Guggulsterone Activates Adipocyte Beiging through Direct Effects on 3T3-L1 Adipocytes and Indirect Effects Mediated through RAW264.7 Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Lipid Quantification
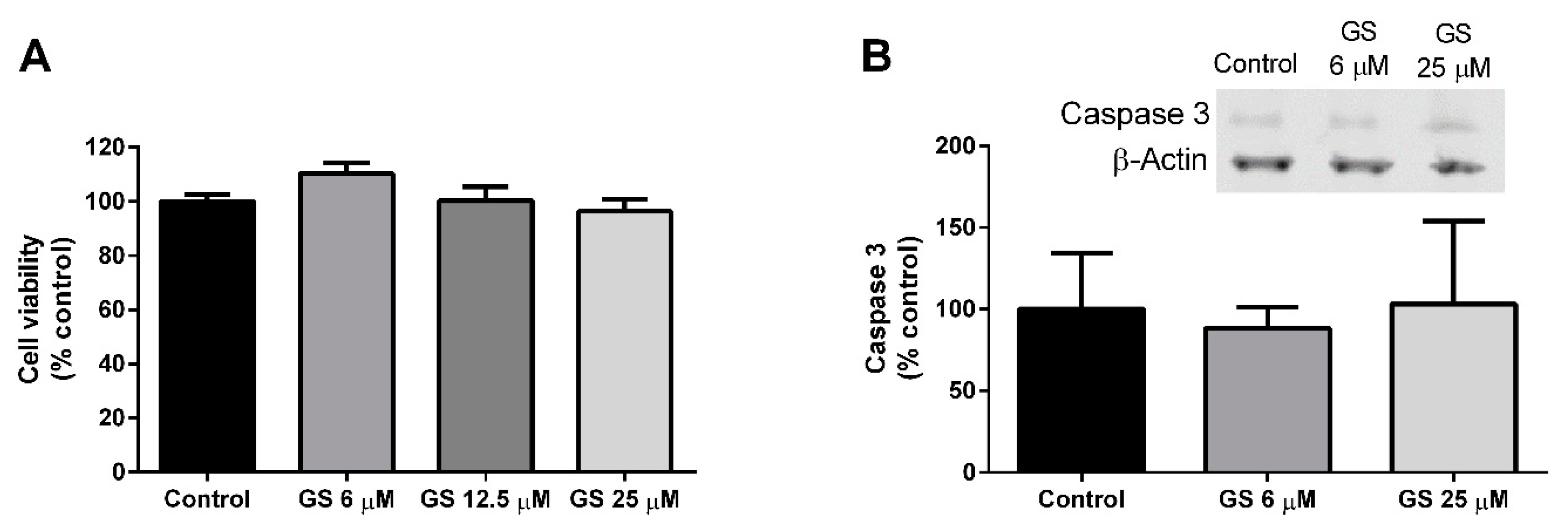
2.3. Cell Viability
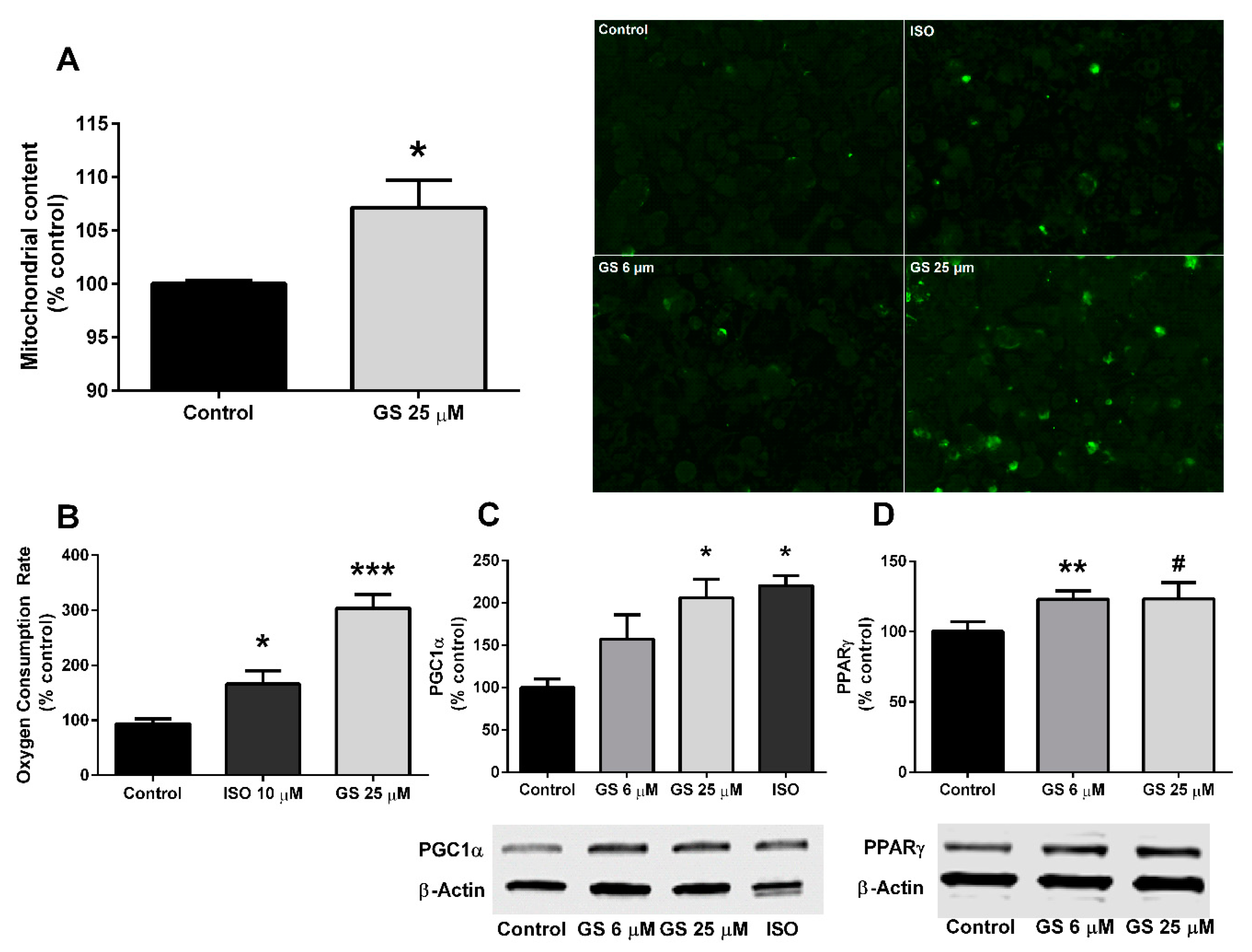
2.4. Mitochondrial Biogenesis
2.5. Oxygen Consumption
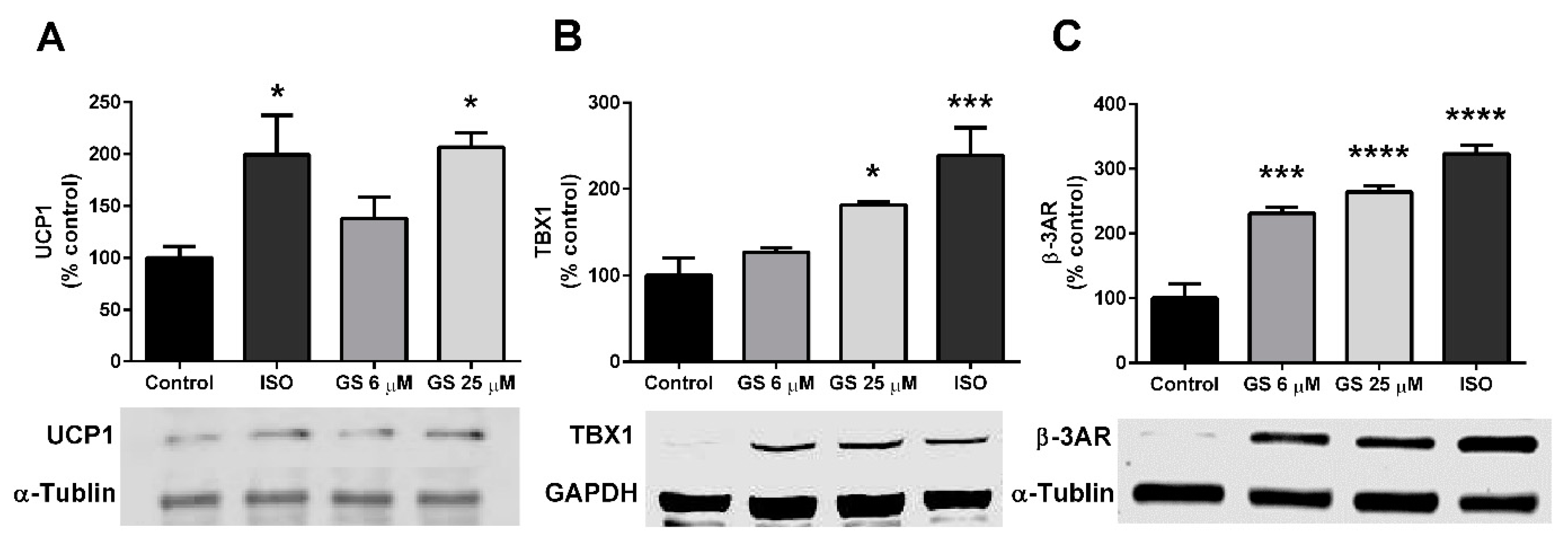
2.6. Immunoblot Analysis
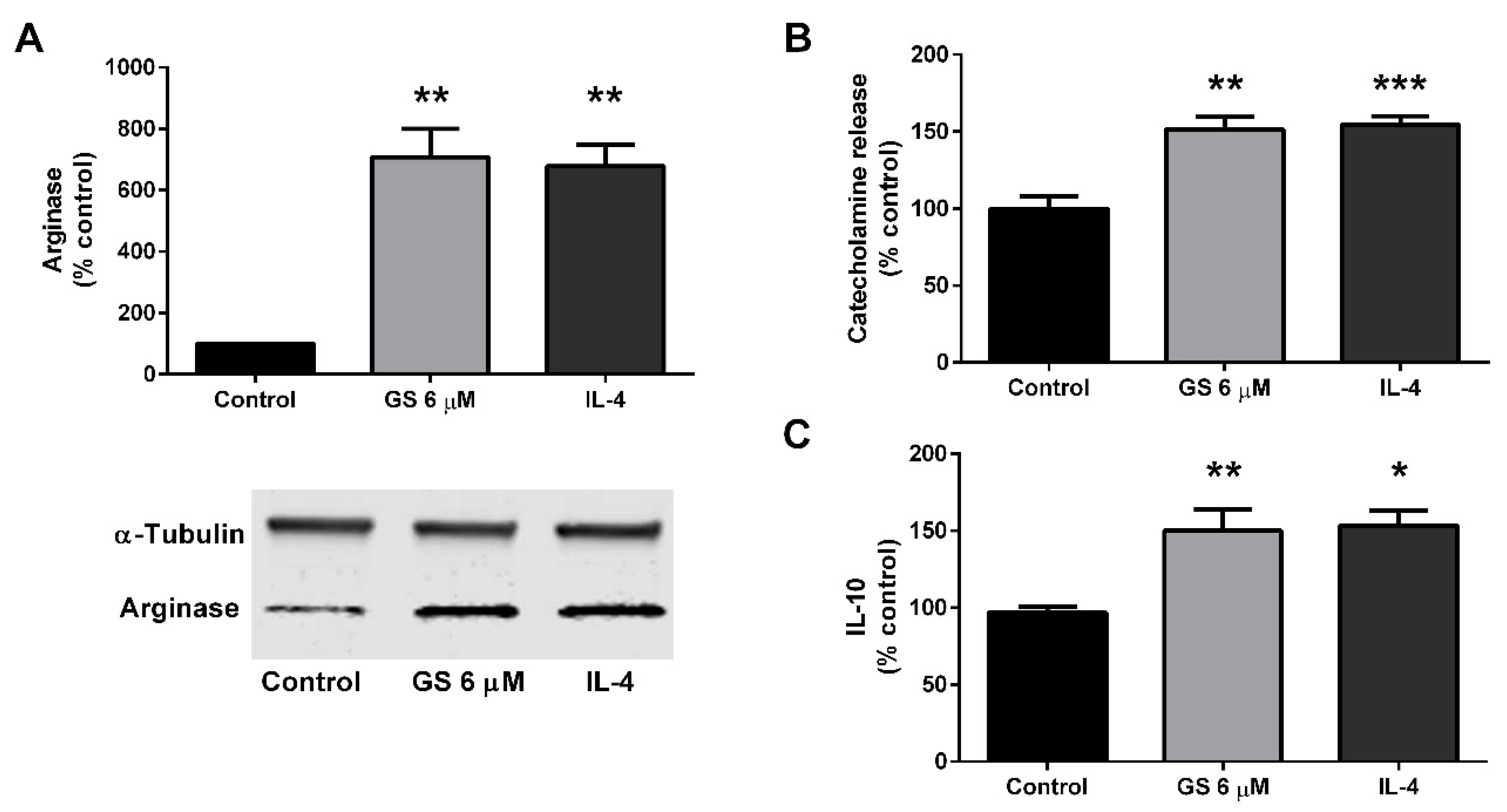
2.7. Catecholamine Assay
2.8. Interleukin-10 (IL-10) ELISA
2.9. Data Analysis
3. Results
3.1. GS inhibits Adipogenesis in 3T3-L1 Adipocytes
3.2. Effect of GS on 3T3-L1 Viability and Apoptosis
3.3. Effect of GS on Mitochondrial Biogenesis and Oxygen Consumption
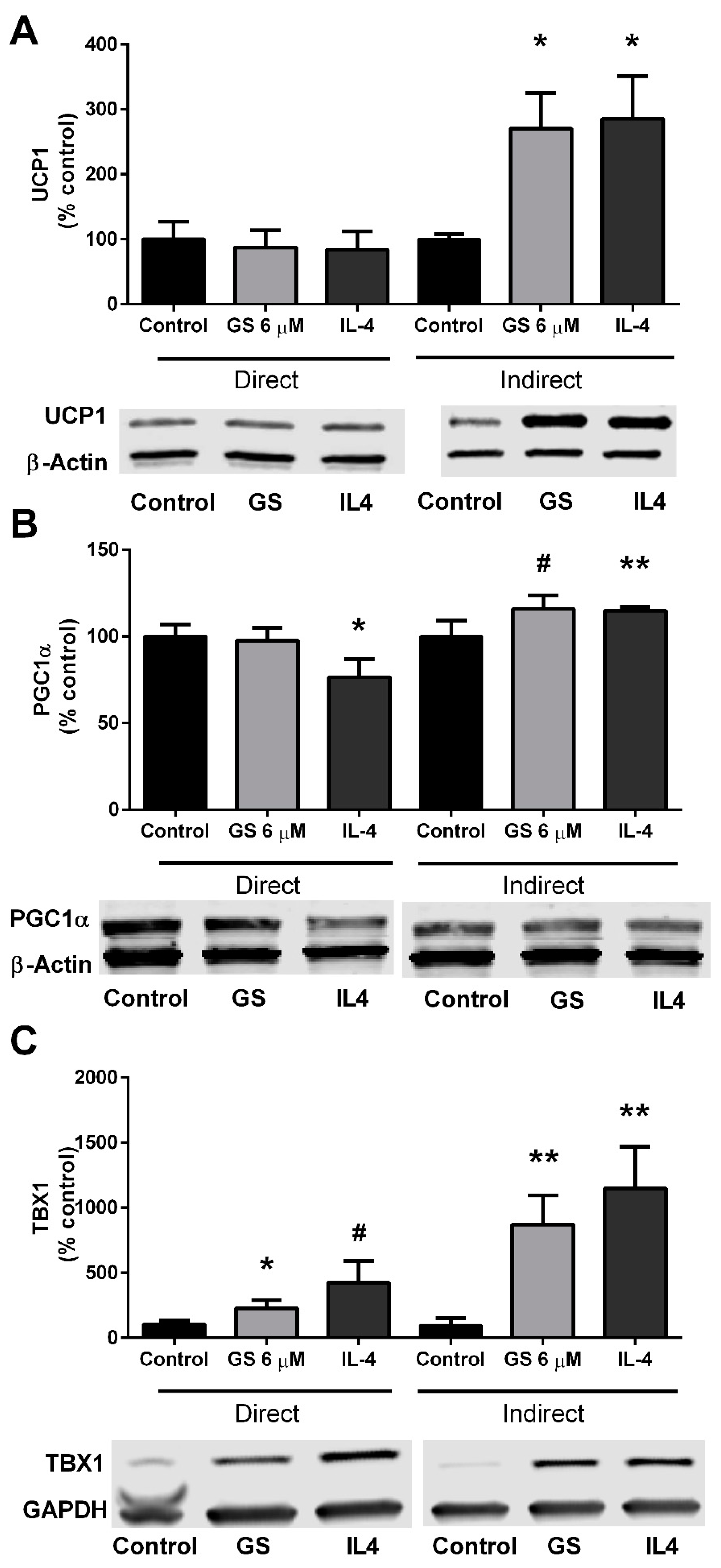
3.4. GS-Induced Effects on Markers of Beige Adipocytes
3.5. Effect of GS on M2 Polarization of Macrophages
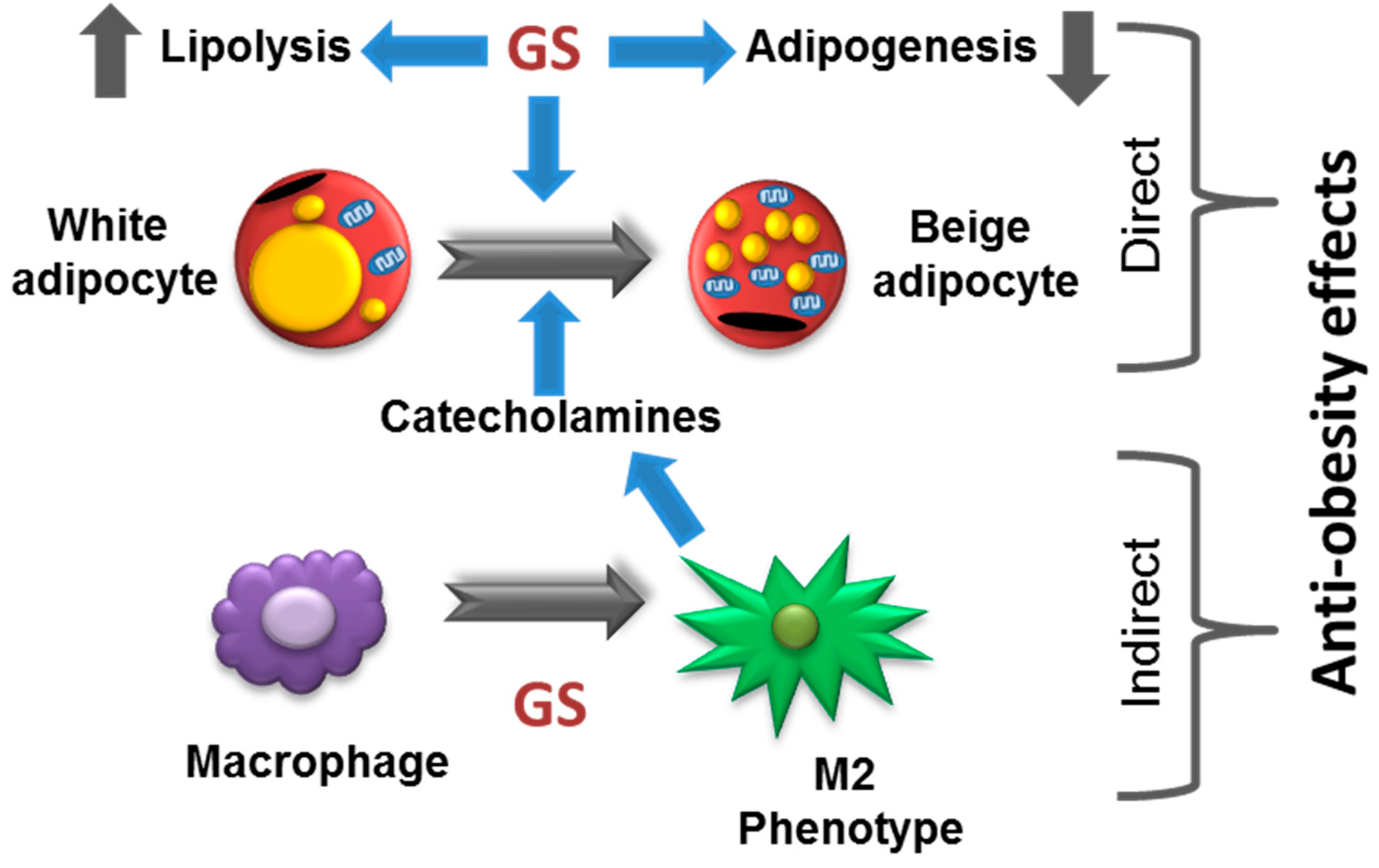
3.6. Role of M2 Macrophage Polarization on GS-Induced on Adipocyte Beiging
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [PubMed]
- Okamatsu-Ogura, Y.; Fukano, K.; Tsubota, A.; Uozumi, A.; Terao, A.; Kimura, K.; Saito, M. Thermogenic ability of uncoupling protein 1 in beige adipocytes in mice. PLoS ONE 2013, 8, e84229. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, K.; Nogusa, Y.; Shinoda, K.; Suzuki, K.; Bannai, M.; Kajimura, S. A Synergistic Antiobesity Effect by a Combination of Capsinoids and Cold Temperature Through Promoting Beige Adipocyte Biogenesis. Diabetes 2016, 65, 1410–1423. [Google Scholar] [CrossRef] [PubMed]
- Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Phytochemicals and regulation of the adipocyte life cycle. J. Nutr. Biochem. 2008, 19, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Kujawska-Luczak, M.; Szulinska, M.; Kregielska-Narozna, M.; Skrypnik, D.; Suliburska, J.; Skrypnik, P.; Regula, J.; Bogdanski, P. Beneficial dose-independent influence of Camellia sinensis supplementation on lipid profile, glycemic, and insulin resistance in an NaCl-induced hypertensive rat model. J. Physiol. Pharmacol. 2018, 69, 275–282. [Google Scholar]
- Azhar, Y.; Parmar, A.; Miller, C.N.; Samuels, J.S.; Rayalam, S. Phytochemicals as novel agents for the induction of browning in white adipose tissue. Nutr. Metab. 2016, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.M.; Frade, A.C.; Guimaraes, J.B.; Freitas, K.M.; Lopes, M.T.; Guimaraes, A.L.; de Paula, A.M.; Coimbra, C.C.; Santos, S.H. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur. J. Nutr. 2014, 53, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.; Wang, F.; Stieren, E.; Tong, Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J. Biol. Chem. 2005, 280, 13560–13567. [Google Scholar] [CrossRef]
- Yi, C.O.; Jeon, B.T.; Shin, H.J.; Jeong, E.A.; Chang, K.C.; Lee, J.E.; Lee, D.H.; Kim, H.J.; Kang, S.S.; Cho, G.J.; et al. Resveratrol activates AMPK and suppresses LPS-induced NF-kappaB-dependent COX-2 activation in RAW 264.7 macrophage cells. Anat. Cell Biol. 2011, 44, 194–203. [Google Scholar] [CrossRef]
- Huebbe, P.; Giller, K.; de Pascual-Teresa, S.; Arkenau, A.; Adolphi, B.; Portius, S.; Arkenau, C.N.; Rimbach, G. Effects of blackcurrant-based juice on atherosclerosis-related biomarkers in cultured macrophages and in human subjects after consumption of a high-energy meal. Br. J. Nutr. 2012, 108, 234–244. [Google Scholar] [CrossRef]
- Qiu, Y.; Nguyen, K.D.; Odegaard, J.I.; Cui, X.; Tian, X.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and type 2 cytokine signaling in macrophages orchestrate development of functional beige fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, S.Y.; Liang, X.; Zhang, H.; Xu, Z.; Liu, B.; Xu, M.J.; Jiang, C.; Shang, J.; Wang, X. Adrenomedullin 2 Enhances Beiging in White Adipose Tissue Directly in an Adipocyte-autonomous Manner and Indirectly through Activation of M2 Macrophages. J. Biol. Chem. 2016, 291, 23390–23402. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, S.P.; McCann, D.; Desai, M.; Rosenbaum, M.; Leibel, R.L.; Ferrante, A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Investig. 2003, 112, 1796–1808. [Google Scholar] [CrossRef]
