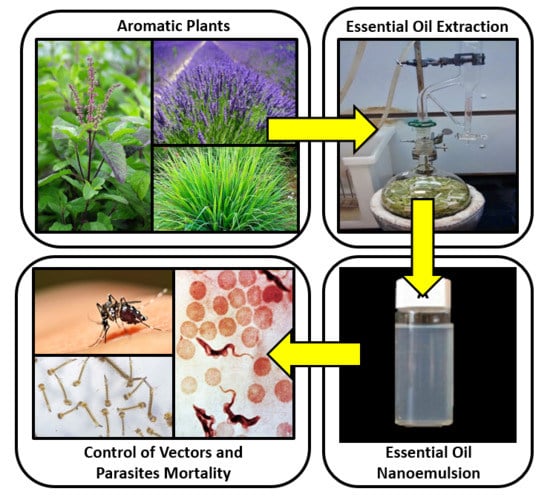
Nanoemulsions of Essential Oils: New Tool for Control of Vector-Borne Diseases and In Vitro Effects on Some Parasitic Agents
Abstract
1. Introduction
1.1. Essential Oils and Biological Activity
1.2. Nanotechnology and Nanoemulsions
1.3. Essential Oil Nanoemulsion
2. Biological Activities of Essential Oil Nanoemulsions
2.1. Larvicidal Activity
2.2. Insecticidal and Repellent Activity
2.3. Acaricidal Activity
2.4. Antiparasitic Activity on Etiological Agents
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Urzúa, A.; Santander, R.; Echeverría, J.; Cabezas, N.; Palacios, S.M.; Rossi, Y. Insecticide properties of the essential oils from Haplopappus foliosus and Bahia ambrosoides against the house fly, Musca domestica L. J. Chil. Chem. Soc. 2010, 55, 392–395. [Google Scholar] [CrossRef]
- Urzúa, A.; Santander, R.; Echeverría, J.; Villalobos, C.; Palacios, S.M.; Rossi, Y. Insecticidal properties of Peumus boldus Mol. essential oil on the house fly, Musca domestica L. Bol. Latinoam Caribe Plantas Med. Aromat. 2010, 9, 465–469. [Google Scholar]
- Urzúa, A.; di Cosmo, D.; Echeverría, J.; Santander, R.; Palacios, S.M.; Rossi, Y. Insecticidal effect of Schinus latifolius essential oil on the housefly, Musca domestica L. Bol. Latinoam Caribe Plantas Med. Aromat. 2011, 10, 470–475. [Google Scholar]
- Espinoza, J.; Urzúa, A.; Bardehle, L.; Quiroz, A.; Echeverría, J.; González-Teuber, M. Antifeedant effects of essential oil, extracts, and isolated sesquiterpenes from Pilgerodendron uviferum (D. Don) Florin heartwood on red clover borer Hylastinus obscurus (Coleoptera: Curculionidae). Molecules 2018, 23, 1282. [Google Scholar] [CrossRef]
- Ferhat, M.A.; Meklati, B.Y.; Chemat, F. Comparison of different isolation methods of essential oil from Citrus fruits: Cold pressing, hydrodistillation and microwave ‘dry’distillation. Flavour Fragr. J. 2007, 22, 494–504. [Google Scholar] [CrossRef]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Mills, C.; Cleary, B.V.; Walsh, J.J.; Gilmer, J.F. Inhibition of acetylcholinesterase by tea tree oil. J. Pharm. Pharmacol. 2004, 56, 375–379. [Google Scholar] [CrossRef]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Sattelle, D.B. Thymol, a constituent of thyme essential oil, is a positive allosteric modulator of human GABAA receptors and a homo-oligomeric GABA receptor from Drosophila melanogaster. Br. J. Pharmacol. 2003, 140, 1363–1372. [Google Scholar] [CrossRef]
- Enan, E.E. Molecular response of Drosophila melanogaster tyramine receptor cascade to plant essential oils. Insect Biochem. Mol. Biol. 2005, 35, 309–321. [Google Scholar] [CrossRef]
- Munro, I.C.; Ford, R.A.; Kennepohl, E.; Sprenger, J.G. Correlation of structural class with no-observed-effect levels: A proposal for establishing a threshold of concern. Food Chem. Toxicol. 1996, 34, 829–867. [Google Scholar] [CrossRef]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Hall, R.L. A procedure for the safety evaluation of natural flavor complexes used as ingredients in food: Essential oils. Food Chem. Toxicol. 2005, 43, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Alarcón, O.; Setzer, W.N. Antiprotozoal activity of essential oils. Agric. Conspec. Sci. 2012, 77, 167–175. [Google Scholar]
