Acupuncture for Relief of Gag Reflex in Patients Undergoing Transoesophageal Echocardiography—A Protocol for a Randomized Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
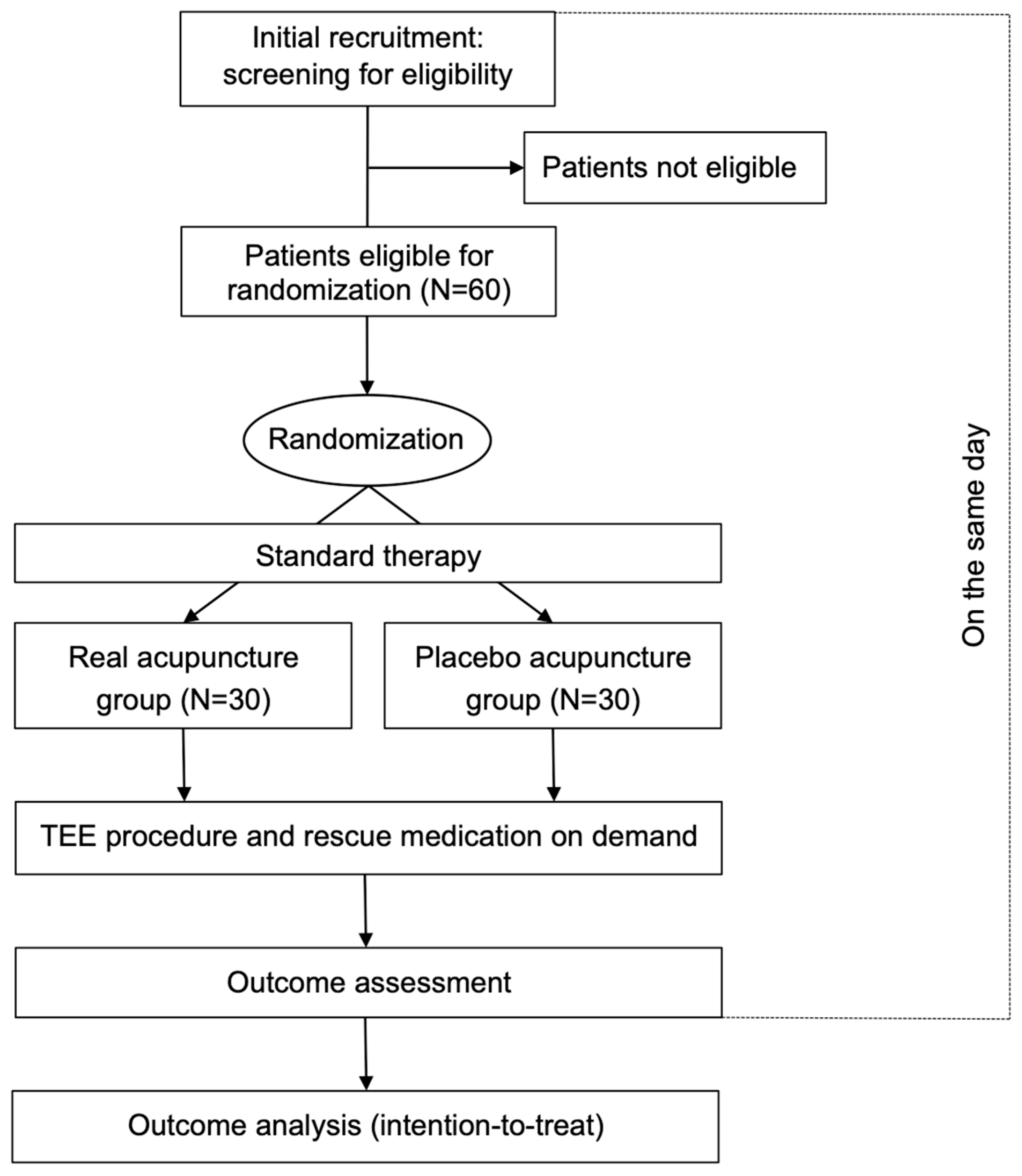
2.1. Study Design
2.2. Eligibility Criteria
2.3. Group Allocation and Randomization
2.4. Current Standard Treatment for TEE Procedure
2.5. Study Procedure
2.6. Outcome Measures
2.7. Sample Size
2.8. Data Analysis
3. Discussion
Trial Status
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davies, A.E.; Kidd, D.; Stone, S.P.; MacMahon, J. Pharyngeal sensation and gag reflex in healthy subjects. Lancet 1995, 345, 487–488. [Google Scholar] [CrossRef]
- Hughes, T.A.T.; Wiles, C.M. Palatal and pharyngeal reflexes in health and motor neuron disease. J. Neurol. Neurosurg. Psychiatry 1996, 61, 96–98. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bassi, G.S.; Humphris, G.M.; Longman, L.P. The etiology and management of gagging: A review of the literature. J. Prosthet. Dent. 2004, 91, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Khongkaew, J.; Sahasthas, D.; Potat, T.; Thammawirat, P. Modified mallampati classification in determining the success of unsedated transesophageal echocardiography procedure in patients with heart disease: Simple but efficient. Cardiovasc. Ultrasound. 2016, 14, 42. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prashanti, E.; Sumanth, K.N.; Renjith George, P.; Karanth, L.; Soe, H.H. Management of gag reflex for patients undergoing dental treatment. Cochrane Database Syst. Rev. 2015, 10, CD011116. [Google Scholar] [CrossRef] [PubMed]
- Fiske, J.; Dickinson, C. The role of acupuncture in controlling the gagging reflex using a review of ten cases. Br. Dent. J. 2001, 190, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Rosted, P.; Bundgaard, M.; Fiske, J.; Pedersen, A.M. The use of acupuncture in controlling the gag