Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination
Abstract
1. Introduction
2. Modes of Use in Recreational and Therapeutic Situations, Pharmacokinetics, and Pharmacodynamics
3. Secondary and Toxic Effects, Dependence, and Tolerance
4. Prevalence and Control Status
5. Therapeutic Indications
5.1. Pain Management
5.2. Epilepsy
5.2.1. Evidence in Animal Models and Basic Pharmacological Mechanisms
5.2.2. Clinical Evidence in Epilepsy
5.3. Neurodegenerative Disorders: Parkinson’s Disease, Alzheimer’s Disease, and Multiple Sclerosis
5.3.1. Parkinson’s Disease
5.3.2. Alzheimer’s Disease
5.3.3. Multiple Sclerosis
5.4. Post-Traumatic Stress Disorder
5.5. Tourette’s Syndrome
5.6. Nausea and Vomiting
5.7. Treatment of Loss of Appetite
5.8. Cutaneous Treatments and Dermatology
5.9. Infectious Diseases
5.10. Glaucoma
5.11. Sleep and Anxiety Disorders
6. Challenges in the Determination of Secondary Metabolites of Cannabis
6.1. Samples used for Determination
6.1.1. Biological Specimens
6.1.2. Non-Biological Specimens
6.2. Sample Preparation
6.3. Analytical Instrumentation
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Verpoorte, R.; Contin, A.; Memelink, J. Biotechnology for the production of plant secondary metabolites. Phytochem. Rev. 2002, 1, 13–25. [Google Scholar] [CrossRef]
- Sirikantaramas, S.; Taura, F.; Morimoto, S.; Shoyama, Y. Recent Advances in Cannabis sativa Research: Biosynthetic Studies and Its Potential in Biotechnology. Curr. Pharm. Biotechnol. 2007, 8, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Cohen, K.; Weinstein, A. The effects of cannabinoids on executive functions: Evidence from cannabis and synthetic cannabinoids—A systematic review. Brain Sci. 2018, 8, 40. [Google Scholar] [CrossRef] [PubMed]
- Solymosi, K.; Kofalvi, A. Cannabis: A Treasure Trove or Pandora’s Box? Mini Rev. Med. Chem. 2017, 17, 1223–1291. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. Prog. Chem. Org. Nat. Prod. 2017, 103, 1–36. [Google Scholar] [PubMed]
- Andre, C.M.; Hausman, J.-F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, R. Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents—Marijuana and the Cannabinoids. In Marijuana and the Cannabinoids; ElSohly, M.A., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 17–49. ISBN 978-1-59259-947-9. [Google Scholar]
- El Sohly, M.A. (Ed.) Marijuana and the Cannabinoids; Humana Press: Totowa, NJ, USA, 2017; ISBN 9781588294562. [Google Scholar]
- Guo, T.T.; Zhang, J.C.; Zhang, H.; Liu, Q.C.; Zhao, Y.; Hou, Y.F.; Bai, L.; Zhang, L.; Liu, X.Q.; Liu, X.Y.; et al. Bioactive spirans and other constituents from the leaves of Cannabis sativa f. sativa. J. Asian Nat. Prod. Res. 2017, 19, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and Medicinal Chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Grof, C.P.L. Cannabis, from plant to pill. Br. J. Clin. Pharmacol. 2018, 84, 2463–2467. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.J.; Galettis, P.; Schneider, J. The pharmacokinetics and the pharmacodynamics of cannabinoids. Br. J. Clin. Pharmacol. 2018, 84, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Newmeyer, M.N.; Swortwood, M.J.; Barnes, A.J.; Abulseoud, O.A.; Scheidweiler, K.B.; Huestis, M.A. Free and Glucuronide Whole Blood Cannabinoids’ Pharmacokinetics after Controlled Smoked, Vaporized, and Oral Cannabis Administration in Frequent and Occasional Cannabis Users: Identification of Recent Cannabis Intake. Clin. Chem. 2016, 62, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Solowij, N.; Broyd, S.J.; van Hell, H.H.; Hazekamp, A. A protocol for the delivery of cannabidiol (CBD) and combined CBD and ∆9-tetrahydrocannabinol (THC) by vaporisation. BMC Pharmacol. Toxicol. 2014, 15, 58. [Google Scholar] [CrossRef] [PubMed]
- Johansson, E.; Norén, K.; Sjövall, J.; Halldin, M.M. Determination of Δ1-tetrahydrocannabinol in human fat biopsies from marihuana users by gas chromatography-mass spectrometry. Biomed. Chromatogr. 1989, 3, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Rosado, T.; Gonçalves, J.; Luís, Â.; Malaca, S.; Soares, S.; Vieira, D.N.; Barroso, M.; Gallardo, E. Synthetic cannabinoids in biological specimens: A review of current analytical methods and sample preparation techniques. Bioanalysis 2018, 10, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A. Pharmacokinetics and Metabolism of the Plant Cannabinoids, Δ9-Tetrahydrocannibinol, Cannabidiol and Cannabinol. In Cannabinoids; Pertwee, R.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 657–690. ISBN 978-3-540-26573-3. [Google Scholar]
- Toennes, S.W.; Ramaekers, J.G.; Theunissen, E.L.; Moeller, M.R.; Kauert, G.F. Comparison of cannabinoid pharmacokinetic properties in occasional and heavy users smoking a marijuana or placebo joint. J. Anal. Toxicol. 2008, 32, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Grotenhermen, F. Pharmacokinetics and Pharmacodynamics of Cannabinoids. Clin. Pharmacokinet. 2003, 42, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Lindgren, J.E.; Andersson, S.; Agurell, S.; Gillespie, H.; Hollister, L.E. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed. Environ. Mass Spectrom. 1986, 13, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Huestis, M.A.; Henningfield, J.E.; Cone, E.J. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J. Anal. Toxicol. 1992, 16, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Kauert, G.F.; Ramaekers, J.G.; Schneider, E.; Moeller, M.R.; Toennes, S.W. Pharmacokinetic Properties of 9-Tetrahydrocannabinol in Serum and Oral Fluid. J. Anal. Toxicol. 2007, 31, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.H.; Schneider, J.; Lucas, C.J.; Galettis, P. Exogenous Cannabinoid Efficacy: Merely a Pharmacokinetic Interaction? Clin. Pharmacokinet. 2018, 57, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Goullé, J.-P.; Saussereau, E.; Lacroix, C. Pharmacocinétique du delta-9-tétrahydrocannabinol (THC). Ann. Pharm. Françaises 2008, 66, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, J.E.; Ohlsson, A.; Agurell, S.; Hollister, L.; Gillespie, H. Clinical effects and plasma levels of delta 9-tetrahydrocannabinol (delta 9-THC) in heavy and light users of cannabis. Psychopharmacology 1981, 74, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Azorlosa, J.L.; Heishman, S.J.; Stitzer, M.L.; Mahaffey, J.M. Marijuana smoking: Effect of varying delta 9-tetrahydrocannabinol content and number of puffs. J. Pharmacol. Exp. Ther. 1992, 261, 114–122. [Google Scholar] [PubMed]
- Agurell, S.; Carlsson, S.; Lindgren, J.E.; Ohlsson, A.; Gillespie, H.; Hollister, L. Interactions of delta 1-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia 1981, 37, 1090–1092. [Google Scholar] [CrossRef] [PubMed]
- Eichler, M.; Spinedi, L.; Unfer-Grauwiler, S.; Bodmer, M.; Surber, C.; Luedi, M.; Drewe, J. Heat Exposure of Cannabis sativa Extracts Affects the Pharmacokinetic and Metabolic Profile in Healthy Male Subjects. Planta Med. 2012, 78, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Grant, K.S.; Petroff, R.; Isoherranen, N.; Stella, N.; Burbacher, T.M. Cannabis use during pregnancy: Pharmacokinetics and effects on child development. Pharmacol. Ther. 2018, 182, 133–151. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, A.; Lindgren, J.-E.; Wahlen, A.; Agurell, S.; Hollister, L.E.; Gillespie, H.K. Plasma delta-9-tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin. Pharmacol. Ther. 1980, 28, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Wall, M.E.; Sadler, B.M.; Brine, D.; Taylor, H.; Perez-Reyes, M. Metabolism, disposition, and kinetics of delta-9-tetrahydrocannabinol in men and women. Clin. Pharmacol. Ther. 1983, 34, 352–363. [Google Scholar] [CrossRef] [PubMed]
- Garrett, E.R.; Hunt, C.A. Physicochemical Properties, Solubility, and Protein Binding of Δ9-Tetrahydrocannabinol. J. Pharm. Sci. 1974, 63, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Law, B.; Mason, P.A.; Moffat, A.C.; Gleadle, R.I.; King, L.J. Forensic aspects of the metabolism and excretion of cannabinoids following oral ingestion of cannabis resin. J. Pharm. Pharmacol. 1984, 36, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Frytak, S.; Moertel, C.G.; Rubin, J. Metabolic studies of delta-9-tetrahydrocannabinol in cancer patients. Cancer Treat. Rep. 1984, 68, 1427–1431. [Google Scholar] [PubMed]
- Ahmed, A.I.A.; van den Elsen, G.A.H.; Colbers, A.; Kramers, C.; Burger, D.M.; van der Marck, M.A.; Olde Rikkert, M.G.M. Safety, pharmacodynamics, and pharmacokinetics of multiple oral doses of delta-9-tetrahydrocannabinol in older persons with dementia. Psychopharmacology 2015, 232, 2587–2595. [Google Scholar] [CrossRef] [PubMed]
- Schwilke, E.W.; Schwope, D.M.; Karschner, E.L.; Lowe, R.H.; Darwin, W.D.; Kelly, D.L.; Goodwin, R.S.; Gorelick, D.A.; Huestis, M.A. Delta9-tetrahydrocannabinol (THC), 11-hydroxy-THC, and 11-nor-9-carboxy-THC plasma pharmacokinetics during and after continuous high-dose oral THC. Clin. Chem. 2009, 55, 2180–2189. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, J.T.; Harvey, D.J.; Bullingham, R.E.; Paton, W.D. Pharmacokinetics of delta 9-tetrahydrocannabinol in rabbits following single or multiple intravenous doses. Drug Metab. Dispos. 1986, 14, 230–238. [Google Scholar] [PubMed]
- Challapalli, P.V.; Stinchcomb, A.L. In vitro experiment optimization for measuring tetrahydrocannabinol skin permeation. Int. J. Pharm. 2002, 241, 329–339. [Google Scholar] [CrossRef]
- Stinchcomb, A.L.; Valiveti, S.; Hammell, D.C.; Ramsey, D.R. Human skin permeation of Δ8-tetrahydrocannabinol, cannabidiol and cannabinol. J. Pharm. Pharmacol. 2004, 56, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Valiveti, S.; Hammell, D.C.; Earles, D.C.; Stinchcomb, A.L. In vitro/in vivo correlation studies for transdermal Δ8-THC development. J. Pharm. Sci. 2004, 93, 1154–1164. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Fabin, B.; Dany, S.; Almog, S. Transdermal delivery of tetrahydrocannabinol. Int. J. Pharm. 1988, 43, 9–15. [Google Scholar] [CrossRef]
