Inhibition of Cyclic Adenosine Monophosphate-Specific Phosphodiesterase by Various Food Plant-Derived Phytotherapeutic Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Cell Lines and Reagents
2.2. Strawberry Tree Fruit Extraction
2.3. Quantification of Arbutin in SFE
2.4. Green Tea Extraction (TXE)
2.5. Quantification of Caffeine in TXE
2.6. Identification of Catechins in TXE
2.7. Artichoke Leaf Extraction (ALE)
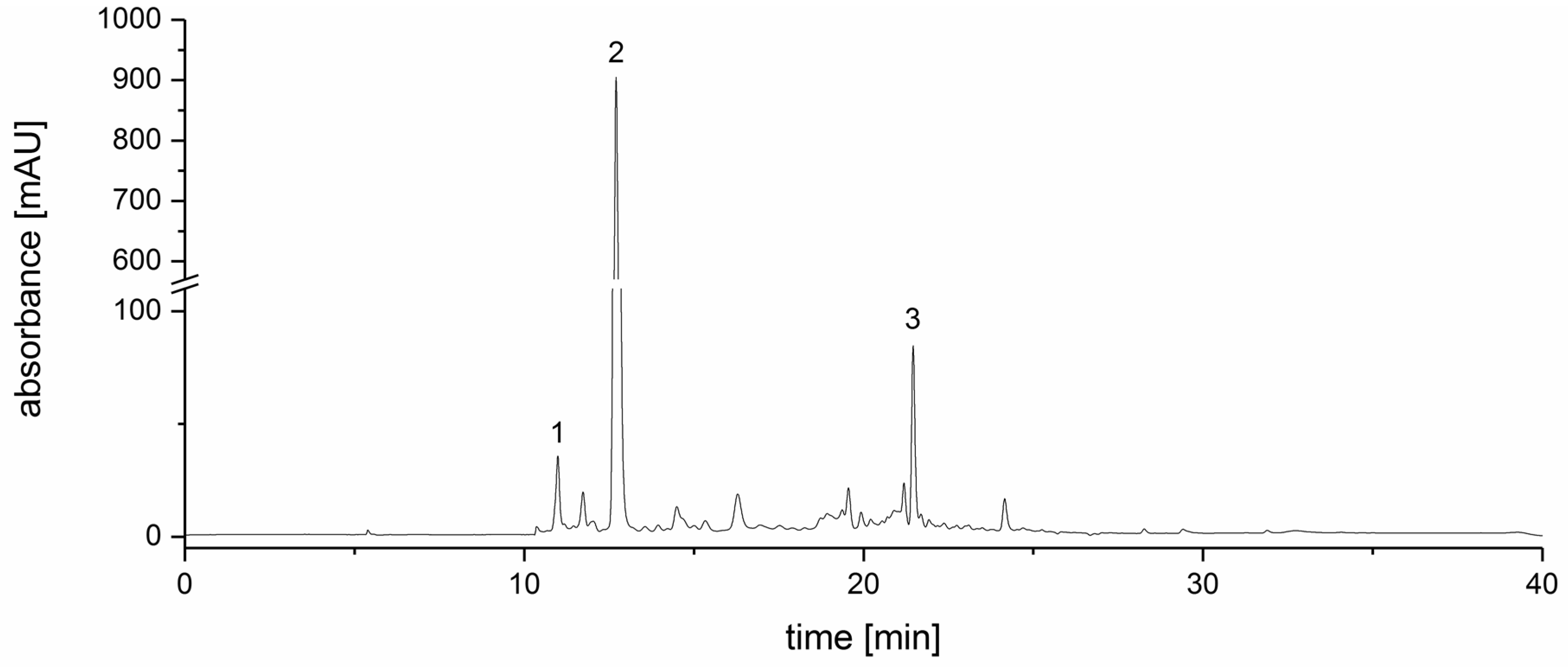
2.8. Identification of Chlorogenic Acids and Flavones in ALE
2.9. Quantification of Dicaffeoylquinic Acids in ALE
2.10. Quantification of Flavones in ALE
2.11. Ginger Extraction and Fractionation
2.12. Quantification of [6]-Gingerol in Ginger Extracts (GPE, GLE, GWE)
2.13. Cell Culture
2.14. cAMP-Specific PDE Activity Assay
2.15. Statistical Analysis
3. Results
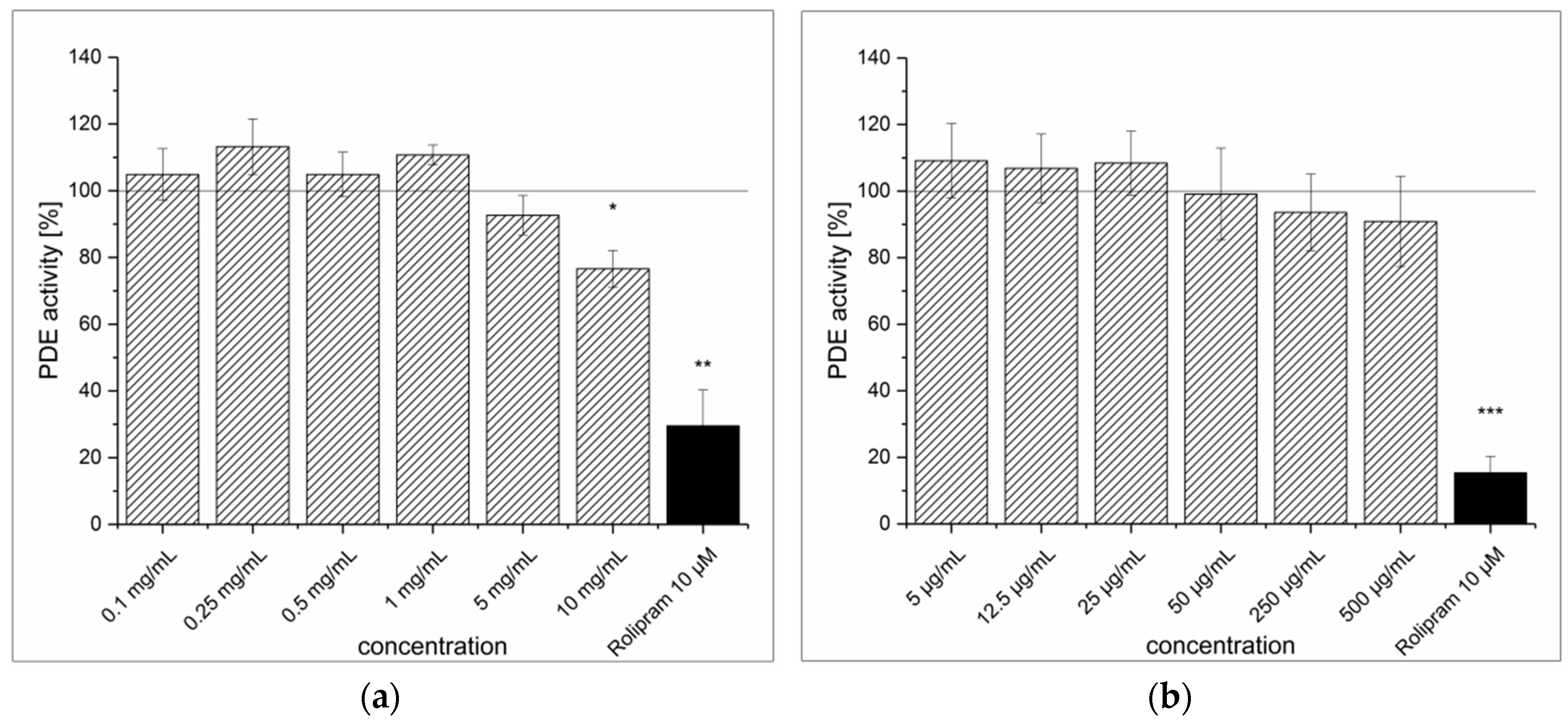
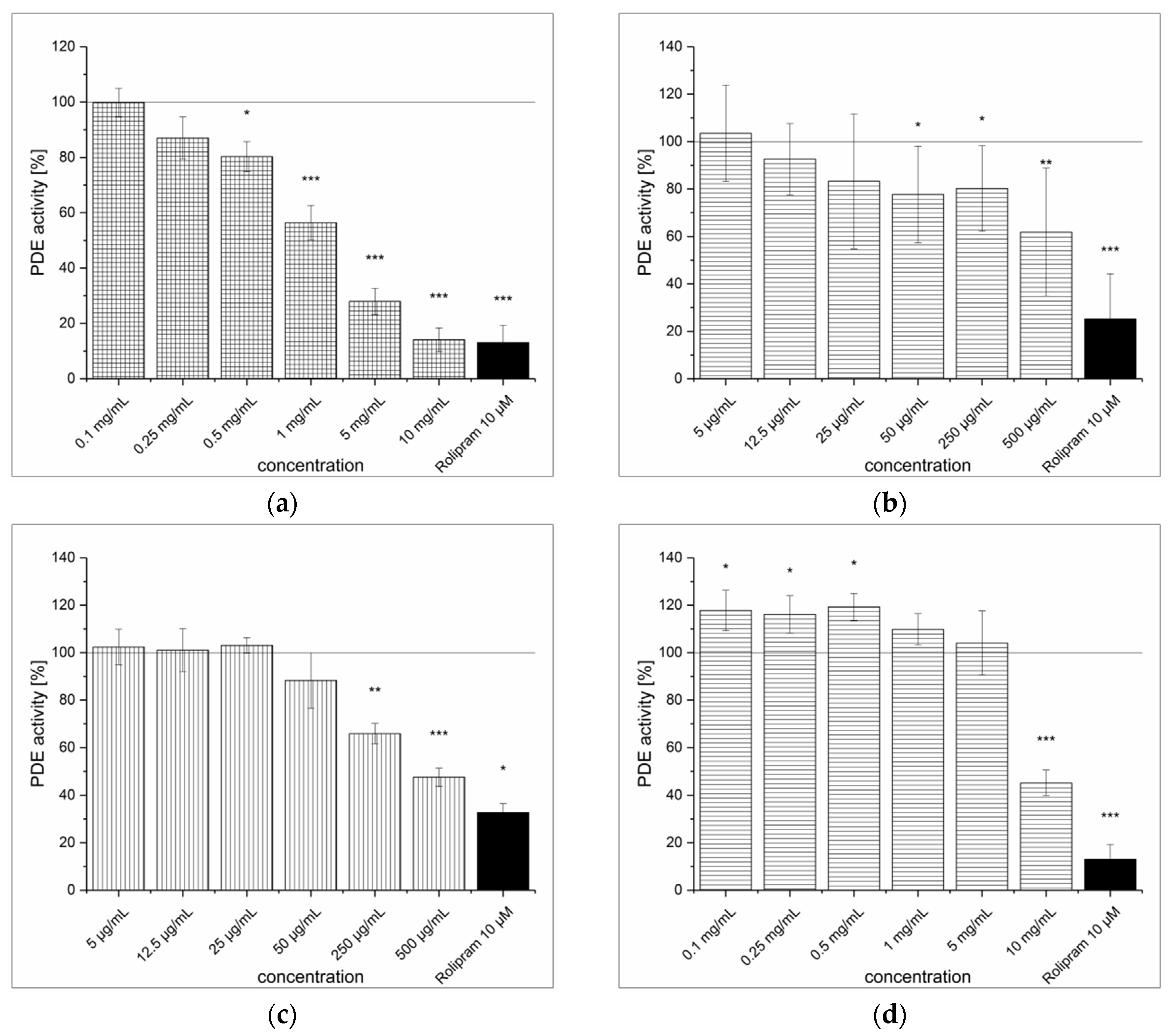
3.1. Strawberry Tree Fruit Extraction (SFE) and Inhibitory Effect on PDE Activity In Vitro
3.2. Green Tea Leaves Extraction (TXE) and Inhibitory Effect on PDE Activity In Vitro
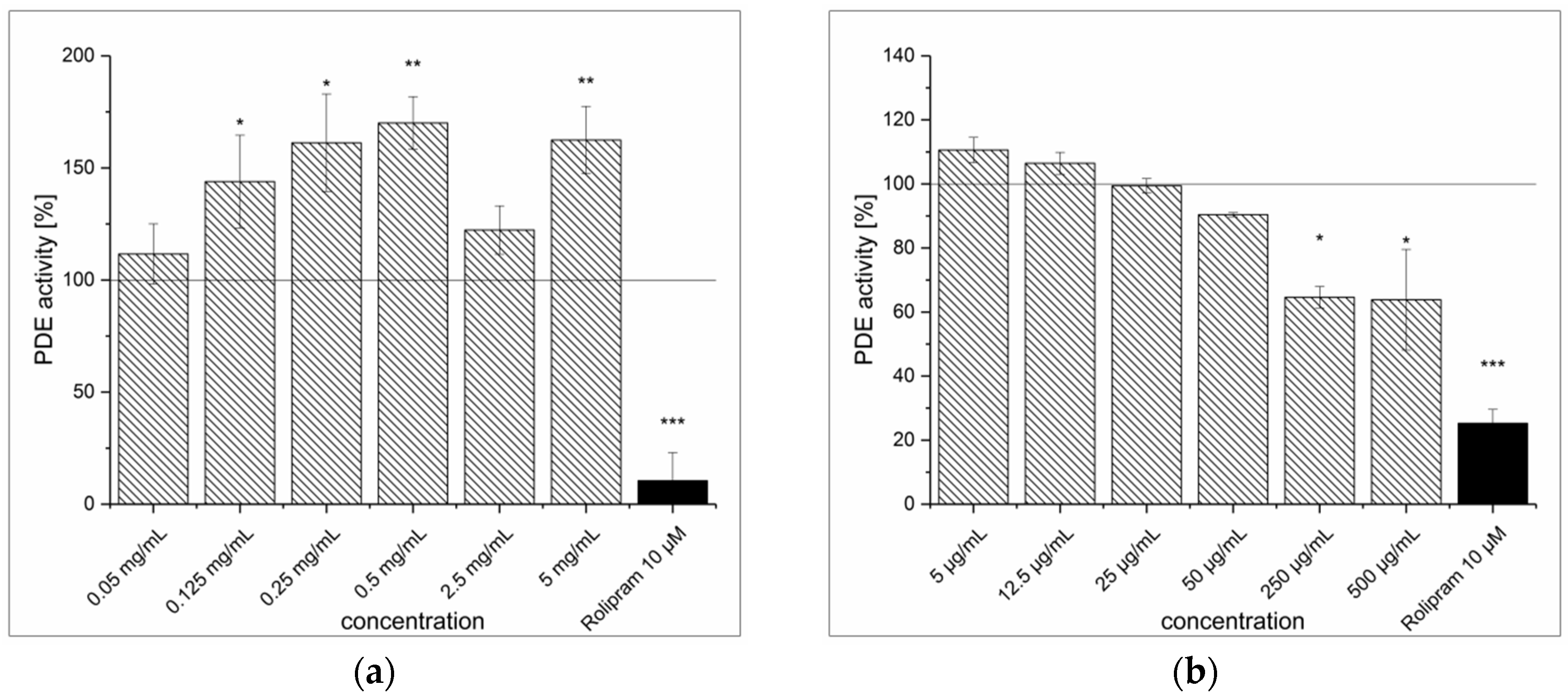
3.3. Artichoke Leaves Extraction (ALE) and Inhibitory Effect on PDE Activity In Vitro
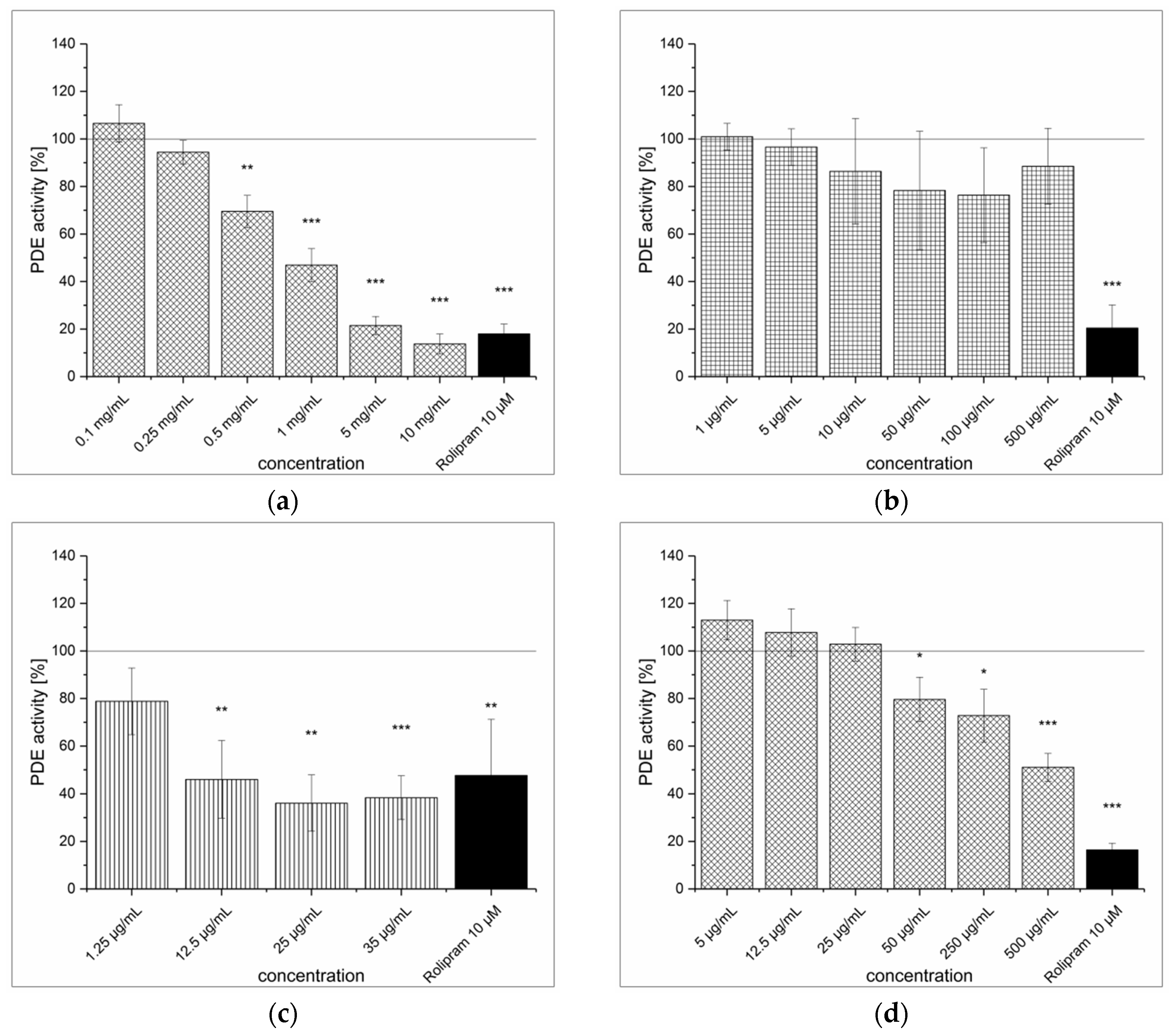
3.4. Dried Ginger Rhizome Extraction (GPE) and Inhibitory Effect on PDE Activity In Vitro
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Boswell-Smith, V.; Spina, D.; Page, C.P. Phosphodiesterase inhibitors. Br. J. Pharmacol. 2006, 147, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Gresele, P.; Momi, S.; Falcinelli, E. Anti-platelet therapy: Phosphodiesterase inhibitors. Br. J. Clin. Pharmacol. 2011, 72, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Rybalkin, S.D.; Yan, C.; Bornfeldt, K.E.; Beavo, J.A. Cyclic GMP phosphodiesterases and regulation of smooth muscle function. Circ. Res. 2003, 93, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Theophylline: New perspectives for an old drug. Am. J. Respir. Crit. Care Med. 2003, 167, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Giembycz, M.A.; Field, S.K. Roflumilast: First phosphodiesterase 4 inhibitor approved for treatment of COPD. Drug Des. Dev. Ther. 2010, 4, 147–158. [Google Scholar] [CrossRef]
- Rall, T.; Sutherland, E.W. Formation of a cyclic adenine ribonucleotide by tissue particles. J. Biol. Chem. 1958, 232, 1065–1076. [Google Scholar] [PubMed]
- Butcher, R.; Sutherland, E. Adenosine 3′,5′-phosphate in biological materials. J. Biol. Chem. 1962, 237, 1244–1250. [Google Scholar] [PubMed]