reflex in patients requiring an upper alginate impression: An audit. Br. Dent. J. 2006, 201, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Jeffrey, S.; Lochhead, V. Use of acupuncture to reduce gagging during insertion of an oral airway. Anaesthesia 2008, 63, 1387–1391. [Google Scholar] [CrossRef]
- Rösler, A.; Otto, B.; Schreiber-Dietrich, D.; Steinmetz, H.; Kessler, K.R. Single-needle acupuncture alleviates gag reflex during transesophageal Echocardiography: A blinded, randomized, controlled pilot trial. J. Alternat. Complement. Med. 2003, 9, 847–849. [Google Scholar] [CrossRef] [PubMed]
- Bilello, G.; Fregapane, A. Gag reflex control through acupuncture: A case series. Acupunct. Med. 2014, 32, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Goel, H.; Mathur, S.; Sandhu, M.; Jhingan, P.; Sachdev, V. Effect of Low-level LASER Therapy on P6 Acupoint to Control Gag Reflex in Children: A Clinical Trial. J. Acupunct. Meridian Stud. 2017, 10, 317–323. [Google Scholar] [CrossRef] [PubMed]
- MacPherson, H.; White, A.; Cummings, M.; Jobst, K.; Rose, K.; Niemtzow, R. STandards for Reporting Interventions in Controlled Trails of Acupuncture. Standards for reporting interventions in controlled trials of acupuncture: The STRICTA recommendations. STandards for Reporting Interventions in Controlled Trails of Acupuncture. Acupunct. Med. 2002, 20, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Usichenko, T.I.; Lysenyuk, V.P.; Groth, M.H.; Pavlovic, D. Detection of ear acupuncture points by measuring the electrical skin resistance in patients before, during and after orthopedic surgery performed under general anesthesia. Acupunct. Electrother. Res. 2003, 28, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Inference for Means: Comparing Two Independent Samples. Available online: https://www.stat.ubc.ca/~rollin/stats/ssize/n2.html (accessed on 29 September 2018).