- Elsohly, M.A.; Stanford, D.F.; Harland, E.C.; Hikal, A.H.; Walker, L.A.; Little, T.L.; Rider, J.N.; Jones, A.B. Rectal Bioavailability of Δ-9-Tetrahydrocannabinol from the Hemisuccinate Ester in Monkeys. J. Pharm. Sci. 1991, 80, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Brenneisen, R.; Egli, A.; Elsohly, M.A.; Henn, V.; Spiess, Y. The effect of orally and rectally administered delta 9-tetrahydrocannabinol on spasticity: A pilot study with 2 patients. Int. J. Clin. Pharmacol. Ther. 1996, 34, 446–452. [Google Scholar] [PubMed]
- Chiang, C.W.; Barnett, G.; Brine, D. Systemic absorption of delta 9-tetrahydrocannabinol after ophthalmic administration to the rabbit. J. Pharm. Sci. 1983, 72, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Galettis, P.; Song, S.; Solowij, N.; Reuter, S.; Schneider, J.; Martin, J. Cannabinoid Disposition After Human Intraperitoneal Use: An Insight Into Intraperitoneal Pharmacokinetic Properties in Metastatic Cancer. Clin. Ther. 2018, 40, 1442–1447. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Friedman, D. Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy Behav. 2017, 70, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.; Jones, R. Tolerance and disposition of tetrahydrocannabinol in man. J. Pharmacol. Exp. Ther. 1980, 215, 35–44. [Google Scholar] [PubMed]
- Huestis, M.A. Human Cannabinoid Pharmacokinetics. Chem. Biodivers. 2007, 4, 1770–1804. [Google Scholar] [CrossRef] [PubMed]
- Blackard, C.; Tennes, K. Human Placental Transfer of Cannabinoids. N. Engl. J. Med. 1984, 311, 797. [Google Scholar] [PubMed]
- Perez-Reyes, M.; Wall, M.E. Presence of Δ9-Tetrahydrocannabinol in Human Milk. N. Engl. J. Med. 1982, 307, 819–820. [Google Scholar] [PubMed]
- Agurell, S.; Halldin, M.; Lindgren, J.E.; Ohlsson, A.; Widman, M.; Gillespie, H.; Hollister, L. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol. Rev. 1986, 38, 21–43. [Google Scholar] [PubMed]
- Matsunaga, T.; Iwawaki, Y.; Watanabe, K.; Yamamoto, I.; Kageyama, T.; Yoshimura, H. Metabolism of delta 9-tetrahydrocannabinol by cytochrome P450 isozymes purified from hepatic microsomes of monkeys. Life Sci. 1995, 56, 2089–2095. [Google Scholar] [CrossRef]
- Narimatsu, S.; Watanabe, K.; Matsunaga, T.; Yamamoto, I.; Imaoka, S.; Funae, Y.; Yoshimura, H. Cytochrome P-450 isozymes involved in the oxidative metabolism of delta 9-tetrahydrocannabinol by liver microsomes of adult female rats. Drug Metab. Dispos. 1992, 20, 79–83. [Google Scholar] [PubMed]
- Watanabe, K.; Matsunaga, T.; Yamamoto, I.; Funae, Y.; Yoshimura, H. Involvement of CYP2C in the metabolism of cannabinoids by human hepatic microsomes from an old woman. Biol. Pharm. Bull. 1995, 18, 1138–1141. [Google Scholar] [CrossRef] [PubMed]
- Widman, M.; Halldin, M.; Martin, B. In vitro metabolism of tetrahydrocannabinol by rhesus monkey liver and human liver. Adv. Biosci. 1979, 22–23, 101–103. [Google Scholar]
- Huestis, M.A.; Cone, E.J. Differentiating new marijuana use from residual drug excretion in occasional marijuana users. J. Anal. Toxicol. 1998, 22, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Zendulka, O.; Dovrtělová, G.; Nosková, K.; Turjap, M.; Šulcová, A.; Hanuš, L.; Juřica, J. Cannabinoids and Cytochrome P450 Interactions. Curr. Drug Metab. 2016, 17, 206–226. [Google Scholar] [CrossRef]
- Harvey, D.J.; Martin, B.R.; Paton, W.D.M. Identification and measurement of cannabinoids and their in vivo metabolites in liver by gas chromatography-mass spectrometry. Marihuana Biol. Eff. 1979, 45–62. [Google Scholar] [CrossRef]
- Consroe, P.; Laguna, J.; Allender, J.; Snider, S.; Stern, L.; Sandyk, R.; Kennedy, K.; Schram, K. Controlled clinical trial of cannabidiol in Huntington’s disease. Pharmacol. Biochem. Behav. 1991, 40, 701–708. [Google Scholar] [CrossRef]
- Maurer, H.H.; Sauer, C.; Theobald, D.S. Toxicokinetics of Drugs of Abuse: Current Knowledge of the Isoenzymes Involved in the Human Metabolism of Tetrahydrocannabinol, Cocaine, Heroin, Morphine, and Codeine. Ther. Drug Monit. 2006, 28, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Halldin, M.M.; Widman, M.; Bahr, C.V.; Lindgren, J.E.; Martin, B.R. Identification of in vitro metabolites of delta 1-tetrahydrocannabinol formed by human livers. Drug Metab. Dispos. 1982, 10, 297–301. [Google Scholar] [PubMed]
- Harvey, D.J. Absorption, Distribution, and Biotransformation of the Cannabinoids. In Marihuana and Medicine; Nahas, G.G., Sutin, K.M., Harvey, D., Agurell, S., Pace, N., Cancro, R., Eds.; Humana Press: Totowa, NJ, USA, 1999; pp. 91–103. ISBN 978-1-59259-710-9. [Google Scholar]
- Kelly, P.; Jones, R.T. Metabolism of tetrahydrocannabinol in frequent and infrequent marijuana users. J. Anal. Toxicol. 1992, 16, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Alburges, M.E.; Peat, M.A. Profiles of Δ9-Tetrahydrocannabinol Metabolites in Urine of Marijuana Users: Preliminary Observations by High Performance Liquid Chromatography-Radioimmunoassay. J. Forensic Sci. 1986, 31, 12302J. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997, 74, 129–180. [Google Scholar] [CrossRef]
- Pertwee, R.G. Pharmacology of cannabinoid receptor ligands. Curr. Med. Chem. 1999, 6, 635–664. [Google Scholar] [PubMed]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.M.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB 1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef] [PubMed]
- McCarberg, B.H.; Barkin, R.L. The Future of Cannabinoids as Analgesic Agents: A Pharmacologic, Pharmacokinetic, and Pharmacodynamic Overview. Am. J. Ther. 2007, 14, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, L.; De Petrocellis, V. Endocannabinoids as Regulators of Transient Receptor Potential (TRP)Channels: A Further Opportunity to Develop New Endocannabinoid-Based Therapeutic Drugs. Curr. Med. Chem. 2010, 17, 1430–1449. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, P.M.; Petersson, J.; Andersson, D.A.; Chuang, H.; Sørgård, M.; Di Marzo, V.; Julius, D.; Högestätt, E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 1999, 400, 452. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Ashton, C.H. Adverse effects of cannabis and cannabinoids. Br. J. Anaesth. 1999, 83, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Hall, W. The health and psychological effects of cannabis use. Curr. Issues Crim. Just. 1994, 6, 208. [Google Scholar] [CrossRef]
- Degenhardt, L.; Hall, W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 2012, 379, 55–70. [Google Scholar] [CrossRef]
- Ameri, A. The effects of cannabinoids on the brain. Prog. Neurobiol. 1999, 58, 315–348. [Google Scholar] [CrossRef]
- Fergusson, D.M.; Horwood, L.J.; Swain-Campbell, N.R. Cannabis dependence and psychotic symptoms in young people. Psychol. Med. 2003, 33, 15–21. [Google Scholar] [CrossRef] [PubMed]
- De Aquino, J.P.; Sherif, M.; Radhakrishnan, R.; Cahill, J.D.; Ranganathan, M.; D’Souza, D.C. The Psychiatric Consequences of Cannabinoids. Clin. Ther. 2018, 40, 1448–1456. [Google Scholar] [CrossRef] [PubMed]
- Swift, W.; Hall, W.; Teesson, M. Characteristics of DSM-IV and ICD-10 cannabis dependence among Australian adults: Results from the National Survey of Mental Health and Wellbeing. Drug Alcohol Depend. 2001, 63, 147–153. [Google Scholar] [CrossRef]
- Swift, W.; Hall, W.; Didcott, P.; Reilly, D. Patterns and correlates of cannabis dependence among long-term users in an Australian rural area. Addiction 1998, 93, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Warner, L.A.; Kessler, R.C. Comparative Epidemiology of Dependence on Tobacco, Alcohol, Controlled Substances, and Inhalants: Basic Findings From the National Comorbidity Survey. Exp. Clin. Psychopharmacol. 1994, 2, 244–268. [Google Scholar] [CrossRef]
- Solowij, N. Do cognitive impairments recover following cessation of cannabis use? Life Sci. 1995, 56, 2119–2126. [Google Scholar] [CrossRef]
- Pope, H.G., Jr.; Gruber, A.J.; Hudson, J.I.; Huestis, M.A.; Yurgelun-Todd, D. Cognitive Measures in Long-Term Cannabis Users. J. Clin. Pharmacol. 2002, 42, 41S–47S. [Google Scholar] [CrossRef]
- Budney, A.J.; Higgins, S.T.; Radonovich, K.J.; Novy, P.L. Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. J. Consult. Clin. Psychol. 2000, 68, 1051–1061. [Google Scholar] [CrossRef] [PubMed]
- European Monitoring Centre for Drugs and Drug Adiction. Cannabis Legislation in Europe. Available online: http://www.emcdda.europa.eu/system/files/publications/4135/TD0217210ENN.pdf (accessed on 22 January 2019).
- European Monitoring Centre for Drugs and Drug Adiction. 2018 European Drug Report. Available online: http://www.emcdda.europa.eu/system/files/publications/8585/20181816_TDAT18001PTN_PDF.pdf (accessed on 11 January 2019).
- Serviço de intervenção nos comportamentos aditivos e nas dependências. Relatório Anual 2016 A Situação do País em Matéria de Drogas e Toxicodependências. Available online: file://turing/users2$/saude/depcmed/mega/Desktop/RelatorioAnual_2016_A_SituacaoDoPaisEmMateriaDeDrogas_e_Toxicodependencias.pdf (accessed on 17 January 2019).
- European Monitoring Centre for Drugs and Drug Addiction. ESPAD Report 2015: Results from the European School Survey Project on Alcohol and Other Drugs. Available online: http://www.espad.org/sites/espad.org/files/ESPAD_report_2015.pdf (accessed on 17 January 2019).
- United Nations. The Single Convention on Narcotic Drugs. Available online: File:///C:/Users/Administrator/Desktop/convention_1961_en.pdf (accessed on 18 January 2019).
- Powell, B. The 7 Countries With The Strictest Weed Laws. Available online: https://www.medicalmarijuana.com.au/medical-marijuana/cannabis/the-7-countries-with-the-strictest-weed-laws (accessed on 17 January 2019).
- European Monitoring Centre for Drugs and Addiction. Medical Use of Cannabis and Cannabinoids: Questions and Answers for Policymaking. Available online: http://www.emcdda.europa.eu/publications/rapid-communications/medical-use-of-cannabis-and-cannabinoids-questions-and-answers-for-policymaking_en (accessed on 22 January 2019).
