, 10, 226–231. [Google Scholar] [PubMed]
- Chidinma, E.I.; Duru, A.C.; Ingwu, J.A.; Arinze, J.C.; Chikeme, P.C.; Kotoye, C.O. Use of traditional medicines in treatment of malaria among pregnant women in two urban slums in Enugu State, Nigeria. J. Public Health Dis. 2019, 30, 24–31. [Google Scholar]
- Khalid, S.A.; Farouk, A.; Geary, T.G.; Jensen, J.B. Potential antimalarial candidates from African plants: An in vitro approach using Plasmodium falciparum. J. Ethnopharmacol. 1986, 15, 201–209. [Google Scholar] [CrossRef]
- Phillipson, J.D.; Wright, C.W. Can ethnopharmacology contribute to the development of antimalarial agents? J. Ethnopharmacol. 1991, 32, 155–165. [Google Scholar] [CrossRef]
- Leaman, D.J.; Arnason, J.T.; Yusuf, R.; Sangat-Roemantyo, H.; Soedjito, H.; Angerhofer, C.K.; Pezzuto, J.M. Malaria remedies of the Kenyah of the Apo Kayan, East Kalimantan, Indonesian Borneo: A quantitative assessment of local consensus as an indicator of biological efficacy. J. Ethnopharmacol. 1995, 49, 1–16. [Google Scholar] [CrossRef]
- Priyadarsini, R.V.; Manikandan, P.; Kumar, G.H.; Nagini, S. The neem limonoids azadirachtin and nimbolide inhibit hamster cheek pouch carcinogenesis by modulating xenobiotic-metabolizing enzymes, DNA damage, antioxidants, invasion and angiogenesis. Free Radic. Res. 2009, 43, 492–504. [Google Scholar] [CrossRef]
- Alzohairy, M.A. Therapeutics Role of Azadirachta indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evid.-Based Complement. Alternat. Med. 2016, 2016, 7382506. [Google Scholar] [CrossRef]
- MacKinnon, S.; Durst, T.; Arnason, J.T.; Angerhofer, C.; Pezzuto, J.; Sanchez-Vindas, P.E.; Poveda, L.J.; Gbeassor, M. Antimalarial activity of tropical Meliaceae extracts and gedunin derivatives. J. Nat. Prod. 1997, 60, 336–341. [Google Scholar] [CrossRef]
- Rochanakij, S.; Thebtaranonth, Y.; Yenjai, C.; Yuthavong, Y. Nimbolide, a constituent of Azadirachta indica, inhibits Plasmodium falciparum in culture. Southeast Asian J. Trop. Med. Public Health. 1985, 16, 66–72. [Google Scholar]
- Amadi, P.U.; Agomuo, E.N.; Adumekwe, C.W. Modulatory properties of cardiac and quercetin glycosides from Dacryodes edulis seeds during L-NAME-induced vascular perturbation. J. Basic Clin. Physiol. Pharmacol. 2020, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Amadi, P.U.; Agomuo, E.N.; Bob-Chile Agada, A.; Njoku, U.C.; Ifeanacho, M.O.; Okereke, C.J.; Iheka, C.U.; Osuoha, J.O. Toxicities selected medicinal plants, and floras of lower phyla. Alex. J. Med. 2018, 54, 587–596. [Google Scholar] [CrossRef]
- Xie, L.H.; Johnson, T.O.; Weina, P.J.; Si, Y.; Haeberle, A.; Upadhyay, R.; Wong, E.; Li, Q. Risk assessment and therapeutic indices of artesunate and artelinate in Plasmodium berghei-infected and uninfected rats. Int. J. Toxicol. 2005, 24, 251–264. [Google Scholar] [CrossRef]
- Shu, W.; Li, H.; Gong, H.; Zhang, M.; Niu, X.; Ma, Y.; Zhang, X.; Cai, W.; Yang, G.; Wei, M.; et al. Evaluation of blood vessel injury, oxidative stress and circulating inflammatory factors in an L-NAME-induced preeclampsia-like rat model. Exp. Ther. Med. 2018, 16, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Ryley, J.; Peters, W. The antimalarial activity of some quinolone esters. Ann. Trop. Med. Parasitol. 1970, 64, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Peters, W. The four-day suppressive in vivo antimalarial test. Ann. Trop. Med. Parasitol. 1975, 69, 155–171. [Google Scholar] [CrossRef]