- Chan, K.L.; Cohen, G.I.; Sochowski, R.A.; Baird, M.G. Complications of transesophageal echocardiography in ambulatory adult patients: Analysis of 1500 consecutive examinations. J. Am. Soc. Echocardiogr. 1991, 4, 577–582. [Google Scholar] [CrossRef]
- Aeschbacher, B.C.; Portner, M.; Fluri, M.; Meier, B.; Lüscher, T.F. Midazolam premedication improves tolerance of transesophageal echocardiography. Am. J. Cardiol. 1998, 81, 1022–1026. [Google Scholar] [CrossRef]
- Maurice-Szamburski, A.; Auquier, P.; Viarre-Oreal, V.; Cuvillon, P.; Carles, M.; Ripart, J.; Honore, S.; Triglia, T.; Loundou, A.; Leone, M.; et al. Effect of sedative premedication on patient experience after general anesthesia: A randomized clinical trial. JAMA 2015, 313, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Cahn, A.M.; Carayon, P.; Hill, C.; Flammant, R. Acupuncture in gastroscopy. Lancet 1978, 28, 182–183. [Google Scholar] [CrossRef]
- Lundeberg, T.; Lund, I.; Näslund, J.; Thomas, M. The Emperors sham—Wrong assumption that sham needling is sham. Acupunct. Med. 2008, 26, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J. Placebo controls in randomized trials of acupuncture. Eval. Health Prof. 2002, 25, 421–435. [Google Scholar] [CrossRef] [PubMed]
| Item | Detailed Items | Description |
|---|---|---|
| 1. Acupuncture rationale | 1a) Style of acupuncture 1b) Reasoning for treatment provided based on historical context, literature sources, and/or consensus methods, with references where appropriate 1c) Extent to which treatment was varied | Western medical acupuncture Acupuncture methods described as effective in previous pilot investigations (references [6,7,8,9,10,11]) Standardized acupuncture for each patient, no individual variation |