- National Alliance for Model State Drug Laws Use of Marijuana for Medicinal Purposes: Map of State Laws. Available online: http://www.namsdl.org/Maps/Use of Marijuana for Medicinal Purposes - Map of State Laws - 1_10_2017 - FINAL.pdf (accessed on 21 January 2019).
- National Conference of State Legislatures. Marijuana Overview. Available online: http://www.ncsl.org/research/civil-and-criminal-justice/marijuana-overview.aspx (accessed on 18 January 2019).
- Bigand, T.; Anderson, C.L.; Roberts, M.L.; Shaw, M.R.; Wilson, M. Benefits and Adverse Effects of Cannabis use among Adults with Persistent Pain. Nurs. Outlook 2018. [Google Scholar] [CrossRef] [PubMed]
- Armour, M.; Sinclair, J.; Chalmers, K.J.; Smith, C.A. Self-management strategies amongst Australian women with endometriosis: A national online survey. BMC Complement. Altern. Med. 2019, 19, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Fitzcharles, M.-A.; Zahedi Niaki, O.; Hauser, W.; Hazlewood, G.; the Canadian Rheumatology Association. Position Statement: A Pragmatic Approach for Medical Cannabis and Patients with Rheumatic Diseases. J. Rheumatol. 2019, 46, 181120. [Google Scholar] [CrossRef] [PubMed]
- Palace, Z.J.; Reingold, D.A. Medical Cannabis in the Skilled Nursing Facility: A Novel Approach to Improving Symptom Management and Quality of Life. J. Am. Med. Dir. Assoc. 2019, 20, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Mitchell, C.; Mannion, K.; Smolyn, J.; Meghani, S.H. An Integrated Review of Cannabis and Cannabinoids in Adult Oncologic Pain Management. Pain Manag. Nurs. 2018. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.; Stockings, E.; Nielsen, S. Understanding the evidence for medical cannabis and cannabis-based medicines for the treatment of chronic non-cancer pain. Eur. Arch. Psychiatry Clin. Neurosci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Perron, B.E.; Holt, K.R.; Yeagley, E.; Ilgen, M. Mental health functioning and severity of cannabis withdrawal among medical cannabis users with chronic pain. Drug Alcohol Depend. 2019, 194, 401–409. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences Engineering and Medicine. Therapeutic Effects of Cannabis and Cannabinoids. In The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; National Academies Press: Washington, DC, USA, 2017; pp. 94–97. [Google Scholar]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. J. Am. Med. Assoc. 2015, 313, 2456–2473. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Artul, S. Medical Cannabis for the Treatment of Fibromyalgia. JCR J. Clin. Rheumatol. 2018, 24, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.; Avisar, I. The Consumption of Cannabis by Fibromyalgia Patients in Israel. Pain Res. Treat. 2018, 2018, 7829427. [Google Scholar] [CrossRef] [PubMed]
- van de Donk, T.; Niesters, M.; Kowal, M.A.; Olofsen, E.; Dahan, A.; van Velzen, M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.; Robinson, D. Effect of Adding Medical Cannabis Treatment (MCT) to Analgesic Treatment in Patients with Low Back Pain related to Fibromyalgia: An Observational Cross-over Single Center Study. Int. J. Anesthesiol. Pain Med. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Koppel, B.S.; Brust, J.C.M.; Fife, T.; Bronstein, J.; Youssof, S.; Gronseth, G.; Gloss, D. Systematic review: Efficacy and safety of medical marijuana in selected neurologic disorders. Neurology 2014, 82, 1556–1563. [Google Scholar] [CrossRef] [PubMed]
- Klumpers, L.E.; Thacker, D.L. A Brief Background on Cannabis: From Plant to Medical Indications. J. AOAC Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Jett, J.; Stone, E.; Warren, G.; Cummings, K.M. Cannabis Use, Lung Cancer, and Related Issues. J. Thorac. Oncol. 2018, 13, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Burnell-Nugent, M.; Lossignol, D.; Ganae-Motan, E.D.; Potts, R.; Fallon, M.T. Multicenter, Double-Blind, Randomized, Placebo-Controlled, Parallel-Group Study of the Efficacy, Safety, and Tolerability of THC:CBD Extract and THC Extract in Patients with Intractable Cancer-Related Pain. J. Pain Symptom Manag. 2010, 39, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Portenoy, R.K.; Ganae-Motan, E.D.; Allende, S.; Yanagihara, R.; Shaiova, L.; Weinstein, S.; McQuade, R.; Wright, S.; Fallon, M.T. Nabiximols for opioid-treated cancer patients with poorly-controlled chronic pain: A randomized, placebo-controlled, graded-dose trial. J. Pain 2012, 13, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.-S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jetté, N. Prevalence and incidence of epilepsy: A systematic review and meta-analysis of international studies. Neurology 2017, 88, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Schuele, S.U.; Lüders, H.O. Intractable epilepsy: Management and therapeutic alternatives. Lancet Neurol. 2008, 7, 514–524. [Google Scholar] [CrossRef]
- Brodie, M.J.; Barry, S.J.E.; Bamagous, G.A.; Norrie, J.D.; Kwan, P. Patterns of treatment response in newly diagnosed epilepsy. Neurology 2012, 78, 1548–1554. [Google Scholar] [CrossRef] [PubMed]
- Carlini, E.A.; Leite, J.R.; Tannhauser, M.; Berardi, A.C. Cannabidiol and Cannabis sativa extract protect mice and rats against convulsive agents. J. Pharm. Pharmacol. 1973, 25, 664–665. [Google Scholar] [CrossRef] [PubMed]
- Consroe, P.; Wolkin, A. Cannabidiol–antiepileptic drug comparisons and interactions in experimentally induced seizures in rats. J. Pharmacol. Exp. Ther. 1977, 201, 26–32. [Google Scholar] [PubMed]
- Consroe, P.; Benedito, M.A.C.; Leite, J.R.; Carlini, E.A.; Mechoulam, R. Effects of cannabidiol on behavioral seizures caused by convulsant drugs or current in mice. Eur. J. Pharmacol. 1982, 83, 293–298. [Google Scholar] [CrossRef]
- Wallace, M.J.; Wiley, J.L.; Martin, B.R.; DeLorenzo, R.J. Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur. J. Pharmacol. 2001, 428, 51–57. [Google Scholar] [CrossRef]
- Jones, N.; Hill, T.; Stott, C.; Wright, S. Assessment of the anticonvulsant effects and tolerability of GW Pharmaceuticals’ cannabidiol in the anticonvulsant screening program. In Proceedings of the American Epilepsy Society Annual Meeting, Philadelphia, PA, USA, 3–7 December 2015; pp. 4–8. [Google Scholar]
- Jones, N.A.; Hill, A.J.; Smith, I.; Bevan, S.A.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Cannabidiol displays antiepileptiform and antiseizure properties in vitro and in vivo. J. Pharmacol. Exp. Ther. 2010, 332, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.A.; Glyn, S.E.; Akiyama, S.; Hill, T.D.M.; Hill, A.J.; Weston, S.E.; Burnett, M.D.A.; Yamasaki, Y.; Stephens, G.J.; Whalley, B.J.; et al. Cannabidiol exerts anti-convulsant effects in animal models of temporal lobe and partial seizures. Seizure 2012, 21, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Baillie, G.L.; Phillips, A.M.; Razdan, R.K.; Ross, R.A.; Pertwee, R.G. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 2007, 150, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Puffenbarger, R.A.; Boothe, A.C.; Cabral, G.A. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia 2000, 29, 58–69. [Google Scholar] [CrossRef]
- Facchinetti, F.; Del Giudice, E.; Furegato, S.; Passarotto, M.; Leon, A. Cannabinoids ablate release of TNFα in rat microglial cells stimulated with lypopolysaccharide. Glia 2003, 41, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Molina-Holgado, E.; Vela, J.M.; Arévalo-Martín, A.; Almazán, G.; Molina-Holgado, F.; Borrell, J.; Guaza, C. Cannabinoids Promote Oligodendrocyte Progenitor Survival: Involvement of Cannabinoid Receptors and Phosphatidylinositol-3 Kinase/Akt Signaling. J. Neurosci. 2002, 22, 9742–9753. [Google Scholar] [CrossRef] [PubMed]
- Benito, C.; Tolón, R.M.; Pazos, M.R.; Núñez, E.; Castillo, A.I.; Romero, J. Cannabinoid CB2 receptors in human brain inflammation. Br. J. Pharmacol. 2008, 153, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Ross, H.R.; Napier, I.; Connor, M. Inhibition of recombinant human T-type calcium channels by Delta9-tetrahydrocannabinol and cannabidiol. J. Biol. Chem. 2008, 283, 16124–16134. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B.; Burnett, A.; Hall, B.; Parker, K.K. Agonistic Properties of Cannabidiol at 5-HT1a Receptors. Neurochem. Res. 2005, 30, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.I.; Auchampach, J.A.; Hillard, C.J.; Zhu, G.; Yousufzai, B.; Mian, S.; Khan, S.; Khalifa, Y. Mediation of Cannabidiol Anti-inflammation in the Retina by Equilibrative Nucleoside Transporter and A2A Adenosine Receptor. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5526–5531. [Google Scholar] [CrossRef] [PubMed]