- Peters, W. Drug resistance in Plasmodium bergheiVincke and lips, 1948. I. Chloroquine resistance. Exp. Parasitol. 1965, 17, 80–89. [Google Scholar] [CrossRef]
- Peters, W.; Robinson, B. The chemotherapy of rodent malaria. XLVII. Studies on pyronaridine and other Mannich base antimalarials. Ann. Trop. Med. Parasitol. 1992, 86, 455–465. [Google Scholar] [CrossRef]
- Agomuo, E.N.; Amadi, P.U.; Adumekwe, C.W. Gestational geophagia affects nephrocardiac integrity, ATP-driven proton pumps, renin-angiotensin-aldosterone system and F2-isoprostane status. Med. Sci. 2019, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Horder, M.; Elser, R.C.; Gerhardt, W.; Matthieu, M.; Sampson, E.J. Approved recommendation on IFCC methods for the measurement of catalytic concentration of enzymes. Part 7. IFCC method for creatine kinase (ATP: Creatine N-phosphotransferase, EC 2.7.3.2). Eur. J. Clin. Chem. Clin. Biochem. 1991, 29, 435–456. [Google Scholar]
- Ndyomugyenyi, R.; Magnussen, P.; Clarke, S. Diagnosis and treatment of malaria in peripheral health facilities in Uganda: Findings from an area of low transmission in south-western Uganda. Malar. J. 2007, 6, 39. [Google Scholar] [CrossRef]
- Fall, C.H. Fetal malnutrition and long-term outcomes. In Maternal and Child Nutrition: The First 1000 Days; Bhatia, J., Bhutta, Z.A., Kalhan, S.C., Eds.; Karger Publishers, Basel, Switzerland: 2013; Volume 74, pp. 11–25.
- Ashafa, A.O.; Orekoya, L.O.; Yakubu, M.T. Toxicity profile of ethanolic extract of Azadirachta indica stem bark in male Wistar rats. Asian Pac. J. Trop. Biomed. 2012, 2, 811–817. [Google Scholar] [CrossRef]
- Panti, A.A.; Ekele, B.A.; Nwobodo, E.I.; Yakubu, A. The relationship between the weight of the placenta and birth weight of the neonate in a Nigerian Hospital. Niger. Med. J. 2012, 53, 80–84. [Google Scholar] [CrossRef]
- McNamara, H.; Hutcheon, J.A.; Platt, R.W.; Benjamin, A.; Kramer, M.S. Risk factors for high and low placental weight. Paediatr. Perinat. Epidemiol. 2014, 28, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Baptiste-Roberts, K.; Salafia, C.M.; Nicholson, W.K.; Duggan, A.; Wang, N.Y.; Brancati, F.L. Maternal risk factors for abnormal placental growth: The national collaborative perinatal project. BMC Pregnancy Childbirth 2008, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Zirihi, G.N.; Mambu, L.; Guédé-Guina, F.; Bodo, B.; Grellier, P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J. Ethnopharmacol. 2005, 98, 281–285. [Google Scholar] [CrossRef]
- Zaki, S.A.; Shanbag, P. Increased urinary frequency: An unusual presentation of Plasmodium falciparum malaria. Saudi J. Kidney Dis. Transpl. 2012, 23, 844–845. [Google Scholar] [CrossRef] [PubMed]
- Rulisa, S.; Kaligirwa, N.; Agaba, S.; Karangayire, P.; Mens, P.F.; de Vries, P.J. Fetal and maternal hemodynamics in acute malaria during pregnancy. Int. J. Gynaecol. Obstet. 2012, 119, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Imbert, P.; Rogier, C.; Gerardin, P. Letter to the editor. Am. J. Trop. Med. Hyg. 2003, 68, 380–381. [Google Scholar] [CrossRef]
