β-Selinene-Rich Essential Oils from the Parts of Callicarpa macrophylla and Their Antioxidant and Pharmacological Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Isolation of Essential Oil
2.3. GC Analysis
2.4. GC/MS Analysis
2.5. Antioxidant Assay
2.5.1. Reducing Power Activity
2.5.2. Effect on the Chelating Activity of Fe2+
2.5.3. DPPH Radical Scavenging Activity
2.5.4. NO Radical Scavenging Activity
2.5.5. Super Oxide Radical Scavenging Activity
2.5.6. OH Radical Scavenging Activity
2.6. Evaluation of Pharmacological Activities
2.7. Anti-Inflammatory Activity
2.7.1. Carrageenan-Induced Paw Edema
2.7.2. Formaldehyde-Induced Inflammatory Activity
2.8. Analgesic Activity
Acetic Acid-Induced Abdominal Writhing Test
2.9. Antipyretic Activity
2.10. Assessment of Toxicity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Assay
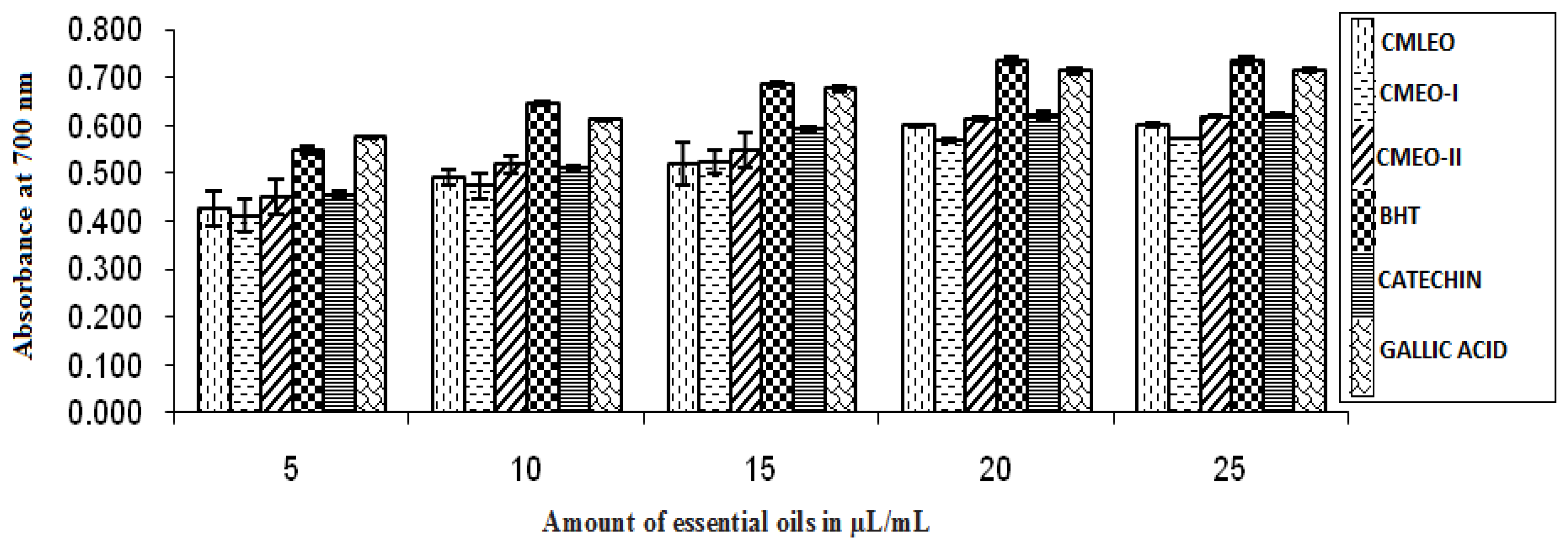
3.1.1. Reducing Power
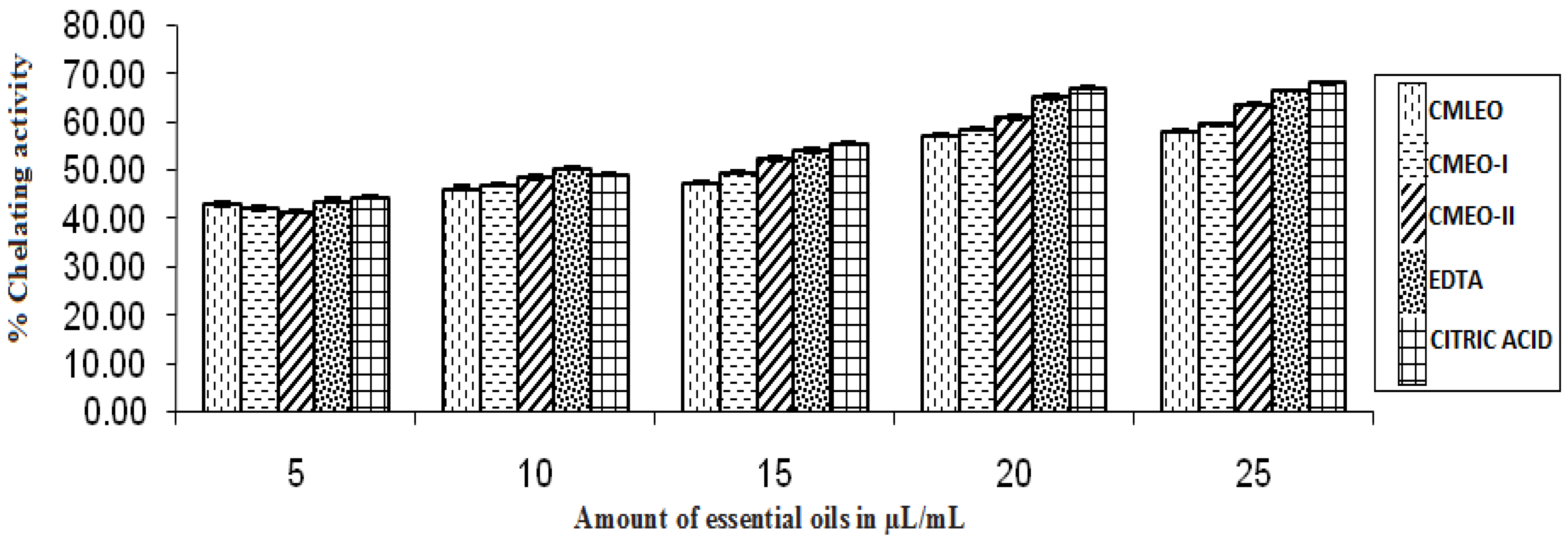
3.1.2. Ability of Chelating Fe2+ Ion
3.1.3. DPPH Radical Scavenging Activity
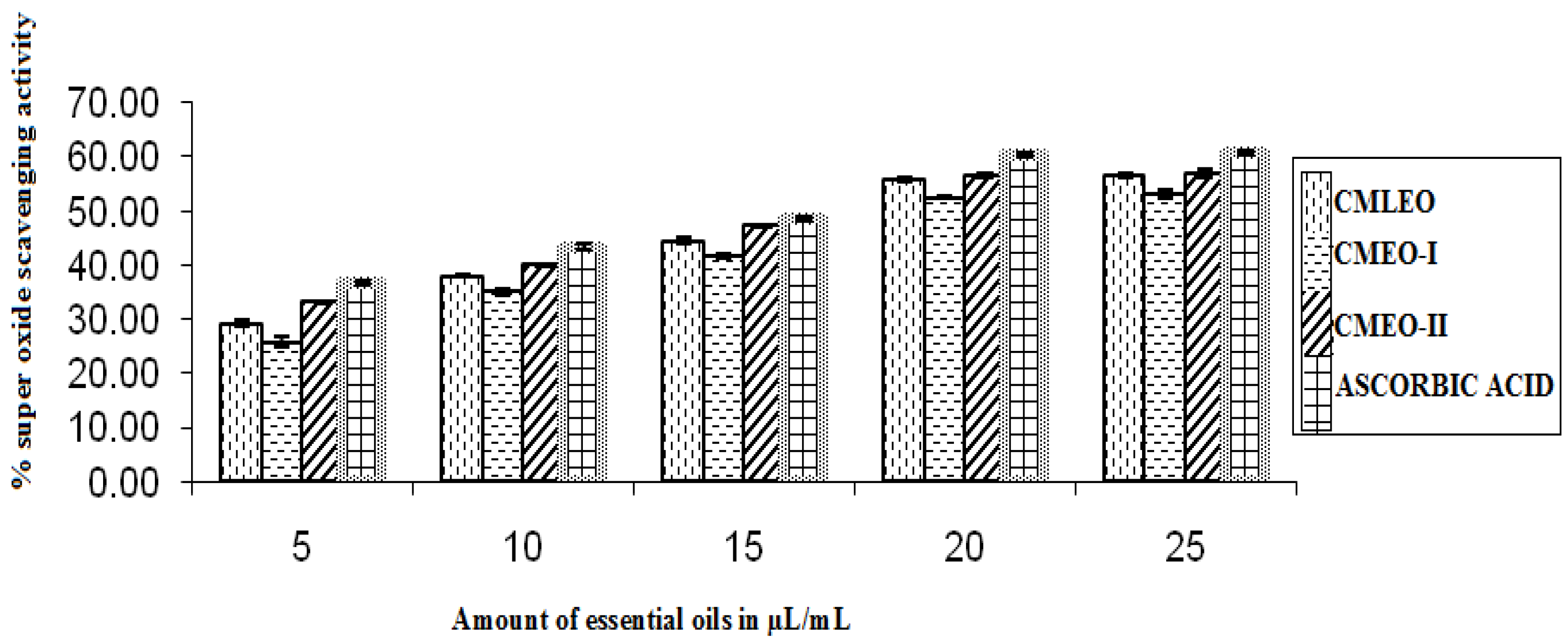
3.1.4. Superoxide Radical Scavenging Activity
3.1.5. NO Radical Scavenging Activity
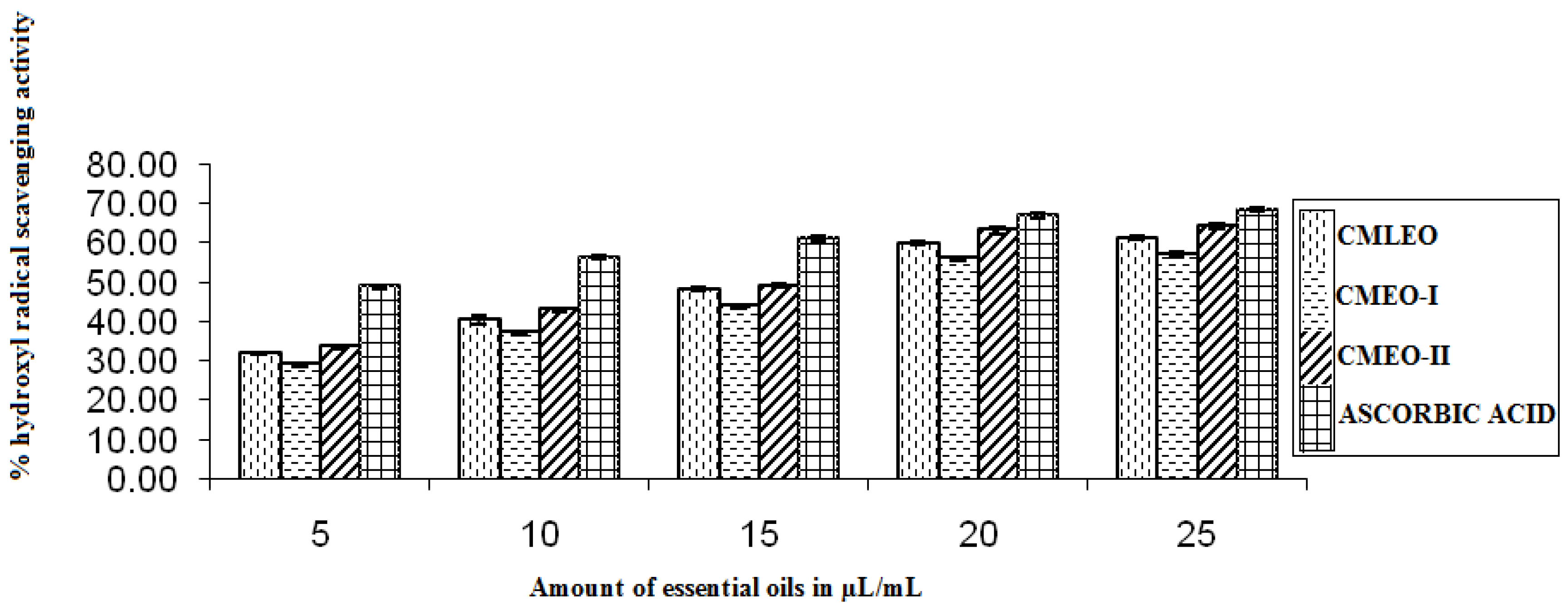
3.1.6. OH Radical Scavenging Activity
3.2. Anti-Inflammatory Activity
3.2.1. Mice Paw Edema (Carrageenan-Induced)
3.2.2. Formaldehyde-Induced Inflammatory Activity
3.3. Analgesic Activity
3.4. Antipyretic Activity
3.5. Acute Toxicity
4. Conclusions
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Babu, C.R. Herbaceous Flora of Dehradun; PID Publication: New Delhi, India, 1977; pp. 395–396. [Google Scholar]
- Yatato, S.; Chunyong, W.; Yanhua, C.; Wenyuan, L.; Feng, F.; Ning, X. Comparative analysis of three Callicarpa herbs using high performance liquid chromatography with diode array detector and electrospray ionization-trap mass spectrometry method. J. Pharm. Biom. Anal. 2013, 75, 239–247. [Google Scholar]
- Megoneitso; Rao, R.R. Enthnobotanical studies in Nagaland-4. Sixty two medicinal plants used by the Agami Nagas. J. Econ. Tax. Bot. 1983, 4, 167–172. [Google Scholar]
- Balodi, V. Introductory note on the enthnobotany of Gori Valley. J. Econ. Tax. Bot. 1998, 12, 453–455. [Google Scholar]
- Singh, A.K.; Pawan, K.A. A diterpenoid from Callicarpa macrophylla. Phytochemistry 1994, 37, 587–588. [Google Scholar] [CrossRef]
- Yadav, V.; Jayalakshmi, S.; Patra, A.; Singla, P.K. Investigation of Analgesic & Anti-Pyretic Potentials of Callicarpa macrophylla Vahl. Leaves Extracts. Int. J. Med. Mol. Med. 2012, 3, 1–7. [Google Scholar]
- Yadav, V.; Jayalakshmi, S.; Singh, R.K.; Patra, A. Preliminary Assessment of Anti-Inflammatory Activity of Callicarpa macrophylla Vahl. Leaves Extracts Indo-Global. J. Pharm. Sci. 2011, 1, 219–222. [Google Scholar]
