Characterisation of Bone Beneficial Components from Australian Wallaby Bone
Abstract
:1. Introduction
2. Material and Methods
2.1. Animal and Bone Samples
2.2. Digestion Procedures for Wallaby Bone
2.3. Inductively Coupled Plasma-Mass Spectrometry
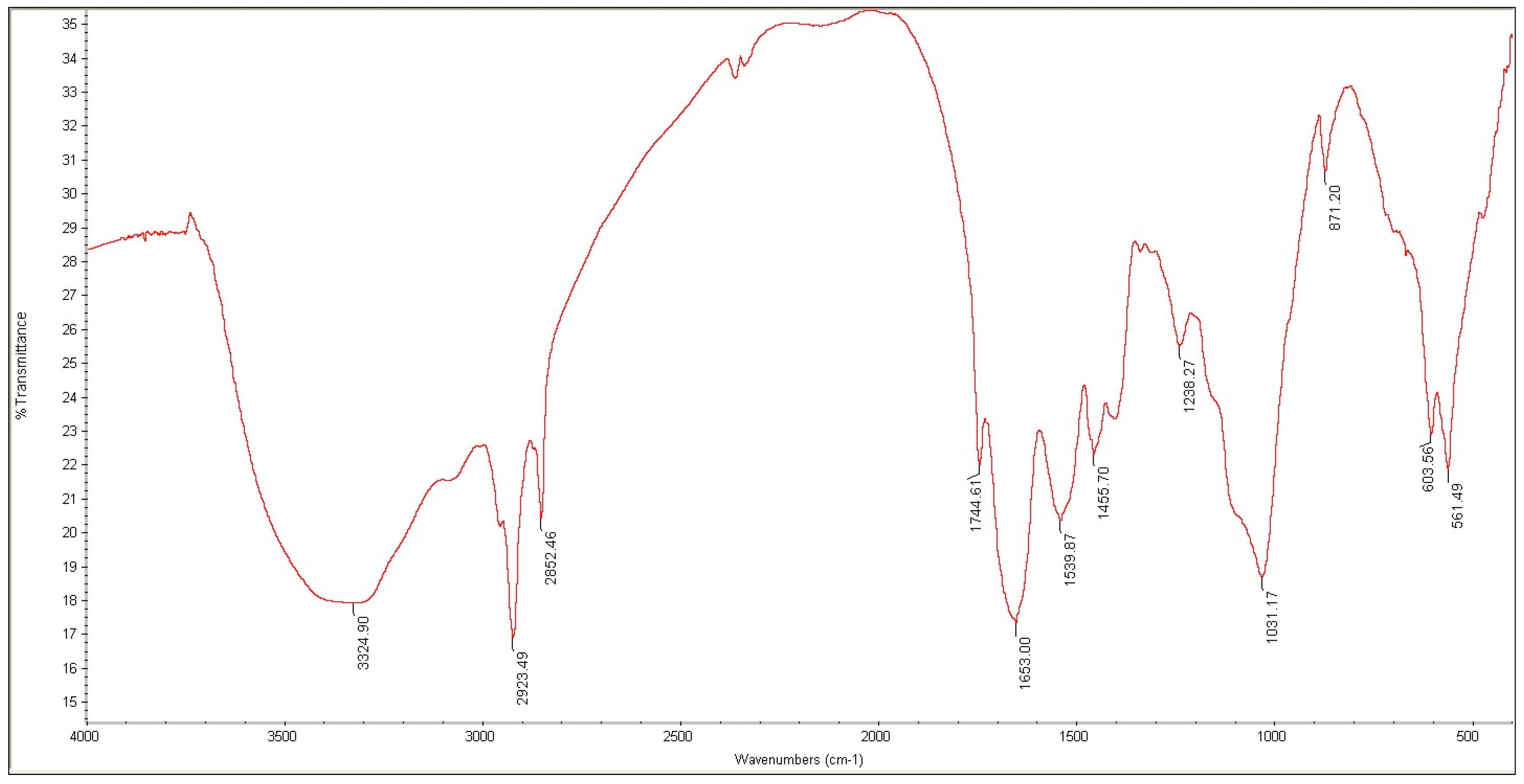
2.4. Fourier Transform Infrared Spectroscopy
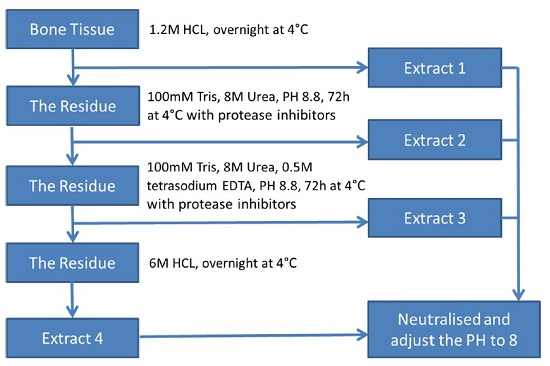
2.5. Protein Extraction from Wallaby Bone
2.6. Protein Determination with Bradford Assay Kit