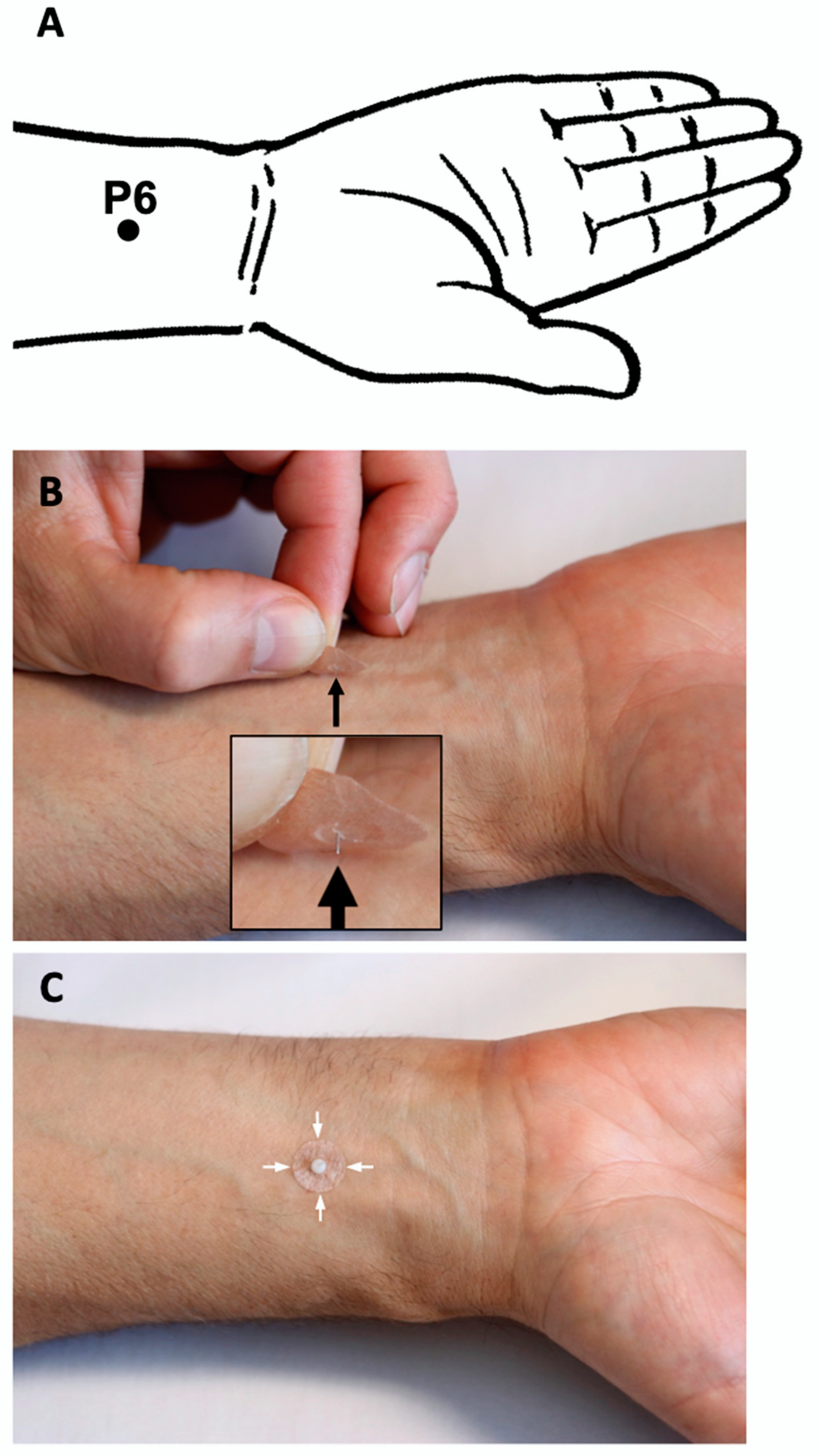
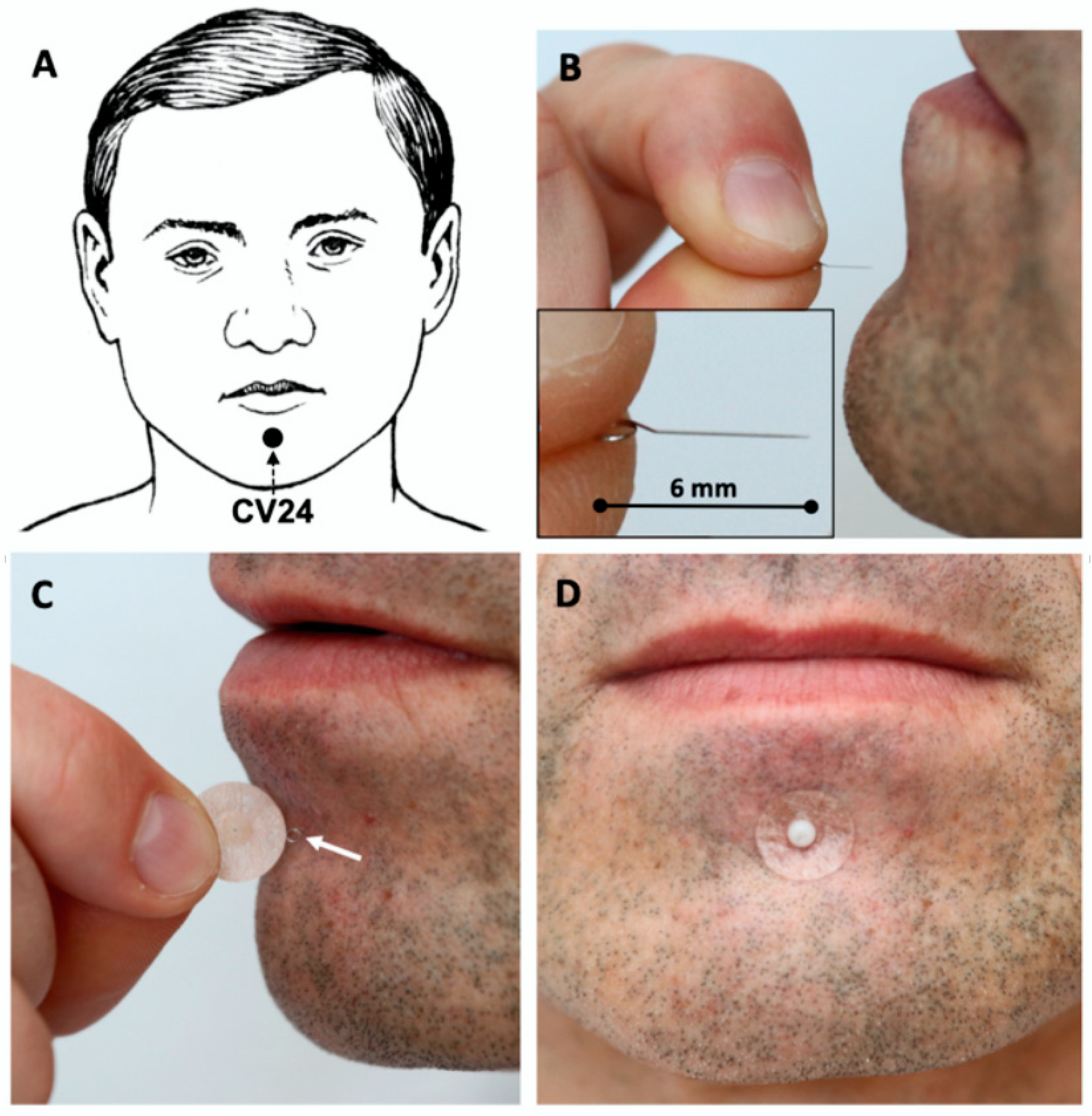
| 2. Details of needling | 2a) Number of needle insertions per subject per session (mean and range where relevant) 2b) Names (or location if no standard name) of points used (uni/bilateral) 2c) Depth of insertion, based on a specified unit of measurement or on a particular tissue level 2d) Response sought 2e) Needle stimulation 2f) Needle retention time 2 g) Needle type (diameter, length, and manufacturer or material) | Three intradermal needles Chengjiang (CV24) midline acupoint Neiguan (PC6) bilateral 5 mm at CV24; 1.5 mm at PC6 No response sought No needle stimulation Until the end of TEE (max. 30 min) Intradermal “Spinex” needle (0.14 mm × 6 mm, Seirin Corp. Japan) at CV24; indwelling ear acupuncture “New Pyonex” needle (0.2 mm × 1.5 mm, Seirin Corp. Japan) at PC6 |
| 3. Treatment regimen | 3a) Number of treatment sessions 3b) Frequency and duration of treatment sessions | One session Once for each patient |
| 4. Other components of treatment | 4a) Details of other interventions administered to the acupuncture group 4b) Setting and context of treatment, including instructions to practitioners, and information and explanations to patients | None Each patient will be informed about acupuncture or placebo procedure against gagging during TEE 1 |
| 5. Practitioner background | 5) Description of participating acupuncturists (qualification or professional affiliation, years in acupuncture practice, other relevant experience) | Licensed medical acupuncturist with more than 10 years of acupuncture practice |
