Past, Present and Future Anti-Obesity Effects of Flavin-Containing and/or Copper-Containing Amine Oxidase Inhibitors
Abstract
1. Introduction
2. Flavin-Containing and Copper-Containing Amine Oxidases
3. Past and Present Observations of Amine Oxidase Inhibitor Effects on Adiposity in Animal Models
4. Reducing the Risk/Benefit Ratio of Semicarbazide by Combining with Other Inhibitors
5. Combining the Inhibition Effect of MAOs and AOCs on Adiposity: The Case of Phenelzine
6. Adipocytes as Targets of Amine-Oxidase-Interacting Agents
7. Future Directions and Combining in Vitro, in Vivo and Clinical Studies
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holtzman, S.G.; Jewett, R.E. The role of brain norepinephrine in the anorexic effects of dextroamphetamine and monoamine oxidase inhibitors in the rat. Psychopharmacologia 1971, 22, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Miller, D.S. Thermogenic drugs for the treatment of obesity: Sympathetic stimulants in animal models. Br. J. Nutr. 1984, 52, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, J.G. Induction of obesity by psychotropic drugs. Ann. N. Y. Acad. Sci. 1987, 499, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Cantu, T.G.; Korek, J.S. Monoamine oxidase inhibitors and weight gain. Drug Intell. Clin. Pharm. 1988, 22, 755–759. [Google Scholar] [CrossRef] [PubMed]
- Dulloo, A.G.; Miller, D.S. Screening of drugs for thermogenic anti-obesity properties: Antidepressants. Ann. Nutr. Metab. 1987, 31, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Nandigama, R.K.; Newton-Vinson, P.; Edmondson, D.E. Phentermine inhibition of recombinant human liver monoamine oxidases A and B. Biochem. Pharmacol. 2002, 63, 865–869. [Google Scholar] [CrossRef]
- Kilpatrick, I.C.; Traut, M.; Heal, D.J. Monoamine oxidase inhibition is unlikely to be relevant to the risks associated with phentermine and fenfluramine: A comparison with their abilities to evoke monoamine release. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 1454–1458. [Google Scholar] [CrossRef]
- Houslay, M.D.; Tipton, K.F. The nature of the electrophoretically separable multiple forms of rat liver monoamine oxidase. Biochem. J. 1973, 135, 173–186. [Google Scholar] [CrossRef]
- Pizzinat, N.; Marti, L.; Remaury, A.; Leger, F.; Langin, D.; Lafontan, M.; Carpéné, C.; Parini, A. High expression of monoamine oxidases in human white adipose tissue: Evidence for their involvement in noradrenaline clearance. Biochem. Pharmacol. 1999, 58, 1735–1742. [Google Scholar] [CrossRef]
- Song, M.S.; Matveychuk, D.; MacKenzie, E.M.; Duchcherer, M.; Mousseau, D.D.; Baker, G.B. An update on amine oxidase inhibitors: Multifaceted drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 44, 118–124. [Google Scholar] [CrossRef]