2.7. Protein Concentration Assay by SDS-PAGE System
2.8. Liquid Chromatography–Mass Spectrometry (LC-MS/MS)
2.9. MS/MS Data Analysis and Protein Identification
2.10. In Vitro Evaluation of Wallaby Bone Extracts on Osteogenic Elements
2.11. Statistical Analysis
3. Results
3.1. Mineral Analysis
3.2. Organic Compounds Analysis
3.3. Total Protein Content from Four Protein Extracts in Different Bone Samples
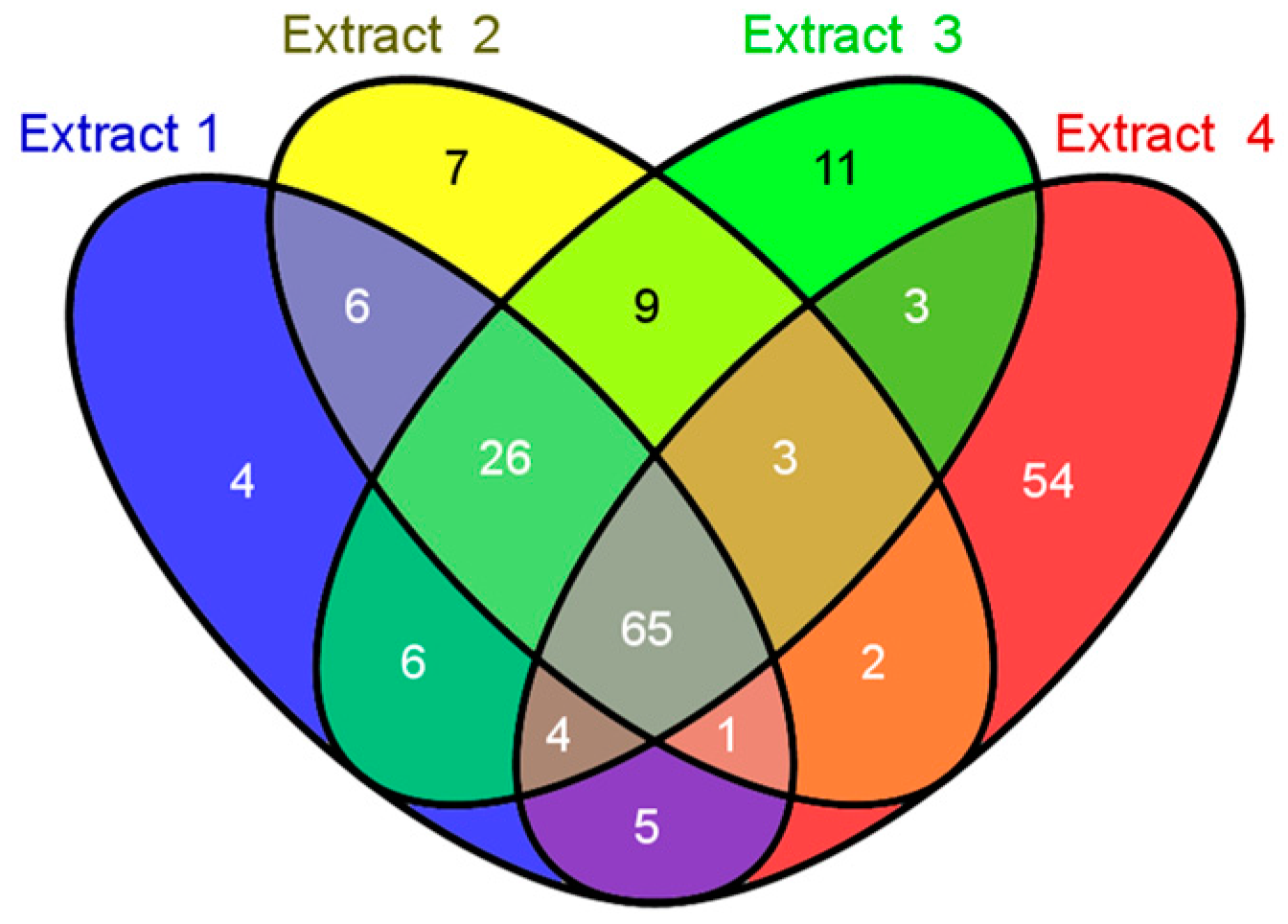