| 6. Control of comparator interventions | 6a) Rationale for the control or comparator in the context of the research question, with sources that justify this choice 6b) Precise description of the control or comparison group. If sham acupuncture or any other type of acupuncture-like control is used, provide details as for items 1 to 3, above | To study the efficacy and safety of acupuncture as an additional therapy in the relief of gagging during a routine TEE procedure Placebo needles will be placed over the same sites in patients from the control group as in the acupuncture group |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usichenko, T.I.; Müller-Kozarez, I.; Knigge, S.; Busch, R.; Busch, M. Acupuncture for Relief of Gag Reflex in Patients Undergoing Transoesophageal Echocardiography—A Protocol for a Randomized Placebo-Controlled Trial. Medicines 2020, 7, 17. https://doi.org/10.3390/medicines7040017
Usichenko TI, Müller-Kozarez I, Knigge S, Busch R, Busch M. Acupuncture for Relief of Gag Reflex in Patients Undergoing Transoesophageal Echocardiography—A Protocol for a Randomized Placebo-Controlled Trial. Medicines. 2020; 7(4):17. https://doi.org/10.3390/medicines7040017
Chicago/Turabian StyleUsichenko, Taras I., Irina Müller-Kozarez, Stephan Knigge, Raila Busch, and Mathias Busch. 2020. "Acupuncture for Relief of Gag Reflex in Patients Undergoing Transoesophageal Echocardiography—A Protocol for a Randomized Placebo-Controlled Trial" Medicines 7, no. 4: 17. https://doi.org/10.3390/medicines7040017
APA StyleUsichenko, T. I., Müller-Kozarez, I., Knigge, S., Busch, R., & Busch, M. (2020). Acupuncture for Relief of Gag Reflex in Patients Undergoing Transoesophageal Echocardiography—A Protocol for a Randomized Placebo-Controlled Trial. Medicines, 7(4), 17. https://doi.org/10.3390/medicines7040017






