HDAC5 Inhibition as a Therapeutic Strategy for Titin Deficiency-Induced Cardiac Remodeling: Insights from Human iPSC Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Public Dataset Acquisition and Data Processing
2.2. Cell Lines, Culture and Reagents for Major Interventions
2.3. Gene Silencing Using siRNA Transfection
2.4. Total RNA Extraction, cDNA Synthesis, and Quantitative Real-Time PCR (qRT PCR)
2.5. Statistical Analysis
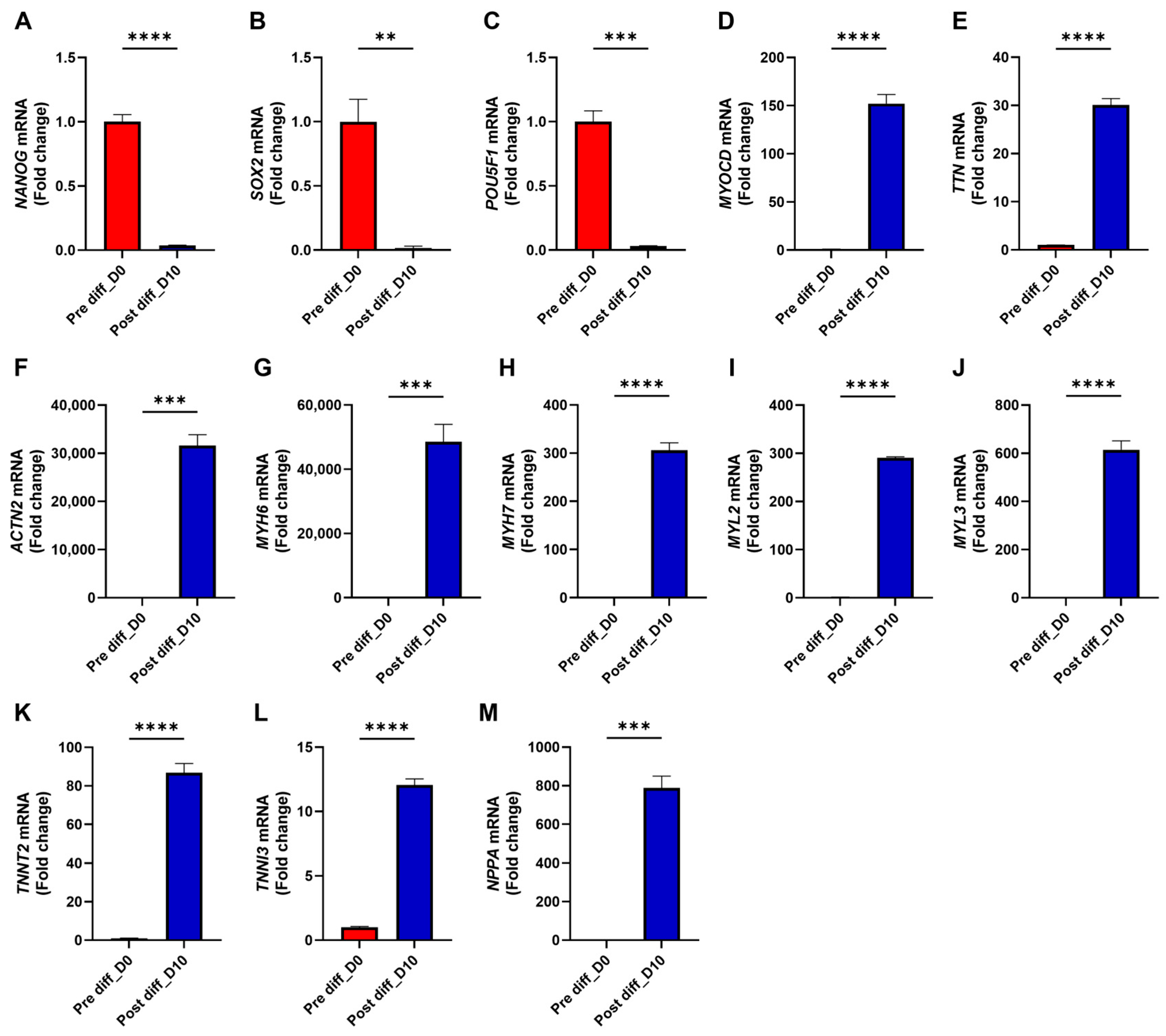
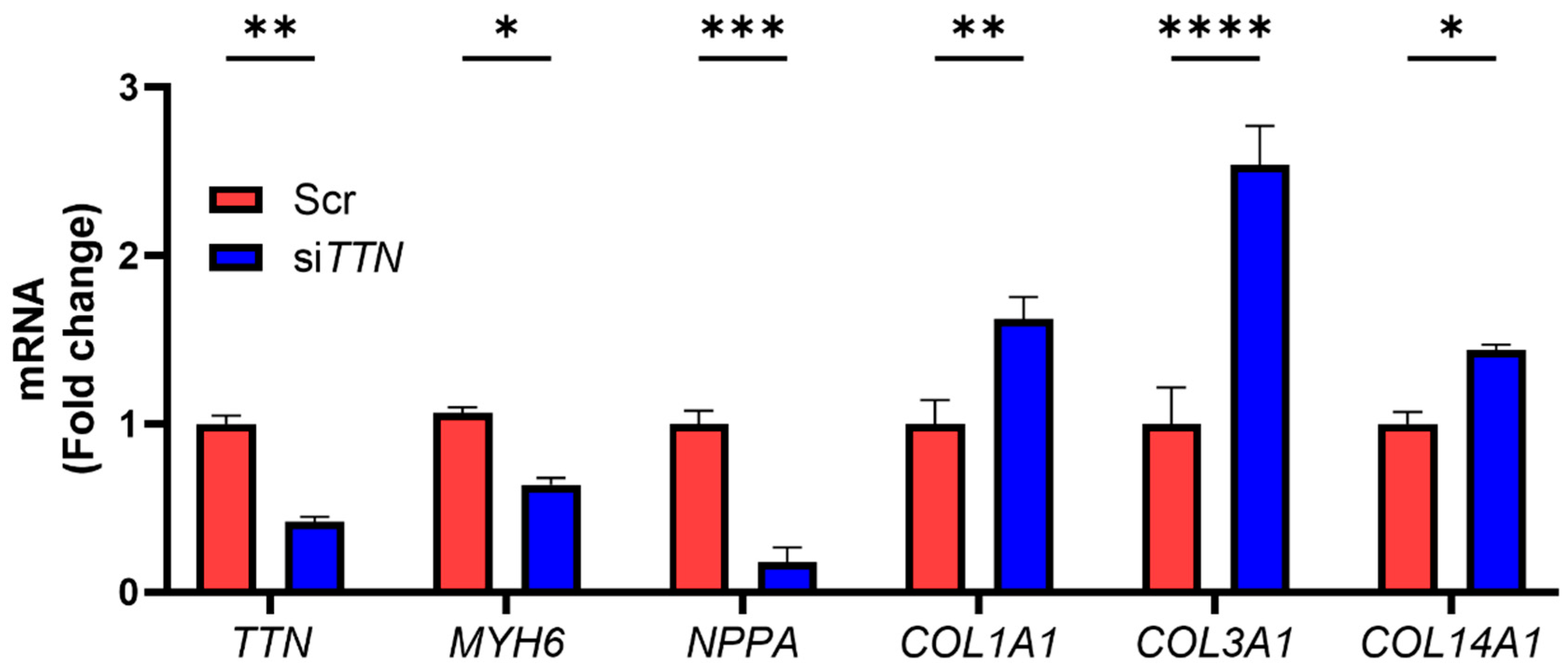
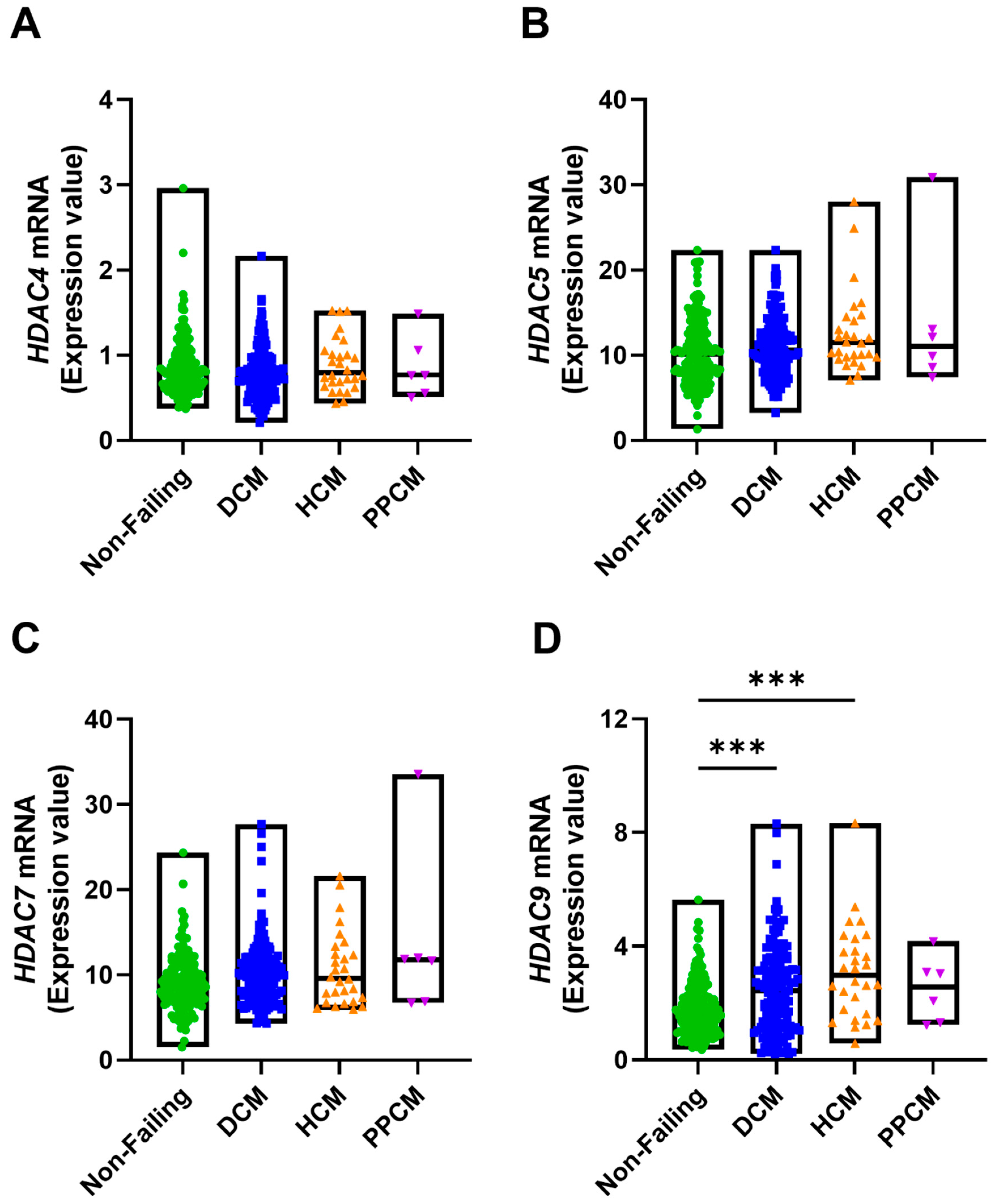
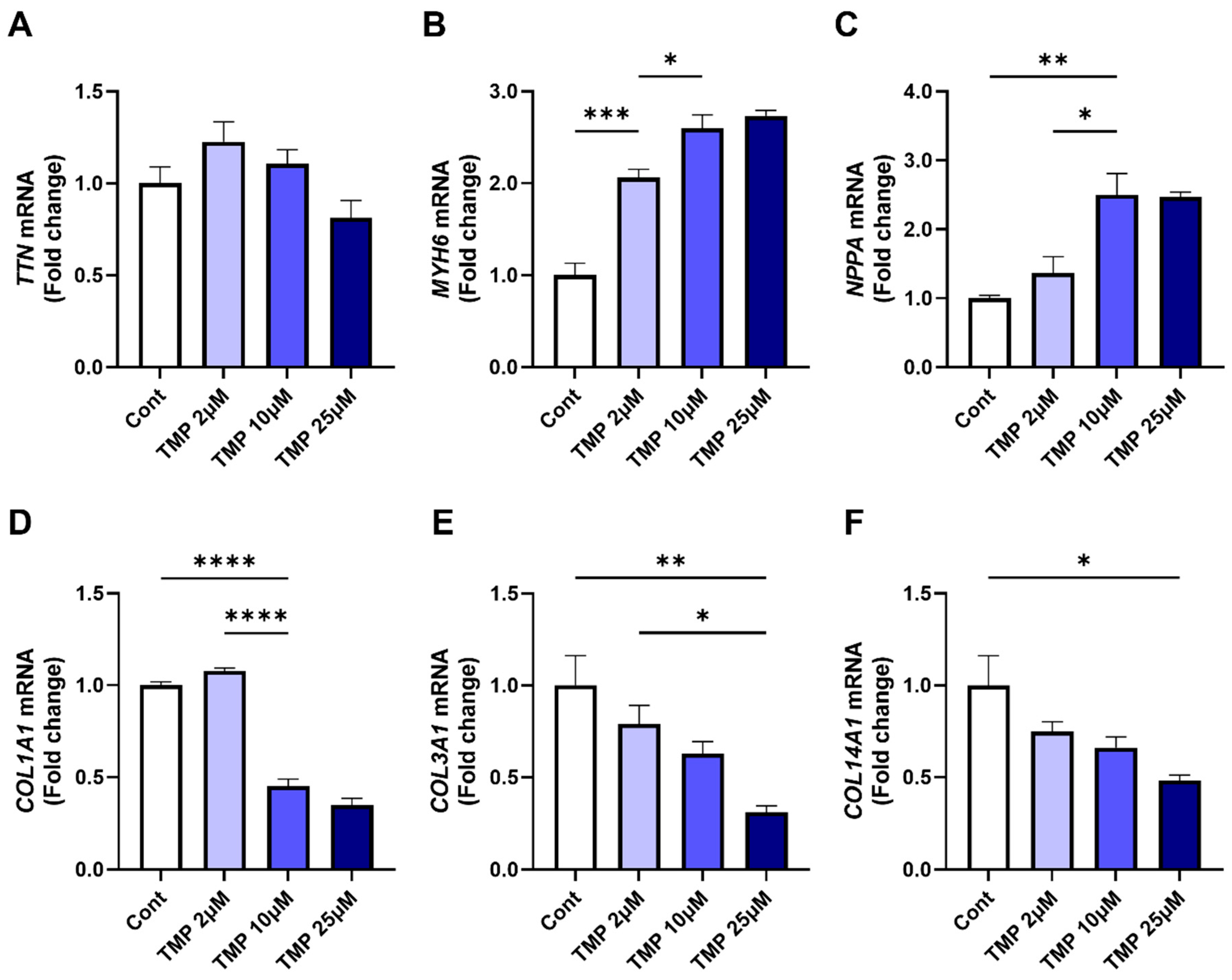
3. Results
3.1. TTN and Associated Gene Expression Alterations in Human Cardiomyopathies
3.2. TTN Deficiency Suppresses Cardiac Functional Genes and Augments Fibrosis-Associated Genes in iPSC-CMs
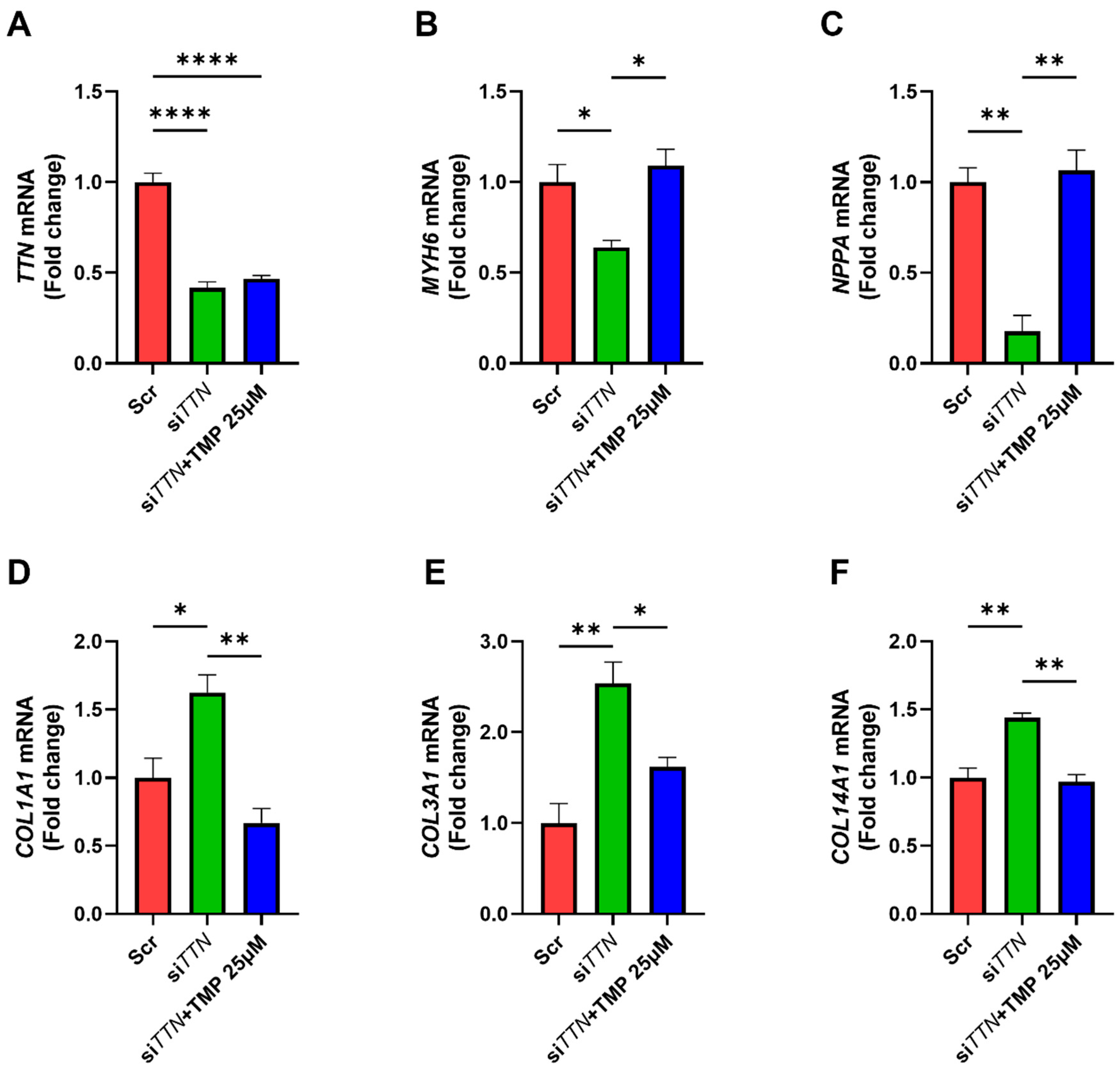
3.3. Class II HDAC Inhibitor TMP-195 Restores Cardiac Functional Genes and Suppresses Fibrosis-Associated Genes
3.4. TMP-195 Restores Cardiac Functional Gene Expression and Suppresses Fibrosis-Associated Gene Following TTN Silencing
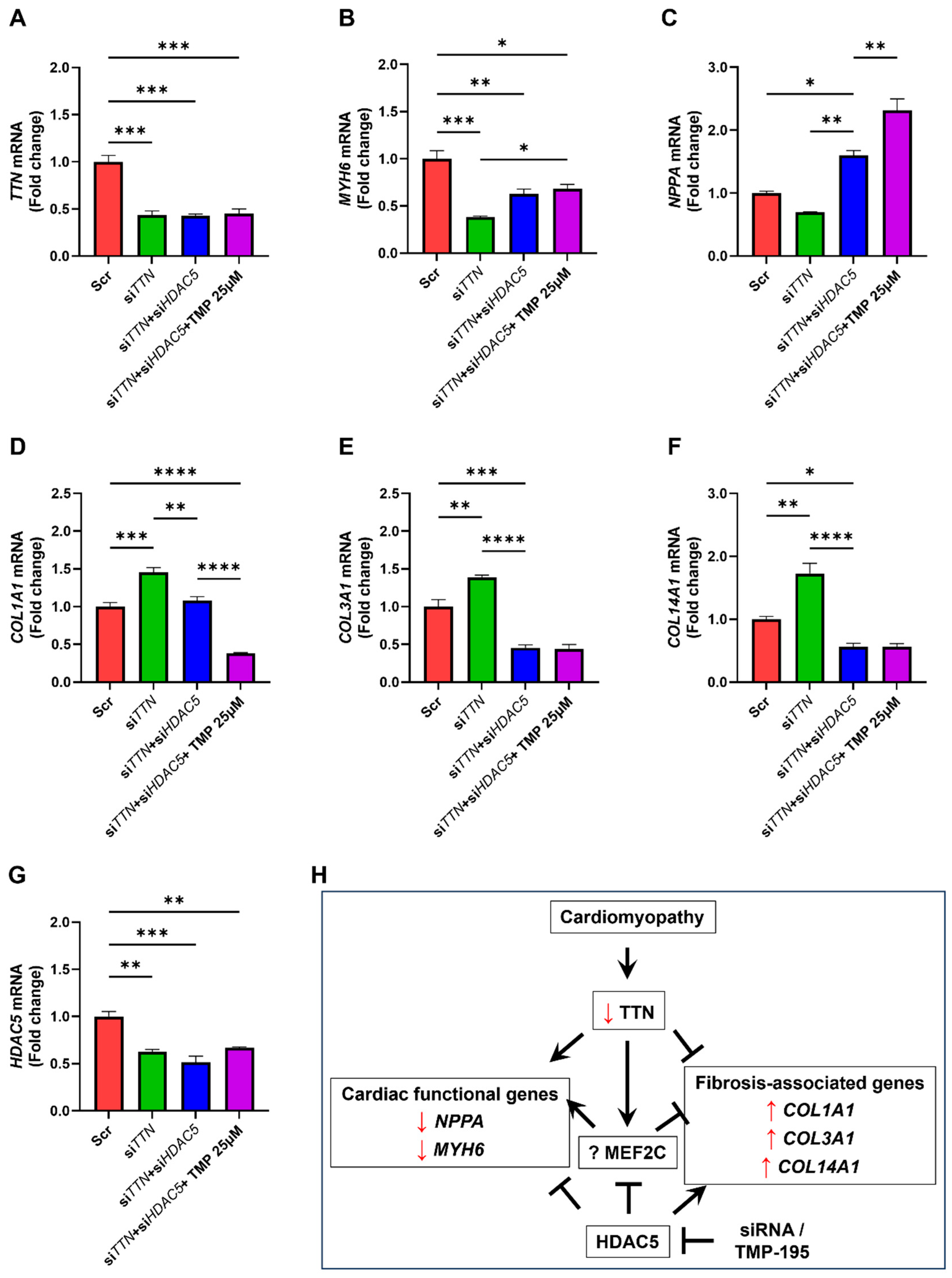
3.5. HDAC5 Inhibition Promotes Cardiac Functional Gene Expression and Suppresses Fibrosis-Associated Genes
3.6. TMP-195 Reverses TTN Deficiency-Induced Gene Alterations Through HDAC5 Inhibition
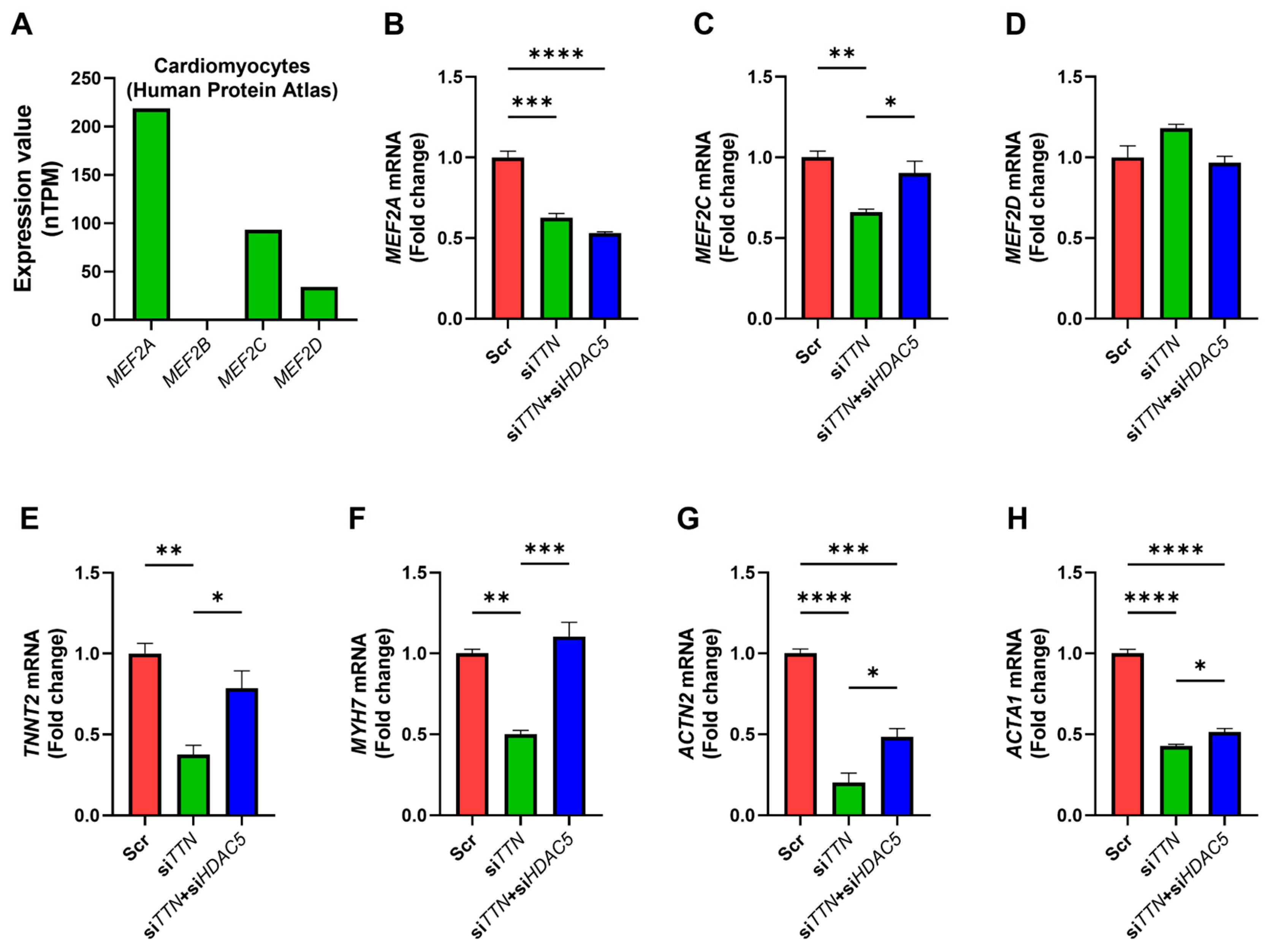
3.7. HDAC5 Inhibition Modulates MEF2-Associated Gene Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, T.; Song, D.; Dong, J.; Zhu, P.; Liu, J.; Liu, W.; Ma, X.; Zhao, L.; Ling, S. Current Understanding of the Pathophysiology of Myocardial Fibrosis and Its Quantitative Assessment in Heart Failure. Front. Physiol. 2017, 8, 238. [Google Scholar] [CrossRef] [PubMed]
- Hershberger, R.E.; Morales, A.; Siegfried, J.D. Clinical and Genetic Issues in Dilated Cardiomyopathy: A Review for Genetics Professionals. Genet. Med. 2010, 12, 655. [Google Scholar] [CrossRef]
- Jin, Y.; Che, W.; Yang, J.; Chang, S.; Bao, W.; Ren, X.; Yu, P.; Hou, A. Classification, Diagnosis, and Prognosis of Cardiomyopathy: A Comprehensive Narrative Review. Rev. Cardiovasc. Med. 2025, 26, 36280. [Google Scholar] [CrossRef]
