Mesenchymal Stromal Cells for Antineoplastic Drug Loading and Delivery
Abstract
1. Introduction
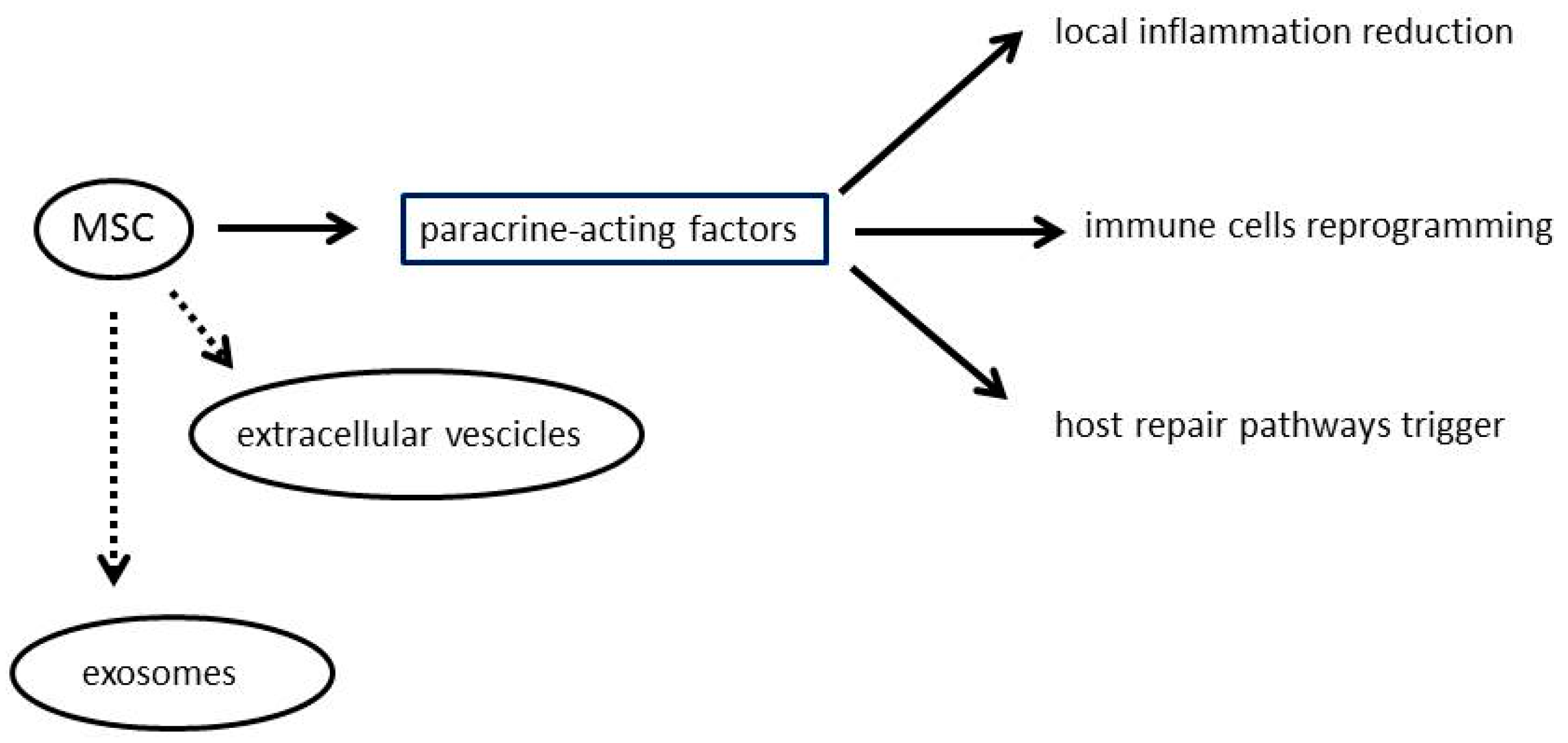
2. Mesenchymal Stromal Cells
3. Drug Loading and Drug Delivery by Mesenchymal Stem Cells
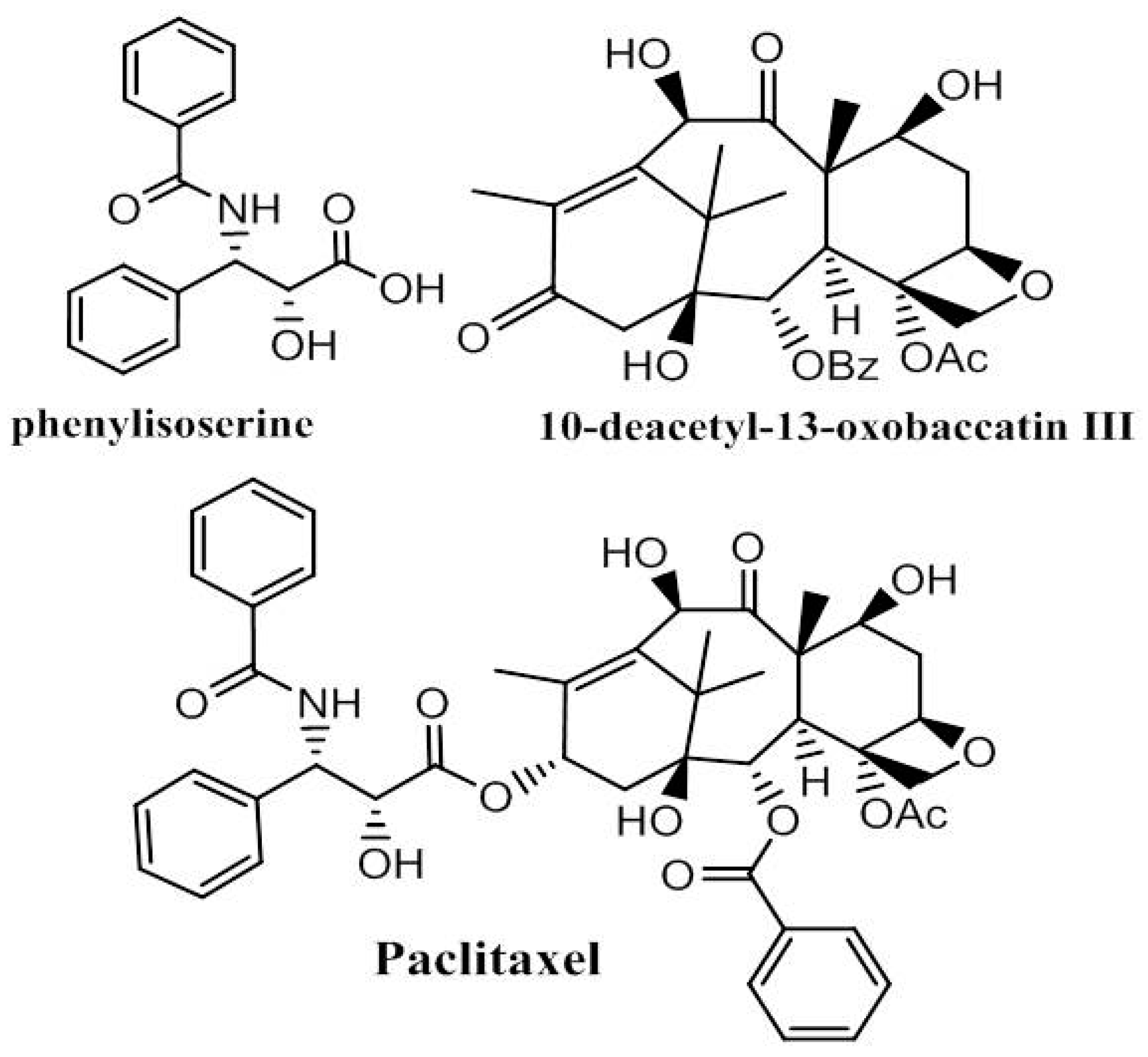
4. Paclitaxel
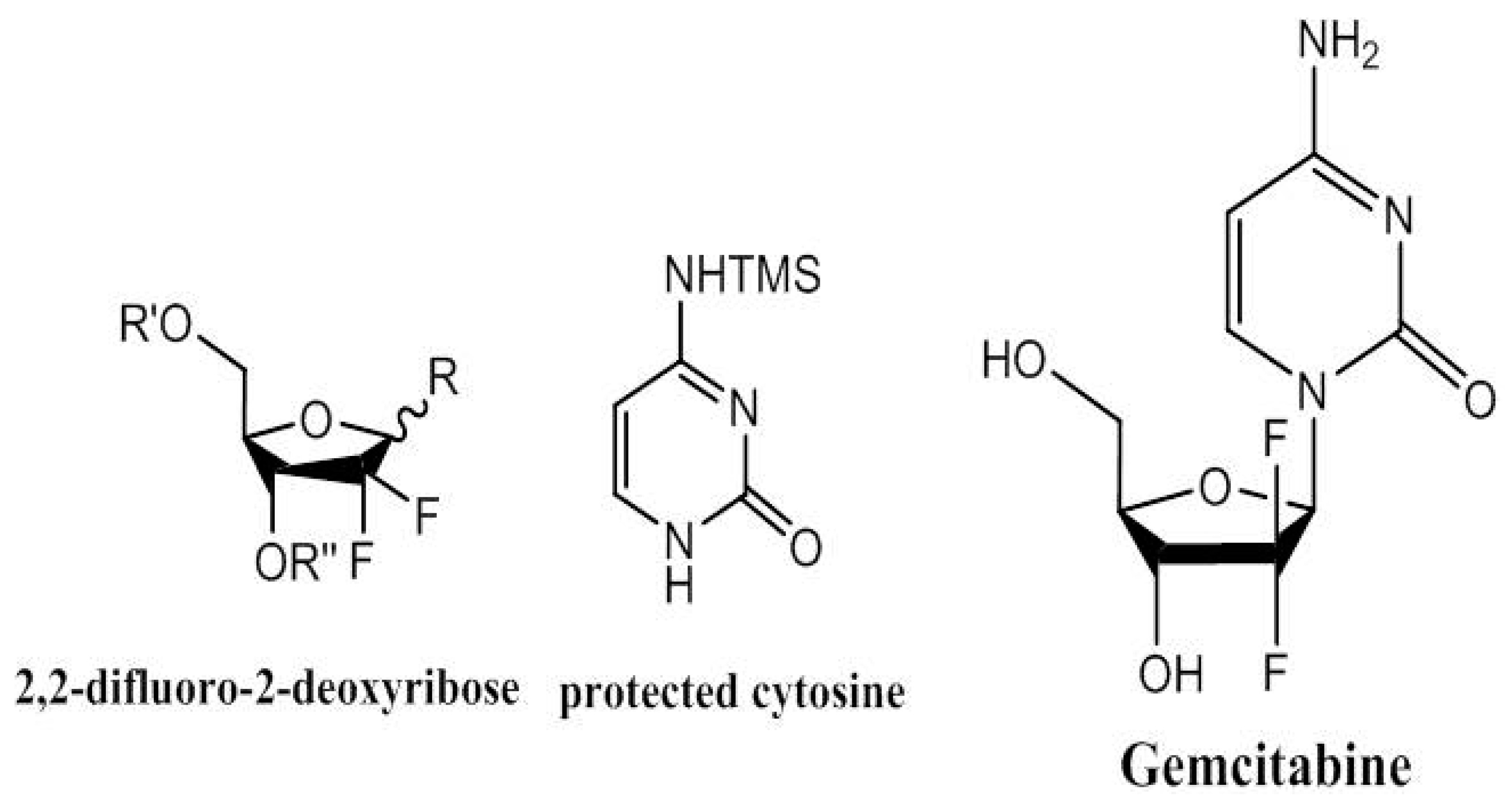
5. Gemcitabine
6. Pemetrexed
7. Other Drugs Potentially Deliverable by MSC
8. Clinical Perspectives
9. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| MSC | mesenchymal stromal cells |
| PDAC | pancreatic ductal adenocarcinoma |
| GCB | gemcitabine |
| GBM | glioblastoma multiforme |
| PTX | paclitaxel |
| BM | bone marrow |
| MPM | malignant pleural mesothelioma |
| PMX | pemetrexed |
References
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Van der Meel, R.; Fens, M.H.A.M.; Vader, P.; van Solinge, W.W.; Eniola-Adefeso, O.; Schiffelers, R.M. Extracellular vesicles as drug delivery systems: Lessons from the liposome field. J. Control. Release 2014, 195, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Rizzo, S.; Borri, A.; Casiraghi, M.; Spaggiari, L. Current Perspectives in Mesenchymal Stromal Cell Therapies for Airway Tissue Defects. Stem Cells Int. 2015, 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Petrella, F.; Nicosia, L.; Bellomi, M.; Rizzo, S. Molecular Imaging of Stem Cell Transplantation for Liver Diseases: Monitoring, Clinical Translation, and Theranostics. Stem Cells Int. 2016, 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Toffalorio, F.; Brizzola, S.; De Pas, T.M.; Rizzo, S.; Barberis, M.; Pelicci, P.; Spaggiari, L.; Acocella, F. Stem Cell Transplantation Effectively Occludes Bronchopleural Fistula in an Animal Model. Ann. Thorac. Surg. 2014, 97, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Acocella, F.; Barberis, M.; Bellomi, M.; Brizzola, S.; Donghi, S.; Giardina, G.; Giordano, R.; Guarize, J.; Lazzari, L.; et al. Airway Fistula Closure after Stem-Cell Infusion. N. Engl. J. Med. 2015, 372, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Petrella, F.; Coccè, V.; Masia, C.; Milani, M.; Salè, E.O.; Alessandri, G.; Parati, E.; Sisto, F.; Pentimalli, F.; Brini, A.T.; et al. Paclitaxel-releasing mesenchymal stromal cells inhibit in vitro proliferation of human mesothelioma cells. Biomed. Pharmacother. 