Toxicological Aspects and Determination of the Main Components of Ayahuasca: A Critical Review
Abstract
1. Introduction
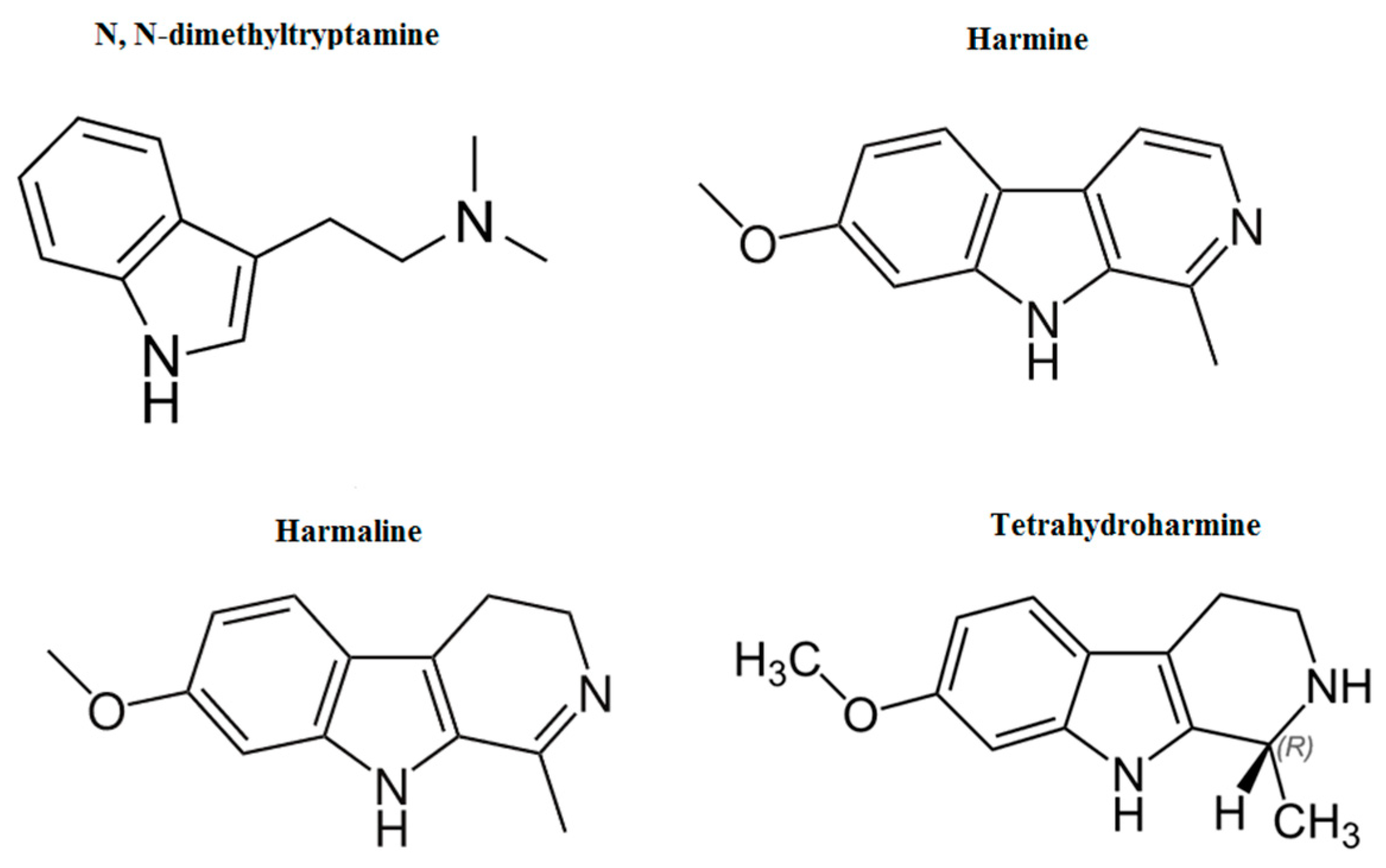
2. N,N-Dimethyltryptamine
2.1. DMT Pharmacokinetics
2.2. DMT Pharmacodynamics
2.3. Adverse Effects of DMT
3. β-Carbolines Alkaloids
4. Methods of Quantification of Ayahuasca
5. In Vivo and In Vitro Studies of Ayahuasca Compounds
6. Conclusions and Further Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-HT receptors | 5-Hydroxytriptamine receptors |
| AChE | acetylcholinesterase |
| B-CA | beta-carbolines alkaloids |
| BT-549 | human breast cancer cell line |
| BuChE | butylcholinesterase |
| cAMP | cyclic adenosine monophosphate |
| CE | capillary electrophoresis |
| COMT | catechol-O-methyl transferase |
| CNS | central nervous system |
| DART-HRMS | direct analysis in real-time–high-resolution mass spectrometry |
| DMT | N, N-dimethyltriptamine |
| EI | electron ionization |
| ESI | electrospray ionization |
| GC | gas chromatography |
| GC-IT-MS | gas chromatography coupled to ion trap mass spectrometry |
| GC-MS | gas chromatography coupled to mass spectrometry |
| GC-NPD | gas chromatography coupled to nitrogen phosphorous detector |
| HEK293 | human embryonic kidney 293 cells |
| HepG2 | human liver cancer cell line |
| HESI | heated electrospray ionization |
| HLOL | harmalol |
| HML | harmaline |
| HMN | harmine |
| HPLC | high-performance liquid chromatography |
| HPLC-FLD | high-performance liquid chromatography coupled to fluorescence detector |
| IS | internal standard |
| KB | human HeLa contaminant carcinoma cell line |
| LC-MS/MS | liquid chromatography coupled to tandem mass spectrometry |
| LD50 | lethal dose 50 |
| LIF | laser-induced fluorescence |
| LLC-PK11 | pig kidney’s epithelial cells |
| LLE | liquid–liquid extraction |
| LOD | limit of detection |
| LOQ | limit of quantification |
| LSD | lysergic acid diethylamide |
| MAO | monoamine oxidase |
| n.a. | not available |
| NMR | nuclear magnetic resonance of proton |
| MS | mass spectrometry |
| R2/r2 | determination coefficient |
| SERT | serotonin transporter |
| SK-MEL | human melanoma cell Line |
| SPE | solid-phase extraction |
| SPME | solid-phase microextraction |
| TAAR1 | trace amine-associated receptor 1 |
| THH | tetrahydroharmine |
| UDV | União do Vegetal |
| UHPLC-MS/MS | ultra-high-pressure liquid chromatography–tandem mass spectrometry |
| VERO | monkey kidney cell line |
| VMAT | vesicular monoamine transporter |
References
- Labate, B.C.; Feeney, K. Ayahuasca and the process of regulation in Brazil and internationally: Implications and challenges. Int. J. Drug Policy 2012, 23, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Andrade, T.S.; de Oliveira, R.; da Silva, M.L.; Von Zuben, M.V.; Grisolia, C.K.; Domingues, I.; Caldas, E.D.; Pic-Taylor, A. Exposure to ayahuasca induces developmental and behavioral alterations on early life stages of zebrafish. Chem. Biol. Interact. 2018, 293, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Gaujac, A.; Dempster, N.; Navickiene, S.; Brandt, S.D.; Andrade, J.B. de Determination of N,N-dimethyltryptamine in beverages consumed in religious practices by headspace solid-phase microextraction followed by gas chromatography ion trap mass spectrometry. Talanta 2013, 106, 394–398. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.J. Clinical investigations of the therapeutic potential of ayahuasca: Rationale and regulatory challenges. Pharmacol. Ther. 2004, 102, 111–129. [Google Scholar] [CrossRef]
- Callaway, J.C.; McKenna, D.J.; Grob, C.S.; Brito, G.S.; Raymon, L.P.; Poland, R.E.; Andrade, E.N.; Andrade, E.O.; Mash, D.C. Pharmacokinetics of Hoasca alkaloids in healthy humans. J. Ethnopharmacol. 1999, 65, 243–256. [Google Scholar] [CrossRef]
- Grob, C.S.; McKenna, D.J.; Callaway, J.C.; Brito, G.S.; Neves, E.S.; Oberlaender, G.; Saide, O.L.; Labigalini, E.; Tacla, C.; Miranda, C.T.; et al. Human psychopharmacology of hoasca, a plant hallucinogen used in ritual context in Brazil. J. Nerv. Ment. Dis. 1996, 184, 86–94. [Google Scholar] [CrossRef]
- Anderson, B.T.; Labate, B.C.; Meyer, M.; Tupper, K.W.; Barbosa, P.C.R.; Grob, C.S.; Dawson, A.; McKenna, D. Statement on ayahuasca. Int. J. Drug Policy 2012, 23, 173–175. [Google Scholar] [CrossRef]
- Malcolm, B.J.; Lee, K.C. Ayahuasca: An ancient sacrament for treatment of contemporary psychiatric illness? Ment. Heal. Clin. 2018, 7, 39–45. [Google Scholar] [CrossRef]