- Abdalla, S.; Pasvol, G. Platelets and blood coagulation in human malaria. In The Haemotology of Malaria; Newton, P.N., Essien, E., White, N.J., Eds.; Imperial College Press: London, UK, 2004; pp. 249–276. [Google Scholar]
- Erhabor, O.; Jeremiah, Z.A.; Adias, T.C.; Hart, M.L. Thrombocytopenia in plasmodium parasitized pregnant women in the Niger Delta of Nigeria. Pathol. Lab. Med. Int. 2010, 2, 1–5. [Google Scholar]
- Iyare, E.E.; Obaji, N.N. Effects of aqueous leaf extract of azadirachta indica on some haematological parameters and blood glucose level in female rats. Niger. J. Exp. Clin. Biosci. 2014, 2, 54–58. [Google Scholar] [CrossRef]
- Dias, R.M.; Vieira, J.L.; Cabral, B.D.; da Silva, I.R.; Brasil, L.M.; Araújo, E.D.; de Andrade, M.A. Lipid Profile of Children with Malaria by Plasmodium vivax. J. Trop. Med. 2016, 2016, 9052612. [Google Scholar] [CrossRef]
- Fleming, S.M.; O’Gorman, T.; Finn, J.; Grimes, H.; Daly, K.; Morrison, J.J. Cardiac troponin I in pre-eclampsia and gestational hypertension. BJOG 2000, 107, 1417–1420. [Google Scholar] [CrossRef] [PubMed]
- Jaiswar, S.P.; Gupta, A.; Sachan, R.; Natu, S.N.; Shaili, M. Lactic dehydrogenase: A biochemical marker for preeclampsia-eclampsia. J. Obstet. Gynaecol. India 2011, 61, 645–648. [Google Scholar] [CrossRef][Green Version]
- Horjus, D.L.; Bokslag, A.; Hooijberg, F.; Hutten, B.A.; Middeldorp, S.; de Groot, C.J.M. Creatine kinase and blood pressure in women with a history of early-onset preeclampsia. Pregnancy Hypertens. 2019, 15, 118–122. [Google Scholar] [CrossRef]
- Roberts, J.M.; Rajakumar, A. Preeclampsia and soluble fms-like tyrosine kinase 1. J. Clin. Endocrinol. Metab. 2009, 94, 2252–2254. [Google Scholar] [CrossRef]
- Agrawal, S.; Shinar, S.; Cerdeira, A.S.; Redman, C.; Vatish, M. Predictive Performance of PlGF (Placental Growth Factor) for Screening Preeclampsia in Asymptomatic Women: A Systematic Review and Meta-Analysis. Hypertension 2019, 74, 1124–1135. [Google Scholar] [CrossRef]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Schlembach, D.; Hund, M.; Schroer, A.; Wolf, C. Economic assessment of the use of the sFlt-1/PlGF ratio test to predict preeclampsia in Germany. BMC Health Serv. Res. 2018, 18, 603. [Google Scholar] [CrossRef] [PubMed]
- Nikuei, P.; Rajaei, M.; Roozbeh, N.; Mohseni, F.; Poordarvishi, F.; Azad, M.; Haidari, S. Diagnostic accuracy of sFlt1/PlGF ratio as a marker for preeclampsia. BMC Pregnancy Childbirth 2020, 20, 80. [Google Scholar] [CrossRef] [PubMed]
| Groups | Maternal Mortality (%) | Feed Intake (g) | Weight Gain (g) | Placenta Weight (g) |
|---|---|---|---|---|
| -ve ctrl | 0 | 498.6 ± 18.7 | 32.4 ± 4.3 | 0.44 ± 0.02 |
| Inf + CQ | 0 | 680.0 ± 26.2 | 75.6 ± 4.8 | 0.53 ± 0.01 |
| Inf + AI | 0 | 701.8 ± 22.0 a | 82.2 ± 4.1 | 0.53 ± 0.02 c |
| Inf + PS | 0 | 632.6 ± 30.8 | 71.6 ± 3.2 c | 0.53 ± 0.02 c |
| Inf + AI + PS | 0 | 733.8 ± 28.3 b | 88.0 ± 2.6 | 0.54 ± 0.03 c |
| Baseline | 0 | 756.4 ± 15.1 | 76.8 ± 5.9 | 0.54 ± 0.03 |
| Groups | Systolic BP (mm/Hg) | Diastolic BP (mm/Hg) | HBR (Beats/min) | 24 h Urine Protein Excretion (mg) | Platelets Counts (× 103 µL) |