- Musialik, M.; Szulinska, M.; Hen, K.; Skrypnik, D.; Bogdanski, P. The relation between osteoprotegerin, inflammatory processes, and atherosclerosis in patients with metabolic syndrome. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4379–4385. [Google Scholar] [PubMed]
- Shishodia, S.; Harikumar, K.B.; Dass, S.; Ramawat, K.G.; Aggarwal, B.B. The guggul for chronic diseases: Ancient medicine, modern targets. Anticancer Res. 2008, 28, 3647–3664. [Google Scholar] [PubMed]
- Deng, R. Therapeutic effects of guggul and its constituent guggulsterone: Cardiovascular benefits. Cardiovasc. Drug Rev. 2007, 25, 375–390. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.Y.; Della-Fera, M.A.; Baile, C.A. Guggulsterone inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 cells. Obesity 2008, 16, 16–22. [Google Scholar] [CrossRef]
- Yang, J.Y.; Della-Fera, M.A.; Rayalam, S.; Ambati, S.; Baile, C.A. Enhanced pro-apoptotic and anti-adipogenic effects of genistein plus guggulsterone in 3T3-L1 adipocytes. BioFactors 2007, 30, 159–169. [Google Scholar] [CrossRef]
- Sharma, B.; Salunke, R.; Srivastava, S.; Majumder, C.; Roy, P. Effects of guggulsterone isolated from Commiphora mukul in high fat diet induced diabetic rats. Food Chem. Toxicol. 2009, 47, 2631–2639. [Google Scholar] [CrossRef]