- Mozaina, K.; Mario, R.T.; Franck, E.D.; Stephen, O.D. Phytotoxicity and volatile constituents from leaves of Callicarpa japonica Thunb. Phytochemistry 2002, 61, 37–40. [Google Scholar]
- Chandra, M.; Prakash, O.; Punetha, H.; Bhushan, B.; Bachetti, R.K.; Kumar, M.; Pant, A.K. An analgesic and anti-inflammatory activity of hydro-alcoholic leaves extracts of some indigenous herbs growing in Uttarakhand, India. Int. J. Inst. Pharm. Life Sci. 2015, 5, 116–123. [Google Scholar]
- Singh, A.K.; Chanotiya, C.S.; Yadav, A.; Kalra, A. Volatile of Callicarpa macropylla: A rich source of selinene isomer. Nat. Prod. Commun. 2010, 5, 269–272. [Google Scholar] [PubMed]
- Chatterjee, A.; Desmukh, S.K.; Chandrasekharan, S. Diterpenoid constituents of Callicarpa macrophylla Vahl: The structures and stereochemistry of calliterpenone and calliterpenonemonoacetate. Tetrahedron 1972, 28, 4319–4323. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Business Media Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Kumar, R.; Prakash, O.; Pant, A.K.; Isidorov, V.A.; Mathela, C.S. Chemical composition, antioxidant and myorelaxant activity of essential oils of Globba sessiliflora Sims. J. Essent. Oil Res. 2012, 24, 385–391. [Google Scholar] [CrossRef]
- Naskar, S.; Islam, A.; Mazumdar, U.K.; Saha, P.P.; Haldar, K.; Gupta, M. In vitro and in vivo antioxidant Potential of hydromethanolic extract of Phoenix dactylifera fruits. J. Sci. Res. 2010, 2, 144–157. [Google Scholar] [CrossRef]
- Fu, W.; Chen, J.L.; Cai, Y.L.; Lei, Y.F.; Chen, L.M.; Pei, L.; Zhou, D.N.; Liang, X.F.; Ruan, J.L. Antioxidant, free radical scavenging, antiinflammatoryand hepatoprotective potential of the extract from Parathelypteris nipponica (Franch.etSav.) Ching. J. Ethnopharm. 2010, 130, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Olabinri, B.M.; Odedire, O.O.; Olaleye, M.T.; Adekunle, A.S.; Ehigie, L.O.; Olabinri, P.F. Evaluation of hydroxyl and nitric oxide radical scavenging activities. Res. J. Biol. Sci. 2010, 5, 102–105. [Google Scholar]
- Chandra, M.; Parakash, O.; Bachheti, R.K.; Kumar, M.; Pant, A.K. Essential oil composition and pharmacological activities of Micromeria biflora (Buch.-Ham. Ex D. Don) Benth. collected from Uttarakhand region of India. J. Med. Res. 2013, 7, 2538–2544. [Google Scholar]
- Italenti, A.; Ianaro, A.; Mancada, S.; Di Rosa, M. Modulation of acute inflammation by endogenous nitric oxide. Eur. J. Pharmacol. 1995, 211, 177–182. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on product of browning reaction prepared from glucose amine Japan. J. Nutr. 1986, 44, 307–315. [Google Scholar]