- Ramos-Lopez, M.A.; Sanchez-Mir, E.; Fresan-Orozco, M.C.; Perez-Ramos, J. Antiprotozoa activity of some essential oils. J. Med. Plants Res. 2012, 6, 2901–2908. [Google Scholar] [CrossRef]
- Rossi-Bergmann, B. A nanotecnologia: Da saúde para além do determinismo tecnológico. Ciênc. Cult. 2008, 60, 54–57. [Google Scholar]
- De Souza Marcone, G.P. Nanotecnologia e nanociência: Aspectos gerais, aplicações e perspectivas no contexto do Brasil. Rev. Eletrôn. Perspect. Ciênc. Tecnol. 2016, 7, 1–24. [Google Scholar]
- De Melo, C.P.; Pimenta, M. Nanociências e nanotecnologia. Parcerias Estratég. 2010, 9, 9–22. [Google Scholar]
- Dúran, N. Nanotecnologia: Introdução, Preparação e Caracterização de Nanomateriais e Exemplos de Aplicação; Artliber: São Paulo, Brazil, 2006; ISBN 8588098334. [Google Scholar]
- Pimentel, L.F.; Jácome Júnior, A.T.; Mosqueira, V.C.F.; Santos-Magalhães, N.S. Nanotecnologia farmacêutica aplicada ao tratamento da malária. Rev. Bras. Ciênc. Farm. 2007, 43, 503–514. [Google Scholar] [CrossRef]
- Craparo, E.F.; Bondì, M.L.; Pitarresi, G.; Cavallaro, G. Nanoparticulate systems for drug delivery and targeting to the central nervous system. CNS Neurosci. Ther. 2011, 17, 670–677. [Google Scholar] [CrossRef]
- Salager, J.-L.; Antón, R.E.; Andérez, J.M.; Aubry, J.M. Formulation des micro-émulsions par la méthode HLD. In Techniques de l’Ingénieur, 1st ed.; Editions T.I.: Paris, France, 2001; pp. 1–20. [Google Scholar]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Forgiarini, A.; Esquena, J.; González, C.; Solans, C. Studies of the relation between phase behavior and emulsification methods with nanoemulsion formation. In Trends in Colloid and Interface Science XIV; Springer: Berlin/Heidelberg, Germany, 2000; pp. 36–39. [Google Scholar] [CrossRef]
- Chime, S.A.; Kenechukwu, F.C.; Attama, A.A. Nanoemulsions—advances in formulation, characterization and applications in drug delivery. In Application of Nanotechnology in Drug Delivery; InTechOpen: London, UK, 2014. [Google Scholar]
- De Campos, V.E.B.; Ricci-Júnior, E.; Mansur, C.R.E. Nanoemulsions as delivery systems for lipophilic drugs. J. Nanosci. Nanotechnol. 2012, 12, 2881–2890. [Google Scholar] [CrossRef] [PubMed]
- Trommer, H.; Neubert, R.H.H. Overcoming the stratum corneum: The modulation of skin penetration. Skin Pharmacol. Physiol. 2006, 19, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, X.; Zhang, G.; Dong, J.; Eastoe, J. Oil-in-water nanoemulsions for pesticide formulations. J. Colloid Interface Sci. 2007, 314, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Hazra, D.K. Nano-formulations: High definition liquid engineering of pesticides for nano-formulations: High definition liquid engineering of pesticides for advanced crop protection in agriculture. Adv. Plant. Agric. Res. 2017, 6, 1–2. [Google Scholar] [CrossRef]
- Mishra, P.; Balaji, A.P.B.; Tyagi, B.K.; Mukherjee, A.; Chandrasekaran, N. Nanopesticides: A boon towards the control of dreadful vectors of lymphatic filariasis. In Lymphatic Filariasis; Springer: Singapore, 2018; pp. 247–257. [Google Scholar]
- Mishra, P.; Balaji, A.P.B.; Mukherjee, A.; Chandrasekaran, N. Bio-based nanoemulsions: An eco-safe approach towards the eco-toxicity problem. In Handbook of Ecomaterials; Springer: Singapore, 2018; pp. 1–23. [Google Scholar]