2017, 87, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Igura, K.; Zhang, X.; Takahashi, K.; Mitsuru, A.; Yamaguchi, S.; Takahashi, T.A. Isolation and characterization of mesenchymal progenitor cells from chorionic villi of human placenta. Cytotherapy 2004, 6, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Baiguera, S.; Jungebluth, P.; Mazzanti, B.; Macchiarini, P. Mesenchymal stromal cells for tissue-engineered tissue and organ replacements. Transpl. Int. 2012, 25, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Kyurkchiev, D.; Bochev, I.; Ivanova-Todorova, E.; Mourdjeva, M.; Oreshkova, T.; Belemezova, K.; Kyurkchiev, S. Secretion of immunoregulatory cytokines by mesenchymal stem cells. World J. Stem Cells 2014, 6, 552–570. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.-C.; Shyu, W.-C.; Lin, S.-Z. Mesenchymal Stem Cells. Cell Transplant. 2011, 20, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Scott, P.G.; Tredget, E.E. Mesenchymal Stem Cells Enhance Wound Healing Through Differentiation and Angiogenesis. Stem Cells 2007, 25, 2648–2659. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, A.; Sordi, V.; Dugnani, E.; Ceserani, V.; Dossena, M.; Coccè, V.; Cavicchini, L.; Ciusani, E.; Bondiolotti, G.; Piovani, G.; et al. Gemcitabine-releasing mesenchymal stromal cells inhibit in vitro proliferation of human pancreatic carcinoma cells. Cytotherapy 2015, 17, 1687–1695. [Google Scholar] [CrossRef] [PubMed]
- Pessina, A.; Bonomi, A.; Coccè, V.; Invernici, G.; Navone, S.; Cavicchini, L.; Sisto, F.; Ferrari, M.; Viganò, L.; Locatelli, A.; et al. Mesenchymal Stromal Cells Primed with Paclitaxel Provide a New Approach for Cancer Therapy. PLoS ONE 2011, 6, e28321. [Google Scholar] [CrossRef] [PubMed]
- Mooney, R.; Weng, Y.; Garcia, E.; Bhojane, S.; Smith-Powell, L.; Kim, S.U.; Annala, A.J.; Aboody, K.S.; Berlin, J.M. Conjugation of pH-responsive nanoparticles to neural stem cells improves intratumoral therapy. J. Control. Release 2014, 191, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Morshed, R.; Cheng, S.-H.; Tobias, A.; Auffinger, B.; Wainwright, D.A.; Zhang, L.; Yunis, C.; Han, Y.; Chen, C.-T.; et al. Nanoparticle-Programmed Self-Destructive Neural Stem Cells for Glioblastoma Targeting and Therapy. Small 2013, 9, 4123–4129. [Google Scholar] [CrossRef] [PubMed]
