Dual Antibiotic-Infused Liposomes to Control Methicillin-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Material and Methods
2.1. Liposome Preparation
2.2. DLS
2.3. Encapsulation Efficacy
2.4. Antibiotics Release Kinetics
2.5. FTIR
2.6. SEM
2.7. Bacterial Strain and Antibacterial Assay
2.8. MIC
2.9. Biofilm Eradication
2.10. Leakage Assay
2.11. Statistical Analysis
3. Results and Discussion
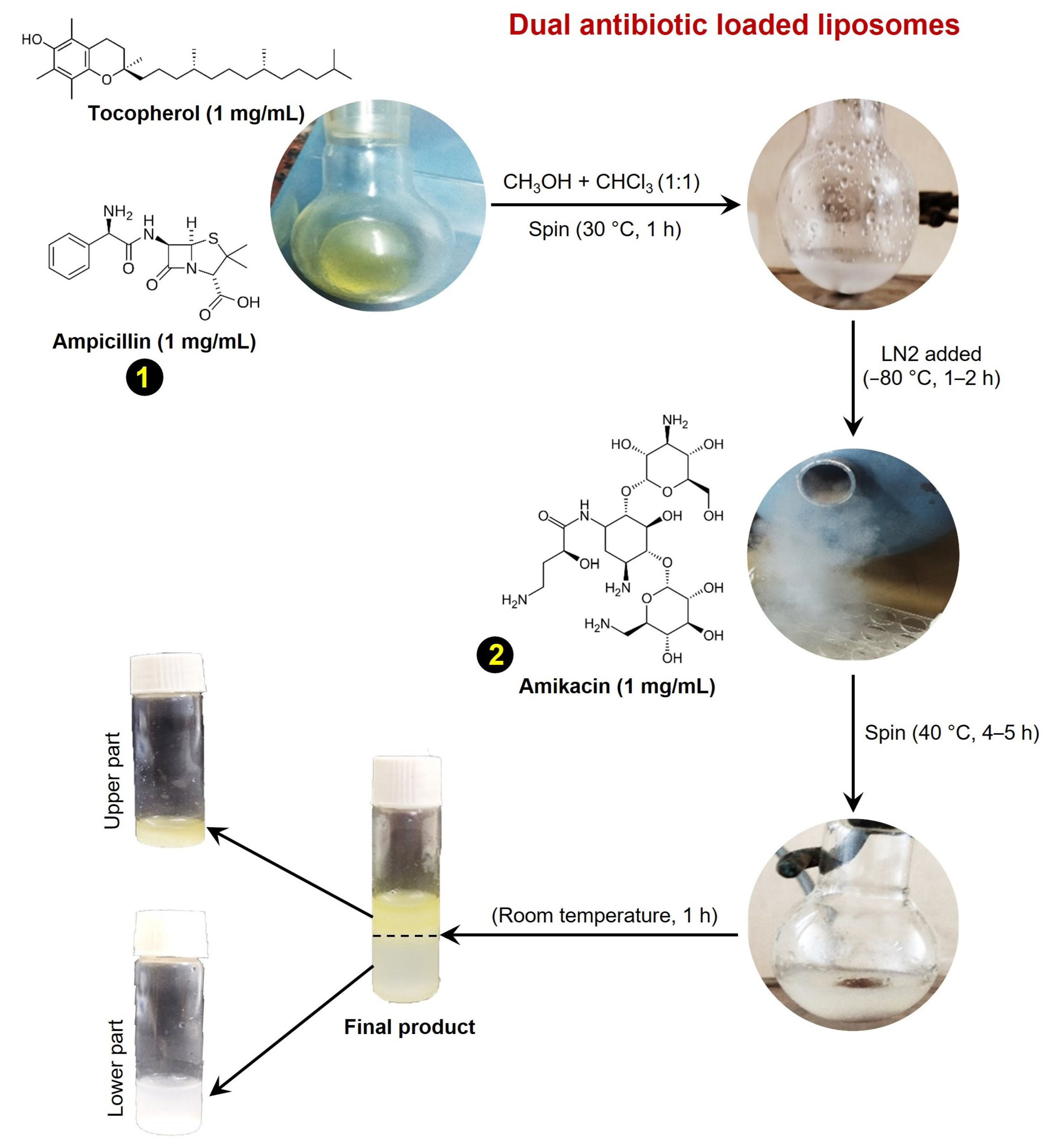
3.1. Synthesis of Dual Antibiotic-Loaded Tocopherol Conjugated Liposomes
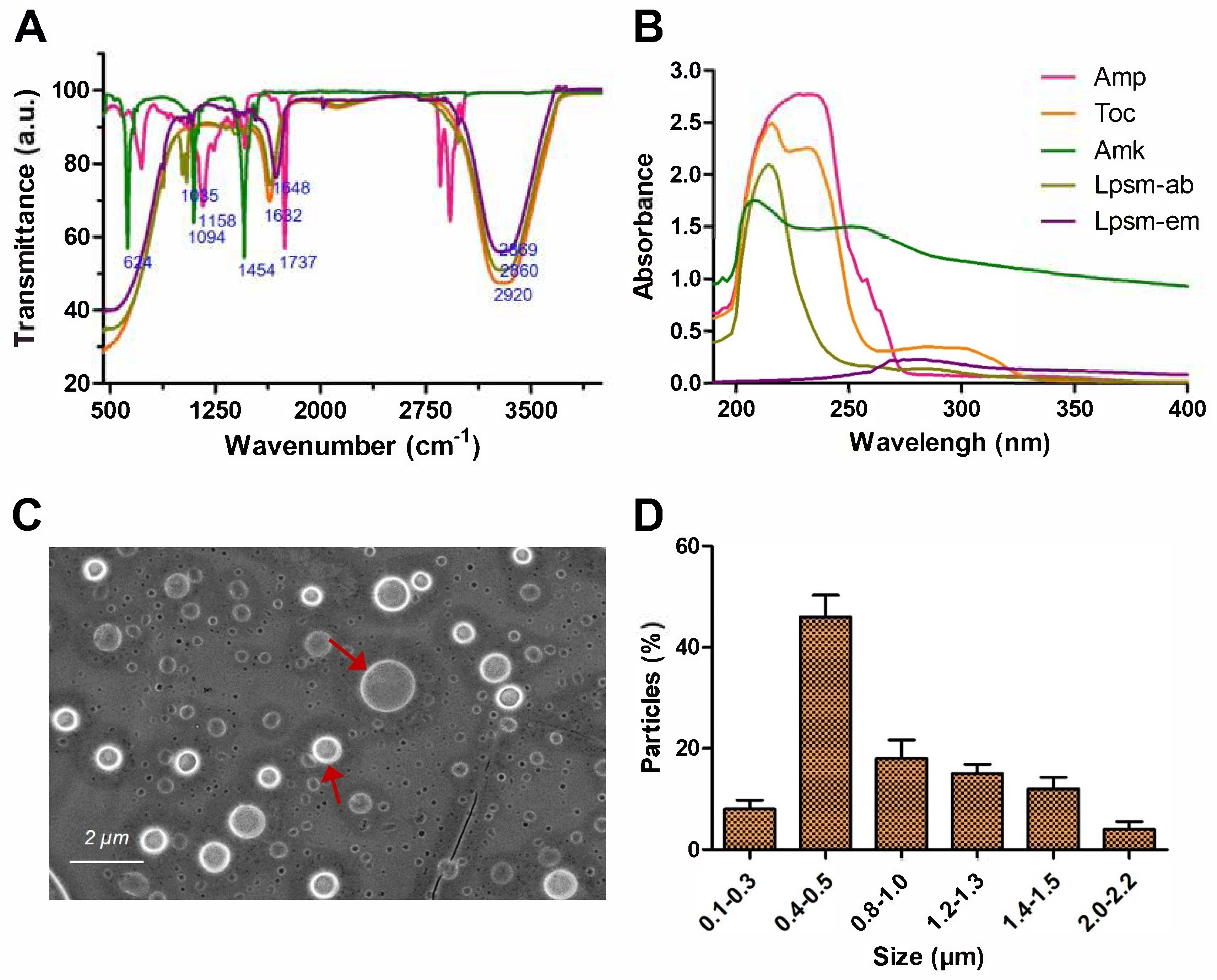
3.2. Spectroscopic Analysis
3.3. Microscopic Size Analysis of Liposomes
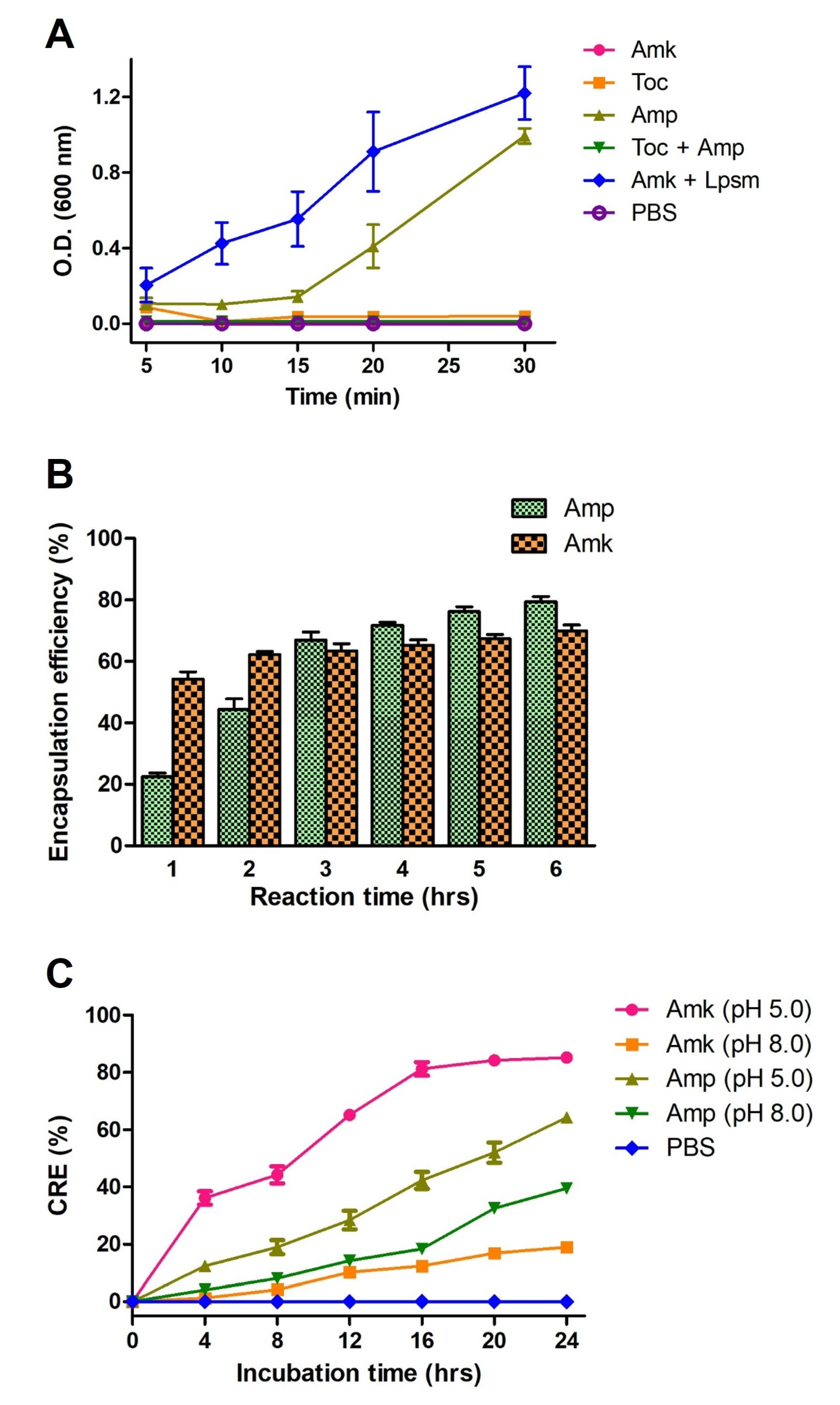
3.4. Encapsulation and Release Kinetics Liposomes
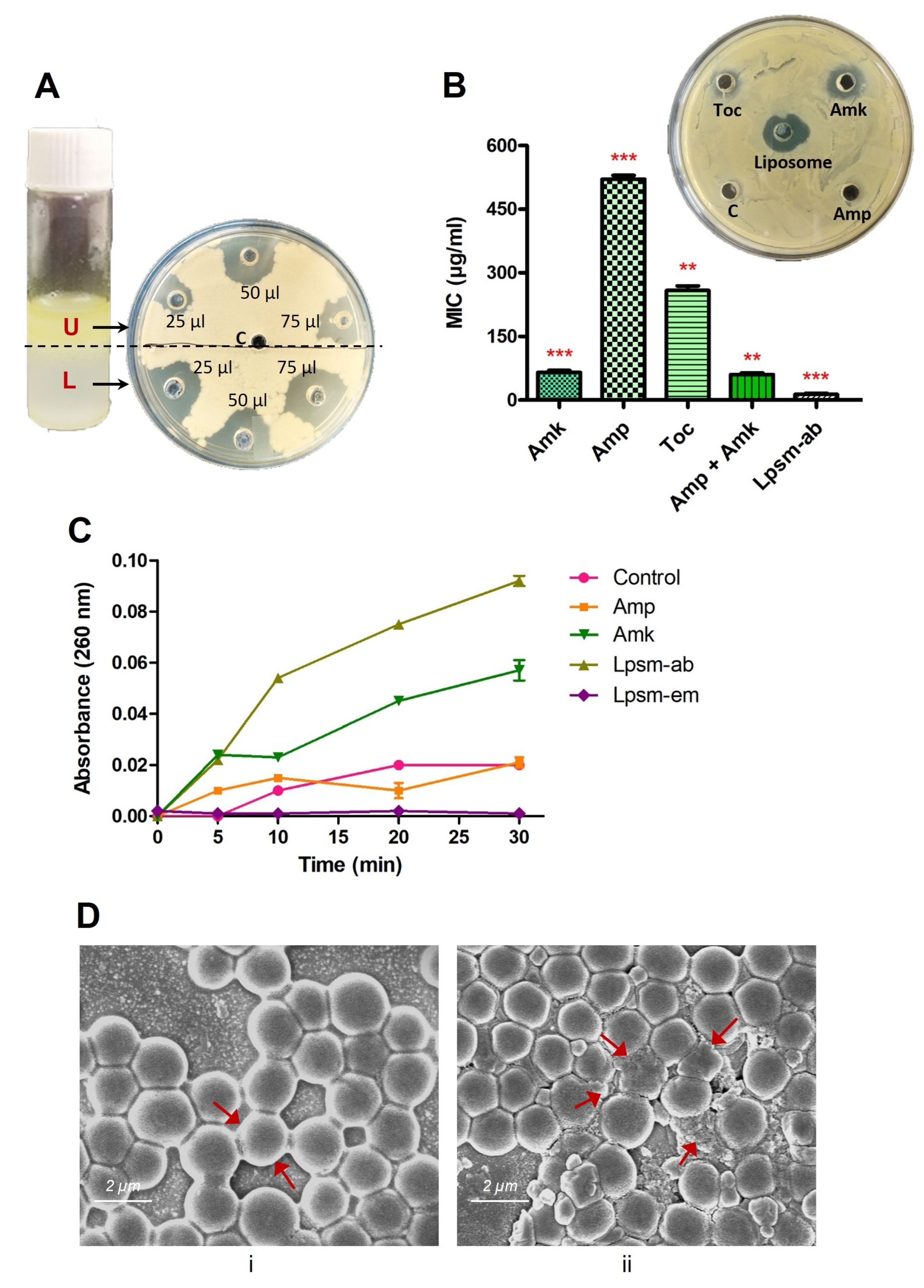
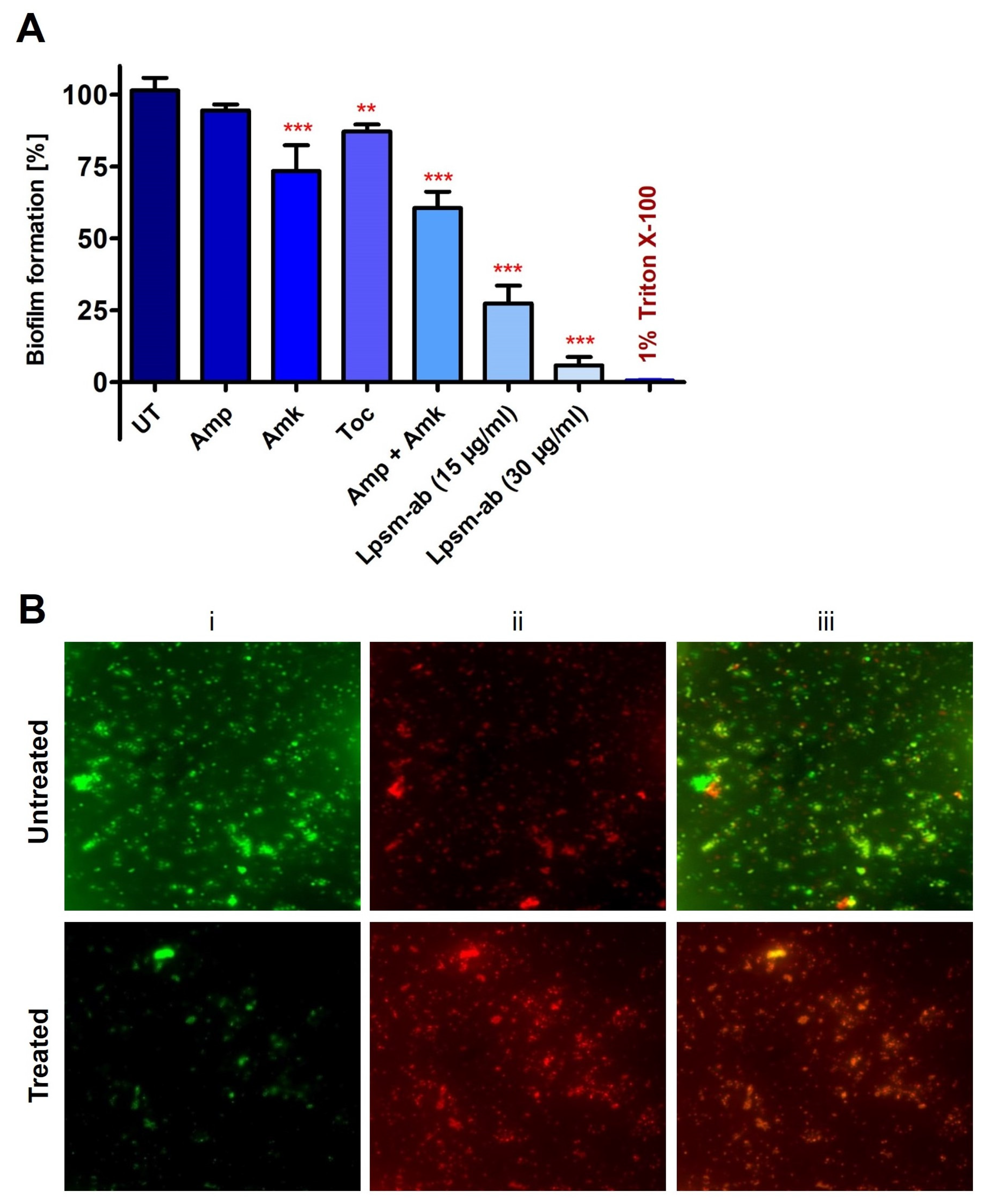
3.5. Antimicrobial and Antibiofilm Potential
3.6. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Hasanpour, A.H.; Sepidarkish, M.; Mollalo, A.; Ardekani, A.; Almukhtar, M.; Mechaal, A.; Hosseini, S.R.; Bayani, M.; Javanian, M.; Rostami, A. The global prevalence of methicillin-resistant Staphylococcus aureus colonization in residents of elderly care centers: A systematic review and meta-analysis. Antimicrob. Resist. Infect. Control 2023, 12, 4. [Google Scholar] [CrossRef]