- Sugumar, S.; Clarke, S.K.; Nirmala, M.J.; Tyagi, B.K.; Mukherjee, A.; Chandrasekaran, N. Nanoemulsion of Eucalyptus oil and its larvicidal activity against Culex quinquefasciatus. Bull. Entomol. Res. 2014, 104, 393–402. [Google Scholar] [CrossRef]
- Ghosh, V.; Sugumar, S.; Mukherjee, A.; Chandrasekaran, N. Cinnamon oil nanoemulsion formulation by ultrasonic emulsification: Investigation of Its bactericidal activity. J. Nanosci. Nanotechnol. 2013, 13, 114–122. [Google Scholar] [CrossRef]
- Chang, C.L.; Kyu Cho, I.; Li, Q.X. Insecticidal activity of basil oil, trans-anethole, estragole, and linalool to adult fruit flies of Ceratitis capitata, Bactrocera dorsalis, and Bactrocera cucurbitae. J. Econ. Entomol. 2009, 102, 203–209. [Google Scholar] [CrossRef]
- Duarte, J.L.; Amado, J.R.R.; Oliveira, A.E.M.F.M.; Cruz, R.A.S.; Ferreira, A.M.; Souto, R.N.P.; Falcão, D.Q.; Carvalho, J.C.T.; Fernandes, C.P. Evaluation of larvicidal activity of a nanoemulsion of Rosmarinus officinalis essential oil. Rev. Bras. Farmacogn. 2015, 25, 189–192. [Google Scholar] [CrossRef]
- Conti, B.; Canale, A.; Bertoli, A.; Gozzini, F.; Pistelli, L. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2010, 107, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Canale, A.; Cioni, P.L.; Flamini, G. Repellence of essential oils from tropical and mediterranean lamiaceae against Sitophilus zeamais. Bull. Insectol. 2010, 63, 197–202. [Google Scholar]
- Prajapati, V.; Tripathi, A.K.; Aggarwal, K.K.; Khanuja, S.P.S. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 2005, 96, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Volpato, A.; Baretta, D.; Zortéa, T.; Campigotto, G.; Galli, G.M.; Glombowsky, P.; Santos, R.C.V.; Quatrin, P.M.; Ourique, A.F.; Baldissera, M.D. Larvicidal and insecticidal effect of Cinnamomum zeylanicum oil (pure and nanostructured) against mealworm (Alphitobius diaperinus) and its possible environmental effects. J. Asia Pac. Entomol. 2016, 19, 1159–1165. [Google Scholar] [CrossRef]
- Botas, G.d.S.; Cruz, R.A.S.; de Almeida, F.B.; Duarte, J.L.; Araújo, R.S.; Souto, R.N.P.; Ferreira, R.; Carvalho, J.C.T.; Santos, M.G.; Rocha, L.; et al. Baccharis reticularia DC. and limonene nanoemulsions: Promising larvicidal agents for Aedes aegypti (Diptera: Culicidae) control. Molecules 2017, 22, 1990. [Google Scholar] [CrossRef]
- Balasubramani, S.; Rajendhiran, T.; Moola, A.; Kumari, B. Development of nanoemulsion from Vitex negundo L. essential oil and their efficacy of antioxidant, antimicrobial and larvicidal activities (Aedes aegypti L.). Environ. Sci. Pollut. Res. 2017, 24, 15125–15133. [Google Scholar] [CrossRef]
- Osanloo, M.; Amani, A.; Sereshti, H.; Abai, M.R.; Esmaeili, F.; Sedaghat, M.M. Preparation and optimization nanoemulsion of tarragon (Artemisia dracunculus) essential oil as effective herbal larvicide against Anopheles stephensi. Ind. Crops Prod. 2017, 109, 214–219. [Google Scholar] [CrossRef]
- Osanloo, M.; Sereshti, H.; Sedaghat, M.M.; Amani, A. Nanoemulsion of dill essential oil as a green and potent larvicide against Anopheles stephensi. Environ. Sci. Pollut. Res. 2018, 25, 6466–6473. [Google Scholar] [CrossRef]