- Layek, B.; Sadhukha, T.; Prabha, S. Glycoengineered mesenchymal stem cells as an enabling platform for two-step targeting of solid tumors. Biomaterials 2016, 88, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Sadhukha, T.; O’Brien, T.D.; Prabha, S. Nano-engineered mesenchymal stem cells as targeted therapeutic carriers. J. Control. Release 2014, 196, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Shin, J.Y.; Kim, H.N.; Oh, S.H.; Song, S.K.; Lee, P.H. Mesenchymal stem cells stabilize the blood–brain barrier through regulation of astrocytes. Stem Cell Res. Ther. 2015, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Fei, Y.; Xu, C.; Zhao, Y.; Pan, Y. Bone marrow mesenchymal stem cells ameliorate neurological deficits and blood-brain barrier dysfunction after intracerebral hemorrhage in spontaneously hypertensive rats. Int. J. Clin. Exp. Pathol. 2015, 8, 4715–4724. [Google Scholar] [PubMed]
- Pacioni, S.; D’Alessandris, Q.G.; Giannetti, S.; Morgante, L.; Coccè, V.; Bonomi, A.; Buccarelli, M.; Pascucci, L.; Alessandri, G.; Pessina, A.; et al. Human mesenchymal stromal cells inhibit tumor growth in orthotopic glioblastoma xenografts. Stem Cell Res. Ther. 2017, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Khakoo, A.Y.; Pati, S.; Anderson, S.A.; Reid, W.; Elshal, M.F.; Rovira, I.I.; Nguyen, A.T.; Malide, D.; Combs, C.A.; Hall, G.; et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J. Exp. Med. 2006, 203, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Xu, Z.-L.; Zhao, T.-J.; Ye, L.-H.; Zhang, X.-D. Dkk-1 secreted by mesenchymal stem cells inhibits growth of breast cancer cells via depression of Wnt signalling. Cancer Lett. 2008, 269, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Xu, Z.; Zhao, T.; Zhao, Z.; Shi, M.; Zhao, R.C.; Ye, L.; Zhang, X. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008, 18, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Wani, M.C.; Taylor, H.L.; Wall, M.E.; Coggon, P.; McPhail, A.T. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J. Am. Chem. Soc. 1971, 93, 2325–2327. [Google Scholar] [CrossRef] [PubMed]