- Montoya, G.A.; Bakuradze, T.; Eirich, M.; Erk, T.; Baum, M.; Habermeyer, M.; Eisenbrand, G.; Richling, E. Modulation of 3′,5′-cyclic AMP homeostasis in human platelets by coffee and individual coffee constituents. Br. J. Nutr. 2014, 112, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Riedel, A.; Dieminger, N.; Bakuradze, T.; Lang, R.; Montoya Parra, G.A.; Hochkogler, C.M.; Winkler, S.; Bytof, G.; Lantz, I.; Stiebitz, H.; et al. A 4-week consumption of medium roast and dark roast coffees affects parameters of energy status in healthy subjects. Food Res. Int. 2014, 63(Part C), 409–419. [Google Scholar] [CrossRef]
- Röhrig, T.; Liesenfeld, D.; Richling, E. Identification of a Phosphodiesterase-Inhibiting Fraction from Roasted Coffee (Coffea arabica) through Activity-Guided Fractionation. J. Agric. Food Chem. 2017, 65, 3792–3800. [Google Scholar] [CrossRef] [PubMed]
- Marko, D.; Puppel, N.; Tjaden, Z.; Jakobs, S.; Pahlke, G. The substitution pattern of anthocyanidins affects different cellular signaling cascades regulating cell proliferation. Mol. Nutr. Food Res. 2004, 48, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Ko, W.-C.; Shih, C.-M.; Lai, Y.-H.; Chen, J.-H.; Huang, H.-L. Inhibitory effects of flavonoids on phosphodiesterase isozymes from guinea pig and their structure-activity relationships. Biochem. Pharmacol. 2004, 68, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Beretz, A.; Anton, R.; Stoclet, J. Flavonoid compounds are potent inhibitors of cyclic AMP phosphodiesterase. Experientia 1978, 34, 1054–1055. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, U.; Das, N. Effects of flavonoids on cyclic AMP phosphodiesterase and lipid mobilization in rat adipocytes. Biochem. Pharmacol. 1992, 44, 1307–1315. [Google Scholar] [CrossRef]
- Dallas, C.; Gerbi, A.; Tenca, G.; Juchaux, F.; Bernard, F.-X. Lipolytic effect of a polyphenolic citrus dry extract of red orange, grapefruit, orange (SINETROL) in human body fat adipocytes. Mechanism of action by inhibition of cAMP-phosphodiesterase (PDE). Phytomedicine 2008, 15, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Dell’Agli, M.; Galli, G.V.; Vrhovsek, U.; Mattivi, F.; Bosisio, E. In vitro inhibition of human cGMP-specific phosphodiesterase-5 by polyphenols from red grapes. J. Agric. Food Chem. 2005, 53, 1960–1965. [Google Scholar] [CrossRef] [PubMed]
- Ziyyat, A.; Legssyer, A.; Mekhfi, H.; Dassouli, A.; Serhrouchni, M.; Benjelloun, W. Phytotherapy of hypertension and diabetes in oriental Morocco. J. Ethnopharmacol. 1997, 58, 45–54. [Google Scholar] [CrossRef]