- Soares, J.R.; Dins, T.C.P.; Cunha, A.P.; Ameida, L.M. Antioxidant activity of some extracts of Thymus zygis. Free Rad. Res. 1997, 26, 469–478. [Google Scholar] [CrossRef]
- Floyd, R.A. Nitric oxide and cancer development. J. Toxi. Pathol. 2007, 20, 77–92. [Google Scholar] [CrossRef][Green Version]
- Manjamalai, A.; Berlin Grace, V.M. Antioxidant Activity of Essential Oils from Wedelia chinensis (Osbeck) in vitro and in vivo Lung Cancer Bearing C57BL/6 Mice. Asian Pac. J. Cancer Prev. 2012, 13, 3065–3071. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Mishra, T.K.; Ghosal, M. Free radical scavenging activity and phytochemical analysis in the leaf and stem of Drymaria diandra Glume. Indian J. Tuberc. 2009, 7, 80–84. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free radicals in biology and medicine. In Free Radicals, Ageing, and Disease; Clarendron Press: Oxford, UK, 1985; Volume 2, pp. 279–315. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–277. [Google Scholar] [CrossRef]
- Gutteridge, M.C. Reactivity of hydroxyl and hydroxyl-like radicals discriminated by release of thiobarbituric acid reactive material from deoxy sugars, nucleosides and benzoate. Biochem. J. 1984, 224, 761–767. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Jenner, A.O.I. Aroma Intense oxidative DNA damage promoted by L-DOPA and its metabolites, implications for neurodegenerative disease. FEBS Lett. 1994, 353, 246–250. [Google Scholar] [CrossRef]
- Sanchez, M.C.; Larrauri, J.A.; Saura, C.F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res. Int. 1999, 32, 407–412. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Gani, A.Z.D.F. Antinociceptive, anti-inflammatory, and antipyretic properties of an aqueous extract of Dicranopteris linearis leaves in experimental animal models. J. Nat. Med. 2008, 62, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Collier, H.D.J.; Dinnin, L.C.C.; Johnson, A.; Schneider, C. The abdominal response and its suppression by analgesic drugs in mouse. Br. J. Pharm. 1968, 32, 295–310. [Google Scholar] [CrossRef]
- Brazezinska, S.E. Fever induced oxidative stress. The effect on thyroid status and the S’ monodeiodinase activity protective role of selenium vitamin. Eur. J. Phys. Pharm. 2001, 52, 275–284. [Google Scholar]
- Devi, B.P.; Boominathan, R.; Mandal, S.C. Evalution of antipyreticpotential of Cleome viscose Linn. (Capparidaceae) extract in rats. J. Ethnopharmacol. 2003, 87, 11–13. [Google Scholar]
- Dannhardt, G.; Kiefer, W. Cyclooxygenase inhibitors—Current status and future prospects. Eur. J. Med. Chem. 2001, 36, 109–126. [Google Scholar] [CrossRef]