- Pic-Taylor, A.; da Motta, L.G.; de Morais, J.A.; Junior, W.M.; Santos Ade, F.; Campos, L.A.; Mortari, M.R.; von Zuben, M.V.; Caldas, E.D. Behavioural and neurotoxic effects of ayahuasca infusion (Banisteriopsis caapi and Psychotria viridis) in female Wistar rat. Behav. Processes 2015, 118, 102–110. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Bouso, J.C.; Hallak, J.E.C. Ayahuasca, dimethyltryptamine, and psychosis: A systematic review of human studies. Ther. Adv. Psychopharmacol. 2017, 7, 141–157. [Google Scholar] [CrossRef]
- Ramachandran, P.; Zhang, N.; McLaughlin, W.B.; Luo, Y.; Handy, S.; Duke, J.A.; Vasquez, R.; Ottesen, A. Sequencing the vine of the soul: Full Chloroplast genome sequence of Banisteriopsis caapi. Genome Announc. 2018, 6, e00203-18. [Google Scholar] [CrossRef] [PubMed]
- Frison, G.; Favretto, D.; Zancanaro, F.; Fazzin, G.; Ferrara, S.D. A case of beta-carboline alkaloid intoxication following ingestion of Peganum harmala seed extract. Forensic Sci. Int. 2008, 179, e37–e43. [Google Scholar] [CrossRef] [PubMed]
- Sklerov, J.; Levine, B.; Moore, K.A.; King, T.; Fowler, D. A fatal intoxication following the ingestion of 5-methoxy-N,N-dimethyltryptamine in an ayahuasca preparation. J. Anal. Toxicol. 2005, 29, 838–841. [Google Scholar] [CrossRef] [PubMed]
- Morales-García, J.A.; de la Fuente Revenga, M.; Alonso-Gil, S.; Rodríguez-Franco, M.I.; Feilding, A.; Perez-Castillo, A.; Riba, J. The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro. Sci. Rep. 2017, 7, 5309. [Google Scholar] [CrossRef]
- McKenna, D.J. Ayahuasca: An ethnopharmacologic history. In Sacred Vine of Spirits: Ayahuasca; Park Street Press: Rochester, VT, USA, 1999; pp. 40–62. [Google Scholar]
- Rivier, L.; Lindgren, J.-E. “Ayahuasca,” the South American hallucinogenic drink: An ethnobotanical and chemical investigation. Econ. Bot. 1972, 26, 101–129. [Google Scholar] [CrossRef]
- da Motta, L.G.; de Morais, J.A.; Tavares, A.C.A.M.; Vianna, L.M.S.; Mortari, M.R.; Amorim, R.F.B.; Carvalho, R.R.; Paumgartten, F.J.R.; Pic-Taylor, A.; Caldas, E.D. Maternal and developmental toxicity of the hallucinogenic plant-based beverage ayahuasca in rats. Reprod. Toxicol. 2018, 77, 143–153. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Osório, F.L.; Crippa, J.A.S.; Hallak, J.E.C.; dos Santos, R.G.; Osório, F.L.; Crippa, J.A.S.; Hallak, J.E.C. Antidepressive and anxiolytic effects of ayahuasca: A systematic literature review of animal and human studies. Rev. Bras. Psiquiatr. 2016, 38, 65–72. [Google Scholar] [CrossRef]
- Osório Fde, L.; Sanches, R.F.; Macedo, L.R.; dos Santos, R.G.; Maia-de-Oliveira, J.P.; Wichert-Ana, L.; de Araujo, D.B.; Riba, J.; Crippa, J.A.; Hallak, J.E.; et al. Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: A preliminary report. Rev. Bras. Psiquiatr. 2015, 37, 13–20. [Google Scholar]
- Nunes, A.A.; dos Santos, R.G.; Osório, F.L.; Sanches, R.F.; Crippa, J.A.S.; Hallak, J.E.C. Effects of Ayahuasca and its Alkaloids on Drug Dependence: A Systematic Literature Review of Quantitative Studies in Animals and Humans. J. Psychoactive Drugs 2016, 48, 195–205. [Google Scholar] [CrossRef]
- Thomas, G.; Lucas, P.; Capler, N.; Tupper, K.; Martin, G. Ayahuasca-Assisted Therapy for Addiction: Results from a Preliminary Observational Study in Canada. Curr. Drug Abuse Rev. 2013, 6, 30–42. [Google Scholar] [CrossRef]
- Oliveira-Lima, A.J.; Santos, R.; Hollais, A.W.; Gerardi-Junior, C.A.; Baldaia, M.A.; Wuo-Silva, R.; Yokoyama, T.S.; Costa, J.L.; Malpezzi-Marinho, E.L.A.; Ribeiro-Barbosa, P.C.; et al. Effects of ayahuasca on the development of ethanol-induced behavioral sensitization and on a post-sensitization treatment in mice. Physiol. Behav. 2015, 142, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Da Silveira, D.X.; Grob, C.S.; de Rios, M.D.; Lopez, E.; Alonso, L.K.; Tacla, C.; Doering-Silveira, E. Ayahuasca in Adolescence: A Preliminary Psychiatric Assessment. J. Psychoactive Drugs 2005, 37, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.C.R.; Cazorla, I.M.; Giglio, J.S.; Strassman, R. A six-month prospective evaluation of personality traits, psychiatric symptoms and quality of life in ayahuasca-naïve subjects. J. Psychoactive Drugs 2009, 41, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Cameron, L.P.; Olson, D.E. Dark Classics in Chemical Neuroscience: N, N -Dimethyltryptamine (DMT). ACS Chem. Neurosci. 2018, 9, 2344–2357. [Google Scholar] [CrossRef] [PubMed]