- Wilding, B.; Vesela, A.B.; Perry, J.J.B.; Black, G.W.; Zhang, M.; Martinkova, L.; Klempier, N. An investigation of nitrile transforming enzymes in the chemo-enzymatic synthesis of the taxol sidechain. Org. Biomol. Chem. 2015, 13, 7803–7812. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Xu, X.; Jiang, L.; Prajapati, D.; Hu, W. A Strategy to Synthesize Taxol Side Chain and (−)-epi Cytoxazone via Chiral Bronsted Acid-Rh2(OAc)4 Co-catalyzed Enantioselective Three-Component Reactions. J. Org. Chem. 2010, 75, 7483–7486. [Google Scholar] [CrossRef] [PubMed]
- Rimoldi, I.; Pellizzoni, M.; Facchetti, G.; Molinari, F.; Zerla, D.; Gandolfi, R. Chemo- and biocatalytic strategies to obtain phenylisoserine, a lateral chain of Taxol by asymmetric reduction. Tetrahedron Asymmetry 2011, 22, 2110–2116. [Google Scholar] [CrossRef]
- Torssell, S.; Somfai, P. 1,3-Dipolar Cycloadditions of Carbonyl Ylides to Aldimines: Scope, Limitations and Asymmetric Cycloadditions. Adv. Synth. Catal. 2006, 348, 2421–2430. [Google Scholar] [CrossRef]
- Kasaei, A.; Mobini-Dehkordi, M.; Mahjoubi, F.; Saffar, B. Isolation of Taxol-Producing Endophytic Fungi from Iranian Yew Through Novel Molecular Approach and Their Effects on Human Breast Cancer Cell Line. Curr. Microbiol. 2017, 74, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, J.; Naghavi, M.R.; Alizadeh, H.; Moghadam, M.R.F. Seasonal-based temporal changes fluctuate expression patterns of TXS, DBAT, BAPT and DBTNBT genes alongside production of associated taxanes in Taxus baccata. Plant Cell Rep. 2016, 35, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Dang, P.H.; Nguyen, H.X.; Duong, T.T.T.; Tran, T.K.T.; Nguyen, P.T.; Vu, T.K.T.; Vuong, H.C.; Phan, N.H.T.; Nguyen, M.T.T.; Nguyen, N.T.; et al. α-Glucosidase Inhibitory and Cytotoxic Taxane Diterpenoids from the Stem Bark of Taxus wallichiana. J. Nat. Prod. 2017, 80, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Qiu, Y.; Li, X.; Liu, N.; Yao, Z. Biological evaluation of new antitumor taxoids: Alteration of substitution at the C-7 and C-10 of docetaxel. Bioorg. Med. Chem. Lett. 2014, 24, 855–859. [Google Scholar] [CrossRef] [PubMed]
- Hertel, L.W.; Kroin, J.S.; Misner, J.W.; Tustin, J.M. Synthesis of 2-deoxy-2,2-difluoro-d-ribose and 2-deoxy-2,2′-difluoro-d-ribofuranosyl nucleosides. J. Org. Chem. 1988, 53, 2406–2409. [Google Scholar] [CrossRef]