- Rao, C.V.; Kartik, R.; Ojha, S.K.; Amresh Rao, G.M.M. Anti-inflammatory and antinociceptive activity of stem juice powder of Tinospora cordifolia Miers. in experimental animals. Hamdard Med. 2005, XLVIII, 102–106. [Google Scholar]
- Adedapo, A.A.; Sofidiya, M.O.; Maphosa, V.; Moyo, B.; Masika, P.J.; Afolayan, A.J. Anti-inflammatory and analgesic activities of the aqueous extract of Cussonia paniculata stem Bark. Rec. Nat. Prod. 2008, 2, 46–53. [Google Scholar]


| S.N. | Compounds Name | KI/RI | FID % | ||
|---|---|---|---|---|---|
| CMLEO | CMOE-I | CMEO-II | |||
| 1 | hex-2E-enal | 850 | 0.20 | - | - |
| 2 | α-pinene | 933 | 0.06 | - | 0.40 |
| 3 | β-pinene | 943 | 0.53 | 1.07 | 2.32 |
| 4 | 1-octene-3one | 943 | 0.02 | 0.12 | 0.02 |
| 5 | 3-octanone | 952 | 0.10 | 0.12 | 0.06 |
| 6 | banzaldehide | 960 | 0.14 | - | - |
| 7 | sabinene | 972 | 0.17 | 0.40 | 0.25 |
| 8 | hexanoic acid | 979 | - | 0.47 | - |
| 9 | myrcene | 991 | - | - | 0.04 |
| 10 | hex-3Z-ethyl acetate | 1008 | 0.01 | - | - |
| 11 | p-cymene | 1025 | 0.13 | 0.41 | 0.14 |
| 12 | limonene | 1030 | - | 0.15 | 0.12 |
| 13 | 1,8-cineole | 1032 | 2.23 | 3.10 | 1.62 |
| 14 | β-ocimene | 1046 | - | - | 0.31 |
| 15 | 2-nonanone | 1052 | 0.02 | - | - |
| 16 | trans-2-octenal | 1067 | - | 0.27 | - |
| 17 | cis linalool oxide | 1069 | - | 0.21 | - |
| 18 | trans linalool oxide | 1086 | - | 0.15 | - |
| 19 | linalool | 1101 | 0.20 | 0.86 | - |
| 20 | nopinone | 1139 | - | 0.50 | - |
| 21 | sabina ketone | 1154 | 0.03 | - | - |
| 22 | pinocarvone | 1164 | - | 0.64 | 0.45 |
| 23 | terpinen-4-ol | 1180 | - | 0.43 | - |
| 24 | myrtenal | 1197 | 0.23 | 0.74 | 0.20 |
| 25 | 1-butyryl-1,2,3,6-tetrahydropyridine | 1249 | 0.05 | ||
| 26 | 3,9-dodecadiyn | 1249 | 0.37 | - | - |
| 27 | bornyl acetate | 1285 | - | 0.42 | - |
| 28 | leden oxide (I) | 1293 | - | 0.40 | - |
| 29 | myrtenal acetate | 1326 | - | 0.19 | - |
| 30 | α-copaene | 1375 | 0.35 | - | 0.27 |
| 31 | β-elemene | 1390 | 0.98 | 0.82 | 0.61 |
| 32 | β-cubebene | 1392 | 0.10 | 0.09 | 0.48 |
| 33 | α-gurjunene | 1406 | 0.50 | 0.95 | 0.15 |
| 34 | nopyl acetate | 1413 | - | 0.26 | - |
| 35 | β-caryophelline | 1424 | 3.26 | - | 1.84 |
| 36 | (E) caryophellene | 1424 | 0.44 | - | 0.11 |
| 37 | aromadandrene | 1438 | - | 2.14 | 0.19 |
| 38 | 4-camphenylbutan-2-one | 1451 | - | 0.80 | - |
| 39 | α-humulene | 1454 | - | - | 0.14 |
| 40 | aromadendrene oxide II | 1462 | 0.43 | 0.46 | 0.25 |
| 41 | 9E-epi-caryophelline | 1464 | 6.23 | 1.27 | 3.43 |
| 42 | α-selinene | 1474 | 0.26 | ||
| 43 | α-cubebene | 1480 | 0.11 | - | - |
| 44 | ar-curcumene | 1480 | 0.19 | 0.10 | 0.14 |
| 45 | β-selinene | 1492 | 37.51 | 44.66 | 57.01 |
| 46 | amorphene | 1502 | 0.41 | - | - |
| 47 | perhydropyrene | 1502 | 0.37 | ||