- Strassman, R.J.; Qualls, C.R. Dose-response study of N,N-dimethyltryptamine in humans. I. Neuroendocrine, autonomic, and cardiovascular effects. Arch. Gen. Psychiatry 1994, 51, 85–97. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.G.; Bouso, J.C.; Alcázar-Córcoles, M.Á.; Hallak, J.E.C. Efficacy, tolerability, and safety of serotonergic psychedelics for the management of mood, anxiety, and substance-use disorders: a systematic review of systematic reviews. Expert Rev. Clin. Pharmacol. 2018, 11, 889–902. [Google Scholar] [CrossRef]
- Horák, M.; Novák, P.; Vozáryová, W. Legal Aspects of the Ayahuasca Consumption in the European Union. In Sborník Prípevku z Mezinárodní Vedecké Konference Region v Rozvoji Spolecnosti; Mendel University: Brno, Czech Republic, 2016; pp. 276–283. [Google Scholar]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx 2005, 2, 541–553. [Google Scholar] [CrossRef]
- Ott, J. Pharmacotheon: Entheogenic Drugs, Their Plant Sources and History, 2nd ed.; Natural Products Co.: Kennewick, WA, USA, 1993; ISBN 0961423439. [Google Scholar]
- Halpern, J.H. Hallucinogens and dissociative agents naturally growing in the United States. Pharmacol. Ther. 2004, 102, 131–138. [Google Scholar] [CrossRef]
- Gable, R.S. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction 2007, 102, 24–34. [Google Scholar] [CrossRef]
- Callaway, J.C.; Brito, G.S.; Neves, E.S. Phytochemical analyses of Banisteriopsis Caapi and Psychotria Viridis. J. Psychoact. Drugs 2005, 37, 145–150. [Google Scholar] [CrossRef]
- Cakic, V.; Potkonyak, J.; Marshall, A. Dimethyltryptamine (DMT): Subjective effects and patterns of use among Australian recreational users. Drug Alcohol Depend. 2010, 111, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A.; Monti, J.A.; Christian, S.T. N,N-dimethyltryptamine: An endogenous hallucinogen. Int. Rev. Neurobiol. 1981, 22, 83–110. [Google Scholar] [PubMed]
- Sitaram, B.R.; Lockett, L.; Talomsin, R.; Blackman, G.L.; McLeod, W.R. In vivo metabolism of 5-methoxy-N, N-dimethyltryptamine and N,N-dimethyltryptamine in the rat. Biochem. Pharmacol. 1987, 36, 1509–1512. [Google Scholar] [CrossRef]
- Barbosa, P.C.R.; Mizumoto, S.; Bogenschutz, M.P.; Strassman, R.J. Health status of ayahuasca users. Drug Test. Anal. 2012, 4, 601–609. [Google Scholar] [CrossRef]
- Riba, J.; Valle, M.; Urbano, G.; Yritia, M.; Morte, A.; Barbanoj, M.J. Human pharmacology of ayahuasca: Subjective and cardiovascular effects, monoamine metabolite excretion, and pharmacokinetics. J. Pharmacol. Exp. Ther. 2003, 306, 73–83. [Google Scholar] [CrossRef]
- Riba, J.; McIlhenny, E.H.; Valle, M.; Bouso, J.C.; Barker, S.A. Metabolism and disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test. Anal. 2012, 4, 610–616. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Balthazar, F.M.; Bouso, J.C.; Hallak, J.E. The current state of research on ayahuasca: A systematic review of human studies assessing psychiatric symptoms, neuropsychological functioning, and neuroimaging. J. Psychopharmacol. 2016, 30, 1230–1247. [Google Scholar] [CrossRef]
- Helsley, S.; Fiorella, D.; Rabin, R.A.; Winter, J.C. A comparison of N,N-dimethyltryptamine, harmaline, and selected congeners in rats trained with LSD as a discriminative stimulus. Prog. Neuro Psychopharmacol. Biol. Psychiatry 1998, 22, 649–663. [Google Scholar] [CrossRef]
- Appel, J.B.; West, W.B.; Rolandi, W.G.; Alici, T.; Pechersky, K. Increasing the selectivity of drug discrimination procedures. Pharmacol. Biochem. Behav. 1999, 64, 353–358. [Google Scholar] [CrossRef]
- Smith, R.L.; Canton, H.; Barrett, R.J.; Sanders-Bush, E. Agonist Properties of N,N-dimethyltryptamine at serotonin 5-HT2A and 5-HT2C receptors. Pharmacol. Biochem. Behav. 1998, 61, 323–330. [Google Scholar] [CrossRef]
- Gatch, M.B.; Rutledge, M.A.; Carbonaro, T.; Forster, M.J. Comparison of the discriminative stimulus effects of dimethyltryptamine with different classes of psychoactive compounds in rats. Psychopharmacology 2009, 204, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Cameron, C.; Kelly, S.; Hsieh, S.-C.; Murphy, M.; Chen, L.; Kotb, A.; Peterson, J.; Coyle, D.; Skidmore, B.; Gomes, T.; et al. Triptans in the acute treatment of migraine: A systematic review and network meta-analysis. Headache J. Head Face Pain 2015, 55, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Karila, D.; Freret, T.; Bouet, V.; Boulouard, M.; Dallemagne, P.; Rochais, C. Therapeutic potential of 5-HT6 receptor agonists. J. Med. Chem. 2015, 58, 7901–7912. [Google Scholar] [CrossRef] [PubMed]