- Yang, B.; Jinnouchi, A.; Suemune, H.; Aso, M. Difluoro-C4′-oxidized Abasic Site for Efficient Amine Modification in Biological Systems. Org. Lett. 2012, 14, 5852–5855. [Google Scholar] [CrossRef] [PubMed]
- Colombel, S.; Van Hijfte, N.; Poisson, T.; Leclerc, E.; Pannecoucke, X. Addition of Electrophilic Radicals to 2-Benzyloxyglycals: Synthesis and Functionalization of Fluorinated α-C-Glycosides and Derivatives. Chem. Eur. J. 2013, 19, 12778–12787. [Google Scholar] [CrossRef] [PubMed]
- Hamon, N.; Quintiliani, M.; Balzarini, J.; McGuigan, C. Synthesis and biological evaluation of prodrugs of 2-fluoro-2-deoxyribose-1-phosphate and 2,2-difluoro-2-deoxyribose-1-phosphate. Bioorg. Med. Chem. Lett. 2013, 23, 2555–2559. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, H.; Zhao, D.; Ding, D.; Sun, M.; Kou, L.; Luo, C.; Zhang, D.; Yi, X.; Dong, J.; et al. Combination of l-Carnitine with Lipophilic Linkage-Donating Gemcitabine Derivatives as Intestinal Novel Organic Cation Transporter 2-Targeting Oral Prodrugs. J. Med. Chem. 2017, 60, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Peifer, M.; Berger, R.; Shurtleff, V.W.; Conrad, J.C.; MacMillan, D.W.C. A General and Enantioselective Approach to Pentoses: A Rapid Synthesis of PSI-6130, the Nucleoside Core of Sofosbuvir. J. Am. Chem. Soc. 2014, 136, 5900–5903. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.; Weymouth-Wilson, A.; Linclau, B. A linear synthesis of gemcitabine. Carbohydr. Res. 2015, 406, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ku, T.C.; Seley-Radtke, K.L. Thiophene-expanded guanosine analogues of Gemcitabine. Bioorg. Med. Chem. Lett. 2015, 25, 4274–4276. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.C.; Kuhnt, D.; Shih, C.; Rinzel, S.M.; Grindey, G.B.; Barredo, J.; Jannatipour, M.; Moran, R.G. A dideazatetrahydrofolate analog lacking a chiral center at C-6: N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5yl)ethyl[benzoyl]-l-glutamic acid is an inhibitor of thymidylate synthase. J. Med. Chem. 1992, 35, 4450–4454. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.C.; Liu, B. A simple and concise synthesis of LY231514(MTA). Tetrahedron Lett. 1999, 40, 4023–4026. [Google Scholar] [CrossRef]