|---|---|---|---|---|---|
| -ve ctrl | 136.0 ± 4.1 | 89.0 ± 2.2 | 405.8 ± 17.8 | 0.83 ± 0.03 | 214.1 ± 19.3 |
| Inf + CQ | 122.0 ± 2.7 | 84.0 ± 4.1 | 332.0 ± 8.6 | 0.79 ± 0.01 | 314.6 ± 20.4 |
| Inf + AI | 123.0 ± 2.7 c | 83.0 ± 2.7 c | 366.4 ± 6.8 | 0.77 ± 0.01 a | 364.5 ± 14.9 b |
| Inf + PS | 123.0 ± 2.7 c | 80.0 ± 0.0 c | 319.6 ± 5.7 c | 0.62 ± 0.05 b | 249.9 ± 17.0 |
| Inf + AI + PS | 122.0 ± 2.7 c | 81.0 ± 2.2 c | 332.0 ± 10.6 c | 0.67 ± 0.02 | 351.2 ± 19.1 b |
| Baseline | 122.0 ± 2.7 | 78.0 ± 2.7 | 321.6 ± 6.0 | 0.59 ± 0.02 | 356.6 ± 26.9 |
| Groups | Total No. of Pups | No. of Live Pups | No. of Still Born | Av. Pup Weight (g) | Eye Opening (Days) | Appearance of Fur (Days) | Crown Rump Length (cm) |
|---|---|---|---|---|---|---|---|
| Baseline | 12.0 | 11.0 | 1.0 | 3.6 ± 0.2 | 15.0 ± 1.0 | 5.6 ± 0.8 | 3.3 ± 0.1 |
| -ve ctrl | 9.2 ± 1.4 b | 4.6 ± 1.1 b | 4.6 ± 0.8 b | 2.5 ± 0.2 b | 15.0 ± 0.7 a | 5.6 ± 0.8 | 2.6 ± 0.1 b |
| Inf + CQ | 12.4 ± 1.1 | 11.4 ± 1.1 | 1.0 ± 0.2 | 3.1 ± 0.2 | 15.0 ± 1.0 | 6.0 ± 0.7 | 3.3 ± 0.1 b |
| Inf + AI | 12.0 ± 1.8 a | 10.8 ± 1.3 a | 1.2 ± 0.2 a | 3.9 ± 0.3 c | 15.0 ± 0.7 a | 5.4 ± 0.5 | 4.1 ± 0.2 |
| Inf + PS | 11.8 ± 0.8 a | 9.4 ± 1.6 a | 2.4 ± 0.4 | 2.8 ± 0.1 a | 15.2 ± 0.8 a | 5.6 ± 0.8 | 3.2 ± 0.2 a |
| Inf + AI + PS | 12.6 ± 1.1 a | 10.6 ± 1.6 a | 2.0 ± 0.5 | 3.9 ± 0.1 c | 14.8 ± 0.8 a | 5.8 ± 0.8 | 4.1 ± 0.2 |
| Groups | TC (mg/dl) | TG (mg/dl) | LDL (mg/dl) | HDL (mg/dl) | CRR | AC |
|---|---|---|---|---|---|---|
| -ve ctrl | 92.7 ± 5.2 | 70.0 ± 4.9 | 62.0 ± 6.4 | 16.6 ± 2.0 | 5.64 ± 0.8 | 4.64 ± 0.8 |
| Inf + CQ | 83.6 ± 6.9 | 55.8 ± 5.5 | 45.3 ± 7.8 | 27.1 ± 2.5 c | 3.10 ± 0.4 | 2.10 ± 0.4 |
| Inf + AI | 80.1 ± 5.0 a | 54.1 ± 5.7 c | 39.1 ± 3.5 a | 30.1 ± 4.0 c | 2.68 ± 0.2 b | 1.68 ± 0.2 b |
| Inf + PS | 65.9 ± 4.3 b | 56.5 ± 5.6 c | 25.2 ± 7.4 b | 29.3 ± 3.7 c | 2.29 ± 0.4 b | 1.29 ± 0.4 b |
| Inf + AI + PS | 72.7 ± 6.6 b | 58.6 ± 5.1 c | 31.4 ± 7.1 b | 29.5 ± 2.6 c | 2.47 ± 0.3 b | 1.47 ± 0.3 b |
| Baseline | 70.4 ± 5.0 | 57.2 ± 6.6 | 28.1 ± 8.8 | 30.8 ± 4.3 | 2.32 ± 0.3 | 1.32 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amadi, P.U.; Agomuo, E.N.; Ukaga, C.N.; Njoku, U.C.; Amadi, J.A.; Nwaekpe, C.G. Preclinical Trial of Traditional Plant Remedies for the Treatment of Complications of Gestational Malaria. Medicines 2021, 8, 79. https://doi.org/10.3390/medicines8120079
Amadi PU, Agomuo EN, Ukaga CN, Njoku UC, Amadi JA, Nwaekpe CG. Preclinical Trial of Traditional Plant Remedies for the Treatment of Complications of Gestational Malaria. Medicines. 2021; 8(12):79. https://doi.org/10.3390/medicines8120079
Chicago/Turabian StyleAmadi, Peter Uchenna, Emmanuel Nnabugwu Agomuo, Chinyere Nneka Ukaga, Uche Chinedu Njoku, Joy Adaku Amadi, and Chinweuba Godswill Nwaekpe. 2021. "Preclinical Trial of Traditional Plant Remedies for the Treatment of Complications of Gestational Malaria" Medicines 8, no. 12: 79. https://doi.org/10.3390/medicines8120079
APA StyleAmadi, P. U., Agomuo, E. N., Ukaga, C. N., Njoku, U. C., Amadi, J. A., & Nwaekpe, C. G. (2021). Preclinical Trial of Traditional Plant Remedies for the Treatment of Complications of Gestational Malaria. Medicines, 8(12), 79. https://doi.org/10.3390/medicines8120079