- Uddin, T.M.; Chakraborty, A.J.; Khusro, A.; Zidan, B.R.M.; Mitra, S.; Bin Emran, T.; Dhama, K.; Ripon, M.K.H.; Gajdács, M.; Sahibzada, M.U.K.; et al. Antibiotic resistance in microbes: History, mechanisms, therapeutic strategies and future prospects. J. Infect. Public Health 2021, 14, 1750–1766. [Google Scholar] [CrossRef]
- Bo, L.; Sun, H.; Li, Y.D.; Zhu, J.; Wurpel, J.N.D.; Lin, H.; Chen, Z.S. Combating antimicrobial resistance: The silent war. Front. Pharmacol. 2024, 15, 1347750. [Google Scholar] [CrossRef]
- Mondal, S.K.; Chakraborty, S.; Manna, S.; Mandal, S.M. Antimicrobial nanoparticles: Current landscape and future challenges. RSC Pharm. 2024, 1, 388–402. [Google Scholar] [CrossRef]
- Baindara, P.; Mandal, S.M. Gut-Antimicrobial Peptides: Synergistic Co-Evolution with Antibiotics to Combat Multi-Antibiotic Resistance. Antibiotics 2023, 12, 1732. [Google Scholar] [CrossRef]
- Aflakian, F.; Mirzavi, F.; Aiyelabegan, H.T.; Soleimani, A.; Gholizadeh Navashenaq, J.; Karimi-Sani, I.; Rafati Zomorodi, A.; Vakili-Ghartavol, R. Nanoparticles-based therapeutics for the management of bacterial infections: A special emphasis on FDA approved products and clinical trials. Eur. J. Pharm. Sci. 2023, 188, 106515. [Google Scholar] [CrossRef]