- Schultheiss, H.P.; Fairweather, D.L.; Caforio, A.L.P.; Escher, F.; Hershberger, R.E.; Lipshultz, S.E.; Liu, P.P.; Matsumori, A.; Mazzanti, A.; McMurray, J.; et al. Dilated Cardiomyopathy. Nat. Rev. Dis. Prim. 2019, 5, 32. [Google Scholar] [CrossRef] [PubMed]
- Micaglio, E.; Monasky, M.M.; Bernardini, A.; Mecarocci, V.; Borrelli, V.; Ciconte, G.; Locati, E.T.; Piccoli, M.; Ghiroldi, A.; Anastasia, L.; et al. Clinical Considerations for a Family with Dilated Cardiomyopathy, Sudden Cardiac Death, and a Novel TTN Frameshift Mutation. Int. J. Mol. Sci. 2021, 22, 670. [Google Scholar] [CrossRef]
- Panjiyar, B.K.; Changlani, N.; Jha, S.K.; Khan, S.W.; Khan, O. Titin As the Culprit Behind Dilated Cardiomyopathy: A Case Series of Three Cases and a Comprehensive Literature Review. Cureus 2024, 16, e67489. [Google Scholar] [CrossRef]
- Hershberger, R.E.; Cowan, J.; Jordan, E.; Kinnamon, D.D. The Complex and Diverse Genetic Architecture of Dilated Cardiomyopathy. Circ. Res. 2021, 128, 1514. [Google Scholar] [CrossRef]
- Jansen, M.; Baas, A.F.; van Spaendonck-Zwarts, K.Y.; Ummels, A.S.; van den Wijngaard, A.; Jongbloed, J.D.H.; van Slegtenhorst, M.A.; Lekanne Deprez, R.H.; Wessels, M.W.; Michels, M.; et al. Mortality Risk Associated With Truncating Founder Mutations in Titin. Circ. Genom. Precis. Med. 2019, 12, e002436. [Google Scholar] [CrossRef] [PubMed]
- Hinson, J.T.; Campbell, S.G. TTN Truncation Variants Produce Sarcomere-Integrating Proteins of Uncertain Functional Significance. J. Clin. Investig. 2024, 134, e175206. [Google Scholar] [CrossRef]
- Kellermayer, D.; Tordai, H.; Kiss, B.; Török, G.; Péter, D.M.; Sayour, A.A.; Pólos, M.; Hartyánszky, I.; Szilveszter, B.; Labeit, S.; et al. Truncated Titin Is Structurally Integrated into the Human Dilated Cardiomyopathic Sarcomere. J. Clin. Investig. 2024, 134, e169753. [Google Scholar] [CrossRef]
- Schlittler, M.; Pramstaller, P.P.; Rossini, A.; De Bortoli, M. Myocardial Fibrosis in Hypertrophic Cardiomyopathy: A Perspective from Fibroblasts. Int. J. Mol. Sci. 2023, 24, 14845. [Google Scholar] [CrossRef]