3.4. Identification of Peptides and Proteins by Proteome Analysis
3.5. Biological Process Classification of the Identified Proteins
3.6. Cellular Component of the Identified Proteins
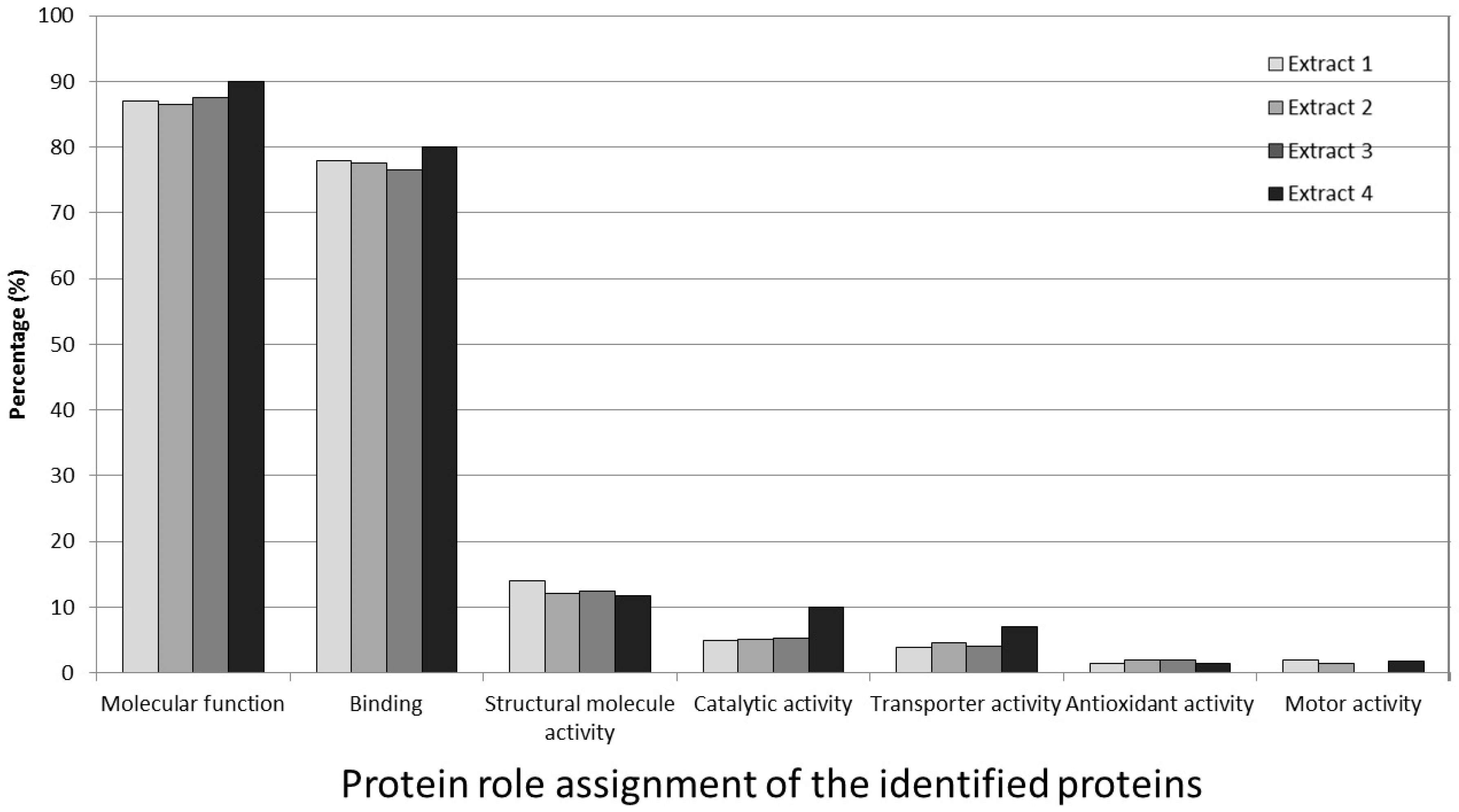
3.7. Protein Role Assignment of the Identified Proteins
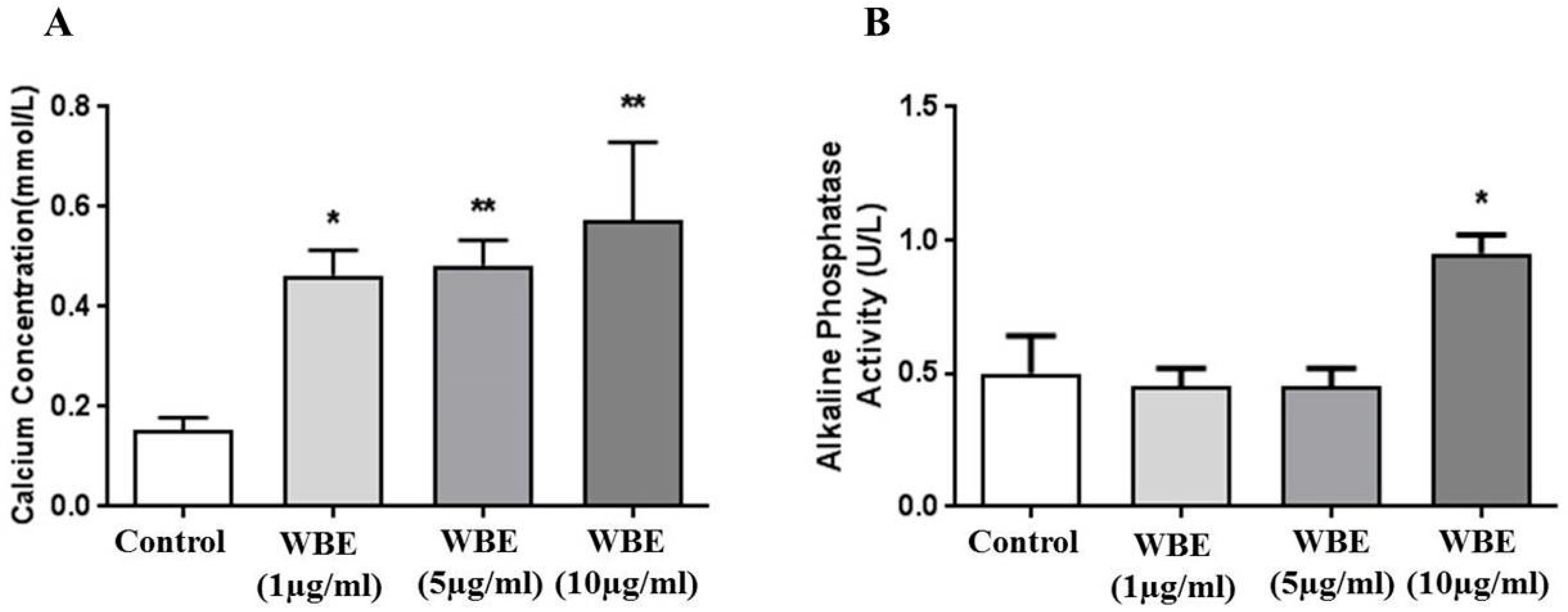
3.8. Calcium Concentration and Alkaline Phosphate Activity in WBE Treated Osteogenic Cells
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Christodoulou, C.; Cooper, C. What is osteoporosis. Postgrad. Med. J. 2003, 79, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Cole, Z.A.; Dennison, E.M.; Cooper, C. Osteoporosis epidemiology update. Curr. Rheumatol. Rep. 2008, 10, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Australian Government Department of Health and Aged Care, Whitepaper 2001, Costing Osteoporosis in Australia, 2001. Available online: http://www.health.gov.au (accessed on 24 August 2016).