- Ziyyat, A.; Mekhfi, H.; Bnouham, M.; Tahri, A.; Legssyer, A.; Hoerter, J.; Fischmeister, R. Arbutus unedo induces endothelium-dependent relaxation of the isolated rat aorta. Phytother. Res. 2002, 16, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Afkir, S.; Nguelefack, T.B.; Aziz, M.; Zoheir, J.; Cuisinaud, G.; Bnouham, M.; Mekhfi, H.; Legssyer, A.; Lahlou, S.; Ziyyat, A. Arbutus unedo prevents cardiovascular and morphological alterations in L-NAME-induced hypertensive rats Part I: Cardiovascular and renal hemodynamic effects of Arbutus unedo in L-NAME-induced hypertensive rats. J. Ethnopharmacol. 2008, 116, 288–295. [Google Scholar] [CrossRef] [PubMed]
- El Haouari, M.; López, J.J.; Mekhfi, H.; Rosado, J.A.; Salido, G.M. Antiaggregant effects of Arbutus unedo extracts in human platelets. J. Ethnopharmacol. 2007, 113, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Mekhfi, H.; ElHaouari, M.; Bnouham, M.; Aziz, M.; Ziyyat, A.; Legssyer, A. Effects of extracts and tannins from Arbutus unedo leaves on rat platelet aggregation. Phytother. Res. 2006, 20, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Pallauf, K.; Rivas-Gonzalo, J.; Del Castillo, M.; Cano, M.; de Pascual-Teresa, S. Characterization of the antioxidant composition of strawberry tree (Arbutus unedo L.) fruits. J. Food Compost. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; De Leo, M.; Braca, A. Phenolics of Arbutus unedo L.(Ericaceae) fruits: Identification of anthocyanins and gallic acid derivatives. J. Agric. Food Chem. 2006, 54, 10234–10238. [Google Scholar] [CrossRef] [PubMed]
- Pimpão, R.C.; Dew, T.; Oliveira, P.B.; Williamson, G.; Ferreira, R.B.; Santos, C.N. Analysis of phenolic compounds in Portuguese wild and commercial berries after multienzyme hydrolysis. J. Agric. Food Chem. 2013, 61, 4053–4062. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, R.; Lakušićć, B.; Došlov-Kokoruš, Z.; Kovačević, N. Arbutin content and antioxidant activity of some Ericaceae species. Die Pharm. Int. J. Pharm. Sci. 2009, 64, 656–659. [Google Scholar] [CrossRef]
- Astill, C.; Birch, M.R.; Dacombe, C.; Humphrey, P.G.; Martin, P.T. Factors affecting the caffeine and polyphenol contents of black and green tea infusions. J. Agric. Food Chem. 2001, 49, 5340–5347. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-Y.; Zhu, Q.Y.; Tsang, D.; Huang, Y. Degradation of green tea catechins in tea drinks. J. Agric. Food Chem. 2001, 49, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Perva-Uzunalić, A.; Škerget, M.; Knez, Ž.; Weinreich, B.; Otto, F.; Grüner, S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- Sharangi, A. Medicinal and therapeutic potentialities of tea (Camellia sinensis L.)—A review. Food Res. Int. 2009, 42, 529–535. [Google Scholar] [CrossRef]