- Goli, R.; Li, J.; Brandimarto, J.; Levine, L.D.; Riis, V.; McAfee, Q.; Depalma, S.; Haghighi, A.; Seidman, J.G.; Seidman, C.E.; et al. Genetic and Phenotypic Landscape of Peripartum Cardiomyopathy. Circulation 2021, 143, 1852–1862. [Google Scholar] [CrossRef]
- Jolfayi, A.G.; Kohansal, E.; Ghasemi, S.; Naderi, N.; Hesami, M.; MozafaryBazargany, M.H.; Moghadam, M.H.; Fazelifar, A.F.; Maleki, M.; Kalayinia, S. Exploring TTN Variants as Genetic Insights into Cardiomyopathy Pathogenesis and Potential Emerging Clues to Molecular Mechanisms in Cardiomyopathies. Sci. Rep. 2024, 14, 5313. [Google Scholar] [CrossRef]
- Davis, K.; Azarcon, P.; Hickenlooper, S.; Bia, R.; Horiuchi, E.; Szulik, M.W.; Franklin, S. The Role of Demethylases in Cardiac Development and Disease. J. Mol. Cell. Cardiol. 2021, 158, 89–100. [Google Scholar] [CrossRef]
- Lau, E.; Cao, Q.; Lam, M.P.Y.; Wang, J.; Ng, D.C.M.; Bleakley, B.J.; Lee, J.M.; Liem, D.A.; Wang, D.; Hermjakob, H.; et al. Integrated Omics Dissection of Proteome Dynamics during Cardiac Remodeling. Nat. Commun. 2018, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- BaniHani, H.A.; Khaled, L.H.; Al Sharaa, N.M.; A Al Saleh, R.; Bin Ghalaita, A.K.; Bin Sulaiman, A.S.; Holeihel, A. Causes, Diagnosis, Treatment, and Prognosis of Cardiac Fibrosis: A Systematic Review. Cureus 2025, 17, e81264. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Y.; Chen, S.; Zhou, J.; Li, J.; Cheng, Y. Epigenetics-Based Therapeutics for Myocardial Fibrosis. Life Sci. 2021, 271, 119186. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.R.; Meredith, G.W.; Entcheva, E. Human IPSC-Cardiomyocytes as an Experimental Model to Study Epigenetic Modifiers of Electrophysiology. Cells 2022, 11, 200. [Google Scholar] [CrossRef]
- Grimaldi, V.; De Pascale, M.R.; Zullo, A.; Soricelli, A.; Infante, T.; Mancini, F.P.; Napoli, C. Evidence of Epigenetic Tags in Cardiac Fibrosis. J. Cardiol. 2017, 69, 401–408. [Google Scholar] [CrossRef]
- Zhang, C.L.; McKinsey, T.A.; Chang, S.; Antos, C.L.; Hill, J.A.; Olson, E.N. Class II Histone Deacetylases Act as Signal-Responsive Repressors of Cardiac Hypertrophy. Cell 2002, 110, 479–488. [Google Scholar] [CrossRef]