- Mithila, M.V.; Khanum, F. The appetite regulatory effect of guggulsterones in rats: A repertoire of plasma hormones and transmitters. J. Diet. Suppl. 2014, 11, 262–271. [Google Scholar] [CrossRef]
- Zietak, M.; Kozak, L.P. Bile acids induce uncoupling protein 1-dependent thermogenesis and stimulate energy expenditure at thermoneutrality in mice. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E346–E354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Shangguan, Z.S.; Chen, C.; Zhang, H.J.; Lin, Y. Anti-inflammatory effects of guggulsterone on murine macrophage by inhibiting LPS-induced inflammatory cytokines in NF-kappaB signaling pathway. Drug Des. Dev. Ther. 2016, 10, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Asano, H.; Kanamori, Y.; Higurashi, S.; Nara, T.; Kato, K.; Matsui, T.; Funaba, M. Induction of beige-like adipocytes in 3T3-L1 cells. J. Vet. Med. Sci./Jpn. Soc. Vet. Sci. 2014, 76, 57–64. [Google Scholar] [CrossRef]
- Miller, C.N.; Yang, J.Y.; England, E.; Yin, A.; Baile, C.A.; Rayalam, S. Isoproterenol Increases Uncoupling, Glycolysis, and Markers of Beiging in Mature 3T3-L1 Adipocytes. PLoS ONE 2015, 10, e0138344. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Aggarwal, B.B. Guggulsterone inhibits NF-kappaB and IkappaBalpha kinase activation, suppresses expression of anti-apoptotic gene products, and enhances apoptosis. J. Biol. Chem. 2004, 279, 47148–47158. [Google Scholar] [CrossRef] [PubMed]