- Laster, A.J.; Tanner, S.B. Duration of treatment in postmenopausal osteoporosis: How long to treat and what are the consequences of cessation of treatment? Rheum. Dis. Clin. N. Am. 2011, 37, 323–336. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Gang, O.Y. The present research and prospect of complementary and alternative medicine for prevention and treatment of osteoporosis. Chin. J. Osteoporos. 2014, 20, 1371–1374. [Google Scholar]
- United States Government, Complementary and Alternative Medicine Products and their Regulation by the Food and Drug Administration, December, 2006. Available online: http://www.fda.gov (accessed on 24 August 2016).
- Ge, Z.; Zhou, T. The similar comprehensive analysis of amino acid composition between tiger bone and sika deer, red deer, pig, sheep, dog bones. J. Math. Med. 1999, 12, 131–132. [Google Scholar]
- Australian Government Department of Foreign Affairs and Trade, 2008. Available online: http://www.dfat.gov.au/facts/wallabys.html (accessed on 24 August 2016).
- Kelly, J. Wallaby Industry Background, Wallaby Industries Association of Australia, 2008. Available online: http://www.wallaby-industry.asn.au/morinfo/BACKGR1.HTM (accessed on 24 August 2016).
- Zhang, J.; Wu, L.S.; Sun, S.C. Clinical study on effect of JINTIANGE capsules for treatment of primary osteoporosis. Chin. J. Osteoporos. 2005, 4, 490–495. [Google Scholar]
- Lloyd, S.A.; Loiselle, A.E.; Zhang, Y.; Donahue, H.J. Connexin 43 deficiency desensitizes bone to the effects of mechanical unloading through modulation of both arms of bone remodeling. Bone 2013, 57, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Ye, M.; Jiang, X.; Liu, G.; Feng, S.; Cui, L.; Zou, H. Method development of efficient protein extraction in bone tissue for proteome analysis. J. Proteome Res. 2007, 6, 2287–2294. [Google Scholar]
- Pokharel, D.; Padula, M.P.; Lu, J.F.; Tacchi, J.L.; Luk, F.; Djordjevic, S.P.; Bebawy, M. Proteome analysis of multidrug-resistant, breast cancer-derived microparticles. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, R.; Carpi, D.; Airoldi, L.; Chiabrando, C.; Bagnati, R.; Fanelli, R.; Moverare, S.; Ohlsson, C. Proteome analysis for the identification of in vivo estrogen-regulated proteins in bone. Proteomics 2005, 5, 4936–4945. [Google Scholar] [CrossRef] [PubMed]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.; Nesvizhskii, A.I.; Kolker, E.; Aebersold, R. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 2002, 74, 5383–5392. [Google Scholar] [CrossRef] [PubMed]
- Nesvizhskii, A.I.; Keller, A.; Kolker, E.; Aebersold, R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003, 75, 4646–4658. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.A.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-derived stem cells: Isolation, expansion and differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency, Committee for Medicinal Products for Human Use (CHMP). Guideline on the Specification Limits for Residues of Metal Catalysts or Metal Reagents. 2008. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003587.pdf (accessed on 24 August 2016).