- Zhu, C.; Piao, Z.; Jin, L. HDAC5 Inhibition Attenuates Ventricular Remodeling and Cardiac Dysfunction. Orphanet J. Rare Dis. 2023, 18, 266. [Google Scholar] [CrossRef]
- Bai, L.; Kee, H.J.; Choi, S.Y.; Seok, Y.M.; Kim, G.R.; Kee, S.J.; Kook, H.; Jeong, M.H. HDAC5 Inhibition Reduces Angiotensin II-Induced Vascular Contraction, Hypertrophy, and Oxidative Stress in a Mouse Model. Biomed. Pharmacother. 2021, 134, 111162. [Google Scholar] [CrossRef]
- Desjardins, C.A.; Naya, F.J. The Function of the MEF2 Family of Transcription Factors in Cardiac Development, Cardiogenomics, and Direct Reprogramming. J. Cardiovasc. Dev. Dis. 2016, 3, 26. [Google Scholar] [CrossRef]
- Materna, S.C.; Sinha, T.; Barnes, R.M.; Lammerts van Bueren, K.; Black, B.L. Cardiovascular Development and Survival Require Mef2c Function in the Myocardial but Not the Endothelial Lineage. Dev. Biol. 2018, 445, 170. [Google Scholar] [CrossRef]
- Muncie-Vasic, J.M.; Sinha, T.; Clark, A.P.; Brower, E.F.; Saucerman, J.J.; Black, B.L.; Bruneau, B.G. MEF2C Controls Segment-Specific Gene Regulatory Networks That Direct Heart Tube Morphogenesis. Genes Dev. 2025. [Google Scholar] [CrossRef]
- Tan, W.L.W.; Anene-Nzelu, C.G.; Wong, E.; Lee, C.J.M.; Tan, H.S.; Tang, S.J.; Perrin, A.; Wu, K.X.; Zheng, W.; Ashburn, R.J.; et al. Epigenomes of Human Hearts Reveal New Genetic Variants Relevant for Cardiac Disease and Phenotype. Circ. Res. 2020, 127, 761. [Google Scholar] [CrossRef] [PubMed]
- Flam, E.; Jang, C.; Murashige, D.; Yang, Y.; Morley, M.P.; Jung, S.; Kantner, D.S.; Pepper, H.; Bedi, K.C.; Brandimarto, J.; et al. Integrated Landscape of Cardiac Metabolism in End-Stage Human Nonischemic Dilated Cardiomyopathy. Nat. Cardiovasc. Res. 2022, 1, 817. [Google Scholar] [CrossRef] [PubMed]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Hasan, A.U.; Serada, S.; Sato, S.; Obara, M.; Hirata, S.; Nagase, Y.; Kondo, Y.; Taira, E. KDM4B Histone Demethylase Inhibition Attenuates Tumorigenicity of Malignant Melanoma Cells by Overriding the P53-Mediated Tumor Suppressor Pathway. J. Cell. Biochem. 