- Zheng, Y.C.; Yu, B.; Jiang, G.Z.; Feng, X.J.; He, P.X.; Chu, X.Y.; Zhao, W.; Liu, H.M. Irreversible LSD1 Inhibitors: Application of tranylcypromine and its derivatives in cancer treatment. Curr. Top. Med. Chem. 2016, 16, 2179–2188. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Wynder, C.; Schmidt, D.M.; McCafferty, D.G.; Shiekhattar, R. Histone H3 lysine 4 demethylation is a target of nonselective antidepressive medications. Chem. Biol. 2006, 13, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Casero, R.A.; Pegg, A.E. Polyamine catabolism and disease. Biochem. J. 2009, 421, 323–338. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. Vascular Adhesion Protein-1: A Cell Surface Amine Oxidase in Translation. Antioxid. Redox Signal. 2017. [Google Scholar] [CrossRef]
- Weston, C.J.; Shepherd, E.L.; Claridge, L.C.; Rantakari, P.; Curbishley, S.M.; Tomlinson, J.W.; Hubscher, S.G.; Reynolds, G.M.; Aalto, K.; Anstee, Q.M.; et al. Vascular adhesion protein-1 promotes liver inflammation and drives hepatic fibrosis. J. Clin. Investig. 2015, 125, 501–520. [Google Scholar] [CrossRef] [PubMed]
- Morin, N.; Lizcano, J.M.; Fontana, E.; Marti, L.; Smih, F.; Rouet, P.; Prévot, D.; Zorzano, A.; Unzeta, M.; Carpéné, C. Semicarbazide-sensitive amine oxidase substrates stimulate glucose transport and inhibit lipolysis in human adipocytes. J. Pharmacol. Exp. Ther. 2001, 297, 563–572. [Google Scholar] [PubMed]
- Mercier, N.; El Hadri, K.; Osborne-Pellegrin, M.; Nehme, J.; Perret, C.; Labat, C.; Regnault, V.; Lamaziere, J.M.; Challande, P.; Lacolley, P.; et al. Modifications of arterial phenotype in response to amine oxidase inhibition by semicarbazide. Hypertension 2007, 50, 234–241. [Google Scholar] [CrossRef]
- Schwelberger, H.G. Structural organization of mammalian copper-containing amine oxidase genes. Inflamm. Res. 2010, 59 (Suppl. 2), S223–S225. [Google Scholar] [CrossRef]
- Olivieri, A.; Rico, D.; Khiari, Z.; Henehan, G.; O’Sullivan, J.; Tipton, K. From caffeine to fish waste: Amine compounds present in food and drugs and their interactions with primary amine oxidase. J. Neural Transm. (Vienna) 2011, 118, 1079–1089. [Google Scholar] [CrossRef]
- Trackman, P.C. Enzymatic and non-enzymatic functions of the lysyl oxidase family in bone. Matrix Biol. 2016, 52–54, 7–18. [Google Scholar] [CrossRef]
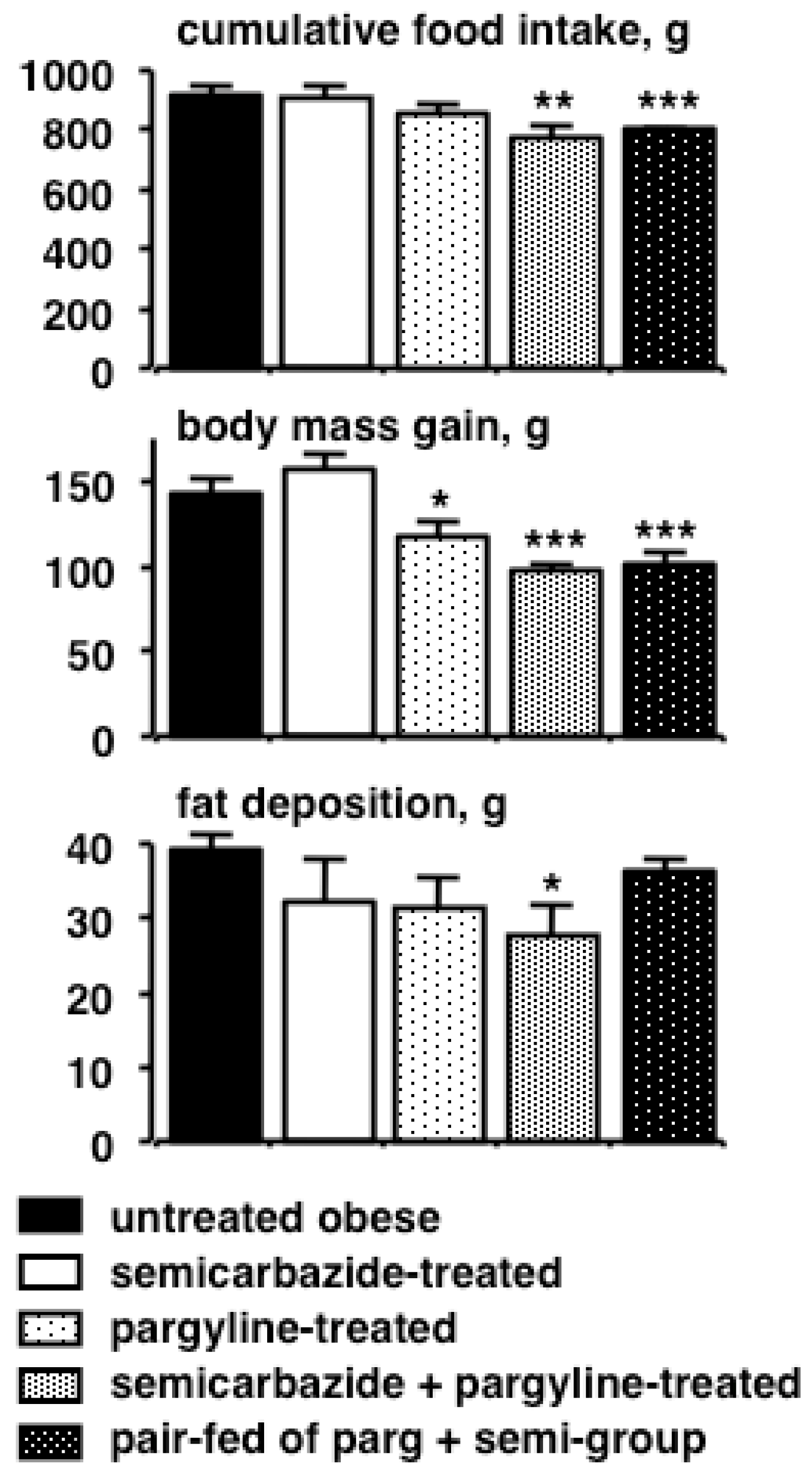
- Carpéné, C.; Iffiu-Soltesz, Z.; Bour, S.; Prevot, D.; Valet, P. Reduction of fat deposition by combined inhibition of monoamine oxidases and semicarbazide-sensitive amine oxidases in obese Zucker rats. Pharmacol. Res. 2007, 56, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Mercader, J.; Le Gonidec, S.; Schaak, S.; Mialet-Perez, J.; Zakaroff-Girard, A.; Galitzky, J. Body fat reduction without cardiovascular changes in mice after oral treatment by the MAO inhibitor phenelzine. Br. J. Pharmacol. 2018, 175, 2428–2440. [Google Scholar] [CrossRef] [PubMed]