| 48 | caryophelline oxide | 1507 | 7.34 | 8.74 | 5.0 |
| 49 | δ-cadinine | 1518 | 0.85 | 0.41 | 0.59 |
| 50 | trans-calamene | 1527 | 0.35 | 0.15 | 0.31 |
| 51 | globulol | 1530 | 0.42 | - | - |
| 52 | Z-α-bisaboline epoxide | 1531 | 0.21 | ||
| 53 | α-agarofuron | 1548 | - | 0.40 | - |
| 54 | (E)-nerolidol | 1561 | - | 0.20 | - |
| 55 | longicamphenylone | 1563 | - | 3.08 | - |
| 56 | longipinocarvone | 1569 | 4.96 | 1.17 | 2.0 |
| 57 | sphathulenol | 1576 | 1.06 | 2.10 | 0.30 |
| 58 | β-copaen-4 α-ol | 1590 | 1.03 | - | - |
| 59 | trans longipinocarveol | 1590 | 0.63 | 0.71 | - |
| 60 | fokienol | 1596 | 0.38 | 0.31 | - |
| 61 | salvial-4 (14)-en-1-one | 1596 | 0.73 | - | - |
| 62 | β-oplopanone | 1607 | 0.33 | ||
| 63 | humulene epoxide II | 1613 | 0.21 | - | - |
| 64 | Z-3-hexadecane-7-yne | 1637 | - | 0.31 | - |
| 65 | solavetivone | 1645 | 0.99 | 0.30 | 0.40 |
| 66 | cedren-13-ol | 1646 | - | 0.52 | - |
| 67 | vulgarone | 1649 | 2.92 | 1.02 | 0.40 |
| 68 | α-muurolol | 1651 | 1.76 | - | - |
| 69 | cadalene | 1677 | - | 0.32 | - |
| 70 | khusinol | 1679 | - | - | 0.10 |
| 71 | juniper camphor | 1696 | 3.13 | 3.03 | - |
| 72 | cis-lanceol | 1760 | 0.12 | - | - |
| 73 | 14-oxy α-muurolene | 1767 | 2.50 | - | - |
| 74 | phyllocladene | 1789 | 9.76 | 5.80 | 12.38 |
| 75 | cupressene | 1880 | - | 0.47 | - |
| 76 | 5-octen-2-one | 1932 | 0.53 | ||
| 77 | androsta-4,16-dien-3-one | 1933 | 0.71 | 0.50 | 0.70 |
| 78 | androsta-3,5-dien-7-one | 1933 | 0.55 | 0.26 | 0.32 |
| 79 | 6-androstanone | 1940 | 0.13 | ||
| 80 | n-hexadecanoic acid | 1977 | 0.43 | ||
| 81 | 9Z,12Z,15Z-octadecatrien-1-ol | 2077 | 0.20 | ||
| 82 | pimara-7,15-dien-3-one | 2097 | 0.36 | 0.16 | 0.24 |
| 83 | thunbergol | 2211 | - | 0.98 | 0.23 |
| 84 | andrographolide | 2944 | 0.84 | 0.74 | |
| Total | 96.55 | 94.56 | 95.35 | ||
| Extracts | (IC 50 in (µL/µg)/mL)/R2 | % Absorbance (Reducing Power) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radical scavenging Activities | Chelating Activity | At lower Dose Level (5 µL/µg)/mL | At Higher Dose Level (25 µL/µg)/mL | |||||||||
| DPPH Scavenging | NO scavenging | Super Oxide Scavenging | OH Scavenging | |||||||||
| IC50 | R2 | IC50 | R2 | IC50 | R2 | IC50 | R2 | IC50 | R2 | |||
| CMLEO | 18.35 ± 0.18 | 0.933 ± 0.003 | 10.61 ± 0.02 | 0.941 ± 0.000 | 18.58 ± 0.19 | 0.956 ± 0.006 | 16.06 ± 0.16 | 0.963 ± 0.006 | 14.38 ± 0.27 | 0.913 ± 0.009 | 0.426 ± 0.004 | 0.602 ± 0.000 |
| CMEO-I | 20.29 ± .11 | 0.960 ± 0.06 | 11.18 ± 0.06 | 0.95 ± 0.004 | 20.79 ± 0.30 | 0.954 ± 0.005 | 18.59 ± 0.25 | 0.960 ± 0.006 | 13.42 ± 0.17 | 0.950 ± 0.004 | 0.411 ± 0.003 | 0.573 ± 0.000 |