- Amer, A.; Mehlhorn, H. Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol. Res. 2006, 99, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, B.; Moola, A.K.; Vivek, K.; Kumari, B.D.R. Formulation of nanoemulsion from leaves essential oil of Ocimum basilicum L. and its antibacterial, antioxidant and larvicidal activities (Culex quinquefasciatus). Microb. Pathog. 2018, 125, 475–485. [Google Scholar] [CrossRef]
- Ramar, M.; Manonmani, P.; Arumugam, P.; Kannam, S.K.; Erusan, R.R.; Baskaran, N.; Murugan, K. Nano-insecticidal formulations from essential oil (Ocimum sanctum) and fabricated in filter paper on adult of Aedes aegypti and Culex quinquefasciatus. J. Entomol. Zool. Stud. 2017, 5, 1769–1774. [Google Scholar]
- Sakulku, U.; Nuchuchua, O.; Uawongyart, N.; Puttipipatkhachorn, S.; Soottitantawat, A.; Ruktanonchai, U. Characterization and mosquito repellent activity of citronella oil nanoemulsion. Int. J. Pharm. 2009, 372, 105–111. [Google Scholar] [CrossRef]
- Sritabutra, D.; Soonwera, M.; Waltanachanobon, S.; Poungjai, S. Evaluation of herbal essential oil as repellents against Aedes aegypti (L.) and Anopheles dirus Peyton & Harrion. Asian Pac. J. Trop. Biomed. 2011, 1, S124–S128. [Google Scholar] [CrossRef]
- Nuchuchua, O.; Sakulku, U.; Uawongyart, N.; Puttipipatkhachorn, S.; Soottitantawat, A.; Ruktanonchai, U. In vitro characterization and mosquito (Aedes aegypti) repellent activity of essential-oils-loaded nanoemulsions. Aaps Pharmscitech. 2009, 10, 1234. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.S.; Boito, J.P.; Santos, R.C.V.; Quatrin, P.M.; Ourique, A.F.; dos Reis, J.H.; Gebert, R.R.; Glombowsky, P.; Klauck, V.; Boligon, A.A. Nanostructured cinnamon oil has the potential to control Rhipicephalus microplus ticks on cattle. Exp. Appl. Acarol. 2017, 73, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.M.; Volpato, V.; Santos, R.C.V.; Gebert, R.R.; Quatrin, P.; Ourique, A.F.; Klein, B.; Wagner, R.; Tonin, A.A.; Baldissera, M.D. Effects of essential oil of Eucalyptus globulus loaded in nanoemulsions and in nanocapsules on reproduction of cattle tick (Rhipicephalus microplus). Arch. Zootec. 2018, 67, 494–498. [Google Scholar] [CrossRef]
- Monteiro, I.N.; dos Santos Monteiro, O.; Costa-Junior, L.M.; da Silva Lima, A.; de Aguiar Andrade, E.H.; Maia, J.G.S.; Mouchrek Filho, V.E. Chemical composition and acaricide activity of an essential oil from a rare chemotype of Cinnamomum verum Presl on Rhipicephalus microplus (Acari: Ixodidae). Vet. Parasitol. 2017, 238, 54–57. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Da Silva, A.S.; Oliveira, C.B.; Zimmermann, C.E.P.; Vaucher, R.A.; Santos, R.C.V.; Rech, V.C.; Tonin, A.A.; Giongo, J.L.; Mattos, C.B. Trypanocidal activity of the essential oils in their conventional and nanoemulsion forms: In vitro tests. Exp. Parasitol. 2013, 134, 356–361. [Google Scholar] [CrossRef]
- Ziaei Hezarjaribi, H.; Nadeali, N.; Saeedi, M.; Soosaraei, M.; Jorjani, O.N.; Momeni, Z.; Fakhar, M. The effect of lavender essential oil and nanoemulsion on Trichomonas vaginalis in vitro. Feyz J. Kashan Univ. Med. Sci. 2017, 21, 326–334. [Google Scholar]