- Pazos, A.; Probst, A.; Palacios, J.M. Serotonin receptors in the human brain—III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 1987, 21, 97–122. [Google Scholar] [CrossRef]
- Sotelo, C.; Cholley, B.; El Mestikawy, S.; Gozlan, H.; Hamon, M. Direct immunohistochemical evidence of the existence of 5-HT1A autoreceptors on serotoninergic neurons in the midbrain raphe nuclei. Eur. J. Neurosci. 1990, 2, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Keiser, M.J.; Setola, V.; Irwin, J.J.; Laggner, C.; Abbas, A.I.; Hufeisen, S.J.; Jensen, N.H.; Kuijer, M.B.; Matos, R.C.; Tran, T.B.; et al. Predicting new molecular targets for known drugs. Nature 2009, 462, 175–181. [Google Scholar] [CrossRef]
- Dos Santos, R.G.; Osório, F.L.; Crippa, J.A.S.; Riba, J.; Zuardi, A.W.; Hallak, J.E.C. Antidepressive, anxiolytic, and antiaddictive effects of ayahuasca, psilocybin and lysergic acid diethylamide (LSD): A systematic review of clinical trials published in the last 25 years. Ther. Adv. Psychopharmacol. 2016, 6, 193–213. [Google Scholar] [CrossRef]
- Domínguez-Clavé, E.; Soler, J.; Elices, M.; Pascual, J.C.; Álvarez, E.; de la Fuente Revenga, M.; Friedlander, P.; Feilding, A.; Riba, J. Ayahuasca: Pharmacology, neuroscience and therapeutic potential. Brain Res. Bull. 2016, 126, 89–101. [Google Scholar] [CrossRef]
- Carbonaro, T.M.; Eshleman, A.J.; Forster, M.J.; Cheng, K.; Rice, K.C.; Gatch, M.B. The role of 5-HT2A, 5-HT2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology 2015, 232, 275–284. [Google Scholar] [CrossRef]
- Aghajanian, G.; Marek, G. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology 1997, 36, 589–599. [Google Scholar] [CrossRef]
- Aghajanian, G.K.; Marek, G.J. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999, 825, 161–171. [Google Scholar] [CrossRef]
- Mckenna, D.J.; Repke, D.B.; Lo, L.; Peroutka, S.J. Differential interactions of indolealkylamines with 5-hydroxytryptamine receptor subtypes. Neuropharmacology 1990, 29, 193–198. [Google Scholar] [CrossRef]
- Pierce, P.A.; Peroutka, S.J. Hallucinogenic drug interactions with neurotransmitter receptor binding sites in human cortex. Psychopharmacology 1989, 97, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Heuring, R.E.; Peroutka, S.J. Characterization of a novel 3H-5-hydroxytryptamine binding site subtype in bovine brain membranes. J. Neurosci. 1987, 7, 894–903. [Google Scholar] [CrossRef]
- Bunzow, J.R.; Sonders, M.S.; Arttamangkul, S.; Harrison, L.M.; Zhang, G.; Quigley, D.I.; Darland, T.; Suchland, K.L.; Pasumamula, S.; Kennedy, J.L.; et al. Amphetamine, 3,4-methylenedioxymethamphetamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001, 60, 1181–1188. [Google Scholar] [CrossRef]
- Saavedra, J.M.; Axelrod, J.; Hajipour, A.R.; Cozzi, N.V.; Jackson, M.B.; Ruoho, A.E. Psychotomimetic N-methylated tryptamines: Formation in brain in vivo and in vitro. Science 1972, 175, 1365–1366. [Google Scholar] [CrossRef]
- Szabo, A.; Kovacs, A.; Riba, J.; Djurovic, S.; Rajnavolgyi, E.; Frecska, E. The endogenous hallucinogen and trace amine N,N-dimethyltryptamine (DMT) displays potent protective effects against hypoxia via sigma-1 receptor activation in human primary iPSC-derived cortical neurons and microglia-like immune cells. Front. Neurosci. 2016, 10, 423. [Google Scholar] [CrossRef]
- Hayashi, T. Sigma-1 receptor: The novel intracellular target of neuropsychotherapeutic drugs. J. Pharmacol. Sci. 2015, 127, 2–5. [Google Scholar] [CrossRef]
- Rickli, A.; Moning, O.D.; Hoener, M.C.; Liechti, M.E. Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur. Neuropsychopharmacol. 2016, 26, 1327–1337. [Google Scholar] [CrossRef]
- Smith, T.L. Increased synthesis of striatal dopamine by N,N-dimethyltryptamine. Life Sci. 1977, 21, 1597–1601. [Google Scholar] [CrossRef]
- Haubrich, D.R.; Wang, P.F.L. N,N-dimethyltryptamine lowers rat brain acetylcholine and dopamine. Brain Res. 1977, 131, 158–161. [Google Scholar] [CrossRef]
- Riba, J.; Anderer, P.; Morte, A.; Urbano, G.; Jané, F.; Saletu, B.; Barbanoj, M.J. Topographic pharmaco-EEG mapping of the effects of the South American psychoactive beverage Ayahuasca in healthy volunteers. Br. J. Clin. Pharmacol. 2002, 53, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Sangiah, S.; Gomez, M.V.; Domino, E.F. Accumulation of N,N-dimethyltryptamine in rat brain cortical slices. Biol. Psychiatry 1979, 14, 925–936. [Google Scholar] [PubMed]