| CMEO-II | 15.66 ± 0.03 | 0.961 ± 00.005 | 7.37 ± 0.11 | 0.922 ± 0.002 | 17.49 ± 0.13 | 0.954 ± 0.015 | 14. 59 ± 0.18 | 0.956 ± 0.016 | 11.49 ± 0.87 | 0.979 ± 0.006 | 0.450 ± 0.004 | 0.418 ± 0.000 |
| BHT | 8.55 ± 0.10 | 0.947 ± 0.005 | NA | NA | NA | NA | NA | NA | NA | NA | 0.55 ± 0.008 | 0.735 ± 0.009 |
| Catechin | 8.18 ± 0.11 | 0.950 ± 0.004 | NA | NA | NA | NA | NA | NA | NA | NA | 0.455 ± 0.006 | 0.623 ± 0.004 |
| Gallic Acid | 7.95 ± 0.11 | 0.964 ± 0.004 | NA | NA | NA | NA | NA | NA | NA | NA | 0.575 ± 0.003 | 0.715 ± 0.003 |
| Ascorbic acid | NA | NA | 7.72 ± 0.19 | 0.942 ± 0.002 | 15.03 ± 0.13 | 0.951 ± 0.007 | 11.22 ± 0.30 | 0.960 ± 0.017 | NA | NA | NA | NA |
| EDTA | NA | NA | NA | NA | NA | NA | NA | NA | 9.27 ± 0.11 | 0.955 ± 0.003 | NA | NA |
| Citric Acid | NA | NA | NA | NA | NA | NA | NA | NA | 9.42 ± 0.95 | 0.981 ± 0.021 | NA | NA |
| Group | Treatment | Doses (0.2 mL) | Paw Volume (in mm3) | % Inhibition | |||
|---|---|---|---|---|---|---|---|
| 0 h | 4 h | 24 h | 4 h | 24 h | |||
| I | CMLEO | 5% | 2.34 ± 0.01 | 2.29 ± 0.02b | 2.23 ± 0.02 ab | 2.14 | 4.7 |
| II | CMLEO | 10% | 2.31 ± 0.03 a | 2.21 ± 0.02 ab | 2.12 ± 0.01 ab | 4.33 | 8.23 |
| III | CMLEO | 20% | 2.25 ± 0.02 ab | 2.05 ± 0.02 ab | 1.85 ± 0.03 ab | 8.89 | 17.78 |
| IV | CMEO-II | 5% | 2.29 ± 0.01 a | 2.19 ± 0.02 ab | 2.14 ± 0.03 ab | 4.37 | 6.55 |
| V | CMEO-II | 10% | 2.38 ± 0.02 | 2.30 ± 0.02 b | 2.21 ± 0.02 ab | 3.36 | 7.14 |
| VI | CMEO-II | 20% | 2.41 ± 0.02 | 2.17 ± 0.02 ab | 1.90 ± 0.05 ab | 9.96 | 21.16 |
| VII | CMEO-I | 5% | 2.32 ± 0.03 a | 2.29 ± 0.03 b | 2.21 ± 0.02 ab | 1.29 | 4.74 |
| VIII | CMEO-I | 10% | 2.26 ± 0.02 ab | 2.20 ± 0.02 ab | 2.15 ± 0.03 ab | 2.65 | 4.87 |
| IX | CMEO-I | 20% | 2.27 ± 0.02 a | 2.08 ± 0.03a b | 1.90 ± 0.03 ab | 8.37 | 16.3 |
| X | Control | - | 2.40 ± 0.02 | 2.33 ± 0.01 | 2.32 ± 0.01 | 2.92 | 3.33 |
| XI | Ibuprofen | 40 mg/kg b. wt. | 2.34 ± 0.01 a | 1.73 ± 0.02 a | 1.47 ± 0.02 a | 26.07 | 37.17 |
| Group | Treatment | Dose | Volume of Paw Edema (in mm3) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 Day | 1 Day | 2 Day | 3 Day | 4 Day | 5 Day | 6 Day | 7 Day | 8 Day | 9 Day | 10 Day | |||
| I | CMLEO | 5% (0.2 mL) | 2.28 ± 0.02 | 2.36 ± 0.02 ab | 2.37 ± 0.04 b | 2.42 ± 0.04 b | 2.41 ±0.04 b | 2.40 ± 0.04 b | 2.40 ± 0.04 b | 2.39 ± 0.04 b | 2.39 ± 0.03 b | 2.36 ± 0.03 b | 2.36 ± 0.04 b |
| II | CMLEO | 10% (0.2 mL) | 2.23 ± 0.03 | 2.26 ± 0.02 ab | 2.31 ± 0.03 b | 2.35 ± 0.04 a | 2.34 ±0.03 b | 2.32 ± 0.03 b | 2.32 ± 0.03 b | 2.31 ± 0.03 b | 2.31 ± 0.03 b | 2.31 ± 0.03 b | 2.30 ± 0.03 b |
| III | CMLEO | 20% (0.2 mL) | 2.23 ± 0. 02 | 2.25 ± 0.02 ab | 2.29 ± 0.02 b | 2.30 ± 0.04 a | 2.29 ± 0.04 ab | 2.27 ± 0.03 a | 2.26 ± 0.03 ab | 2.25 ± 0. 02 ab | 2.24 ± 0.02 a | 2.22 ± 0.02 a | 2.22 ± 0.02 b |