- Byun, Y.; Park, J.; Hong, S.H.; Han, M.H.; Park, S.; Jung, H.I.; Noh, M. The opposite effect of isotype-selective monoamine oxidase inhibitors on adipogenesis in human bone marrow mesenchymal stem cells. Bioorg. Med. Chem. Lett. 2013, 23, 3273–3276. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Abello, V.; Iffiu-Soltesz, Z.; Mercier, N.; Fève, B.; Valet, P. Limitation of adipose tissue enlargement in rats chronically treated with semicarbazide-sensitive amine oxidase and monoamine oxidase inhibitors. Pharmacol. Res. 2008, 57, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Nagy, C.T.; Koncsos, G.; Varga, Z.V.; Baranyai, T.; Tuza, S.; Kassai, F.; Ernyey, A.J.; Gyertyan, I.; Kiraly, K.; Olah, A.; et al. Selegiline reduces adiposity induced by high-fat, high-sucrose diet in male rats. Br. J. Pharmacol. 2018, 175, 3713–3726. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, A.; Abdulla, A.; Yang, F.; Chua, S.C.; Pessin, J.E.; Zong, H. The antidepressant trans-2-phenylcyclopropylamine protects mice from high-fat-diet-induced obesity. PLoS ONE 2014, 9, e89199. [Google Scholar] [CrossRef]
- Takahashi, M.; Yoshida, M.; Inoue, K.; Morikawa, T.; Nishikawa, A. A ninety-day toxicity study of semicarbazide hydrochloride in Wistar Hannover GALAS rats. Food Chem. Toxicol. 2009, 47, 2490–2498. [Google Scholar] [CrossRef]
- Mercader, J.; Iffiu-Soltesz, Z.; Bour, S.; Carpéné, C. Oral administration of semicarbazide limits weight gain together with inhibition of fat deposition and of primary amine oxidase activity in adipose tissue. J. Obes. 2011, 2011, 475786. [Google Scholar] [CrossRef]
- Prévot, D.; Soltesz, Z.; Abello, V.; Wanecq, E.; Valet, P.; Unzeta, M.; Carpéné, C. Prolonged treatment with aminoguanidine strongly inhibits adipocyte semicarbazide-sensitive amine oxidase and slightly reduces fat deposition in obese Zucker rats. Pharmacol. Res. 2007, 56, 70–79. [Google Scholar] [CrossRef]
- Carroll, J.F.; King, J.W.; Cohen, J.S. Hydralazine treatment alters body composition in the rabbit model of obesity. Acta Physiol. Scand. 2004, 181, 183–191. [Google Scholar] [CrossRef]
- Yu, P.H.; Wang, M.; Fan, H.; Deng, Y.; Gubisne-Haberle, D. Involvement of SSAO-mediated deamination in adipose glucose transport and weight gain in obese diabetic KKAy mice. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E634–E641. [Google Scholar] [CrossRef] [PubMed]
- Miana, M.; Galan, M.; Martinez-Martinez, E.; Varona, S.; Jurado-Lopez, R.; Bausa-Miranda, B.; Antequera, A.; Luaces, M.; Martinez-Gonzalez, J.; Rodriguez, C.; et al. The lysyl oxidase inhibitor beta-aminopropionitrile reduces body weight gain and improves the metabolic profile in diet-induced obesity in rats. Dis. Model. Mech. 2015, 8, 543–551. [Google Scholar] [CrossRef]
- Fitzgerald, D.H.; Tipton, K.F. Inhibition of monoamine oxidase modulates the behaviour of semicarbazide-sensitive amine oxidase (SSAO). J. Neural Transm. (Vienna) 2002, 109, 251–265. [Google Scholar] [CrossRef]