- Quarta, C.; Mazza, R.; Pasquali, R.; Pagotto, U. Role of sex hormones in modulation of brown adipose tissue activity. J. Mol. Endocrinol. 2012, 49, R1–R7. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.S.; Frank, A.P.; Fatima, L.A.; Palmer, B.F.; Oz, O.K.; Clegg, D.J. Activation of estrogen receptor alpha induces beiging of adipocytes. Mol. Metab. 2018, 18, 51–59. [Google Scholar] [CrossRef]
- van Dam, A.D.; Kooijman, S.; Schilperoort, M.; Rensen, P.C.; Boon, M.R. Regulation of brown fat by AMP-activated protein kinase. Trends Mol. Med. 2015, 21, 571–579. [Google Scholar] [CrossRef]
- Velazquez-Villegas, L.A.; Perino, A.; Lemos, V.; Zietak, M.; Nomura, M.; Pols, T.W.H.; Schoonjans, K. TGR5 signalling promotes mitochondrial fission and beige remodelling of white adipose tissue. Nat. Commun. 2018, 9, 245. [Google Scholar] [CrossRef]
- Che, X.; Park, K.C.; Park, S.J.; Kang, Y.H.; Jin, H.A.; Kim, J.W.; Seo, D.H.; Kim, D.K.; Kim, T.I.; Kim, W.H.; et al. Protective effects of guggulsterone against colitis are associated with the suppression of TREM-1 and modulation of macrophages. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G128–G139. [Google Scholar] [CrossRef]