- Ferreira, M.; Ogren, M.; Dias, J.N.R.; Silva, M.; Gil, S.; Tavares, L.; Aires-Da-silva, F.; Gaspar, M.M.; Aguiar, S.I. Liposomes as antibiotic delivery systems: A promising nanotechnological strategy against antimicrobial resistance. Molecules 2021, 26, 2047. [Google Scholar] [CrossRef]
- Ghosh, R.; De, M. Liposome-Based Antibacterial Delivery: An Emergent Approach to Combat Bacterial Infections. ACS Omega 2023, 8, 35442–35451. [Google Scholar] [CrossRef]
- Baindara, P.; Roy, D.; Mandal, S.M. CycP: A Novel Self-Assembled Vesicle-Forming Cyclic Antimicrobial Peptide to Control Drug-Resistant S. aureus. Bioengineering 2024, 11, 855. [Google Scholar] [CrossRef] [PubMed]
- Karaz, S.; Senses, E. Liposomes Under Shear: Structure, Dynamics, and Drug Delivery Applications. Adv. NanoBiomed Res. 2023, 3, 2200101. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, H.; Pang, S.; Ya, M.; Qiu, F.; Qin, P.; Zeng, C.; Lu, Y. Liposomes as Multifunctional Nano-Carriers for Medicinal Natural Products. Front. Chem. 2022, 10, 963004. [Google Scholar] [CrossRef]