- Shokri, A.; Saeedi, M.; Fakhar, M.; Morteza-Semnani, K.; Keighobadi, M.; Teshnizi, S.H.; Kelidari, H.R.; Sadjadi, S. Antileishmanial activity of Lavandula angustifolia and Rosmarinus officinalis essential oils and nano-emulsions on Leishmania major (MRHO/IR/75/ER). Iran. J. Parasitol. 2017, 12, 622–631. [Google Scholar]
- Bouyahya, A.; Et-Touys, A.; Bakri, Y.; Talbaui, A.; Fellah, H.; Abrini, J.; Dakka, N. Chemical composition of Mentha pulegium and Rosmarinus officinalis essential oils and their antileishmanial, antibacterial and antioxidant activities. Microb. Pathog. 2017, 111, 41–49. [Google Scholar] [CrossRef]
- Moazeni, M.; Borji, H.; Darbandi, M.S.; Saharkhiz, M.J. In vitro and in vivo antihydatid activity of a nano emulsion of Zataria multiflora essential oil. Res. Vet. Sci. 2017, 114, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudvand, H.; Mirbadie, S.R.; Sadooghian, S.; Harandi, M.F.; Jahanbakhsh, S.; Saedi Dezaki, E. Chemical composition and scolicidal activity of Zataria multiflora Boiss essential oil. J. Essent. Oil Res. 2017, 29, 42–47. [Google Scholar] [CrossRef]
- Moazeni, M.; Larki, S.; Saharkhiz, M.J.; Oryan, A.; Lari, M.A.; Alavi, A.M. Efficacy of the aromatic water of Zataria multiflora on hydatid cysts: An In vivo study. Antimicrob. Agents Chemother. 2014, 58, 6003–6008. [Google Scholar] [CrossRef] [PubMed]
- Elissondo, M.C.; Albani, C.M.; Gende, L.; Eguaras, M.; Denegri, G. Efficacy of thymol against Echinococcus granulosus protoscoleces. Parasitol. Int. 2008, 57, 185–190. [Google Scholar] [CrossRef]
- Yones, D.A.; Taher, G.A.; Ibraheim, Z.Z. In vitro effects of some herbs used in Egyptian traditional medicine on viability of protoscolices of hydatid cysts. Korean J. Parasitol. 2011, 49, 255. [Google Scholar] [CrossRef]
- Elissondo, M.C.; Pensel, P.E.; Denegri, G.M. Could thymol have effectiveness on scolices and germinal layer of hydatid cysts? Acta Trop. 2013, 125, 251–257. [Google Scholar] [CrossRef]
- Schummer, J. Multidisciplinarity, interdisciplinarity, and patterns of research collaboration in nanoscience and nanotechnology. Scientometrics 2004, 59, 425–465. [Google Scholar] [CrossRef]
- Choi, H.; Mody, C.C.M. The long history of molecular electronics: Microelectronics origins of nanotechnology. Soc. Stud. Sci. 2009, 39, 11–50. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P.R. A review on prospects of essential oils as biopesticide in insect-pest management. J. Pharmacogn. Phyther. 2009, 1, 52–63. [Google Scholar]
- Meira, C.S.; Menezes, L.R.A.; dos Santos, T.B.; Macedo, T.S.; Fontes, J.E.N.; Costa, E.V.; Pinheiro, M.L.B.; da Silva, T.B.; Teixeira Guimarães, E.; Soares, M.B.P. Chemical composition and antiparasitic activity of essential oils from leaves of Guatteria friesiana and Guatteria pogonopus (Annonaceae). J. Essent. Oil Res. 2017, 29, 156–162. [Google Scholar] [CrossRef]
- Dos Santos Sales, V.; Monteiro, Á.B.; de Araújo Delmondes, G.; do Nascimento, E.P. Antiparasitic activity and essential oil chemical analysis of the Piper tuberculatum Jacq fruit. Iran. J. Pharm. Res. IJPR 2018, 17, 268–275. [Google Scholar]
| Specie | Common Name | Main Essential Oil Compound(s) | Emulsificant | Insect | References |
|---|---|---|---|---|---|
| Anethum graveolens | Dill | p--Cymene and α-phellandrene | Tween 20 | Anopheles stephensi | [42] |
| Artemisia dracunculus | Tarragon | p-Allylanisole | Tween 20 | Anopheles stephensi | [41] |