2024, 126, e30643. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.U.; Ohmori, K.; Hashimoto, T.; Kamitori, K.; Yamaguchi, F.; Noma, T.; Igarashi, J.; Tsuboi, K.; Tokuda, M.; Nishiyama, A.; et al. GPR120 in Adipocytes Has Differential Roles in the Production of Pro-Inflammatory Adipocytokines. Biochem. Biophys. Res. Commun. 2017, 486, 76–82. [Google Scholar] [CrossRef]
- Hasan, A.U.; Obara, M.; Sato, S.; Kondo, Y.; Taira, E. CD146/MCAM Links Doxorubicin-Induced Epigenetic Dysregulation to the Impaired Fatty Acid Transportation in H9c2 Cardiomyoblasts. Biochem. Biophys. Res. Commun. 2024, 693, 149370. [Google Scholar] [CrossRef]
- Akhtar, M.M.; Lorenzini, M.; Cicerchia, M.; Ochoa, J.P.; Hey, T.M.; Sabater Molina, M.; Restrepo-Cordoba, M.A.; Dal Ferro, M.; Stolfo, D.; Johnson, R.; et al. Clinical Phenotypes and Prognosis of Dilated Cardiomyopathy Caused by Truncating Variants in the TTN Gene. Circ. Heart Fail. 2020, 13, e006832. [Google Scholar] [CrossRef]
- Gi, W.T.; Haas, J.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Tappu, R.; Lehmann, D.H.; Samani, O.S.; Wisdom, M.; Keller, A.; Katus, H.A.; et al. Epigenetic Regulation of Alternative MRNA Splicing in Dilated Cardiomyopathy. J. Clin. Med. 2020, 9, 1499. [Google Scholar] [CrossRef]
- Tharp, C.A.; Haywood, M.E.; Sbaizero, O.; Taylor, M.R.G.; Mestroni, L. The Giant Protein Titin’s Role in Cardiomyopathy: Genetic, Transcriptional, and Post-Translational Modifications of TTN and Their Contribution to Cardiac Disease. Front. Physiol. 2019, 10, 1436. [Google Scholar] [CrossRef]
- Fullenkamp, D.E. Regulation of TTN as a Mechanism of and Treatment for Heart Failure. J. Clin. Investig. 2025, 135, e189335. [Google Scholar] [CrossRef]
- Stroik, D.; Gregorich, Z.R.; Raza, F.; Ge, Y.; Guo, W. Titin: Roles in Cardiac Function and Diseases. Front. Physiol. 2024, 15, 1385821. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; Ghahremani, S.; Zimmerman, T.; Legere, N.; Thakar, K.; Ladha, F.A.; Pettinato, A.M.; Hinson, J.T. Reading Frame Repair of TTN Truncation Variants Restores Titin Quantity and Functions. Circulation 2021, 145, 194. [Google Scholar] [CrossRef] [PubMed]