- Oda, A.; Watanabe, C.; Aoki, N.; Yanagisawa, M. Liposomal adhesion: Via electrostatic interactions and osmotic deflation increase membrane tension and lipid diffusion coefficient. Soft Matter 2020, 16, 4549–4554. [Google Scholar] [CrossRef]
- Gould-Fogerite, S.; Mannino, R.J. Rotary dialysis: Its application to the preparation of large liposomes and large proteoliposomes (protein-lipid vesicles) with high encapsulation efficiency and efficient reconstitution of membrane proteins. Anal. Biochem. 1985, 148, 15–25. [Google Scholar] [CrossRef]
- Di Prima, G.; Librizzi, F.; Carrotta, R. Light scattering as an easy tool to measure vesicles weight concentration. Membranes 2020, 10, 222. [Google Scholar] [CrossRef]
- Gonzalez Gomez, A.; Syed, S.; Marshall, K.; Hosseinidoust, Z. Liposomal Nanovesicles for Efficient Encapsulation of Staphylococcal Antibiotics. ACS Omega 2019, 4, 10866–10876. [Google Scholar] [CrossRef]
- Kozik, V.; Pentak, D.; Paździor, M.; Zięba, A.; Bąk, A. From Design to Study of Liposome-Driven Drug Release Part 1: Impact of Temperature and pH on Environment. Int. J. Mol. Sci. 2023, 24, 11686. [Google Scholar] [CrossRef]