- Taylor, E.C.; Liu, B. A New and Efficient Synthesis of Pyrrolo[2,3-d]pyrimidine Anticancer Agents: Alimta (LY231514, MTA), Homo-Alimta, TNP-351, and Some Aryl 5-Substituted Pyrrolo[2,3-d]pyrimidines. J. Org. Chem. 2003, 68, 9938–9947. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Wen, J.; Li, L.; Bai, R.; Chen, L.; Wang, D. An Efficient Synthesis of Pemetrexed Disodium. J. Heterocycl. Chem. 2015, 52, 1565–1569. [Google Scholar] [CrossRef]
- Palacios, F.; Vicario, J.; Aparicio, D. Efficient Synthesis of 1-Azadienes Derived from α-Aminoesters. Regioselective Preparation of α-Dehydroamino Acids, Vinylglycines, and α-Amino Acids. J. Org. Chem. 2006, 71, 7690–7696. [Google Scholar] [CrossRef] [PubMed]
- McKerrow, J.D.; Al-Rawi, J.M.A.; Brooks, P. Use of Diphenyliodonium Bromide in the Synthesis of Some N-Phenyl α-Amino Acids. Synth. Commun. 2010, 40, 1161–1179. [Google Scholar] [CrossRef]
- Zaware, N.; Kisliuk, R.; Bastian, A.; Ihnat, M.A.; Gangjee, A. Synthesis and evaluation of 5-(arylthio)-9H-pyrimido[4,5-b]indole-2,4-diamines as receptor tyrosine kinase and thymidylate synthase inhibitors and as antitumor agents. Bioorg. Med. Chem. Lett. 2017, 27, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhang, Z.; Zhou, S.; Yuan, M.; Wang, X.; Liu, J. Synthesis, Antifolate and Anticancer Activities of N5-Substituted 8,10-Dideazatetrahydrofolate Analogues. Chem. Biol. Drug Des. 2016, 87, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, M.; Zhang, H.; Yuan, J.; Zhang, C.; Zhang, K.; Guo, H.; Zhao, L.; Du, Y.; Wang, L.; et al. Design, synthesis and biological evaluation of 6-substituted pyrrolo[2,3-d]pyrimidines as dual inhibitors of TS and AICARFTase and as potential antitumor agents. Eur. J. Med. Chem. 2016, 115, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Mitchell-Ryan, S.; Wang, Y.; Raghavan, S.; Ravindra, M.P.; Hales, E.; Orr, S.; Cherian, C.; Hou, Z.; Matherly, L.H.; Gangjee, A. Discovery of 5-Substituted Pyrrolo[2,3-d]pyrimidine Antifolates as Dual-Acting Inhibitors of Glycinamide Ribonucleotide Formyltransferase and 5-Aminoimidazole-4-carboxamide Ribonucleotide Formyltransferase in De Novo Purine Nucleotide Biosynthesis: Implications of Inhibiting 5-Aminoimidazole-4-carboxamide Ribonucleotide Formyltransferase to AMPK Activation and Antitumor Activity. J. Med. Chem. 2013, 56, 10016–10032. [Google Scholar] [PubMed]