- McAfee, Q.; Caporizzo, M.A.; Uchida, K.; Bedi, K.C.; Margulies, K.B.; Arany, Z.; Prosser, B.L. Truncated Titin Protein in Dilated Cardiomyopathy Incorporates into the Sarcomere and Transmits Force. J. Clin. Investig. 2024, 134, e170196. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Nie, J.; Wang, D.W.; Ni, L. Mechanism of Histone Deacetylases in Cardiac Hypertrophy and Its Therapeutic Inhibitors. Front. Cardiovasc. Med. 2022, 9, 931475. [Google Scholar] [CrossRef]
- Man, J.; Barnett, P.; Christoffels, V.M. Structure and Function of the Nppa–Nppb Cluster Locus during Heart Development and Disease. Cell. Mol. Life Sci. 2018, 75, 1435. [Google Scholar] [CrossRef]
- Guerriero, J.L.; Sotayo, A.; Ponichtera, H.E.; Castrillon, J.A.; Pourzia, A.L.; Schad, S.; Johnson, S.F.; Carrasco, R.D.; Lazo, S.; Bronson, R.T.; et al. Class IIa HDAC Inhibition Reduces Breast Tumors and Metastases via Anti-Tumor Macrophages. Nature 2017, 543, 428. [Google Scholar] [CrossRef]
- Hu, T.; Schreiter, F.C.; Bagchi, R.A.; Tatman, P.D.; Hannink, M.; McKinsey, T.A. HDAC5 Catalytic Activity Suppresses Cardiomyocyte Oxidative Stress and NRF2 Target Gene Expression. J. Biol. Chem. 2019, 294, 8640. [Google Scholar] [CrossRef]
- Wu, C.P.; Lusvarghi, S.; Wang, J.C.; Hsiao, S.H.; Huang, Y.H.; Hung, T.H.; Ambudkar, S.V. The Selective Class IIa Histone Deacetylase Inhibitor TMP195 Resensitizes ABCB1- and ABCG2-Overexpressing Multidrug-Resistant Cancer Cells to Cytotoxic Anticancer Drugs. Int. J. Mol. Sci. 2019, 21, 238. [Google Scholar] [CrossRef]
- Zhang, W.; Guan, Y.; Bayliss, G.; Zhuang, S. Class IIa HDAC Inhibitor TMP195 Alleviates Lipopolysaccharide-Induced Acute Kidney Injury. Am. J. Physiol. Ren. Physiol. 2020, 319, F1015–F1026. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Yang, F.; Liu, X.; Zhan, P.; Wu, J.; Wang, X.; Wang, Z.; Tang, W.; Sun, Y.; et al. HDAC9-Mediated Epithelial Cell Cycle Arrest in G2/M Contributes to Kidney Fibrosis in Male Mice. Nat. Commun. 2023, 14, 3007. [Google Scholar] [CrossRef]