- Enrique-Tarancon, G.; Marti, L.; Morin, N.; Lizcano, J.M.; Unzeta, M.; Sevilla, L.; Camps, M.; Palacin, M.; Testar, X.; Carpéné, C.; et al. Role of semicarbazide-sensitive amine oxidase on glucose transport and GLUT4 recruitment to the cell surface in adipose cells. J. Biol. Chem. 1998, 273, 8025–8032. [Google Scholar] [CrossRef] [PubMed]
- Barrand, M.A.; Callingham, B.A. The interaction of hydralazine with a semicarbazide-sensitive amine oxidase in brown adipose tissue of the rat. Its use as a radioactive ligand for the enzyme. Biochem. J. 1985, 232, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Vidrio, H.; Medina, M.; Fernandez, G.; Lorenzana-Jimenez, M.; Campos, A.E. Enhancement of hydralazine hypotension by low doses of isoniazid. Possible role of semicarbazide-sensitive amine oxidase inhibition. Gen. Pharmacol. 2000, 35, 195–204. [Google Scholar] [CrossRef]
- Cioni, L.; De Siena, G.; Ghelardini, C.; Sernissi, O.; Alfarano, C.; Pirisino, R.; Raimondi, L. Activity and expression of semicarbazide-sensitive benzylamine oxidase in a rodent model of diabetes: Interactive effects with methylamine and alpha-aminoguanidine. Eur. J. Pharmacol. 2006, 529, 179–187. [Google Scholar] [CrossRef]
- Mercier, N.; Kakou, A.; Challande, P.; Lacolley, P.; Osborne-Pellegrin, M. Comparison of the effects of semicarbazide and beta-aminopropionitrile on the arterial extracellular matrix in the Brown Norway rat. Toxicol. Appl. Pharmacol. 2009, 239, 258–267. [Google Scholar] [CrossRef]
- Yu, M.; Feng, Y.; Zhang, X.; Wang, J.; Tian, H.; Wang, W.; Ru, S. Semicarbazide disturbs the reproductive system of male zebrafish (Danio rerio) through the GABAergic system. Reprod. Toxicol. 2017, 73, 149–157. [Google Scholar] [CrossRef]
- Yue, Z.; Yu, M.; Zhang, X.; Wang, J.; Ru, S. The anti-androgenic effect of chronic exposure to semicarbazide on male Japanese flounder (Paralichthys olivaceus) and its potential mechanisms. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 210, 30–34. [Google Scholar] [CrossRef]
- Richards, V.E.; Chau, B.; White, M.R.; McQueen, C.A. Hepatic gene expression and lipid homeostasis in C57BL/6 mice exposed to hydrazine or acetylhydrazine. Toxicol. Sci. 2004, 82, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Kleno, T.G.; Leonardsen, L.R.; Kjeldal, H.O.; Laursen, S.M.; Jensen, O.N.; Baunsgaard, D. Mechanisms of hydrazine toxicity in rat liver investigated by proteomics and multivariate data analysis. Proteomics 2004, 4, 868–880. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Bour, S.; Visentin, V.; Pellati, F.; Benvenuti, S.; Iglesias-Osma, M.C.; Garcia-Barrado, M.J.; Valet, P. Amine oxidase substrates for impaired glucose tolerance correction. J. Physiol. Biochem. 2005, 61, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Iffiú-Soltész, Z. Monoaminergic systems and anti-obesity drug discovery: Chronic administration of sympathicomimetic amines, re-uptake inhibitors, or amine oxidase inhibitors? In Anti-Obesity Drug Discovery and Development; Rahman, A., Choudhary, M.I., Eds.; Bentham Sciences Publishers: Sharjah, United Arab Emirates, 2011; Volume 1, pp. 114–130. [Google Scholar]