- Rimmerman, N.; Ben-Hail, D.; Porat, Z.; Juknat, A.; Kozela, E.; Daniels, M.P.; Connelly, P.S.; Leishman, E.; Bradshaw, H.B.; Shoshan-Barmatz, V.; et al. Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: A novel mechanism for cannabinoid-induced cell death. Cell Death Dis. 2013, 4, e949. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Mercier, M.S.; Hill, T.D.M.; Glyn, S.E.; Jones, N.A.; Yamasaki, Y.; Futamura, T.; Duncan, M.; Stott, C.G.; Stephens, G.J.; et al. Cannabidivarin is anticonvulsant in mouse and rat. Br. J. Pharmacol. 2012, 167, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Hill, T.D.M.; Cascio, M.-G.; Romano, B.; Duncan, M.; Pertwee, R.G.; Williams, C.M.; Whalley, B.J.; Hill, A.J. Cannabidivarin-rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor-independent mechanism. Br. J. Pharmacol. 2013, 170, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Weston, S.E.; Jones, N.A.; Smith, I.; Bevan, S.A.; Williamson, E.M.; Stephens, G.J.; Williams, C.M.; Whalley, B.J. Δ9-Tetrahydrocannabivarin suppresses in vitro epileptiform and in vivo seizure activity in adult rats. Epilepsia 2010, 51, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Carlini, E.A. Toward drugs derived from cannabis. Naturwissenschaften 1978, 65, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Cunha, J.M.; Carlini, E.A.; Pereira, A.E.; Ramos, O.L.; Pimentel, C.; Gagliardi, R.; Sanvito, W.L.; Lander, N.; Mechoulam, R. Chronic Administration of Cannabidiol to Healthy Volunteers and Epileptic Patients. Pharmacology 1980, 21, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Gloss, D.; Vickrey, B. Cannabinoids for epilepsy. Cochrane Database Syst. Rev. 2012, 6, CD009270. [Google Scholar]
- Szaflarski, J.P.; Bebin, E.M.; Comi, A.M.; Patel, A.D.; Joshi, C.; Checketts, D.; Beal, J.C.; Laux, L.C.; De Boer, L.M.; Wong, M.H.; et al. Long-term safety and treatment effects of cannabidiol in children and adults with treatment-resistant epilepsies: Expanded access program results. Epilepsia 2018, 59, 1540–1548. [Google Scholar] [CrossRef] [PubMed]
- Thiele, E.A.; Marsh, E.D.; French, J.A.; Mazurkiewicz-Beldzinska, M.; Benbadis, S.R.; Joshi, C.; Lyons, P.D.; Taylor, A.; Roberts, C.; Sommerville, K.; et al. Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 1085–1096. [Google Scholar] [CrossRef]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F.; et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Nabbout, R.; Miller, I.; Laux, L.; Zolnowska, M.; Wright, S.; Roberts, C. Long-term cannabidiol treatment in patients with Dravet syndrome: An open-label extension trial. Epilepsia 2019, 60, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox–Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Gaston, T.E.; Bebin, E.M.; Cutter, G.R.; Liu, Y.; Szaflarski, J.P. Interactions between cannabidiol and commonly used antiepileptic drugs. Epilepsia 2017, 58, 1586–1592. [Google Scholar] [CrossRef] [PubMed]
- Geffrey, A.L.; Pollack, S.F.; Bruno, P.L.; Thiele, E.A. Drug–drug interaction between clobazam and cannabidiol in children with refractory epilepsy. Epilepsia 2015, 56, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Patel, A.D.; Thiele, E.A.; Wong, M.H.; Appleton, R.; Harden, C.L.; Greenwood, S.; Morrison, G.; Sommerville, K.; Group, G.P.A.S. Randomized, dose-ranging safety trial of cannabidiol in Dravet syndrome. Neurology 2018, 90, e1204–e1211. [Google Scholar] [CrossRef] [PubMed]
- Maa, E.; Figi, P. The case for medical marijuana in epilepsy. Epilepsia 2014, 55, 783–786. [Google Scholar] [CrossRef] [PubMed]
- Porter, B.E.; Jacobson, C. Report of a parent survey of cannabidiol-enriched cannabis use in pediatric treatment-resistant epilepsy. Epilepsy Behav. 2013, 29, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.A.; Zhou, R.; Jacobson, C.; Weng, J.; Cheng, E.; Lay, J.; Hung, P.; Lerner, J.T.; Sankar, R. Perceived efficacy of cannabidiol-enriched cannabis extracts for treatment of pediatric epilepsy: A potential role for infantile spasms and Lennox–Gastaut syndrome. Epilepsy Behav. 2015, 47, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Press, C.A.; Knupp, K.G.; Chapman, K.E. Parental reporting of response to oral cannabis extracts for treatment of refractory epilepsy. Epilepsy Behav. 2015, 45, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Hausman-Kedem, M.; Menascu, S.; Kramer, U. Efficacy of CBD-enriched medical cannabis for treatment of refractory epilepsy in children and adolescents—An observational, longitudinal study. Brain Dev. 2018, 40, 544–551. [Google Scholar] [CrossRef] [PubMed]
- McCoy, B.; Wang, L.; Zak, M.; Al-Mehmadi, S.; Kabir, N.; Alhadid, K.; McDonald, K.; Zhang, G.; Sharma, R.; Whitney, R.; et al. A prospective open-label trial of a CBD/THC cannabis oil in dravet syndrome. Ann. Clin. Transl. Neurol. 2018, 5, 1077–1088. [Google Scholar] [CrossRef] [PubMed]
- Tzadok, M.; Uliel-Siboni, S.; Linder, I.; Kramer, U.; Epstein, O.; Menascu, S.; Nissenkorn, A.; Yosef, O.B.; Hyman, E.; Granot, D.; et al. CBD-enriched medical cannabis for intractable pediatric epilepsy: The current Israeli experience. Seizure 2016, 35, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s disease. Subcell. Biochem. 2012, 65, 389–455. [Google Scholar] [PubMed]
- Zipp, F.; Aktas, O. The brain as a target of inflammation: Common pathways link inflammatory and neurodegenerative diseases. Trends Neurosci. 2006, 29, 518–527. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E. Endocannabinoids in basal ganglia circuits Implications for Parkinson disease. Neurology 2007, 69, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Van Sickle, M.D.; Duncan, M.; Kingsley, P.J.; Mouihate, A.; Urbani, P.; Mackie, K.; Stella, N.; Makriyannis, A.; Piomelli, D.; Davison, J.S.; et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 2005, 310, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Nunez, E.; Benito, C.; Tolon, R.M.; Hillard, C.J.; Griffin, W.S.T.; Romero, J. Glial expression of cannabinoid CB2 receptors and fatty acid amide hydrolase are beta amyloid–linked events in Down’s syndrome. Neuroscience 2008, 151, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lastres-Becker, I.; Molina-Holgado, F.; Ramos, J.A.; Mechoulam, R.; Fernández-Ruiz, J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson’s disease. Neurobiol. Dis. 2005, 19, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Peres, F.F.; Levin, R.; Suiama, M.A.; Diana, M.C.; Gouvêa, D.A.; Almeida, V.; Santos, C.M.; Lungato, L.; Zuardi, A.W.; Hallak, J.E.C. Cannabidiol prevents motor and cognitive impairments induced by reserpine in rats. Front. Pharmacol. 2016, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- García-Arencibia, M.; García, C.; Kurz, A.; Rodríguez-Navarro, J.A.; Gispert-Sánchez, S.; Mena, M.A.; Auburger, G.; de Yébenes, J.G.; Fernández-Ruiz, J. Cannabinoid CB 1 receptors are early downregulated followed by a further upregulation in the basal ganglia of mice with deletion of specific park genes. In Birth, Life and Death of Dopaminergic Neurons in the Substantia Nigra; Springer: New York, NY, USA, 2009; pp. 269–275. [Google Scholar]
- Consroe, P.; Sandyk, R.; Snider, S.R. Open label evaluation of cannabidiol in dystonic movement disorders. Int. J. Neurosci. 1986, 30, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W.; Crippa, J.A.S.; Hallak, J.E.C.; Pinto, J.P.; Chagas, M.H.N.; Rodrigues, G.G.R.; Dursun, S.M.; Tumas, V. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J. Psychopharmacol. 2009, 23, 979–983. [Google Scholar] [CrossRef] [PubMed]
- Chagas, M.H.N.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; dos Santos, A.C.; Teixeira, A.L.; Hallak, J.E.C.; Crippa, J.A.S. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J. Psychopharmacol. 2014, 28, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Venderová, K.; Růžička, E.; Voříšek, V.; Višňovský, P. Survey on cannabis use in Parkinson’s disease: Subjective improvement of motor symptoms. Mov. Disord. 2004, 19, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Balash, Y.; Schleider, L.B.-L.; Korczyn, A.D.; Shabtai, H.; Knaani, J.; Rosenberg, A.; Baruch, Y.; Djaldetti, R.; Giladi, N.; Gurevich, T. Medical Cannabis in Parkinson Disease: Real-Life Patients’ Experience. Clin. Neuropharmacol. 2017, 40, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Lotan, I.; Treves, T.A.; Roditi, Y.; Djaldetti, R. Cannabis (medical marijuana) treatment for motor and non–motor symptoms of Parkinson disease: An open-label observational study. Clin. Neuropharmacol. 2014, 37, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Kindred, J.H.; Li, K.; Ketelhut, N.B.; Proessl, F.; Fling, B.W.; Honce, J.M.; Shaffer, W.R.; Rudroff, T. Cannabis use in people with Parkinson’s disease and Multiple Sclerosis: A web-based investigation. Complement. Ther. Med. 2017, 33, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Shohet, A.; Khlebtovsky, A.; Roizen, N.; Roditi, Y.; Djaldetti, R. Effect of medical cannabis on thermal quantitative measurements of pain in patients with Parkinson’s disease. Eur. J. Pain 2017, 21, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Frankel, J.P.; Hughes, A.; Lees, A.J.; Stern, G.M. Marijuana for parkinsonian tremor. J. Neurol. Neurosurg. Psychiatry 1990, 53, 436. [Google Scholar] [CrossRef] [PubMed]
- Carroll, C.B.; Bain, P.G.; Teare, L.; Liu, X.; Joint, C.; Wroath, C.; Parkin, S.G.; Fox, P.; Wright, D.; Hobart, J. Cannabis for dyskinesia in Parkinson disease: A randomized double-blind crossover study. Neurology 2004, 63, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Clinicaltrials.gov Cannabis Oil for Pain in Parkinson’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT03639064 (accessed on 20 January 2019).