- Cozzi, N.V.; Gopalakrishnan, A.; Anderson, L.L.; Feih, J.T.; Shulgin, A.T.; Daley, P.F.; Ruoho, A.E. Dimethyltryptamine and other hallucinogenic tryptamines exhibit substrate behavior at the serotonin uptake transporter and the vesicle monoamine transporter. J. Neural Transm. 2009, 116, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Riba, J.; Rodríguez-Fornells, A.; Urbano, G.; Morte, A.; Antonijoan, R.; Montero, M.; Callaway, J.C.; Barbanoj, M.J. Subjective effects and tolerability of the South American psychoactive beverage Ayahuasca in healthy volunteers. Psychopharmacology 2001, 154, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Checkley, S.A.; Murray, R.M.; Oon, M.C.; Rodnight, R.; Birley, J.L. A longitudinal study of urinary excretion of N,N,-dimethyltryptamine in psychotic patients. Br. J. Psychiatry 1980, 137, 236–239. [Google Scholar] [CrossRef]
- Lipinski, J.F.; Mandel, L.R.; Ahn, H.S.; Vanden Heuvel, W.J.; Walker, R.W. Blood dimethyltryptamine concentrations in psychotic disorders. Biol. Psychiatry 1974, 9, 89–91. [Google Scholar]
- Ciprian-Ollivier, J.; Cetkovich-Bakmas, M.G. Altered consciousness states and endogenous psychoses: A common molecular pathway? Schizophr. Res. 1997, 28, 257–265. [Google Scholar] [CrossRef]
- Jacob, M.S.; Presti, D.E. Endogenous psychoactive tryptamines reconsidered: An anxiolytic role for dimethyltryptamine. Med. Hypotheses 2005, 64, 930–937. [Google Scholar] [CrossRef]
- Gillin, J.C.; Kaplan, J.; Stillman, R.; Wyatt, R.J. The psychedelic model of schizophrenia: The case of N,N- dimethyltryptamine. Am. J. Psychiatry 1976, 133, 203–208. [Google Scholar]
- Santos, R.G.; Landeira-Fernandez, J.; Strassman, R.J.; Motta, V.; Cruz, A.P.M. Effects of Ayahuasca on psychometric measures of anxiety, panic-like and hopelessness in Santo Daime members. J. Ethnopharmacol. 2007, 112, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, J.J.; Réus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Stertz, L.; Kapczinski, F.; Pinto, J.P.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; et al. Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2009, 33, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, J.J.; Réus, G.Z.; Kirsch, T.R.; Stringari, R.B.; Fries, G.R.; Kapczinski, F.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Quevedo, J. Effects of β-carboline harmine on behavioral and physiological parameters observed in the chronic mild stress model: Further evidence of antidepressant properties. Brain Res. Bull. 2010, 81, 491–496. [Google Scholar] [CrossRef] [PubMed]
- De Lima Osório, F.; Ribeiro, L.; De Macedo, H.; Machado De Sousa, J.P.; Pinto, J.P.; Quevedo, J.; Alexandre De Souza Crippa, J.; Hallak, J.E.C. 5. The Therapeutic Potential of Harmine and Ayahuasca in Depression: Evidence From Exploratory Animal and Human Studies; Rafael dos Santos, Ed.; Transworld Research Network: Kerala, India, 2011; ISBN 9788178955261. [Google Scholar]
- Kim, H.; Sablin, S.O.; Ramsay, R.R. Inhibition of monoamine oxidase A by β-Carboline derivatives. Arch. Biochem. Biophys. 1997, 337, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Moura, D.J.; Richter, M.F.; Boeira, J.M.; Pêgas Henriques, J.A.; Saffi, J. Antioxidant properties of beta-carboline alkaloids are related to their antimutagenic and antigenotoxic activities. Mutagenesis 2007, 22, 293–302. [Google Scholar] [CrossRef] [PubMed]
- McKenna, D.J.; Towers, G.H.N. Biochemistry and pharmacology of tryptamines and β-carbolines A minireview. J. Psychoactive Drugs 1984, 16, 347–358. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Mikaili, P.; Aghajanshakeri, S.; Asghari, M.; Shayegh, J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacogn. Rev. 2013, 7, 199. [Google Scholar] [CrossRef]
- Asgarpanah, J.; Ramezanloo, F. Chemistry, pharmacology and medicinal properties of Peganum harmala L. Afr. J. Pharm. Pharmacol. 2012, 6, 1573–1580. [Google Scholar] [CrossRef]
- Ott, J. Pharmahuasca: Human pharmacology of oral DMT plus harmine. J. Psychoact. Drugs 1999, 31, 171–177. [Google Scholar] [CrossRef]
- Liester, M.B.; Prickett, J.I. Hypotheses Regarding the mechanisms of Ayahuasca in the treatment of addictions. J. Psychoact. Drugs 2012, 44, 200–208. [Google Scholar] [CrossRef]