- Halberg, N.; Khan, T.; Trujillo, M.E.; Wernstedt-Asterholm, I.; Attie, A.D.; Sherwani, S.; Wang, Z.V.; Landskroner-Eiger, S.; Dineen, S.; Magalang, U.J.; et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell. Biol. 2009, 29, 4467–4483. [Google Scholar] [CrossRef] [PubMed]
- Salter-Cid, L.M.; Wang, E.; O’Rourke, A.M.; Miller, A.; Gao, H.; Huang, L.; Garcia, A.; Linnik, M.D. Anti-inflammatory effects of inhibiting the amine oxidase activity of semicarbazide-sensitive amine oxidase. J. Pharmacol. Exp. Ther. 2005, 315, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Kleineke, J.; Peters, H.; Soling, H.D. Inhibition of hepatic gluconeogenesis by phenethylhydrazine (phenelzine). Biochem. Pharmacol. 1979, 28, 1379–1389. [Google Scholar] [CrossRef]
- Chiche, F.; Le Guillou, M.; Chetrite, G.; Lasnier, F.; Dugail, I.; Carpéné, C.; Moldes, M.; Fève, B. Antidepressant phenelzine alters differentiation of cultured human and mouse preadipocytes. Mol. Pharmacol. 2009, 75, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Grès, S.; Rascalou, S. The amine oxidase inhibitor phenelzine limits lipogenesis in adipocytes without inhibiting insulin action on glucose uptake. J. Neural Transm. (Vienna) 2013, 120, 997–1003. [Google Scholar] [CrossRef]
- Carpéné, C.; Gomez-Zorita, S.; Gupta, R.; Grès, S.; Rancoule, C.; Cadoudal, T.; Mercader, J.; Gomez, A.; Bertrand, C.; Iffiu-Soltesz, Z. Combination of low dose of the anti-adipogenic agents resveratrol and phenelzine in drinking water is not sufficient to prevent obesity in very-high-fat diet-fed mice. Eur. J. Nutr. 2014, 53, 1625–1635. [Google Scholar] [CrossRef]
- Carpéné, C.; Gomez-Zorita, S.; Chaplin, A.; Mercader, J. Metabolic Effects of Oral Phenelzine Treatment on High-Sucrose-Drinking Mice. Int. J. Mol. Sci. 2018, 19, 2904. [Google Scholar] [CrossRef]
- Moldes, M.; Fève, B.; Pairault, J. Molecular cloning of a major mRNA species in murine 3T3 adipocyte lineage. differentiation-dependent expression, regulation, and identification as semicarbazide-sensitive amine oxidase. J. Biol. Chem. 1999, 274, 9515–9523. [Google Scholar] [CrossRef] [PubMed]
- Fontana, E.; Boucher, J.; Marti, L.; Lizcano, J.M.; Testar, X.; Zorzano, A.; Carpéné, C. Amine oxidase substrates mimic several of the insulin effects on adipocyte differentiation in 3T3 F442A cells. Biochem. J. 2001, 356, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.H.; Wertz, D.L.; Klinman, J.P. Implication for functions of the ectopic adipocyte copper amine oxidase (AOC3) from purified enzyme and cell-based kinetic studies. PLoS ONE 2012, 7, e29270. [Google Scholar] [CrossRef] [PubMed]