- Wang, J.; Gu, B.J.; Masters, C.L.; Wang, Y.-J. A systemic view of Alzheimer disease—Insights from amyloid-β metabolism beyond the brain. Nat. Rev. Neurol. 2017, 13, 612. [Google Scholar] [CrossRef] [PubMed]
- Eubanks, L.M.; Rogers, C.J.; Beuscher IV, A.E.; Koob, G.F.; Olson, A.J.; Dickerson, T.J.; Janda, K.D. A molecular link between the active component of marijuana and Alzheimer’s disease pathology. Mol. Pharm. 2006, 3, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on β-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; De Filippis, D.; Maiuri, M.C.; De Stefano, D.; Carnuccio, R.; Iuvone, T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in β-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-κB involvement. Neurosci. Lett. 2006, 399, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Scuderi, C.; Savani, C.; Steardo Jr, L.; De Filippis, D.; Cottone, P.; Iuvone, T.; Cuomo, V.; Steardo, L. Cannabidiol in vivo blunts β-amyloid induced neuroinflammation by suppressing IL-1β and iNOS expression. Br. J. Pharmacol. 2007, 151, 1272–1279. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Spiro, A.S.; Jenner, A.M.; Garner, B.; Karl, T. Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J. Alzheimer’s Dis. 2014, 42, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Walther, S.; Mahlberg, R.; Eichmann, U.; Kunz, D. Delta-9-tetrahydrocannabinol for nighttime agitation in severe dementia. Psychopharmacology 2006, 185, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Shelef, A.; Barak, Y.; Berger, U.; Paleacu, D.; Tadger, S.; Plopsky, I.; Baruch, Y. Safety and efficacy of medical cannabis oil for behavioral and psychological symptoms of dementia: An-open label, add-on, pilot study. J. Alzheimer’s Dis. 2016, 51, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Walther, S.; Schüpbach, B.; Seifritz, E.; Homan, P.; Strik, W. Randomized, controlled crossover trial of dronabinol, 2.5 mg, for agitation in 2 patients with dementia. J. Clin. Psychopharmacol. 2011, 31, 256–258. [Google Scholar] [CrossRef] [PubMed]
- van den Elsen, G.A.H.; Ahmed, A.I.A.; Verkes, R.-J.; Feuth, T.; van der Marck, M.A.; Rikkert, M.G.M.O. Tetrahydrocannabinol in behavioral disturbances in dementia: A crossover randomized controlled trial. Am. J. Geriatr. Psychiatry 2015, 23, 1214–1224. [Google Scholar] [CrossRef] [PubMed]
- Volicer, L.; Stelly, M.; Morris, J.; McLAUGHLIN, J.; Volicer, B.J. Effects of dronabinol on anorexia and disturbed behavior in patients with Alzheimer’s disease. Int. J. Geriatr. Psychiatry 1997, 12, 913–919. [Google Scholar] [CrossRef]
- Mahlberg, R.; Walther, S. Actigraphy in agitated patients with dementia. Z. Gerontol. Geriatr. 2007, 40, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Woodward, M.R.; Harper, D.G.; Stolyar, A.; Forester, B.P.; Ellison, J.M. Dronabinol for the treatment of agitation and aggressive behavior in acutely hospitalized severely demented patients with noncognitive behavioral symptoms. Am. J. Geriatr. Psychiatry 2014, 22, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.A. Diagnosis and management of multiple sclerosis. Am. Fam. Phys. 2004, 70, 1935–1944. [Google Scholar]
- Hauser, S.L.; Goodwin, D.S. Multiple sclerosis and other demyelinating diseases. In Harrison’s Principles of Internal Medicine; Fauci, A.S., Braunwald, E., Kasper, D.L., Hauser, S., Eds.; McGraw-Hill Medical: New York, NY, USA, 2008; pp. 2611–2621. [Google Scholar]
- Global, regional, and national burden of neurological disorders during 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet. Neurol. 2017, 16, 877–897. [CrossRef]
- Comi, G.; Radaelli, M.; Soelberg Sorensen, P. Evolving concepts in the treatment of relapsing multiple sclerosis. Lancet 2017, 389, 1347–1356. [Google Scholar] [CrossRef]
- Brownlee, W.J.; Hardy, T.A.; Fazekas, F.; Miller, D.H. Diagnosis of multiple sclerosis: Progress and challenges. Lancet 2017, 389, 1336–1346. [Google Scholar] [CrossRef]
- Pertwee, R.G. Cannabinoids and multiple sclerosis. Mol. Neurobiol. 2007, 36, 45–59. [Google Scholar] [CrossRef] [PubMed]
- Lyman, W.D.; Sonett, J.R.; Brosnan, C.F.; Elkin, R.; Bornstein, M.B. Delta 9-tetrahydrocannabinol: A novel treatment for experimental autoimmune encephalomyelitis. J. Neuroimmunol. 1989, 23, 73–81. [Google Scholar] [CrossRef]
- Baker, D.; Pryce, G.; Croxford, J.L.; Brown, P.; Pertwee, R.G.; Huffman, J.W.; Layward, L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature 2000, 404, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Maresz, K.; Pryce, G.; Ponomarev, E.D.; Marsicano, G.; Croxford, J.L.; Shriver, L.P.; Ledent, C.; Cheng, X.; Carrier, E.J.; Mann, M.K.; et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat. Med. 2007, 13, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Pryce, G.; Baker, D. Control of spasticity in a multiple sclerosis model is mediated by CB1, not CB2, cannabinoid receptors. Br. J. Pharmacol. 2007, 150, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Croxford, J.L.; Pryce, G.; Jackson, S.J.; Ledent, C.; Giovannoni, G.; Pertwee, R.G.; Yamamura, T.; Baker, D. Cannabinoid-mediated neuroprotection, not immunosuppression, may be more relevant to multiple sclerosis. J. Neuroimmunol. 2008, 193, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Jackson, S.J.; Pryce, G. Cannabinoid control of neuroinflammation related to multiple sclerosis. Br. J. Pharmacol. 2007, 152, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.J.; Garcia-Merino, A. Neuroprotective agents: Cannabinoids. Clin. Immunol. 2012, 142, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Cofield, S.S.; Salter, A.R.; Tyry, T.; Mcneal, S.; Cutter, G.R.; Marrie, R.A.; Fox, R.J. Current Marijuana Usage By MS Status and Disability in the NARCOMS Registry. Int. J. Mult. Scler. Care 2015, 17 (Suppl. S1), 1–9. [Google Scholar]
- Chong, M.S.; Wolff, K.; Wise, K.; Tanton, C.; Winstock, A.; Silber, E. Cannabis use in patients with multiple sclerosis. Mult. Scler. 2006, 12, 646–651. [Google Scholar] [CrossRef] [PubMed]
- GW Pharmaceuticals Plc. Sativex® (Delta-9-Tetrahydrocannibinol and Cannabidiol in the EU) (Nabiximols in the USA). Available online: https://www.gwpharm.com/healthcare-professionals/sativex/patient-information (accessed on 20 January 2019).
- Torres-Moreno, M.C.; Papaseit, E.; Torrens, M.; Farré, M. Assessment of Efficacy and Tolerability of Medicinal Cannabinoids in Patients With Multiple Sclerosis: A Systematic Review and Meta-analysisAssessment of Efficacy and Tolerability of Cannabinoids in Patients With Multiple SclerosisAssessment of Efficacy a. JAMA Netw. Open 2018, 1, e183485. [Google Scholar] [CrossRef] [PubMed]
- Kansagara, D.; O’Neil, M.; Nugent, S.; Freeman, M.; Low, A.; Kondo, K.; Elven, C.; Zakher, B.; Motu’apuaka, M.; Paynter, R. Benefits and Harms of Cannabis in Chronic Pain or Post-Traumatic Stress Disorder: A Systematic Review; Department of Veterans Affairs: Washington, DC, USA, 2017.
- O’neil, M.E.; Nugent, S.M.; Morasco, B.J.; Freeman, M.; Low, A.; Kondo, K.; Zakher, B.; Elven, C.; Motu’apuaka, M.; Paynter, R. Benefits and harms of plant-based cannabis for posttraumatic stress disorder: A systematic review. Ann. Intern. Med. 2017, 167, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, R.M.; Takahashi, R.N. Cannabidiol as a therapeutic alternative for post-traumatic stress disorder: From bench research to confirmation in human trials. Front. Neurosci. 2018, 12, 502. [Google Scholar] [CrossRef] [PubMed]
- Elms, L.; Shannon, S.; Hughes, S.; Lewis, N. Cannabidiol in the Treatment of Post-Traumatic Stress Disorder: A Case Series. J. Altern. Complement. Med. 2018. [Google Scholar] [CrossRef] [PubMed]
- Steenkamp, M.M.; Blessing, E.M.; Galatzer-Levy, I.R.; Hollahan, L.C.; Anderson, W.T. Marijuana and other cannabinoids as a treatment for posttraumatic stress disorder: A literature review. Depress. Anxiety 2017, 34, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Bruun, R.D.; Caine, E.; Cohen, D.J.; Comings, D.E.; Como, P.G.; Conneally, P.M.; Gancher, S.T.; Goetz, C.; Golden, G.S.; et al. Definitions and Classification of Tic Disorders. Arch. Neurol. 1993, 50, 1013–1016. [Google Scholar]
- Gerard, E.; Peterson, B.S. Developmental processes and brain imaging studies in Tourette syndrome. J. Psychosom. Res. 2003, 55, 13–22. [Google Scholar] [CrossRef]
- Mink, J.W. Neurobiology of basal ganglia circuits in Tourette syndrome: Faulty inhibition of unwanted motor patterns? Adv. Neurol. 2001, 85, 113–122. [Google Scholar] [PubMed]
- Mechoulam, R.; Parker, L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef] [PubMed]
- Abrahamov, A.; Abrahamov, A.; Mechoulam, R. An efficient new cannabinoid antiemetic in pediatric oncology. Life Sci. 1995, 56, 2097–2102. [Google Scholar] [CrossRef]
- Giuffrida, A.; Parsons, L.H.; Kerr, T.M.; de Fonseca, F.R.; Navarro, M.; Piomelli, D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat. Neurosci. 1999, 2, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Hill, M.P.; Bisogno, T.; Crossman, A.R.; Brotchie, J.M. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinson’s disease. FASEB J. 2000, 14, 1432–1438. [Google Scholar] [PubMed]
- Müller-Vahl, K.R. Cannabinoids reduce symptoms of Tourette’s syndrome. Expert Opin. Pharmacother. 2003, 4, 1717–1725. [Google Scholar] [CrossRef] [PubMed]
- Sandyk, R.; Awerbuch, G. Marijuana and Tourette’s syndrome. J. Clin. Psychopharmacol. 1988, 8, 444–445. [Google Scholar] [CrossRef] [PubMed]
- Hemming, M.; Yellowlees, P.M. Effective treatment of Tourette’s syndrome with marijuana. J. Psychopharmacol. 1993, 7, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Müller-Vahl, K.R.; Kolbe, H.; Schneider, U.; Emrich, H.M. Cannabinoids: Possible role in patho-physiology and therapy of Gilles de la Tourette syndrome. Acta Psychiatr. Scand. 1998, 98, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Müller-Vahl, K.; Schneider, U.; Koblenz, A.; Jöbges, M.; Kolbe, H.; Daldrup, T.; Emrich, H. Treatment of Tourette’s Syndrome with Δ9-Tetrahydrocannabinol (THC): A Randomized Crossover Trial. Pharmacopsychiatry 2002, 35, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Muller-Vahl, K.R.; Schneider, U.; Prevedel, H.; Theloe, K.; Kolbe, H.; Daldrup, T.; Emrich, H.M. Delta 9-Tetrahydrocannabinol (THC) is Effective in the Treatment of Tics in Tourette Syndrome. J. Clin. Psychiatry 2003, 64, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Abi-Jaoude, E.; Chen, L.; Cheung, P.; Bhikram, T.; Sandor, P. Preliminary Evidence on Cannabis Effectiveness and Tolerability for Adults With Tourette Syndrome. J. Neuropsychiatry Clin. Neurosci. 2017, 29, 391–400. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov Safety and Efficacy of Cannabis in Tourette Syndrome. Available online: https://clinicaltrials.gov/ct2/show/NCT03247244. (accessed on 20 January 2019).
- ClinicalTrials.gov CANNAbinoids in the Treatment of TICS (CANNA-TICS)—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03087201?term=cannabis&cond=Tourette+Syndrome (accessed on 20 January 2019).
- ClinicalTrials.gov Efficacy of a Therapeutic Combination of Dronabinol and PEA for Tourette Syndrome—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03066193?term=THC&cond=Tourette+syndrome (accessed on 20 January 2019).
- ClinicalTrials.gov A Study to Examine the Efficacy of a Therapeutic THX-110 for Tourette Syndrome—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT03651726?term=THC&cond=Tourette+syndrome (accessed on 20 January 2019).
- Navari, R.M. Pharmacological Management of Chemotherapy-Induced Nausea and Vomiting. Drugs 2009, 69, 515–533. [Google Scholar] [CrossRef] [PubMed]
- Hornby, P.J. Central neurocircuitry associated with emesis. Am. J. Med. 2001, 111, 106–112. [Google Scholar] [CrossRef]
- Darmani, N.A.; Janoyan, J.J.; Crim, J.; Ramirez, J. Receptor mechanism and antiemetic activity of structurally-diverse cannabinoids against radiation-induced emesis in the least shrew. Eur. J. Pharmacol. 2007, 563, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.A.; Rock, E.M.; Limebeer, C.L. Regulation of nausea and vomiting by cannabinoids. Br. J. Pharmacol. 2011, 163, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Darmani, N.A. The cannabinoid CB1 receptor antagonist SR 141716A reverses the antiemetic and motor depressant actions of WIN 55, 212-2. Eur. J. Pharmacol. 2001, 430, 49–58. [Google Scholar] [CrossRef]
- Barann, M.; Molderings, G.; Brüss, M.; Bönisch, H.; Urban, B.W.; Göthert, M. Direct inhibition by cannabinoids of human 5-HT3A receptors: Probable involvement of an allosteric modulatory site. Br. J. Pharmacol. 2002, 137, 589–596. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. MARINOL (Dronabinol) Capsules, for Oral Use; US Food and Drug Administration: Silver Spring, MD, USA, 2017.