- Ahmad, K.; Shaheen, M.; Khan, Z.; Bashir, H. Heavy metals contamination of soil and fodder: A possible risk to livestock. Sci. Technol. Dev. 2013, 32, 140–148. [Google Scholar]
- Currey, J.D.; Brear, K.; Zioupos, P. The effects of ageing and changes in mineral content in degrading the toughness of human femora. J. Biomech. 1996, 29, 257–260. [Google Scholar] [CrossRef]
- Fonseca, B.M.; Paquete, C.M.; Salgueiro, C.A.; Louro, R.O. The role of intramolecular interactions in the functional control of multiheme cytochromes c. FEBS Lett. 2012, 586, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Tabe, H.; Tanaka, Y. Artificial metalloenzymes constructed from hierarchically-assembled proteins. Chemistry 2013, 8, 1646–1660. [Google Scholar] [CrossRef] [PubMed]
- Dermauw, V.; Yisehak, K.; Belay, D.; Van Hecke, T. Mineral deficiency status of ranging zebu (Bos indicus) cattle around the Gilgel Gibe catchment, Ethiopia. Trop. Anim. Health Prod. 2013, 45, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.; Richards, R.; Schneider, S. Animal models for implant biomaterial research in bone: A review. Eur. Cell Mater. 2007, 13, 1–10. [Google Scholar] [PubMed]
- Zhang, J.H.; Li, P.F.; Dong, C.S. The composition analysis and evaluation of Alpaca bone. Shanghai J. Anim. Husb. Vet. Med. 2013, 4, 40–41. [Google Scholar]
- Movahedi Najafabadi, B.; Abnosi, M.H. Boron induces early matrix mineralization via calcium deposition and elevation of alkaline phosphatase activity in differentiated rat bone marrow mesenchymal stem cells. Cell J. 2016, 18, 62–73. [Google Scholar] [PubMed]



| Bone Samples | Water (%) | Ca (%) | p (%) |
|---|---|---|---|
| Femur | 54.44 ± 4.09 | 22.72 ± 8.14 | 12.36 ± 4.46 |
| Fibula | 34.05 ± 5.90 | 27.54 ± 1.46 | 16.47 ± 6.24 |
| Tibia | 35.23 ± 6.34 | 27.33 ± 3.16 | 14.92 ± 1.16 |
| Average | 41.24 ± 11.45 | 26.21 ± 6.87 | 14.72 ± 4.64 |
| Bone Samples | Na | Mg | Al | Fe | Sr | Ba |
|---|---|---|---|---|---|---|
| (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |
| Femur | 5.48 × 104 ± 0.53 | 0.99 × 103 ± 0.03 | 238.77 ± 22.99 | 135.04 ± 3.08 | 495.00 ± 15.71 | 89.16 ± 13.54 |
| Fibula | 5.16 × 104 ± 0.24 | 0.78 × 103 ± 0.14 | 292.71 ± 13.37 | 166.64 ± 1.38 | 611.83 ± 23.96 | 92.96 ± 14.00 |
| Tibia | 4.40 × 104 ± 0.81 | 0.83 × 103 ± 0.05 | 261.84 ± 14.85 | 153.08 ± 4.00 | 570.75 ± 11.59 | 84.62 ± 9.10 |
| Average | 5.01 × 104 ± 1.55 | 0.87 × 103 ± 0.21 | 264.44 ± 27.06 | 151.59 ± 15.85 | 559.19 ± 59.26 | 88.91 ± 4.18 |
| Bone Samples | Pb | Ni | Mn | Cu | Cr | Se | Zn |
|---|---|---|---|---|---|---|---|