- Kosaka, H.; Ichikawa, T.; Kurozumi, K.; Kambara, H.; Inoue, S.; Maruo, T.; Nakamura, K.; Hamada, H.; Date, I. Therapeutic effect of suicide gene-transferred mesenchymal stem cells in a rat model of glioma. Cancer Gene Ther. 2012, 19, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.H.; Park, K.Y.; Kim, S.M.; Jeong, C.H.; Woo, J.S.; Hou, Y.; Jeun, S.-S. Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma. Biochem. Biophys. Res. Commun. 2012, 421, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guan, Y.; Liu, H.; Hao, N.; Liu, T.; Meng, X.; Fu, C.; Li, Y.; Qu, Q.; Zhang, Y.; et al. Silica Nanorattle–Doxorubicin-Anchored Mesenchymal Stem Cells for Tumor-Tropic Therapy. ACS Nano 2011, 5, 7462–7470. [Google Scholar] [CrossRef] [PubMed]
- Roger, M.; Clavreul, A.; Huynh, N.T.; Passirani, C.; Schiller, P.; Vessières, A.; Montero-Menei, C.; Menei, P. Ferrociphenol lipid nanocapsule delivery by mesenchymal stromal cells in brain tumor therapy. Int. J. Pharm. 2012, 423, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Rachakatla, R.S.; Balivada, S.; Seo, G.-M.; Myers, C.B.; Wang, H.; Samarakoon, T.N.; Dani, R.; Pyle, M.; Kroh, F.O.; Walker, B.; et al. Attenuation of Mouse Melanoma by A/C Magnetic Field after Delivery of Bi-Magnetic Nanoparticles by Neural Progenitor Cells. ACS Nano 2010, 4, 7093–7104. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.; Maisonneuve, P.; Lowenfels, A.B. Epidemiology of pancreatic cancer: An overview. Nat. Rev. Gastroenterol. Hepatol. 2009, 6, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Xie, K.; Wolff, R.; Abbruzzese, J.L. Pancreatic cancer. Lancet 2004, 363, 1049–1057. [Google Scholar] [CrossRef]
- Hines, O.J.R. Howard A, Pancreatic surgery. Curr. Opin. Gastroenterol. 2008, 24, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.W.; Kim, E.; Kim, T.M.; Park, C.K.; Kim, J.W.; Choi, S.H.; Yoo, R.E.; Lee, S.T.; Kim, I.H. Impact of interim progression during the surgery-to-radiotherapy interval and its predictors in glioblastoma treated with temozolomide-based radiochemotherapy. J. Neurooncol. 2017, 134, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Pessina, A.; Leonetti, C.; Artuso, S.; Benetti, A.; Dessy, E.; Pascucci, L.; Passeri, D.; Orlandi, A.; Berenzi, A.; Bonomi, A.; et al. Drug-releasing mesenchymal cells strongly suppress B16 lung metastasis in a syngeneic murine model. J. Exp. Clin. Cancer Res. 2015, 34, 82. [Google Scholar] [CrossRef] [PubMed]