- Baindara, P.; Singh, N.; Ranjan, M.; Nallabelli, N.; Chaudhry, V.; Pathania, G.L.; Sharma, N.; Kumar, A.; Patil, P.B.; Korpole, S. Laterosporulin10: A novel defensin like class iid bacteriocin from brevibacillus sp. strain SKDU10 with inhibitory activity against microbial pathogens. Microbiology 2016, 162, 1286–1299. [Google Scholar] [CrossRef]
- Hussain, M.B.; Kamel, Y.M.; Ullah, Z.; Jiman-Fatani, A.A.M.; Ahmad, A.S. In vitro evaluation of methicillin-resistant and methicillin-sensitive Staphylococcus aureus susceptibility to Saudi honeys. BMC Complement. Altern. Med. 2019, 19, 185. [Google Scholar] [CrossRef]
- Singh, P.K.; Chittpurna; Ashish; Sharma, V.; Patil, P.B.; Korpole, S. Identification, purification and characterization of laterosporulin, a novel bacteriocin produced by Brevibacillus sp. strain GI-9. PLoS ONE 2012, 7, e31498. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Baindara, P.; Sharma, P.; Jose, T.A.; V, K.; Manoharan, R.; Mandal, S.M. Anti-Biofilm Action of Cineole and Hypericum perforatum to Combat Pneumonia-Causing Drug-Resistant P. aeruginosa. Antibiotics 2024, 13, 689. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Li, X.; Bi, W.; Jiang, C. Relationship between membrane damage, leakage of intracellular compounds, and inactivation of Escherichia coli treated by pressurized CO2. J. Basic Microbiol. 2014, 54, 858–865. [Google Scholar] [CrossRef]