| IV | CMEO -II | 5% (0.2 mL) | 2.27 ± 0.03 | 2.30 ± 0.02 ab | 2.36 ± 0.04 b | 2.40 ± 0.05 ab | 2.39 ± 0.05 b | 2.38 ± 0.05 b | 2.38 ± 0.04 b | 2.38 ± 0.03 b | 2.37 ± 0.03 b | 2.36 ± 0.03 b | 2.36 ± 0.03 a |
| V | CMEO-II | 10% (0.2 mL) | 2.24 ± 0.03 | 2.27 ± 0.02 ab | 2.34 ± 0.04 b | 2.38 ± 0.04 ab | 2.37 ± 0.04 b | 2.36 ± 0.03 b | 2.35 ± 0.03 b | 2.33 ± 0.03 b | 2.32 ± 0.02 b | 2.31 ± 0.02 b | 2.30 ± 0.02 b |
| VI | CMEO-II | 20% (0.2 mL) | 2.23 ± 0.02 | 2.24 ± 0.02 ab | 2.26 ± 0.02 a | 2.28 ± 0.03 a | 2.26 ± 0.03 a | 2.25 ± 0.02 a | 2.24 ± 0.02 ab | 2.24 ± 0.02 ab | 2.22 ± 0.03 a | 2.21 ± 0.02 a | 2.20 ± 0.03 a |
| VII | CMEO-I | 5% (0.2 mL) | 2.28 ± 0.02 | 2.29 ± 0.02 ab | 2.37 ± 0.03 b | 2.42 ± 0.04 b | 2.41 ± 0.03 b | 2.41 ± 0.03 b | 2.41 ± 0.03 b | 2.41 ± 0.02 b | 2.41 ± 0.02 b | 2.41 ± 0.02 b | 2.40 ± 0.02 b |
| VIII | CMEO-I | 10% (0.2 mL) | 2.26 ± 0.03 | 2.29 ± 0.02 ab | 2.32 ± 0.03 b | 2.36 ± 0.04 a | 2.35 ± 0.03 b | 2.35 ±0.03 b | 2.34 ± 0.02 b | 2.33 ± 0.03 b | 2.35 ± 0.03 b | 2.33 ± 0.03 b | 2.33 ± 0.03 b |
| IX | CMEO-I | 20% (0.2 mL) | 2.26 ± 0.02 | 2.27 ± 0.02 ab | 2.29 ± 0.03 b | 2.31 ± 0.03 a | 2.30 ± 0.02 ab | 2.29 ± 0.02 ab | 2.27 ± 0.01 ab | 2.27 ± 0.02 ab | 2.25 ± 0.02 ab | 2.24 ± 0.02 a | 2.24 ± 0.02 ab |
| X | Control | (0.2 mL) | 2.13 ± 0.02 | 2.17 ± 0.04 | 2.38 ± 0.03 | 2.52 ± 0.04 | 2.44 ± 0.02 | 2.39 ± 0.01 | 2.38 ± 0.02 | 2.38 ± 0.01 | 2.36 ± 0.01 | 2.36 ± 0.02 | 2.37 ± 0.03 |
| XI | Ibuprofen | 10 mg/kg | 2.11 ± 0.02 | 2.13 ± 0.02 a | 2.19 ±0.01 a | 2.27 ± 0.01 a | 2.21 ± 0.02 a | 2.19 ± 0.01 a | 2.15 ± 0.01 a | 2.16 ± 0.01 a | 2.17 ± 0.01 a | 2.19 ± 0.01 b | 2.15 ± 0.02 a |
| Group | Treatment | Doses | Writhing Counts | % Writhings | Inhibition (%) |
|---|---|---|---|---|---|
| I | CMLEO | 5% | 115.83 ± 8.06 ab | 83.53 | 16.47 |
| II | CMLEO | 10% | 107.00 ± 4.52 ab | 77.16 | 22.84 |
| III | CMLEO | 20% | 95.00 ± 9.01 ab | 69.95 | 31.49 |
| IV | CMEO -II | 5% | 112.33 ± 5.28 ab | 81 | 18.99 |
| V | CMEO-II | 10% | 104.33 ± 7.09 ab | 75.24 | 24.76 |
| VI | CMEO-II | 20% | 85.33 ± 7.74 a | 61.53 | 38.46 |
| VII | CMEO-I | 5% | 119.67 ± 7.09 ab | 86.3 | 13.7 |
| VIII | CMEO-I | 10% | 111.67 ± 6.12 ab | 80.53 | 19.47 |
| IX | CMEO-I | 20% | 103.00 ± 4.86 ab | 74.28 | 25.72 |
| X | Control | - | 138.67 ± 5.75 | - | - |
| XI | Ibuprofen | 40 mg/kg b. wt. | 77.67 ± 6.86 a | 56.01 | 43.99 |
| Group | Treatment | Dose | Body Temperature (°C) | Body Temperature After Administration of Drug (°C) | % Reduction in Body Temperature | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Injection of Yeast | After 18 h of Yeast Injection | 1 h | 3 h | 24 h | 1 h | 3 h | 24 h | |||