- Bonomi, A.; Steimberg, N.; Benetti, A.; Berenzi, A.; Alessandri, G.; Pascucci, L.; Boniotti, J.; Coccè, V.; Sordi, V.; Pessina, A.; et al. Paclitaxel-releasing mesenchymal stromal cells inhibit the growth of multiple myeloma cells in a dynamic 3D culture system. Hematol. Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Facchetti, G.; Petrella, F.; Spaggiari, L.; Rimoldi, I. Malignant Pleural Mesothelioma: State of the art and advanced cell therapy. Eur. J. Med. Chem. 2017, 142, 266–270. [Google Scholar] [CrossRef] [PubMed]

| Tumor | Standard Care | MSC Action |
|---|---|---|
| Pancreatic ductal adenocarcinoma | Gemcitabine and 5-fluorouracil | “Trojan horse” |
| Glioblastoma multiforme | Surgery followed by chemotherapy and radiation | Reduction of tumour volume, impairment of cell proliferation and vascularization. |
| Malignant melanoma | Surgery and immunotherapy or biologic therapy and radiation or chemotherapy | Inhibition of lung metastasis in a murine melanoma model |
| Multiple myeloma | Chemotherapy | Intense suppression of myeloma cell growth |
| Malignant mesothelioma | Platinum-based doublet containing a third-generation antifolate + surgery + radiotherapy | PTX-loaded MSC strongly inhibit MPM cell proliferation |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrella, F.; Rimoldi, I.; Rizzo, S.; Spaggiari, L. Mesenchymal Stromal Cells for Antineoplastic Drug Loading and Delivery. Medicines 2017, 4, 87. https://doi.org/10.3390/medicines4040087
Petrella F, Rimoldi I, Rizzo S, Spaggiari L. Mesenchymal Stromal Cells for Antineoplastic Drug Loading and Delivery. Medicines. 2017; 4(4):87. https://doi.org/10.3390/medicines4040087
Chicago/Turabian StylePetrella, Francesco, Isabella Rimoldi, Stefania Rizzo, and Lorenzo Spaggiari. 2017. "Mesenchymal Stromal Cells for Antineoplastic Drug Loading and Delivery" Medicines 4, no. 4: 87. https://doi.org/10.3390/medicines4040087
APA StylePetrella, F., Rimoldi, I., Rizzo, S., & Spaggiari, L. (2017). Mesenchymal Stromal Cells for Antineoplastic Drug Loading and Delivery. Medicines, 4(4), 87. https://doi.org/10.3390/medicines4040087