- Andrade, J.C.; Morais-Braga, M.F.B.; Guedes, G.M.M.; Tintino, S.R.; Freitas, M.A.; Menezes, I.R.A.; Coutinho, H.D.M. Enhancement of the antibiotic activity of aminoglycosides by alpha-tocopherol and other cholesterol derivates. Biomed. Pharmacother. 2014, 68, 1065–1069. [Google Scholar] [CrossRef]
- Kaushik, D.; Mohan, M.; Borade, D.M.; Swami, O.C. Ampicillin: Rise fall & resurgence. J. Clin. Diagnostic Res. 2014, 8, ME01. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Amikacin: Uses, resistance, and prospects for inhibition. Molecules 2017, 22, 2267. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef]
- Seenivasan, R.; Halagali, P.; Nayak, D.; Tippavajhala, V.K. Transethosomes: A Comprehensive Review of Ultra-Deformable Vesicular Systems for Enhanced Transdermal Drug Delivery. AAPS PharmSciTech 2025, 26, 41. [Google Scholar] [CrossRef]
- Reiter, E.; Jiang, Q.; Christen, S. Anti-inflammatory properties of α- and γ-tocopherol. Mol. Asp. Med. 2007, 28, 668–691. [Google Scholar] [CrossRef]
- Tadele, D.T.; David, D.; Yim, E.; Mekonnen, T.H. Development and characterization of PVA-zein/α-tocopherol nonwoven mats for functional wound dressing applications. Colloids Surf. B Biointerfaces 2025, 246, 114355. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wu, M.; Yang, K.; Zhao, M.; Wu, D.; Ma, J.; Ding, B.; Sun, W. Effect of nanoliposomal entrapment on antioxidative hydrolysates from goose blood protein. J. Food Sci. 2020, 85, 3034–3042. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.S.; Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Vitamin E as promising adjunct treatment option in the combat of infectious diseases caused by bacterial including multi-drug resistant pathogens—Results from a comprehensive literature survey. Eur. J. Microbiol. Immunol. 2020, 10, 193–201. [Google Scholar] [CrossRef]
- Rani, N.N.I.M.; Chen, X.Y.; Al-Zubaidi, Z.M.; Azhari, H.; Khaitir, T.M.N.; Ng, P.Y.; Buang, F.; Tan, G.C.; Wong, Y.P.; Said, M.M.; et al. Surface-engineered liposomes for dual-drug delivery targeting strategy against methicillin-resistant Staphylococcus aureus (MRSA). Asian J. Pharm. Sci. 2022, 17, 102–119. [Google Scholar] [CrossRef]