- Lin, J.-K.; Lin, C.-L.; Liang, Y.-C.; Lin-Shiau, S.-Y.; Juan, I.-M. Survey of catechins, gallic acid, and methylxanthines in green, oolong, pu-erh, and black teas. J. Agric. Food Chem. 1998, 46, 3635–3642. [Google Scholar] [CrossRef]
- Goto, T.; Yoshida, Y.; Kiso, M.; Nagashima, H. Simultaneous analysis of individual catechins and caffeine in green tea. J. Chromatogr. A 1996, 749, 295–299. [Google Scholar] [CrossRef]
- Bonoli, M.; Pelillo, M.; Toschi, T.G.; Lercker, G. Analysis of green tea catechins: Comparative study between HPLC and HPCE. Food Chem. 2003, 81, 631–638. [Google Scholar] [CrossRef]
- Zuo, Y.; Chen, H.; Deng, Y. Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta 2002, 57, 307–316. [Google Scholar] [CrossRef]
- Imai, K.; Nakachi, K. Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. BMJ 1995, 310, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, S.; Shimazu, T.; Ohmori, K.; Kikuchi, N.; Nakaya, N.; Nishino, Y.; Tsubono, Y.; Tsuji, I. Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: The Ohsaki study. JAMA 2006, 296, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.-S.; Lim, I.-H.; Yuk, D.-Y.; Chung, K.-H.; Park, J.-B.; Yoo, H.-S.; Yun, Y.-P. Antithrombotic activities of green tea catechins and (−)-epigallocatechin gallate. Thromb. Res. 1999, 96, 229–237. [Google Scholar] [CrossRef]
- Ok, W.-J.; Cho, H.-J.; Kim, H.-H.; Lee, D.-H.; Kang, H.-Y.; Kwon, H.-W.; Rhee, M.H.; Kim, M.; Park, H.-J. Epigallocatechin-3-gallate has an anti-platelet effect in a cyclic AMP-dependent manner. J. Atheroscler. Thromb. 2012, 19, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Son, D.-J.; Cho, M.-R.; Jin, Y.-R.; Kim, S.-Y.; Park, Y.-H.; Lee, S.-H.; Akiba, S.; Sato, T.; Yun, Y.-P. Antiplatelet effect of green tea catechins: A possible mechanism through arachidonic acid pathway. Prostaglandins Leukot. Essent. Fatty Acids 2004, 71, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Bundy, R.; Walker, A.F.; Middleton, R.W.; Wallis, C.; Simpson, H.C. Artichoke leaf extract (Cynara scolymus) reduces plasma cholesterol in otherwise healthy hypercholesterolemic adults: A randomized, double blind placebo controlled trial. Phytomedicine 2008, 15, 668–675. [Google Scholar] [CrossRef] [PubMed]
- Englisch, W.; Beckers, C.; Unkauf, M.; Ruepp, M.; Zinserling, V. Efficacy of Artichoke dry extract in patients with hyperlipoproteinemia. Arzneim. Forsch. 2000, 50, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Brausch, I.; Yao, Y.; Förstermann, U. Flavonoids from artichoke (Cynara scolymus L.) up-regulate endothelial-type nitric-oxide synthase gene expression in human endothelial cells. J. Pharmacol. Exp. Ther. 2004, 310, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Zapolska-Downar, D.; Zapolski-Downar, A.; Naruszewicz, M.; Siennicka, A.; Krasnodębska, B.; Kołodziej, B. Protective properties of artichoke (Cynara scolymus) against oxidative stress induced in cultured endothelial cells and monocytes. Life Sci. 2002, 71, 2897–2908. [Google Scholar] [CrossRef]