- Ichikawa, H.; Aggarwal, B.B. Guggulsterone inhibits osteoclastogenesis induced by receptor activator of nuclear factor-kappaB ligand and by tumor cells by suppressing nuclear factor-kappaB activation. Clin. Cancer Res. 2006, 12, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.D.; Qiu, Y.; Cui, X.; Goh, Y.P.; Mwangi, J.; David, T.; Mukundan, L.; Brombacher, F.; Locksley, R.M.; Chawla, A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 2011, 480, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; Ruiz, H.H.; Jhun, K.; Finan, B.; Oberlin, D.J.; van der Heide, V.; Kalinovich, A.V.; Petrovic, N.; Wolf, Y.; Clemmensen, C.; et al. Alternatively activated macrophages do not synthesize catecholamines or contribute to adipose tissue adaptive thermogenesis. Nat. Med. 2017, 23, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Keuper, M.; Sachs, S.; Walheim, E.; Berti, L.; Raedle, B.; Tews, D.; Fischer-Posovszky, P.; Wabitsch, M.; Hrabě de Angelis, M.; Kastenmüller, G.; et al. Activated macrophages control human adipocyte mitochondrial bioenergetics via secreted factors. Mol. Metab. 2017, 6, 1226–1239. [Google Scholar] [CrossRef] [PubMed]
- Chhonker, Y.S.; Chandasana, H.; Mukkavilli, R.; Prasad, Y.D.; Laxman, T.S.; Vangala, S. Assessment of in vitro metabolic stability, plasma protein binding, and pharmacokinetics of E- and Z-guggulsterone in rat. Drug Test. Anal. 2016, 8, 966–975. [Google Scholar] [CrossRef] [PubMed]