- Bour, S.; Daviaud, D.; Grès, S.; Lefort, C.; Prévot, D.; Zorzano, A.; Wabitsch, M.; Saulnier-Blache, J.S.; Valet, P.; Carpéné, C. Adipogenesis-related increase of semicarbazide-sensitive amine oxidase and monoamine oxidase in human adipocytes. Biochimie 2007, 89, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Carpéné, C.; Daviaud, D.; Boucher, J.; Bour, S.; Visentin, V.; Grès, S.; Duffaut, C.; Fontana, E.; Testar, X.; Saulnier-Blache, J.S.; et al. Short- and long-term insulin-like effects of monoamine oxidases and semicarbazide-sensitive amine oxidase substrates in cultured adipocytes. Metabolism 2006, 55, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Hino, S.; Sakamoto, A.; Nagaoka, K.; Anan, K.; Wang, Y.; Mimasu, S.; Umehara, T.; Yokoyama, S.; Kosai, K.; Nakao, M. FAD-dependent lysine-specific demethylase-1 regulates cellular energy expenditure. Nat. Commun. 2012, 3, 758. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ralle, M.; Wolfgang, M.J.; Dhawan, N.; Burkhead, J.L.; Rodriguez, S.; Kaplan, J.H.; Wong, G.W.; Haughey, N.; Lutsenko, S. Copper-dependent amino oxidase 3 governs selection of metabolic fuels in adipocytes. PLoS Biol. 2018, 16, e2006519. [Google Scholar] [CrossRef]
- Bour, S.; Caspar-Bauguil, S.; Iffiu-Soltesz, Z.; Nibbelink, M.; Cousin, B.; Miiluniemi, M.; Salmi, M.; Stolen, C.; Jalkanen, S.; Casteilla, L.; et al. Semicarbazide-sensitive amine oxidase/vascular adhesion protein-1 deficiency reduces leukocyte infiltration into adipose tissue and favors fat deposition. Am. J. Pathol. 2009, 174, 1075–1083. [Google Scholar] [CrossRef]
- Noonan, T.; Lukas, S.; Peet, G.W.; Pelletier, J.; Panzenbeck, M.; Hanidu, A.; Mazurek, S.; Wasti, R.; Rybina, I.; Roma, T.; et al. The oxidase activity of vascular adhesion protein-1 (VAP-1) is essential for function. Am. J. Clin. Exp. Immunol. 2013, 2, 172–185. [Google Scholar]
- Chen, K.; Holschneider, D.P.; Wu, W.; Rebrin, I.; Shih, J.C. A spontaneous point mutation produces monoamine oxidase A/B knock-out mice with greatly elevated monoamines and anxiety-like behavior. J. Biol. Chem. 2004, 279, 39645–39652. [Google Scholar] [CrossRef]
- Shanahan, P.; O’Sullivan, J.; Tipton, K.F.; Kinsella, G.K.; Ryan, B.J.; Henehan, G.T.M. Theobromine and related methylxanthines as inhibitors of Primary Amine Oxidase. J. Food. Biochem. 2018, e12697. [Google Scholar] [CrossRef]
- Dulloo, A.G.; Seydoux, J.; Girardier, L. Potentiation of the thermogenic antiobesity effects of ephedrine by dietary methylxanthines: Adenosine antagonism or phosphodiesterase inhibition? Metabolism 1992, 41, 1233–1241. [Google Scholar] [CrossRef]
- Fernandez-Quintela, A.; Carpéné, C.; Fernandez, M.; Aguirre, L.; Milton-Laskibar, I.; Contreras, J.; Portillo, M.P. Anti-obesity effects of resveratrol: Comparison between animal models and humans. J. Physiol. Biochem. 2016, 73, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Les, F.; Deleruyelle, S.; Cassagnes, L.E.; Boutin, J.A.; Balogh, B.; Arbones-Mainar, J.M.; Biron, S.; Marceau, P.; Richard, D.; Nepveu, F.; et al. Piceatannol and resveratrol share inhibitory effects on hydrogen peroxide release, monoamine oxidase and lipogenic activities in adipose tissue, but differ in their antilipolytic properties. Chem. Biol. Interact. 2016, 258, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Yang, Z.D.; Shi, D.F.; Yao, X.J.; Wang, M.G. Inhibition of monoamine oxidase by stilbenes from Rheum palmatum. Iran. J. Pharm. Res. 2016, 15, 885–892. [Google Scholar] [PubMed]