- Gebhardt, R. Antioxidative and protective properties of extracts from leaves of the artichoke (Cynara scolymus L.) against hydroperoxide-induced oxidative stress in cultured rat hepatocytes. Toxicol. Appl. Pharmacol. 1997, 144, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, S.; Pandino, G.; Mauromicale, G.; Knödler, M.; Carle, R.; Schieber, A. Influence of genotype, harvest time and plant part on polyphenolic composition of globe artichoke [Cynara cardunculus L. var. scolymus (L.) Fiori]. Food Chem. 2010, 119, 1175–1181. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Mauromicale, G.; Williamson, G. Profile of polyphenols and phenolic acids in bracts and receptacles of globe artichoke (Cynara cardunculus var. scolymus) germplasm. J. Food Compost. Anal. 2011, 24, 148–153. [Google Scholar] [CrossRef]
- Schütz, K.; Kammerer, D.; Carle, R.; Schieber, A. Identification and quantification of caffeoylquinic acids and flavonoids from artichoke (Cynara scolymus L.) heads, juice, and pomace by HPLC-DAD-ESI/MSn. J. Agric. Food Chem. 2004, 52, 4090–4096. [Google Scholar] [CrossRef]
- Wang, M.; Simon, J.E.; Aviles, I.F.; He, K.; Zheng, Q.-Y.; Tadmor, Y. Analysis of antioxidative phenolic compounds in artichoke (Cynara scolymus L.). J. Agric. Food Chem. 2003, 51, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Samek, Z.; Holub, M.; Drożdż, B.; Iommi, G.; Corbella, A.; Gariboldi, P. Sesquiterpenic lactones of the Cynara scolymus L. species. Tetrahedron Lett. 1971, 12, 4775–4778. [Google Scholar] [CrossRef]
- Jiang, H.; Sólyom, A.M.; Timmermann, B.N.; Gang, D.R. Characterization of gingerol-related compounds in ginger rhizome (Zingiber officinale Rosc.) by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth, H.; Leach, D.N.; Smith, M.K.; Myers, S.P. Gingerol content of diploid and tetraploid clones of ginger (Zingiber officinale Roscoe). J. Agric. Food Chem. 2005, 53, 5772–5778. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.; Windeck, T.; Ploch, M.; Verspohl, E.J. Mode of action of gingerols and shogaols on 5-HT3 receptors: Binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur. J. Pharmacol. 