- Gaweska, H.; Fitzpatrick, P.F. Structures and mechanism of the monoamine oxidase family. Biomol. Concepts 2011, 2, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Orlefors, H.; Sundin, A.; Fasth, K.J.; Oberg, K.; Långström, B.; Eriksson, B.; Bergström, M. Demonstration of high monoaminoxidase-A levels in neuroendocrine gastroenteropancreatic tumors in vitro and in vivo-tumor visualization using positron emission tomography with 11C-harmine. Nucl. Med. Biol. 2003, 30, 669–679. [Google Scholar] [CrossRef]
- Brierley, D.I.; Davidson, C. Developments in harmine pharmacology—Implications for ayahuasca use and drug-dependence treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2012, 39, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Miller, J.H.; Lea, R.A. Monoamine oxidase and tobacco dependence. Neurotoxicology 2007, 28, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Boulton, S.J.; Keane, P.C.; Morris, C.M.; McNeil, C.J.; Manning, P. Real-time monitoring of superoxide generation and cytotoxicity in neuroblastoma mitochondria induced by 1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline. Redox Rep. 2012, 17, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, P. Estudio comparativo de la harmina, la dietilamida del acido lisérgico (LSD 25) y la mescalina. Rev. Confefderación Médica Panam. 1959, 6, 1–8. [Google Scholar]
- Naranjo, C. Psychotropic properties of the harmala alkaloids. In Ethnopharmacologic Search from Psychoactive Drugs; Efron, D.H., Holmstedt, B., Kline, N.S., Eds.; United States Department of Health and Human Services: Washington, DC, USA, 1967; pp. 385–391. [Google Scholar]
- Yritia, M.; Riba, J.; Ortuño, J.; Ramirez, A.; Castillo, A.; Alfaro, Y.; de la Torre, R.; Barbanoj, M.J. Determination of N,N-dimethyltryptamine and β-carboline alkaloids in human plasma following oral administration of Ayahuasca. J. Chromatogr. B 2002, 779, 271–281. [Google Scholar] [CrossRef]
- Pires, A.P.S.; De Oliveira, C.D.R.; Moura, S.; Dörr, F.A.; Silva, W.A.E.; Yonamine, M. Gas chromatographic analysis of dimethyltryptamine and β -carboline alkaloids in ayahuasca, an amazonian psychoactive plant beverage. Phytochem. Anal. 2009, 20, 149–153. [Google Scholar] [CrossRef]
- Oliveira, C.D.R.; Okai, G.G.; da Costa, J.L.; de Almeida, R.M.; Oliveira-Silva, D.; Yonamine, M. Determination of dimethyltryptamine and β-carbolines (ayahuasca alkaloids) in plasma samples by LC–MS/MS. Bioanalysis 2012, 4, 1731–1738. [Google Scholar] [CrossRef]
- Pichini, S.; Marchei, E.; García-Algar, O.; Gomez, A.; Di Giovannandrea, R.; Pacifici, R. Ultra-high-pressure liquid chromatography tandem mass spectrometry determination of hallucinogenic drugs in hair of psychedelic plants and mushrooms consumers. J. Pharm. Biomed. Anal. 2014, 100, 284–289. [Google Scholar] [CrossRef]
- Callaway, J.C.; Raymon, L.P.; Hearn, W.L.; McKenna, D.J.; Grob, C.S.; Brito, G.S.; Mash, D.C. Quantitation of N,N-dimethyltryptamine and harmala alkaloids in human plasma after oral dosing with ayahuasca. J. Anal. Toxicol. 1996, 20, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.C.Z.; Zandonadi, F.S.; Freitas, D.P.; Tófoli, L.F.F.; Sussulini, A. Validation of an analytical method for the determination of the main ayahuasca active compounds and application to real ayahuasca samples from Brazil. J. Chromatogr. B 2019, 1124, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Gambelunghe, C.; Aroni, K.; Rossi, R.; Moretti, L.; Bacci, M. Identification of N,N-dimethyltryptamine and β-carbolines in psychotropic ayahuasca beverage. Biomed. Chromatogr. 2008, 22, 1056–1059. [Google Scholar] [CrossRef] [PubMed]
- McIlhenny, E.H.; Pipkin, K.E.; Standish, L.J.; Wechkin, H.A.; Strassman, R.; Barker, S.A. Direct analysis of psychoactive tryptamine and harmala alkaloids in the Amazonian botanical medicine ayahuasca by liquid chromatography–electrospray ionization-tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 8960–8968. [Google Scholar] [CrossRef]
- McIlhenny, E.H.; Riba, J.; Barbanoj, M.J.; Strassman, R.; Barker, S.A. Methodology for and the determination of the major constituents and metabolites of the Amazonian botanical medicine ayahuasca in human urine. Biomed. Chromatogr. 2011, 25, 970–984. [Google Scholar] [CrossRef]
- McIlhenny, E.H.; Riba, J.; Barbanoj, M.J.; Strassman, R.; Barker, S.A. Methodology for determining major constituents of ayahuasca and their metabolites in blood. Biomed. Chromatogr. 2012, 26, 301–313. [Google Scholar] [CrossRef]