| I | CMLEO | 5% (0.2 mL) | 37.57 ± 0.05 | 38.57 ± 0.04 | 38.29 ± 0.03 b | 38.18 ± 0.04 ab | 38.13 ± 0.03 b | 28 | 39 | 44.00 |
| II | CMLEO | 10% (0.2 mL) | 37.47 ± 0.03 | 38.49 ± 0.03 b | 38.15 ± 0.03 ab | 38.01 ± 0.04 ab | 37.94 ± 0.04 ab | 33.33 | 47.05 | 53.92 |
| III | CMLEO | 20% (0.2 mL) | 37.56 ± 0.02 a | 38.58 ± 0.04 | 37.99 ± 0.03 ab | 37.86 ± 0.04 ab | 37.77 ± 0.05 ab | 57.84 | 70.59 | 79.41 |
| IV | CMEO-II | 5% (0.2 mL) | 37.55 ± 0.03 | 38.56 ± 0.03 | 38.27 ± 0.02 b | 38.12 ± 0.03 ab | 38.08 ± 0.03 ab | 28.71 | 43.56 | 47.52 |
| V | CMEO-II | 10% (0.2 mL) | 37.49 ± 0.04 | 38.51 ± 0.03 | 38.14 ± 0.04 ab | 37.97 ± 0.04 ab | 37.90 ± 0.04 ab | 36.27 | 52.94 | 59.8 |
| VI | CMEO-II | 20% (0.2 mL) | 37.51 ± 0.04 | 38.55 ± 0.03 | 37.89 ± 0.04 ab | 37.73 ± 0.02 ab | 37.63 ± 0.03 a | 63.46 | 78.85 | 88.46 |
| VII | CMEO-I | 5% (0.2 mL) | 37.48 ± 0.03 | 38.46 ± 0.03 b | 38.19 ± 0.03 ab | 38.12 ± 0.04 ab | 38.06 ± 0.04 ab | 27.55 | 34.69 | 40.82 |
| VIII | CMEO-I | 10% (0.2 mL) | 37.52 ± 0.02 | 38.53 ± 0.03 | 38.20 ± 0.04 ab | 38.07 ± 0.04 ab | 38.00 ± 0.05 ab | 32.67 | 45.54 | 52.47 |
| IX | CMEO-I | 20% (0.2 mL) | 37.49 ± 0.02 | 38.54 ± 0.02 | 37.97 ± 0.02 ab | 37.87 ± 0.02 ab | 37.76 ± 0.05 ab | 54.28 | 63.81 | 74.29 |
| X | Control | (0.2 mL) | 37.46 ± 0.02 | 38.50 ± 0.03 | 38.36 ± 0.04 | 38.30 ± 0.03 | 38.22 ± 0.03 | 13.46 | 19.23 | 26.92 |
| XI | Paracetamol | 33.0 mg/kg | 37.50 ± 0.03 | 38.59 ± 0.04 a | 37.75 ± 0.04 a | 37.61 ± 0.03 a | 37.54 ± 0.04 a | 77.06 | 89.91 | 96.33 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chandra, M.; Prakash, O.; Kumar, R.; Bachheti, R.K.; Bhushan, B.; Kumar, M.; Pant, A.K. β-Selinene-Rich Essential Oils from the Parts of Callicarpa macrophylla and Their Antioxidant and Pharmacological Activities. Medicines 2017, 4, 52. https://doi.org/10.3390/medicines4030052
Chandra M, Prakash O, Kumar R, Bachheti RK, Bhushan B, Kumar M, Pant AK. β-Selinene-Rich Essential Oils from the Parts of Callicarpa macrophylla and Their Antioxidant and Pharmacological Activities. Medicines. 2017; 4(3):52. https://doi.org/10.3390/medicines4030052
Chicago/Turabian StyleChandra, Mahesh, Om Prakash, Ravendra Kumar, Rakesh Kumar Bachheti, Brij Bhushan, Mahesh Kumar, and Anil Kumar Pant. 2017. "β-Selinene-Rich Essential Oils from the Parts of Callicarpa macrophylla and Their Antioxidant and Pharmacological Activities" Medicines 4, no. 3: 52. https://doi.org/10.3390/medicines4030052
APA StyleChandra, M., Prakash, O., Kumar, R., Bachheti, R. K., Bhushan, B., Kumar, M., & Pant, A. K. (2017). β-Selinene-Rich Essential Oils from the Parts of Callicarpa macrophylla and Their Antioxidant and Pharmacological Activities. Medicines, 4(3), 52. https://doi.org/10.3390/medicines4030052