2006, 530, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Heimes, K.; Feistel, B.; Verspohl, E.J. Impact of the 5-HT3 receptor channel system for insulin secretion and interaction of ginger extracts. Eur. J. Pharmacol. 2009, 624, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.-H.; Yue, W.; Chen, W.-H.; Yang, Z.-H.; Liu, Z.-T.; Wang, Y.-X. Effect of gingerol on substance P and NK1 receptor expression in a vomiting model of mink. Chin. Med. J. 2010, 123, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.; Nahrstedt, A.; Petereit, F.; Windeck, T.; Ploch, M.; Verspohl, E. 5-HT3 receptor blocking activity of arylalkanes isolated from the rhizome of Zingiber officinale. Planta Med. 2005, 71, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Riyazi, A.; Hensel, A.; Bauer, K.; Geissler, N.; Schaaf, S.; Verspohl, E. The effect of the volatile oil from ginger rhizomes (Zingiber officinale), its fractions and isolated compounds on the 5-HT3 receptor complex and the serotoninergic system of the rat ileum. Planta Med. 2007, 73, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Tjendraputra, E.; Tran, V.H.; Liu-Brennan, D.; Roufogalis, B.D.; Duke, C.C. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg. Chem. 2001, 29, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Yusof, Y.; Ahmad, N.; Das, S.; Sulaiman, S.; Murad, N. Chemopreventive efficacy of ginger (Zingiber officinale) in ethionine induced rat hepatocarcinogenesis. Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 87–93. [Google Scholar] [CrossRef]
- Adhikari, S.; Priyadarsini, K.I.; Mukherjee, T. Physico-chemical studies on the evaluation of the antioxidant activity of herbal extracts and active principles of some Indian medicinal plants. J. Clin. Biochem. Nutr. 2007, 40, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Patro, B.; Adhikari, S.; Chintalwar, G.; Chattopadhyay, S.; Mukherjee, T. The radioprotection and antioxidant properties of dehydrogingerdione. Res. Chem. Intermed. 2005, 31, 667–678. [Google Scholar] [CrossRef]
- Thein, K.; Myint, W.; Myint, M.M.; Aung, S.P.; Khin, M.; Than, A.; Bwin, M. Preliminary screening of medicinal plants for biological activity based on inhibition of cyclic AMP phosphodiesterase. Pharm. Biol. 1995, 33, 330–333. [Google Scholar] [CrossRef]
- Ghayur, M.N.; Gilani, A.H.; Afridi, M.B.; Houghton, P.J. Cardiovascular effects of ginger aqueous extract and its phenolic constituents are mediated through multiple pathways. Vasc. Pharmacol. 2005, 43, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Shoji, N.; Ohizumi, Y. Gingerol, a novel cardiotonic agent, activates the Ca2+-pumping ATPase in skeletal and cardiac sarcoplasmic reticulum. BBA. Biomembr. 1987, 903, 96–102. [Google Scholar] [CrossRef]