- US Food and Drug Administration. Cesamet FDA Approval; US Food and Drug Administration: Silver Spring, MD, USA, 2006.
- Hesketh, P.J.; Kris, M.G.; Basch, E.; Bohlke, K.; Barbour, S.Y.; Clark-Snow, R.A.; Danso, M.A.; Dennis, K.; Dupuis, L.L.; Dusetzina, S.B.; et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2017, 35, 3240–3261. [Google Scholar] [CrossRef] [PubMed]
- Vinciguerra, V.; Moore, T.; Brennan, E. Inhalation marijuana as an antiemetic for cancer chemotherapy. N. Y. State J. Med. 1988, 88, 525–527. [Google Scholar] [PubMed]
- Musty, R.E.; Rossi, R. Effects of Smoked Cannabis and Oral ∆9-Tetrahydrocannabinol on Nausea and Emesis After Cancer Chemotherapy: A Review of State Clinical Trials. J. Cannabis Ther. 2001, 1, 29–56. [Google Scholar] [CrossRef]
- Söderpalm, A.H. V.; Schuster, A.; de Wit, H. Antiemetic efficacy of smoked marijuana: Subjective and behavioral effects on nausea induced by syrup of ipecac. Pharmacol. Biochem. Behav. 2001, 69, 343–350. [Google Scholar] [CrossRef]
- Roila, F.; Feyer, P.; Hesketh, P.J.; Jordan, K.; Olver, I.; Rapoport, B.L.; Roscoe, J.; Walsh, D.; Warr, D.; van der Wetering, M.; et al. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann. Oncol. 2016, 27, v119–v133. [Google Scholar] [CrossRef] [PubMed]
- Lutge, E.E.; Gray, A.; Siegfried, N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst. Rev. 2013, 30, CD005175. [Google Scholar] [CrossRef] [PubMed]
- Badowski, M.E.; Yanful, P.K. Dronabinol oral solution in the management of anorexia and weight loss in AIDS and cancer. Ther. Clin. Risk Manag. 2018, 14, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Andries, A.; Frystyk, J.; Flyvbjerg, A.; Støving, R.K. Dronabinol in severe, enduring anorexia nervosa: A randomized controlled trial. Int. J. Eat. Disord. 2014, 47, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Scheffler, F.; Kilian, S.; Chiliza, B.; Asmal, L.; Phahladira, L.; du Plessis, S.; Kidd, M.; Murray, R.M.; Di Forti, M.; Seedat, S.; et al. Effects of cannabis use on body mass, fasting glucose and lipids during the first 12months of treatment in schizophrenia spectrum disorders. Schizophr. Res. 2018, 199, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.H.; Friedman, A. The Therapeutic Potential of Cannabinoids in Dermatology. Skin Ther. Lett. 2018, 23, 1–5. [Google Scholar]
- Mounessa, J.S.; Siegel, J.A.; Dunnick, C.A.; Dellavalle, R.P. The role of cannabinoids in dermatology. J. Am. Acad. Dermatol. 2017, 77, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Theroux, Z.; Cropley, T. Cannabis and Dr Piffard—A Century Ahead of the Curve. JAMA Dermatol. 2016, 152, 972. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Akhtar, N. The safety and efficacy of 3% Cannabis seeds extract cream for reduction of human cheek skin sebum and erythema content. Pak. J. Pharm. Sci. 2015, 28, 1389–1395. [Google Scholar] [PubMed]
- Callaway, J.; Schwab, U.; Harvima, I.; Halonen, P.; Mykkänen, O.; Hyvönen, P.; Järvinen, T. Efficacy of dietary hempseed oil in patients with atopic dermatitis. J. Dermatolog. Treat. 2005, 16, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Citti, C.; Pacchetti, B.; Vandelli, M.A.; Forni, F.; Cannazza, G. Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA). J. Pharm. Biomed. Anal. 2018, 149, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Eberlein, B.; Eicke, C.; Reinhardt, H.-W.; Ring, J. Adjuvant treatment of atopic eczema: Assessment of an emollient containing N-palmitoylethanolamine (ATOPA study). J. Eur. Acad. Dermatol. Venereol. 2008, 22, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Chelliah, M.P.; Zinn, Z.; Khuu, P.; Teng, J.M.C. Self-initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr. Dermatol. 2018, 35, e224–e227. [Google Scholar] [CrossRef] [PubMed]
- Maor, Y.; Yu, J.; Kuzontkoski, P.M.; Dezube, B.J.; Zhang, X.; Groopman, J.E. Cannabidiol Inhibits Growth and Induces Programmed Cell Death in Kaposi Sarcoma-Associated Herpesvirus-Infected Endothelium. Genes Cancer 2012, 3, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.L.; Hill, D.S.; McKee, C.S.; Hernandez-Tiedra, S.; Lorente, M.; Lopez-Valero, I.; Eleni Anagnostou, M.; Babatunde, F.; Corazzari, M.; Redfern, C.P.F.; et al. Exploiting Cannabinoid-Induced Cytotoxic Autophagy to Drive Melanoma Cell Death. J. Investig. Dermatol. 2015, 135, 1629–1637. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Kampp, J.T. Review of Common Alternative Herbal “Remedies” for Skin Cancer. Dermatol. Surg. 2019, 45, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Maida, V.; Corban, J. Topical Medical Cannabis: A New Treatment for Wound Pain—Three Cases of Pyoderma Gangrenosum. J. Pain Symptom Manag. 2017, 54, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Eagleston, L.R.M.; Kalani, N.K.; Patel, R.R.; Flaten, H.K.; Dunnick, C.A.; Dellavalle, R.P. Cannabinoids in dermatology: A scoping review. Dermatol. Online J. 2018, 24, 1–17. [Google Scholar]
- Milando, R.; Friedman, A. Cannabinoids: Potential Role in Inflammatory and Neoplastic Skin Diseases. Am. J. Clin. Dermatol. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Brant, F.; Miranda, A.S.; Machado, F.S.; Teixeira, A.L. Cannabidiol increases survival and promotes rescue of cognitive function in a murine model of cerebral malaria. Neuroscience 2015, 289, 166–180. [Google Scholar] [CrossRef] [PubMed]
- Meza, A.; Lehmann, C. Betacaryophyllene—A phytocannabinoid as potential therapeutic modality for human sepsis? Med. Hypotheses 2018, 110, 68–70. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences Engineering and Medicine. The Health Effects of Cannabis and Cannabinoids: The Current State of Evidence and Recommendations for Research; The National Academies Press: Washington, DC, USA, 2017. [Google Scholar]
- Green, K. Marijuana smoking vs cannabinoids for glaucoma therapy. Arch. Ophthalmol. 1998, 116, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Tomida, I.; Azuara-Blanco, A.; House, H.; Flint, M.; Pertwee, R.G.; Robson, P.J. Effect of Sublingual Application of Cannabinoids on Intraocular Pressure: A Pilot Study. J. Glaucoma 2006, 15, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.; Daily, L.; Leishman, E.; Bradshaw, H.; Straiker, A. Δ9-Tetrahydrocannabinol and Cannabidiol Differentially Regulate Intraocular Pressure. Investig. Opthalmol. Vis. Sci. 2018, 59, 5904–5911. [Google Scholar] [CrossRef] [PubMed]
- Belendiuk, K.A.; Babson, K.A.; Vandrey, R.; Bonn-Miller, M.O. Cannabis species and cannabinoid concentration preference among sleep-disturbed medicinal cannabis users. Addict. Behav. 2015, 50, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, A.N.; Turner, C.; Stone, B.M.; Robson, P.J. Effect of Δ-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J. Clin. Psychopharmacol. 2004, 24, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Tringale, R.; Jensen, C. Cannabis and insomnia. Depression 2011, 4, 0–68. [Google Scholar]
- Babson, K.A.; Sottile, J.; Morabito, D. Cannabis, Cannabinoids, and Sleep: A Review of the Literature. Curr. Psychiatry Rep. 2017, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.; Zuardi, A.; Mertín-Santos, R.; Bhattacharyya, S.; Atakan, Z.; McGuire, P.; Fusar-Poli, P. Cannabis and anxiety: A critical review of the evidence. Hum. Psychopharmacol. Clin. Exp. 2009, 24, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, M.M.; Queiroz, R.H.C.; Chagas, M.H.N.; De Oliveira, D.C.G.; De Martinis, B.S.; Kapczinski, F.; Quevedo, J.; Roesler, R.; Schröder, N.; Nardi, A.E.; et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-nave social phobia patients. Neuropsychopharmacology 2011, 36, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Gambelunghe, C.; Fucci, N.; Aroni, K.; Bacci, M.; Marcelli, A.; Rossi, R. Cannabis Use Surveillance by Sweat Analysis. Ther. Drug Monit. 2016, 38, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Singh, R. Microextraction techniques for analysis of cannabinoids. TrAC Trends Anal. Chem. 2016, 80, 156–166. [Google Scholar] [CrossRef]
- De Giovanni, N.; Fucci, N. The Current Status of Sweat Testing For Drugs of Abuse: A Review. Curr. Med. Chem. 2013, 20, 545–561. [Google Scholar] [PubMed]
- Gallardo, E.; Queiroz, J.A. The role of alternative specimens in toxicological analysis. Biomed. Chromatogr. 2008, 22, 795–821. [Google Scholar] [CrossRef] [PubMed]