- Lesiak, A.D.; Musah, R.A. Application of ambient ionization high resolution mass spectrometry to determination of the botanical provenance of the constituents of psychoactive drug mixtures. Forensic Sci. Int. 2016, 266, 271–280. [Google Scholar] [CrossRef]
- Huhn, C.; Neusüß, C.; Pelzing, M.; Pyell, U.; Mannhardt, J.; Pütz, M. Capillary electrophoresis-laser induced fluorescence-electrospray ionization-mass spectrometry: A case study. Electrophoresis 2005, 26, 1389–1397. [Google Scholar] [CrossRef]
- Moura, S.; Carvalho, F.G.; de Oliveira, C.D.R.; Pinto, E.; Yonamine, M. qNMR: An applicable method for the determination of dimethyltryptamine in ayahuasca, a psychoactive plant preparation. Phytochem. Lett. 2010, 3, 79–83. [Google Scholar] [CrossRef]
- Barbosa, P.C.R.; Giglio, J.S.; Dalgalarrondo, P. Altered states of consciousness and short-term psychological after-effects induced by the first time ritual use of ayahuasca in an urban context in Brazil. J. Psychoactive Drugs 2005, 37, 193–201. [Google Scholar] [CrossRef]
- Santos, A.D.F.A.; Vieira, A.L.S.; Pic-Taylor, A.; Caldas, E.D. Reproductive effects of the psychoactive beverage ayahuasca in male Wistar rats after chronic exposure. Rev. Bras. Farmacogn. 2017, 27, 353–360. [Google Scholar] [CrossRef]
- Samoylenko, V.; Rahman, M.M.; Tekwani, B.L.; Tripathi, L.M.; Wang, Y.-H.; Khan, S.I.; Khan, I.A.; Miller, L.S.; Joshi, V.C.; Muhammad, I. Banisteriopsis caapi, a unique combination of MAO inhibitory and antioxidative constituents for the activities relevant to neurodegenerative disorders and Parkinson’s disease. J. Ethnopharmacol. 2010, 127, 357–367. [Google Scholar] [CrossRef] [PubMed]
| Analyte | Matrix | Sample Preparation | Detection Mode | Stationary and Mobile Phase | Recovery (%) | LOD; LOQ | Concentrations of the Compounds | Reference |
|---|---|---|---|---|---|---|---|---|
| DMT, HMN, HML, THH | Plasma | HPLC–NPD: Protein precipitation; GC–NPD: LLE (n-buthylchloride) | HPLC–FLD (HMN, HML, THH); GC–NPD (DMT) | HPLC-NPD: Supercosil LC-DB-8 (15.0 × 4.6 mm i.d., 5 mm); Mobile Phase: Methanol/acetonitrile/ ammonium acetate 0.1M pH = 6.9 (HPLC–FLD); GC–NPD: DB-1 and DB-17 | n.a | 0.5 ng/mL; 5 ng/mL (DMT); 0.1 ng/m; 2.00 ng/mL (HMN) 0.05; 1.00 ng/mL (HML) 0.1 ng/mL; 1.9 ng/mL (THH) | 222.3 ng/mL (HMN); 9.4 ng/mL (HML) 134.5 ng/mL (THH) | [96] |
| DMT, HMN, HML, THH, harmol and HLOL | Plasma | LLE (n-pentane) (DMT); SPE (HMN, HML, THH and THH O-demethylation metabolites) | GC–NPD (DMT); HPLC–FLD (HMN, HML, THH and THH O-demethylation metabolites) | GC–NPD: 5% phenyl-methylsilicone (12 m × 30.2 mm × 0.33μm film thickness) (DMT) HPLC–FLD: Kromasil 100 C18 (5 μm, 150 × 34 mm); Mobile phase: Solvent A: mixture ammonium acetate buffer (50 mM, pH 8.0) (63:37 v/v) and acetonitrile/methanol (20:30 v/v) and Solvent B: mixture of acetonitrile/methanol (20:30 v/v) (HMN, HML and THH); Solvent A: mixture ammonium acetate buffer (50 mM, pH 6.3) (73:27 v/v) and acetonitrile/methanol (20:30 v/v); Solvent B: acetonitrile/methanol (20:30 v/v) (harmol and HLOL) | 74 (DMT); >87 HMN, HML, THH and THH O-demethylation metabolites) | n.a; 1.6 ng/mL (DMT), 0.5 ng/mL (HMN), 0.3 ng/mL (HML), 0.3 ng/mL (harmol and harmala) and 1.0 ng/mL (THH) | 0.53 mg/mL (DMT); 0.9 mg/mL (HMN); 0.06 mg/mL (HML); 0.72 mg/mL (THH) | [92] |
| DMT, THH, HML and HMN | Plasma | SPE (C18) | LC–MS/MS (ESI) | Phenomenex Synergi Hydro-RP80A (50 × 2.0 mm, 4 μm); Mobile phase: Solvent A: mixture of aqueous solution of ammonium formate (5 mmol/L) with formic acid (0.1%); Solvent B: methanol and formic acid (0.1%) | 88.4–107.7 | 0.1 ng/mL; 0.2–0.4 ng/mL | 1.2–19.8 ng/mL (DMT); 1.0–15.6 ng/mL (HMN); 2.7–15.7 ng/mL (HML) and 27.1–71.4 ng/mL (THH) | [94] |
| DMT | Hair | Hydrolysis (M3 reagent) | UHPLC–MS/MS (ESI) | Acquity UHPLC HSS C18 (2.1 mm × 150 mm, 1.8 µm); Mobile phase: solvent A: formic acid in acetonitrile (0.3%), Solvent B: ammonium formate (5 mM, pH 3) | 79.6–97.4 | 0.01–0.02 ng/mg; 0.03–0.05 ng/mg | 5.6 ng/mg | [95] |
| DMT, THH HML and HMN | Ayahuasca preparations | SPE (C18) | GC–NPD | HP Ultra-2 (25 m × 0.2 mm × 0.33 μm) and Solvent A: formic acid in acetonitrile (0.3%); solvent B: ammonium formate (5 mM, pH 3) | 68.4–99 | 10000 ng/mL; 20000 ng/mL | 0.31–0.73 mg/mL (DMT); 0.37–0.83 mg/mL (HMN); 0.64–1.72 mg/mL (HML) and 0.21–0.67 mg/mL (THH) | [93] |