- Chang, T.-T.; Chen, K.-C.; Chang, K.-W.; Chen, H.-Y.; Tsai, F.-J.; Sun, M.-F.; Chen, C.Y.-C. In silico pharmacology suggests ginger extracts may reduce stroke risks. Mol. Biosyst. 2011, 7, 2702–2710. [Google Scholar] [CrossRef] [PubMed]
- Erk, T.; Hauser, J.; Williamson, G.; Renouf, M.; Steiling, H.; Dionisi, F.; Richling, E. Structure- and dose-absorption relationships of coffee polyphenols. Biofactors 2014, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Nakamura, K.; Ma, L.; Li, J.-Z.; Kayahara, H. Analyses of arbutin and chlorogenic acid, the major phenolic constituents in oriental pear. J. Agric. Food Chem. 2005, 53, 3882–3887. [Google Scholar] [CrossRef] [PubMed]
- He, X.-G.; Bernart, M.W.; Lian, L.-Z.; Lin, L.-Z. High-performance liquid chromatography-electrospray mass spectrometric analysis of pungent constituents of ginger. J. Chromatogr. A 1998, 796, 327–334. [Google Scholar] [CrossRef]
- Marko, D.; Pahlke, G.; Merz, K.-H.; Eisenbrand, G. Cyclic 3′,5′-Nucleotide Phosphodiesterases: Potential Targets for Anticancer Therapy. Chem. Res. Toxicol. 2000, 13, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Montoya, G.A. In Vitro and In Vivo Biofunctional Effects of Selected Coffee Compounds, Extracts and Brews on Key Elements of Adenosine Receptor-Mediated Signaling Pathways and on Cellular Heme Oxygenase. Ph.D. Thesis, University of Kaiserslautern, Kaiserslautern, Germany, 25 April 2012. [Google Scholar]
- Li, Z.; Pan, Q.; Cui, X.; Duan, C. Optimization on anthocyanins extraction from wine grape skins using orthogonal test design. Food Sci. Biotechnol. 2010, 19, 1047–1053. [Google Scholar] [CrossRef]
- Ruiz-Rodríguez, B.M.; Sánchez-Moreno, C.; Ancos, B.D.; Cortes, M.; Fernández Ruíz, V.; Cámara, M.; Tardío, J. Wild Arbutus unedo L. and Rubus ulmifolius Schott fruits are underutilized sources of valuable bioactive compounds with antioxidant capacity. Fruits 2014, 69, 435–448. [Google Scholar] [CrossRef]
- Oliveira, I.; Baptista, P.; Malheiro, R.; Casal, S.; Bento, A.; Pereira, J.A. Influence of strawberry tree (Arbutus unedo L.) fruit ripening stage on chemical composition and antioxidant activity. Food Res. Int. 2011, 44, 1401–1407. [Google Scholar] [CrossRef]
- Blaut, M.; Braune, A.; Wunderlich, S.; Sauer, P.; Schneider, H.; Glatt, H. Mutagenicity of arbutin in mammalian cells after activation by human intestinal bacteria. Food Chem. Toxicol. 2006, 44, 1940–1947. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-L.; Wu, R.-T. Quantification of (+)-catechin and (−)-epicatechin in coconut water by LC-MS. Food Chem. 2011, 126, 710–717. [Google Scholar] [CrossRef]