- Wei, J.; Joshi, S.; Speransky, S.; Crowley, C.; Jayathilaka, N.; Lei, X.; Wu, Y.; Gai, D.; Jain, S.; Hoosien, M.; et al. Reversal of Pathological Cardiac Hypertrophy via the MEF2-Coregulator Interface. JCI Insight. 2017, 2, e91068. [Google Scholar] [CrossRef]
- McCalmon, S.A.; Desjardins, D.M.; Ahmad, S.; Davidoff, K.S.; Snyder, C.M.; Sato, K.; Ohashi, K.; Kielbasa, O.M.; Mathew, M.; Ewen, E.P.; et al. Modulation of Angiotensin II–Mediated Cardiac Remodeling by the MEF2A Target Gene Xirp2. Circ. Res. 2010, 106, 952. [Google Scholar] [CrossRef] [PubMed]
- Kubanek, M.; Binova, J.; Piherova, L.; Krebsova, A.; Kotrc, M.; Hartmannova, H.; Hodanova, K.; Musalkova, D.; Stranecky, V.; Palecek, T.; et al. Genotype Is Associated with Left Ventricular Reverse Remodelling and Early Events in Recent-Onset Dilated Cardiomyopathy. ESC Heart Fail. 2024, 11, 4127–4138. [Google Scholar] [CrossRef]
- Xu, J.; Gong, N.L.; Bodi, I.; Aronow, B.J.; Backx, P.H.; Molkentin, J.D. Myocyte Enhancer Factors 2A and 2C Induce Dilated Cardiomyopathy in Transgenic Mice. J. Biol. Chem. 2006, 281, 9152–9162. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.W.; Saul, D.; Neyazi, M.; Schmid, M.; Wakimoto, H.; Slaven, N.; Lee, J.H.; Layton, O.; Wasson, L.K.; et al. Regulation of Sarcomere Formation and Function in the Healthy Heart Requires a Titin Intronic Enhancer. J. Clin. Investig. 2024, 135, e183353. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Andreucci, M.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Bracale, U.M.; Serra, R. Vascular Biology of Arterial Aneurysms. Ann. Vasc. Surg. 2023, 94, 378–389. [Google Scholar] [CrossRef] [PubMed]


| Gene Name | Forward Primer | Reverse Primer |
|---|---|---|
| TTN | AGTCCCCATCGCCCATAAGA | TGGAGATTCTTGCTGCTGGAG |
| HDAC4 | TGTTTCTGCCTTGCTGGGAA | GAACGGACAGCGTTTGCATT |
| HDAC5 | TGCGGAACAAGGAGAAGAGC | GGGCTCCTTTGACTTCGACA |
| HDAC7 | GCTTCAATGTCAATGTGGCCT | GCGATGGGCATCACGACTAT |
| HDAC9 | TCCAGCCACCCTCATGTTAC | GGGAGACTGAGGATGTAAGGG |
| MYH6 | GCCCTTTGACATTCGCACTG | TTCACAGTCACCGTCTTCCC |
| MYH7 | CTCCCTGATCCACTATGCCG | CTGATACAAGCCCACGACAGT |