- Nakabayashi, H.; Hashimoto, T.; Ashida, H.; Nishiumi, S.; Kanazawa, K. Inhibitory effects of caffeine and its metabolites on intracellular lipid accumulation in murine 3T3-L1 adipocytes. Biofactors 2008, 34, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Lasa, A.; Churruca, I.; Eseberri, I.; Andres-Lacueva, C.; Portillo, M.P. Delipidating effect of resveratrol metabolites in 3T3-L1 adipocytes. Mol. Nutr. Food Res. 2012, 56, 1559–1568. [Google Scholar] [CrossRef]
- Iffiu-Soltesz, Z.; Mercader, J.; Daviaud, D.; Boucher, J.; Carpéné, C. Increased primary amine oxidase expression and activity in white adipose tissue of obese and diabetic db-/- mice. J. Neural Transm. (Vienna) 2011, 118, 1071–1077. [Google Scholar] [CrossRef]
- Wanecq, E.; Bour, S.; Verwaerde, P.; Smih, F.; Valet, P.; Carpéné, C. Increased monoamine oxidase and semicarbazide-sensitive amine oxidase activities in white adipose tissue of obese dogs fed a high-fat diet. J. Physiol. Biochem. 2006, 62, 113–123. [Google Scholar] [CrossRef]
- Koborova, I.; Gurecka, R.; Csongova, M.; Volkovova, K.; Szoko, E.; Tabi, T.; Sebekova, K. Association between metabolically healthy central obesity in women and levels of soluble receptor for advanced glycation end products, soluble vascular adhesion protein-1, and the activity of semicarbazide-sensitive amine oxidase. Croat. Med. J. 2017, 58, 106–116. [Google Scholar] [CrossRef]
- Visentin, V.; Prévot, D.; De Saint Front, V.D.; Morin-Cussac, N.; Thalamas, C.; Galitzky, J.; Valet, P.; Zorzano, A.; Carpéné, C. Alteration of amine oxidase activity in the adipose tissue of obese subjects. Obes. Res. 2004, 12, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011, 480, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Pirzgalska, R.M.; Seixas, E.; Seidman, J.S.; Link, V.M.; Sanchez, N.M.; Mahu, I.; Mendes, R.; Gres, V.; Kubasova, N.; Morris, I.; et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 2017, 23, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Ruiz, H.H.; Jhun, K.; Finan, B.; Oberlin, D.J.; van der Heide, V.; Kalinovich, A.V.; Petrovic, N.; Wolf, Y.; Clemmensen, C.; et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 2017, 23, 623–630. [Google Scholar] [CrossRef] [PubMed]
| Amine Oxidase | Substrate(s) | Inhibitor(s) | Effects on BW or Fat Accumulation | References |
|---|---|---|---|---|
| MAO-A | norepinephrine (=noradrenaline) dopamine serotonin tyramine tryptamine | pargyline phenelzine tranylcypromine moclobemide clorgyline toloxatone | ↓ food intake ↓ SCWAT in mice ↓ BW gain mice ↑ adipogenesis | [21] [22] [2] [23] |