- Li, D.-Q.; Qian, Z.-M.; Li, S.-P. Inhibition of three selected beverage extracts on α-glucosidase and rapid identification of their active compounds using HPLC-DAD-MS/MS and biochemical detection. J. Agric. Food Chem. 2010, 58, 6608–6613. [Google Scholar] [CrossRef] [PubMed]
- Zick, S.M.; Djuric, Z.; Ruffin, M.T.; Litzinger, A.J.; Normolle, D.P.; Alrawi, S.; Feng, M.R.; Brenner, D.E. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomark. Prev. 2008, 17, 1930–1936. [Google Scholar] [CrossRef] [PubMed]
| Extracted Plant | Extracted Part | IC50 (mg/mL) | Lead Substance | Content (μg/mg) | IC50 | |
|---|---|---|---|---|---|---|
| Arbutus unedo (strawberry tree) | fruit | n.d. 1 | arbutin | 20.0 ± 1.3 | n.d. 1 | n.d. 1 |
| Camellia sinensis (tea) | dried leaves | n.d. 1 | caffeine | 131.6 ± 5.0 | 0.9 ± 0.1 mg/mL | 4.8 ± 0.6 mM |
| Cynara scolymus (artichoke) | dried leaves | 0.9 ± 0.1 | 3,4-diCQA | 2.5 ± 0.5 | >500 μg/mL (49%) | >1.0 mM |
| luteolin | <0.08 | 11.8 ± 2.9 μg/mL | 41 ± 10 μM | |||
| luteolin-7-O-glucuronide | 2.7 ± 0.3 | n.d. 1 | n.d. 1 | |||
| luteolin-7-O-glucoside | 4.9 ± 1.0 | n.d. 1 | n.d. 1 | |||
| apigenin | <0.08 | n.d. 1 | n.d. 1 | |||
| Zingiber officinale (ginger) | dried rhizome | 1.7 ± 0.2 | [6]-gingerol | 10.0 ± 0.6 | >500 μg/mL (38%) | >1.7 mM |
| lipoid fraction (GLE) | ~50 | 455 ± 19 μg/mL | - | |||
| hydrophilic fraction (GWE) | ~950 | 10.5 ± 1.9 mg/mL | - | |||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Röhrig, T.; Pacjuk, O.; Hernández-Huguet, S.; Körner, J.; Scherer, K.; Richling, E. Inhibition of Cyclic Adenosine Monophosphate-Specific Phosphodiesterase by Various Food Plant-Derived Phytotherapeutic Agents. Medicines 2017, 4, 80. https://doi.org/10.3390/medicines4040080
Röhrig T, Pacjuk O, Hernández-Huguet S, Körner J, Scherer K, Richling E. Inhibition of Cyclic Adenosine Monophosphate-Specific Phosphodiesterase by Various Food Plant-Derived Phytotherapeutic Agents. Medicines. 2017; 4(4):80. https://doi.org/10.3390/medicines4040080
Chicago/Turabian StyleRöhrig, Teresa, Olga Pacjuk, Silvia Hernández-Huguet, Johanna Körner, Katharina Scherer, and Elke Richling. 2017. "Inhibition of Cyclic Adenosine Monophosphate-Specific Phosphodiesterase by Various Food Plant-Derived Phytotherapeutic Agents" Medicines 4, no. 4: 80. https://doi.org/10.3390/medicines4040080
APA StyleRöhrig, T., Pacjuk, O., Hernández-Huguet, S., Körner, J., Scherer, K., & Richling, E. (2017). Inhibition of Cyclic Adenosine Monophosphate-Specific Phosphodiesterase by Various Food Plant-Derived Phytotherapeutic Agents. Medicines, 4(4), 80. https://doi.org/10.3390/medicines4040080