- Gonzalez Gomez, A.; Hosseinidoust, Z. Liposomes for Antibiotic Encapsulation and Delivery. ACS Infect. Dis. 2020, 6, 896–908. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, S.; Baindara, P.; Das, S.; Mondal, S.K.; Sharma, P.; Jose T, A.; V, K.; Manoharan, R.; Mandal, S.M. Dual Antibiotic-Infused Liposomes to Control Methicillin-Resistant Staphylococcus aureus. Medicines 2025, 12, 14. https://doi.org/10.3390/medicines12020014
Chakraborty S, Baindara P, Das S, Mondal SK, Sharma P, Jose T A, V K, Manoharan R, Mandal SM. Dual Antibiotic-Infused Liposomes to Control Methicillin-Resistant Staphylococcus aureus. Medicines. 2025; 12(2):14. https://doi.org/10.3390/medicines12020014
Chicago/Turabian StyleChakraborty, Sourav, Piyush Baindara, Surojit Das, Suresh K. Mondal, Pralay Sharma, Austin Jose T, Kumaravel V, Raja Manoharan, and Santi M. Mandal. 2025. "Dual Antibiotic-Infused Liposomes to Control Methicillin-Resistant Staphylococcus aureus" Medicines 12, no. 2: 14. https://doi.org/10.3390/medicines12020014
APA StyleChakraborty, S., Baindara, P., Das, S., Mondal, S. K., Sharma, P., Jose T, A., V, K., Manoharan, R., & Mandal, S. M. (2025). Dual Antibiotic-Infused Liposomes to Control Methicillin-Resistant Staphylococcus aureus. Medicines, 12(2), 14. https://doi.org/10.3390/medicines12020014