| (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | (mg/kg) | |
| Femur | 0.15 ± 0.01 | 3.97 ± 0.86 | 1.31 ± 0.21 | 1.11 ± 0.33 | 1.08 ± 0.19 | 0.71 ± 0.03 | 71.65 ± 5.52 |
| Fibula | 0.41 ± 0.01 | 1.36 ± 0.45 | 2.82 ± 0.36 | 1.84 ± 1.13 | 1.30 ± 0.25 | 0.76 ± 0.04 | 81.74 ± 6.46 |
| Tibia | 0.16 ± 0.08 | 0.05 ± 0.02 | 1.31 ± 0.24 | 1.12 ± 0.90 | 0.09 ± 0.05 | 0.69 ± 0.05 | 77.44 ± 8.27 |
| Average | 0.24 ± 0.15 | 1.79 ± 1.99 | 2.11 ± 0.76 | 1.36 ± 0.42 | 0.82 ± 0.64 | 0.72 ± 0.12 | 76.94 ± 5.06 |
| Bone Protein Extracts | Femur (mg/g) | Tibia (mg/g) | Fibula (mg/g) |
|---|---|---|---|
| Extract 1 | 0.709 ± 0.07 | 0.611 ± 0.08 | 0.236± 0.03 |
| Extract 2 | 0.922 ± 0.04 | 0.718 ± 0.04 | 0.305 ± 0.02 |
| Extract 3 | 0.152 ± 0.02 | 0.092 ± 0.02 | 0.008 ± 0.05 |
| Extract 4 | 0.70 ± 0.04 | 0.787 ± 0.05 | 0.613 ± 0.01 |
| Total | 2.48 ± 0.1.36 | 2.21 ± 0.11 | 1.23 ± 0.13 |
| Wallaby Bone Protein Extracts | Number of Unique Spectra | Number of Unique Peptides | Number of Unique Proteins | Number of Clusters |
|---|---|---|---|---|
| Extract 1 | 334 | 293 | 117 | 74 |
| Extract 2 | 362 | 311 | 119 | 72 |
| Extract 3 | 409 | 362 | 127 | 79 |
| Extract 4 | 481 | 417 | 137 | 85 |
| Bone Samples | Water (%) | Ca (%) | p (%) |
|---|---|---|---|
| Wallaby bone | 41.24 | 26.21 | 14.72 |
| Alpaca Bone | 41 | 20 | 8.8 |
| Beef Bone | 64.2 | 5.1 | 2 |
| Pork Bone | 66.7 | 4.5 | 2.2 |
| Lamb Bone | 65.1 | 3.4 | 1.6 |
| Chicken Bone | 65.6 | 1 | 0.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lao, W.; Jin, X.; Tan, Y.; Xiao, L.; Padula, M.P.; Bishop, D.P.; Reedy, B.; Ong, M.; Kamal, M.A.; Qu, X. Characterisation of Bone Beneficial Components from Australian Wallaby Bone. Medicines 2016, 3, 23. https://doi.org/10.3390/medicines3030023
Lao W, Jin X, Tan Y, Xiao L, Padula MP, Bishop DP, Reedy B, Ong M, Kamal MA, Qu X. Characterisation of Bone Beneficial Components from Australian Wallaby Bone. Medicines. 2016; 3(3):23. https://doi.org/10.3390/medicines3030023
Chicago/Turabian StyleLao, Weiguo, Xingliang Jin, Yi Tan, Linda Xiao, Matthew P. Padula, David P. Bishop, Brian Reedy, Madeleine Ong, Mohammad A. Kamal, and Xianqin Qu. 2016. "Characterisation of Bone Beneficial Components from Australian Wallaby Bone" Medicines 3, no. 3: 23. https://doi.org/10.3390/medicines3030023
APA StyleLao, W., Jin, X., Tan, Y., Xiao, L., Padula, M. P., Bishop, D. P., Reedy, B., Ong, M., Kamal, M. A., & Qu, X. (2016). Characterisation of Bone Beneficial Components from Australian Wallaby Bone. Medicines, 3(3), 23. https://doi.org/10.3390/medicines3030023