| Baccharis reticularia | Sand-Rosemary | D-limonene | Tween 80 | Aedes aegypti | [39] |
| Cinnamomum zeylanicum | True cinnamon tree | Cinnamaldehyde | Tween 80 | Alphitobius diaperinus | [38] |
| Eucalyptus globulus | Eucalyptus | 1,8-cineole | Tween 80 | Culex quinquefasciatus | [31] |
| Ocimum basilicum | Basil | Methyl-chavicol | Tween 20 | Aedes aegypti | [32] |
| Ocimum basilicum | Basil | trans-β-Guaiene and α-Cadinol | Tween 80 | Culex quinquefasciatus | [44] |
| Rosmarinus officinalis | Rosemary | 1,8-cineole | Tween 20 | Aedes aegypti | [34] |
| Vitex negundo | Chinese chaste tree | 2(R)-acetoxymethyl-1,3,3-trimethyl-4t-(3-methyl-2-buten-1-yl)-1t-cyclohexanol | Tween 80 | Aedes aegypti | [40] |
| Specie | Common Name | Main Essential Oil Compound(s) | Emulsificant | Insect/Parasite | References |
|---|---|---|---|---|---|
| Cinnamomum verum | Cinnamomum | Cinnamaldehyde | Tween 80 | Rhipicephalus microplus (Acaricidal activity) | [49] |
| Cymbopogon nardus | Citronella | D-limonene and citronellal | Montanov 82 | Aedes aegypti (Repellent activity) | [46] |
| Cymbopogon nardus, Ocimum americanum and Vetiveria zizanioides | Citronella, Hairy Basil and Vetiver | D-limonene and citronellal (C. nardus), 3-carene and caryophyllene (O. americanum), vetiveric acid (V. zizanioides) | Montanov 82 | Aedes aegypti (Repellent activity) | [48] |
| Eucalyptus globulus | Eucalyptus | 1,8-cineole | Tween 80 | Rhipicephalus microplus (Acaricidal activity) | [50] |
| Ocimum sanctum | Holy Basil | Not described | Not described | Culex quinquefasciatus and Aedes aegypti (Inseticide activity) | [45] |
| Species | Common Name | Main Essential Oil Compound(s) | Emulsificant | Parasite | References |
|---|---|---|---|---|---|
| Lavandula angustifolia and Rosmarinus officinalis | Lavander and Rosemary | 1,8-cineole and linalool | Tween 80 | Leishmania major | [54] |
| Lavandula officinalis | Lavender | 1,8-cineole | Tween 80 | Trichomonas vaginalis | [53] |
| Schinus molle | Peruvian peppertree | Not Described | Tween 20 | Trypanosoma evansi | [52] |
| Zataria multiflora | Avishan Shirazi | Thymol | Tween 80 | Protoscoleces of the hydatid cysts | [56] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echeverría, J.; Albuquerque, R.D.D.G.d. Nanoemulsions of Essential Oils: New Tool for Control of Vector-Borne Diseases and In Vitro Effects on Some Parasitic Agents. Medicines 2019, 6, 42. https://doi.org/10.3390/medicines6020042
Echeverría J, Albuquerque RDDGd. Nanoemulsions of Essential Oils: New Tool for Control of Vector-Borne Diseases and In Vitro Effects on Some Parasitic Agents. Medicines. 2019; 6(2):42. https://doi.org/10.3390/medicines6020042
Chicago/Turabian StyleEcheverría, Javier, and Ricardo Diego Duarte Galhardo de Albuquerque. 2019. "Nanoemulsions of Essential Oils: New Tool for Control of Vector-Borne Diseases and In Vitro Effects on Some Parasitic Agents" Medicines 6, no. 2: 42. https://doi.org/10.3390/medicines6020042
APA StyleEcheverría, J., & Albuquerque, R. D. D. G. d. (2019). Nanoemulsions of Essential Oils: New Tool for Control of Vector-Borne Diseases and In Vitro Effects on Some Parasitic Agents. Medicines, 6(2), 42. https://doi.org/10.3390/medicines6020042