| NPPA | CAGCCCAGCCCAGAGAGATG | AGCTTGCTTTTTAGGAGGGCA |
| MYOCD | GCCATCCTCCAAGCTTCTCT | TGTAAACCAGCCATCTTTTGC |
| TNNT2 | GCCCAATGGAGGAGTCCAAA | ATCAGCGCCTGCAACTCATT |
| TNNI3 | AGTCACCAAGAACATCACGGA | CCGCTTAAACTTGCCTCGAA |
| MYL2 | ATCATGGACCAGAACAGGGA | TCATTTTTCACGTTCACTCGC |
| MYL3 | TCCAAGAACAAGGACACAGGC | ATTGCCCTCCTTGTCGAAGA |
| ACTN2 | TCTCTTGCTTCTACCACGCT | TCCATTCCAAAAGCTCACTCG |
| ACTA1 | ACCTGTATGCCAACAACGTCA | TGATCTTCATGGTGCTGGGT |
| MEF2A | GCAATGCAGGTGGGATGTTG | TGCACCAGTAGCTCCAATCA |
| MEF2C | ACCAGGCAGCAAGAATACGA | TAGCCAATGACTGAGCCGAC |
| MEF2D | CTGGAGGACAAGTACCGACG | CTCTGATTGGACACGGGCAC |
| NANOG | TGGCCGAAGAATAGCAATGG | GGTTCCCAGTCGGGTTCAC |
| SOX2 | AGAAGGATAAGTACACGCTGCC | TCATGTGCGCGTAACTGTCC |
| POU5F1 | AAACGACCATCTGCCGCTTT | CACGAGGGTTTCTGCTTTGC |
| COL1A1 | GCCAAGACGAAGACATCCCA | CGTCATCGCACAACACCTTG |
| COL3A1 | TGTTCCACGGAAACACTGGT | GGATTGCCGTAGCTAAACTGA |
| COL14A1 | GCTGGGGATGAAAAAGAGATGA | GCCTCTTCTCCAAACATGGC |
| RPLP0 | GAAACTCTGCATTCTCGCTTCC | GACTCGTTTGTACCCGTTGATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, A.U.; Sato, S.; Obara, M.; Kondo, Y.; Taira, E. HDAC5 Inhibition as a Therapeutic Strategy for Titin Deficiency-Induced Cardiac Remodeling: Insights from Human iPSC Models. Medicines 2025, 12, 26. https://doi.org/10.3390/medicines12040026
Hasan AU, Sato S, Obara M, Kondo Y, Taira E. HDAC5 Inhibition as a Therapeutic Strategy for Titin Deficiency-Induced Cardiac Remodeling: Insights from Human iPSC Models. Medicines. 2025; 12(4):26. https://doi.org/10.3390/medicines12040026
Chicago/Turabian StyleHasan, Arif Ul, Sachiko Sato, Mami Obara, Yukiko Kondo, and Eiichi Taira. 2025. "HDAC5 Inhibition as a Therapeutic Strategy for Titin Deficiency-Induced Cardiac Remodeling: Insights from Human iPSC Models" Medicines 12, no. 4: 26. https://doi.org/10.3390/medicines12040026
APA StyleHasan, A. U., Sato, S., Obara, M., Kondo, Y., & Taira, E. (2025). HDAC5 Inhibition as a Therapeutic Strategy for Titin Deficiency-Induced Cardiac Remodeling: Insights from Human iPSC Models. Medicines, 12(4), 26. https://doi.org/10.3390/medicines12040026