- Nityanand, S.; Srivastava, J.S.; Asthana, O.P. Clinical trials with gugulipid: A new hypolipidaemic agent. J. Assoc. Physicians India 1989, 37, 323–328. [Google Scholar] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, C.N.; Samuels, J.S.; Azhar, Y.; Parmar, A.; Shashidharamurthy, R.; Rayalam, S. Guggulsterone Activates Adipocyte Beiging through Direct Effects on 3T3-L1 Adipocytes and Indirect Effects Mediated through RAW264.7 Macrophages. Medicines 2019, 6, 22. https://doi.org/10.3390/medicines6010022
Miller CN, Samuels JS, Azhar Y, Parmar A, Shashidharamurthy R, Rayalam S. Guggulsterone Activates Adipocyte Beiging through Direct Effects on 3T3-L1 Adipocytes and Indirect Effects Mediated through RAW264.7 Macrophages. Medicines. 2019; 6(1):22. https://doi.org/10.3390/medicines6010022
Chicago/Turabian StyleMiller, Colette N., Janaiya S. Samuels, Yusra Azhar, Ashish Parmar, Rangaiah Shashidharamurthy, and Srujana Rayalam. 2019. "Guggulsterone Activates Adipocyte Beiging through Direct Effects on 3T3-L1 Adipocytes and Indirect Effects Mediated through RAW264.7 Macrophages" Medicines 6, no. 1: 22. https://doi.org/10.3390/medicines6010022
APA StyleMiller, C. N., Samuels, J. S., Azhar, Y., Parmar, A., Shashidharamurthy, R., & Rayalam, S. (2019). Guggulsterone Activates Adipocyte Beiging through Direct Effects on 3T3-L1 Adipocytes and Indirect Effects Mediated through RAW264.7 Macrophages. Medicines, 6(1), 22. https://doi.org/10.3390/medicines6010022