| DMT | Ayahuasca beverages | SPME (polydimethylsiloxane/divinylbenzene fiber) | GC–IT-MS (EI) | Supelco SLB-5 MS (30 m × 0.25 mm, 0.25 mm film thickness) | 71–109 | 780 ng/mL; 950 ng/mL | 0.17–1.14 mg/mL | [3] |
| DMT, THH, HMN and HML | Ayahuasca beverages | Dilution with methanol/water (1:1) and direct injection | LC–MS/MS (ESI) | Acquity™ UPLC BEH C18 (50 mm × 2.1 mm, 1.7 μm); Mobile phase: water (90%); solvent B: methanol (10%) | n.a | n.a; 150 ng/mL (DMT); n.a; 350 ng/mL (THH); n.a; 600 ng/mL (HMN) and n.a;100 ng/mL (HML) | 62–340 µg/mL (DMT); 402–3308 µg/mL (THH); 414–1816 µg/mL (HMN); 44–420 µg/mL (HML) | [97] |
| DMT; HML; HMN | Ayahuasca beverage | LLE (10 mL diethyl ether) | GC–MS (ion trap) (EI) | Chrompack CP–SIL 8CB-MS (30 m × 0.25 mm × 0.25 μm) | n.a | n.a | 0.24 mg/mL (DMT); 0.06 mg/mL (HML); 0.34 mg/mL (HMN) | [98] |
| DMT; THH; HMN; HML; HLOL; harmol and metabolites | Ayahuasca preparations | Dilution with mobile phase and direct injection | LC–MS/MS (ESI) | Zorbax Eclipse Plus HT C18 (1.8 µm × 4.6 × 50 mm (i.d.)); Mobile phase: Solvent A: formic acid (0.1% in water); Solvent B: Formic acid (0.1% in acetonitrile) | n.a | 6.4; 210 ng/mL (DMT); 0.5; 210 ng/mL (THH); 0.5; 100 ng/mL (HMN); 2.8; 220 ng/mL (HML); 34.3;510 ng/mL (HLOL) | 0.13–3.19 mg/mL (DMT); 1.22–11.90 mg/mL (THH); 0.91–16.14 mg/mL (HMN); 0.2186–1.55 mg/mL (HML); 0.0026–0.0310 mg/mL (HLOL); 0.0009–0.0633 mg/mL (harmol); 0.0052–0.0313 (N-methyltryptmine) | [99] |
| DMT; THH; HMN; HML; HLOL; harmol and various metabolites | Urine | Enzymatic hydrolysis (B-glucuronidase/sulfatase) of urine, dilution with mobile phase and direct injection | LC–MS/MS (ESI) | Zorbax Eclipse Plus HT C18 (1.8 µm × 4.6 × 50 mm (i.d.)); Mobile phase: Solvent A: formic acid (0.1% in water); Solvent B: Formic acid (0.1% in acetonitrile) | n.a | 0.12; 5.00 ng/mL (DMT); 0.21; 5.00 ng/mL (THH); 0.18; 5.00 ng/mL (HMN); 0.07; 5.00 ng/mL (HML); 0.18; 5.00 ng/mL (HLOL) | 0–0.6 µg/mL (DMT); 0–6.3 µg/mL (THH); 0–0.21 µg/mL (HMN); 0–0.53 µg/mL (HML); 0–14.16 (HLOL); 0.04–126.18 µg/mL (harmol) | [100] |
| DMT; THH; HMN; HML; HLOL; harmol and various metabolites | blood | Protein precipitation 96-well plates, dilution with mobile phase and direct injection | LC–MS/MS (HESI) | Zorbax Eclipse Plus HT C18 (1.8 µm × 4.6 × 50 mm (i.d.)); Mobile phase: Solvent A: formic acid (0.1% in water); Solvent B: Formic acid (0.1% in acetonitrile) | 60.28–76.31 | 0.45; 1 ng/mL (DMT) 0.36; 1 ng/mL (THH); 0.25; 1 ng/mL (HMN); 0.22; 1 ng/mL (HML); 0.38; 1 ng/mL (HLOL); 0.3; 1 ng/mL (harmol) | 0–15.09 ng/mL (DMT); 0–55.44 ng/mL (THH); 0–5.18 ng/mL (HMN); 0–4.53 ng/mL (HML); 0–3.27 ng/mL (HLOL); 0–5.55 ng/mL (harmol) | [101] |
| DMT; THH; HMN; HML | Ayahuasca preparation | direct injection | DART–HRMS | n.a | n.a | n.a | n.a | [102] |
| DMT; THH; HMN; HML | Leaves of Psychotria viridis | n.a | CE–LIF–MS (ESI) | Silica column (7.5 µm ID; 95 cm) | n.a | n.a | n.a | [103] |
| DMT; THH; HMN; HML | Ayahuasca beverage | n.a | NMR | n.a | 70 | 12,500;12,500 ng/mL | 400 µg/mL | [104] |
| DMT; THH; HMN; HML; HLOL; harmol and metabolites | Urine | Dilution with mobile phase and direct injection | LC–MS/MS (ESI) | Zorbax Eclipse Plus HT C18 (1.8 µm × 4.6 × 50 mm (i.d.)); Mobile phase: Solvent A: formic acid (0.1% in water); Solvent B: Formic acid (0.1% in acetonitrile) | n.a | n.a; 5 ng/mL for all compounds | [40] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simão, A.Y.; Gonçalves, J.; Duarte, A.P.; Barroso, M.; Cristóvão, A.C.; Gallardo, E. Toxicological Aspects and Determination of the Main Components of Ayahuasca: A Critical Review. Medicines 2019, 6, 106. https://doi.org/10.3390/medicines6040106
Simão AY, Gonçalves J, Duarte AP, Barroso M, Cristóvão AC, Gallardo E. Toxicological Aspects and Determination of the Main Components of Ayahuasca: A Critical Review. Medicines. 2019; 6(4):106. https://doi.org/10.3390/medicines6040106
Chicago/Turabian StyleSimão, Ana Y., Joana Gonçalves, Ana Paula Duarte, Mário Barroso, Ana Clara Cristóvão, and Eugenia Gallardo. 2019. "Toxicological Aspects and Determination of the Main Components of Ayahuasca: A Critical Review" Medicines 6, no. 4: 106. https://doi.org/10.3390/medicines6040106
APA StyleSimão, A. Y., Gonçalves, J., Duarte, A. P., Barroso, M., Cristóvão, A. C., & Gallardo, E. (2019). Toxicological Aspects and Determination of the Main Components of Ayahuasca: A Critical Review. Medicines, 6(4), 106. https://doi.org/10.3390/medicines6040106