| MAO-B | dopamine benzylamine | pargyline tranylcypromine phenelzine deprenyl (=selegiline) | ↑ effect of SCZ in reducing WAT in rats ↓ adipogenesis ↓ WAT rats | [24] [23] [25] |
| LSD-1 | histones | tranylcypromine | ↓ BW gain mice | [26] |
| LSD-2 | histones | tranylcypromine | ↓ adipogenesis | [23] |
| APAO | N-acetylspermine | MDL 72527 | ||
| SMO | spermine | MDL 72527 | ||
| AOC1 | histamine putrescine cadaverine | semicarbazide (others: see AOC3) | ↓ BW | [17] |
| AOC2 | tyramine tryptamine | Semicarbazide (others: see AOC3) | ↓ BW | [27] |
| AOC3 | benzylamine methylamine aminoacetone | Semicarbazide phenelzine aminoguanidine tranylcypromine phenylhydrazine hydralazine FPFA BTT2052 (=SZE5302) | ↓ BW gain mice ↓ SCWAT rats ↓ BW rabbits ↓ WAT mice | [28] [21] [29] [30] [31] |
| LOX | collagen elastin cadaverine lysine | β-aminopropionitrile semicarbazide tetrathiomolybdate | ↓ BW gain DIO rats | [32] |
| LOXL 1-4 | elastin benzylamine | β-aminopropionitrile |
| Pargyline | 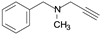 | irreversible MAO-A inhibitor |
| Clorgyline | 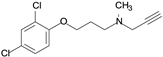 | irreversible inhibitor of MAO-A |
| Moclobemide | 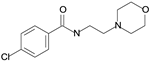 | reversible inhibitor of MAO-A |
| R-deprenyl | 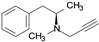 | irreversible inhibitor of MAO-B |
| Tranylcypromine | 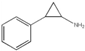 | irreversible MAO inhibitor and AOC inhibitor |
| Phenelzine | 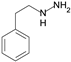 | irreversible MAO inhibitor and AOC inhibitor |
| Semicarbazide | 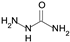 | irreversible inhibitor of AOC 1–3 inhibits LOX |
| Aminoguanidine | 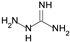 | irreversible of AOC 1–3 inhibits LOX |
| Beta-aminopropionitrile | 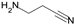 | inhibitor of LOX and LOXL |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpéné, C.; Boulet, N.; Chaplin, A.; Mercader, J. Past, Present and Future Anti-Obesity Effects of Flavin-Containing and/or Copper-Containing Amine Oxidase Inhibitors. Medicines 2019, 6, 9. https://doi.org/10.3390/medicines6010009
Carpéné C, Boulet N, Chaplin A, Mercader J. Past, Present and Future Anti-Obesity Effects of Flavin-Containing and/or Copper-Containing Amine Oxidase Inhibitors. Medicines. 2019; 6(1):9. https://doi.org/10.3390/medicines6010009
Chicago/Turabian StyleCarpéné, Christian, Nathalie Boulet, Alice Chaplin, and Josep Mercader. 2019. "Past, Present and Future Anti-Obesity Effects of Flavin-Containing and/or Copper-Containing Amine Oxidase Inhibitors" Medicines 6, no. 1: 9. https://doi.org/10.3390/medicines6010009
APA StyleCarpéné, C., Boulet, N., Chaplin, A., & Mercader, J. (2019). Past, Present and Future Anti-Obesity Effects of Flavin-Containing and/or Copper-Containing Amine Oxidase Inhibitors. Medicines, 6(1), 9. https://doi.org/10.3390/medicines6010009