- Meier, S.I.; Koelzer, S.C.; Schubert-Zsilavecz, M.; Toennes, S.W. Analysis of drugs of abuse in Cerumen—correlation of postmortem analysis results with those for blood, urine and hair. Drug Test. Anal. 2017, 9, 1572–1585. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Barroso, M.; Queiroz, J.A. LC-MS: A powerful tool in workplace drug testing. Drug Test. Anal. 2009, 1, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Samyn, N.; Van Haeren, C. On-site testing of saliva and sweat with Drugwipe and determination of concentrations of drugs of abuse in saliva, plasma and urine of suspected users. Int. J. Legal Med. 2000, 113, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, J.A.; Gallardo, E.; Barroso, M. What are the recent advances in forensic oral fluid bioanalysis? Bioanalysis 2013, 5, 2077–2079. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Barroso, M.; Queiroz, J.A. Current technologies and considerations for drug bioanalysis in oral fluid. Bioanalysis 2009, 1, 637–667. [Google Scholar] [CrossRef] [PubMed]
- Desrosiers, N.A.; Scheidweiler, K.B.; Huestis, M.A. Quantification of six cannabinoids and metabolites in oral fluid by liquid chromatography-tandem mass spectrometry. Drug Test. Anal. 2015, 7, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Vandrey, R.; Milman, G.; Bergamaschi, M.; Mendu, D.R.; Murray, J.A.; Barnes, A.J.; Huestis, M.A. Oral fluid/plasma cannabinoid ratios following controlled oral THC and smoked cannabis administration. Anal. Bioanal. Chem. 2013, 405, 7269–7279. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Milman, G.; Barnes, A.J.; Goodwin, R.S.; Hirvonen, J.; Huestis, M.A. Oral fluid cannabinoids in chronic, daily cannabis smokers during sustained, monitored abstinence. Clin. Chem. 2011, 57, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Niedbala, R.S.; Kardos, K.W.; Fritch, D.F.; Kunsman, K.P.; Blum, K.A.; Newland, G.A.; Waga, J.; Kurtz, L.; Bronsgeest, M.; Cone, E.J. Passive cannabis smoke exposure and oral fluid testing. II. Two studies of extreme cannabis smoke exposure in a motor vehicle. J. Anal. Toxicol. 2005, 29, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Wtsadik, A.; Scheidweiler, K.B.; Fortner, N.; Takeichi, S.; Huestis, M.A. Validated gas chromatographic-negative ion chemical ionization mass spectrometric method for Δ9-tetrahydrocannabinol in sweat patches. Clin. Chem. 2004, 50, 2083–2090. [Google Scholar] [CrossRef] [PubMed]
- Drummer, O.H. Drug testing in oral fluid. Clin. Biochem. Rev. 2006, 27, 147–159. [Google Scholar] [PubMed]
- Balabanova, S.; Schneider, E. Detection of drugs in sweat. Beitr. Gerichtl. Med. 1990, 48, 45–49. [Google Scholar] [PubMed]
- Samyn, N.; De Boeck, G.; Verstraete, A.G. The use of oral fluid and sweat wipes for the detection of drugs of abuse in drivers. J. Forensic Sci. 2002, 47, 1380–1387. [Google Scholar] [CrossRef] [PubMed]
- De La Torre, R.; Pichini, S. Usefulness of sweat testing for the detection of cannabis smoke. Clin. Chem. 2004, 50, 1961–1962. [Google Scholar] [CrossRef] [PubMed]
- Kieliba, T.; Lerch, O.; Andresen-Streichert, H.; Rothschild, M.A.; Beike, J. Simultaneous quantification of THC-COOH, OH-THC, and further cannabinoids in human hair by gas chromatography–tandem mass spectrometry with electron ionization applying automated sample preparation. Drug Test. Anal. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Gallardo, E. Hair analysis for forensic applications: Is the future bright? Bioanalysis 2013, 6, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Gallardo, E.; Vieira, D.N.; López-Rivadulla, M.; Queiroz, J.A. Hair: A complementary source of bioanalytical information in forensic toxicology. Bioanalysis 2010, 3, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Beasley, E.; Francese, S.; Bassindale, T. Detection and Mapping of Cannabinoids in Single Hair Samples through Rapid Derivatization and Matrix-Assisted Laser Desorption Ionization Mass Spectrometry. Anal. Chem. 2016, 88, 10328–10334. [Google Scholar] [CrossRef] [PubMed]
- Aamir, M.; Hafeez, A.; Ijaz, A.; Khan, S.A.; Chaudhry, N.; Ahmed, N. Development and validation of a liquid chromatography–tandem mass spectrometry method for cannabis detection in hair of chronic cannabis users under surveillance. Pakistan J. Pathol. 2016, 27, 61–70. [Google Scholar]
- Pichini, S.; Marchei, E.; Martello, S.; Gottardi, M.; Pellegrini, M.; Svaizer, F.; Lotti, A.; Chiarotti, M.; Pacifici, R. Identification and quantification of 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid glucuronide (THC-COOH-glu) in hair by ultra-performance liquid chromatography tandem mass spectrometry as a potential hair biomarker of cannabis use. Forensic Sci. Int. 2015, 249, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Concheiro, M.; Huestis, M.A. Drug exposure during pregnancy: Analytical methods and toxicological findings. Bioanalysis 2018, 10, 587–606. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; de Castro, A.; Lendoiro, E.; Cruz-Landeira, A.; López-Rivadulla, M.; Concheiro, M. Detection of in utero cannabis exposure by umbilical cord analysis. Drug Test. Anal. 2018, 10, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Lamy, S.; Hennart, B.; Houivet, E.; Dulaurent, S.; Delavenne, H.; Benichou, J.; Allorge, D.; Marret, S.; Thibaut, F. Assessment of tobacco, alcohol and cannabinoid metabolites in 645 meconium samples of newborns compared to maternal self-reports. J. Psychiatr. Res. 2017, 90, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Chittamma, A.; Marin, S.J.; Williams, J.A.; Clark, C.; McMillin, G.A. Detection of in utero marijuana exposure by GC–MS, ultra-sensitive ELISA and LC–TOF–MS using umbilical cord tissue. J. Anal. Toxicol. 2013, 37, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Prego-Meleiro, P.; Lendoiro, E.; Concheiro, M.; Cruz, A.; López-Rivadulla, M.; de Castro, A. Development and validation of a liquid chromatography tandem mass spectrometry method for the determination of cannabinoids and phase I and II metabolites in meconium. J. Chromatogr. A 2017, 1497, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New methods for the comprehensive analysis of bioactive compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef] [PubMed]
- Richins, R.D.; Rodriguez-Uribe, L.; Lowe, K.; Ferral, R.; O’Connell, M.A.; Prego-Meleiro, P.; Lendoiro, E.; Concheiro, M.; Cruz, A.; López-Rivadulla, M.; et al. Accumulation of bioactive metabolites in cultivated medical Cannabis. PLoS ONE 2018, 113, e0201119. [Google Scholar] [CrossRef] [PubMed]
- Namdar, D.; Mazuz, M.; Ion, A.; Koltai, H. Variation in the compositions of cannabinoid and terpenoids in Cannabis sativa derived from inflorescence position along the stem and extraction methods. Ind. Crops Prod. 2018, 113, 376–382. [Google Scholar] [CrossRef]
- Ciolino, L.A.; Ranieri, T.L.; Taylor, A.M. Commercial cannabis consumer products part 2: HPLC-DAD quantitative analysis of cannabis cannabinoids. Forensic Sci. Int. 2018, 289, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Holness, H.; Furton, K.G.; Almirall, J.R. Recent advances in micro-sample preparation with forensic applications. TrAC Trends Anal. Chem. 2013, 45, 264–279. [Google Scholar] [CrossRef]
- Dulaurent, S.; Gaulier, J.M.; Imbert, L.; Morla, A.; Lachâtre, G. Simultaneous determination of δ9-tetrahydrocannabinol, cannabidiol, cannabinol and 11-nor-δ9-tetrahydrocannabinol-9-carboxylic acid in hair using liquid chromatography-tandem mass spectrometry. Forensic Sci. Int. 2014, 236, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Mackuľak, T.; Brandeburová, P.; Grenčíková, A.; Bodík, I.; Staňová, A.V.; Golovko, O.; Koba, O.; Mackuľaková, M.; Špalková, V.; Gál, M.; et al. Music festivals and drugs: Wastewater analysis. Sci. Total Environ. 2019, 659, 326–334. [Google Scholar] [CrossRef] [PubMed]
- González-Mariño, I.; Thomas, K.V.; Reid, M.J. Determination of cannabinoid and synthetic cannabinoid metabolites in wastewater by liquid–liquid extraction and ultra-high performance supercritical fluid chromatography-tandem mass spectrometry. Drug Test. Anal. 2018, 10, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Petrović, M.; Debeljak, Ž.; Kezić, N.; Džidara, P. Relationship between cannabinoids content and composition of fatty acids in hempseed oils. Food Chem. 2015, 170, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, M.C.; Pellegrini, M.; Martucci, P.; Giacobbe, R.; De Palma, A.; Pacifici, R.; Pichini, S.; Busardò, F.P.; Bisconti, M. Cannabinoids determination in bronchoalveolar lavages of cannabis smokers with lung disease. Clin. Chem. Lab. Med. 2018, in press. [Google Scholar] [CrossRef] [PubMed]
- Ottaviani, G.; Cameriere, R.; Cippitelli, M.; Froldi, R.; Tassoni, G.; Zampi, M.; Cingolani, M. Determination of drugs of abuse in a single sample of human teeth by a gas chromatography-mass spectrometry method. J. Anal. Toxicol. 2017, 41, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, H.; Saito, K. Recent advances in SPME techniques in biomedical analysis. J. Pharm. Biomed. Anal. 2011, 54, 926–950. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Gallardo, E.; Queiroz, J.A. The role of liquid-phase microextraction techniques in bioanalysis. Bioanalysis 2015, 7, 2195–2201. [Google Scholar] [CrossRef] [PubMed]
- Barroso, M.; Moreno, I.; da Fonseca, B.; Queiroz, J.A.; Gallardo, E. Role of microextraction sampling procedures in forensic toxicology. Bioanalysis 2012, 4, 1805–1826. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.J.; Satterfield-Doerr, M.; Parikh, A.R.; Brodbelt, J.S. Determination of cannabinoids in water and human saliva by solid-phase microextraction and quadrupole ion trap gas chromatography/mass spectrometry. Anal. Chem. 1998, 70, 1788–1796. [Google Scholar] [CrossRef] [PubMed]
- Strano-Rossi, S.; Chiarotti, M. Solid-phase microextraction for cannabinoids analysis in hair and its possible application to other drugs. J. Anal. Toxicol. 1999, 23, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Junker, H.P.; Lachenmeier, D.W.; Kroener, L.; Madea, B. Fully automated determination of cannabinoids in hair samples using headspace solid-phase microextraction and gas chromatography-mass spectrometry. J. Anal. Toxicol. 2002, 26, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Emídio, E.S.; de Menezes Prata, V.; Dórea, H.S. Validation of an analytical method for analysis of cannabinoids in hair by headspace solid-phase microextraction and gas chromatography–ion trap tandem mass spectrometry. Anal. Chim. Acta 2010, 670, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Lachenmeier, D.W.; Kroener, L.; Musshoff, F.; Madea, B. Determination of cannabinoids in hemp food products by use of headspace solid-phase microextraction and gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2004, 378, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Racamonde, I.; Villaverde-de-Sáa, E.; Rodil, R.; Quintana, J.B.; Cela, R. Determination of Δ9-tetrahydrocannabinol and 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in water samples by solid-phase microextraction with on-fiber derivatization and gas chromatography–mass spectrometry. J. Chromatogr. A 2012, 1245, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Dizioli Rodrigues de Oliveira, C.; Yonamine, M.; de Moraes Moreau, R.L. Headspace solid-phase microextraction of cannabinoids in human head hair samples. J. Sep. Sci. 2007, 30, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Musshoff, F.; Lachenmeier, D.W.; Kroener, L.; Madea, B. Automated headspace solid-phase dynamic extraction for the determination of cannabinoids in hair samples. Forensic Sci. Int. 2003, 133, 32–38. [Google Scholar] [CrossRef]
- Lachenmeier, D.W.; Kroener, L.; Musshoff, F.; Madea, B. Application of tandem mass spectrometry combined with gas chromatography and headspace solid-phase dynamic extraction for the determination of drugs of abuse in hair samples. Rapid Commun. Mass Spectrom. 2003, 17, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Sergi, M.; Montesano, C.; Odoardi, S.; Rocca, L.M.; Fabrizi, G.; Compagnone, D.; Curini, R. Micro extraction by packed sorbent coupled to liquid chromatography tandem mass spectrometry for the rapid and sensitive determination of cannabinoids in oral fluids. J. Chromatogr. A 2013, 1301, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rosado, T.; Fernandes, L.; Barroso, M.; Gallardo, E. Sensitive determination of THC and main metabolites in human plasma by means of microextraction in packed sorbent and gas chromatography–tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1043, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Yamini, Y.; Baheri, T. Analysis of abuse drugs in urine using surfactant-assisted dispersive liquid-liquid microextraction. J. Sep. Sci. 2011, 34, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Gallardo, E.; Barroso, M. Variations in headspace microextraction procedures and current applications in bioanalysis. Bioanalysis 2015, 7, 2235–2240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Avula, B.; Elsohly, M.A.; Radwan, M.M.; Wang, M.; Wanas, A.S.; Mehmedic, Z.; Khan, I.A. Quantitative Determination of Δ9-THC, CBG, CBD, Their Acid Precursors and Five Other Neutral Cannabinoids by UHPLC-UV-MS. Planta Med. 2018, 84, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Gul, W.; Gul, S.W.; Radwan, M.M.; Wanas, A.S.; Mehmedic, Z.; Khan, I.I.; Sharaf, M.H.M.; ElSohly, M.A. Determination of 11 cannabinoids in biomass and extracts of different varieties of Cannabis using high-performance liquid chromatography. J. AOAC Int. 2015, 98, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Murthy, P.; Bharath, M.M.S. Chemistry, metabolism, and toxicology of cannabis: Clinical implications. Iran. J. Psychiatry 2012, 7, 149. [Google Scholar] [PubMed]
- Citti, C.; Ciccarella, G.; Braghiroli, D.; Parenti, C.; Vandelli, M.A.; Cannazza, G. Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass spectrometry method. J. Pharm. Biomed. Anal. 2016, 128, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Wene, D.; Fan, Z. (Tina) Qualitative and quantitative measurement of cannabinoids in cannabis using modified HPLC/DAD method. J. Pharm. Biomed. Anal. 2017, 146, 15–23. [Google Scholar] [CrossRef] [PubMed]
- De Backer, B.; Debrus, B.; Lebrun, P.; Theunis, L.; Dubois, N.; Decock, L.; Verstraete, A.; Hubert, P.; Charlier, C. Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material. J. Chromatogr. B 2009, 877, 4115–4124. [Google Scholar] [CrossRef] [PubMed]
- Cardenia, V.; Gallina Toschi, T.; Scappini, S.; Rubino, R.C.; Rodriguez-Estrada, M.T. Development and validation of a Fast gas chromatography/mass spectrometry method for the determination of cannabinoids in Cannabis sativa L. J. Food Drug Anal. 2018, 26, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Baciu, T.; Borrull, F.; Aguilar, C.; Calull, M. Recent trends in analytical methods and separation techniques for drugs of abuse in hair. Anal. Chim. Acta 2015, 856, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Rosado, T.; Soares, S.; Malaca, S.; Gonçalves, J.; Barroso, M.; Gallardo, E. The role of liquid chromatography in toxicological analysis. In High-Performance Liquid Chromatography: Types, Parameters and Applications; Lucero, I., Ed.; Nova Science Publishers: New York, NY, USA, 2018; pp. 1–120. ISBN 978-1-53613-543-5. [Google Scholar]
- Calvi, L.; Pentimalli, D.; Panseri, S.; Giupponi, L.; Gelmini, F.; Beretta, G.; Vitali, D.; Bruno, M.; Zilio, E.; Pavlovic, R.; et al. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach. J. Pharm. Biomed. Anal. 2018, 150, 208–219. [Google Scholar] [CrossRef] [PubMed]
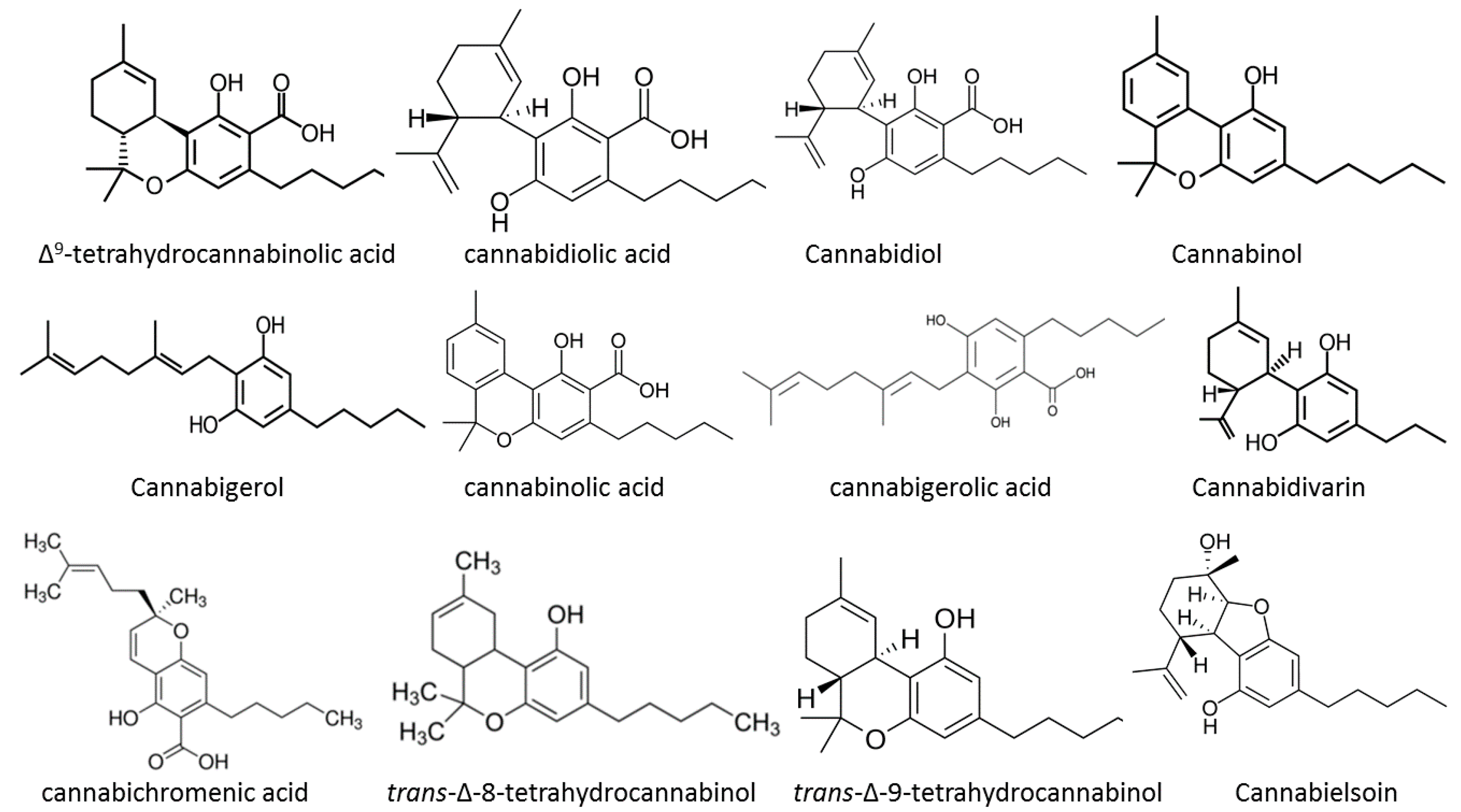
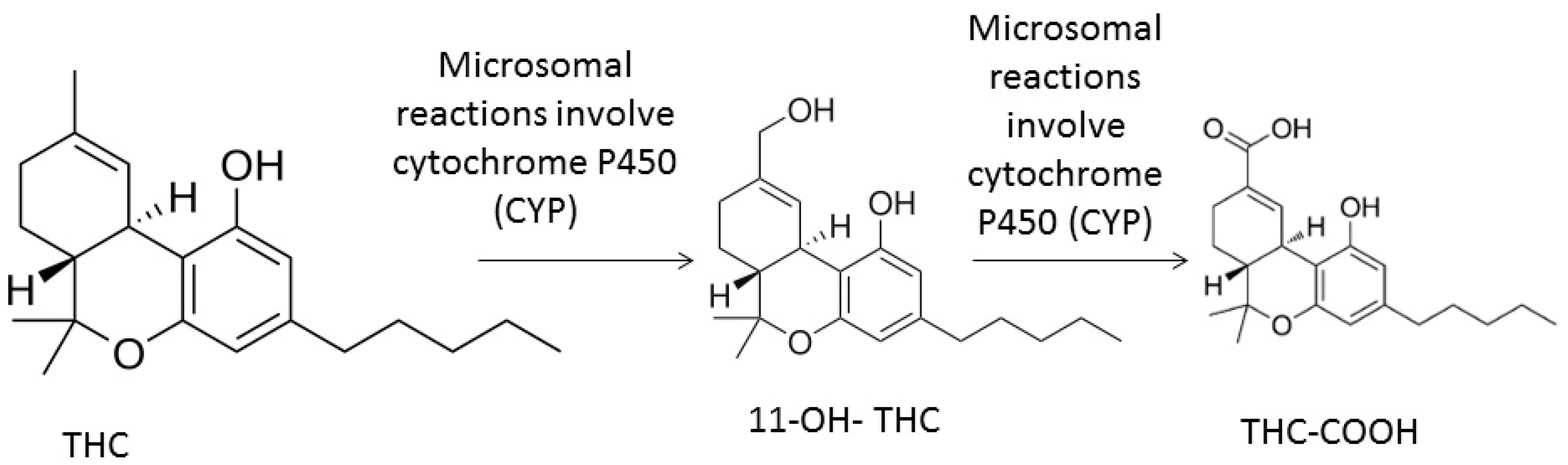
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, J.; Rosado, T.; Soares, S.; Simão, A.Y.; Caramelo, D.; Luís, Â.; Fernández, N.; Barroso, M.; Gallardo, E.; Duarte, A.P. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines 2019, 6, 31. https://doi.org/10.3390/medicines6010031
Gonçalves J, Rosado T, Soares S, Simão AY, Caramelo D, Luís Â, Fernández N, Barroso M, Gallardo E, Duarte AP. Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines. 2019; 6(1):31. https://doi.org/10.3390/medicines6010031
Chicago/Turabian StyleGonçalves, Joana, Tiago Rosado, Sofia Soares, Ana Y. Simão, Débora Caramelo, Ângelo Luís, Nicolás Fernández, Mário Barroso, Eugenia Gallardo, and Ana Paula Duarte. 2019. "Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination" Medicines 6, no. 1: 31. https://doi.org/10.3390/medicines6010031
APA StyleGonçalves, J., Rosado, T., Soares, S., Simão, A. Y., Caramelo, D., Luís, Â., Fernández, N., Barroso, M., Gallardo, E., & Duarte, A. P. (2019). Cannabis and Its Secondary Metabolites: Their Use as Therapeutic Drugs, Toxicological Aspects, and Analytical Determination. Medicines, 6(1), 31. https://doi.org/10.3390/medicines6010031








