Clinical Effectiveness of Mirogabalin Besylate for Trigeminal Neuropathy after Skull Base Surgery: Illustrative Cases
Abstract
1. Introduction
2. Case Description
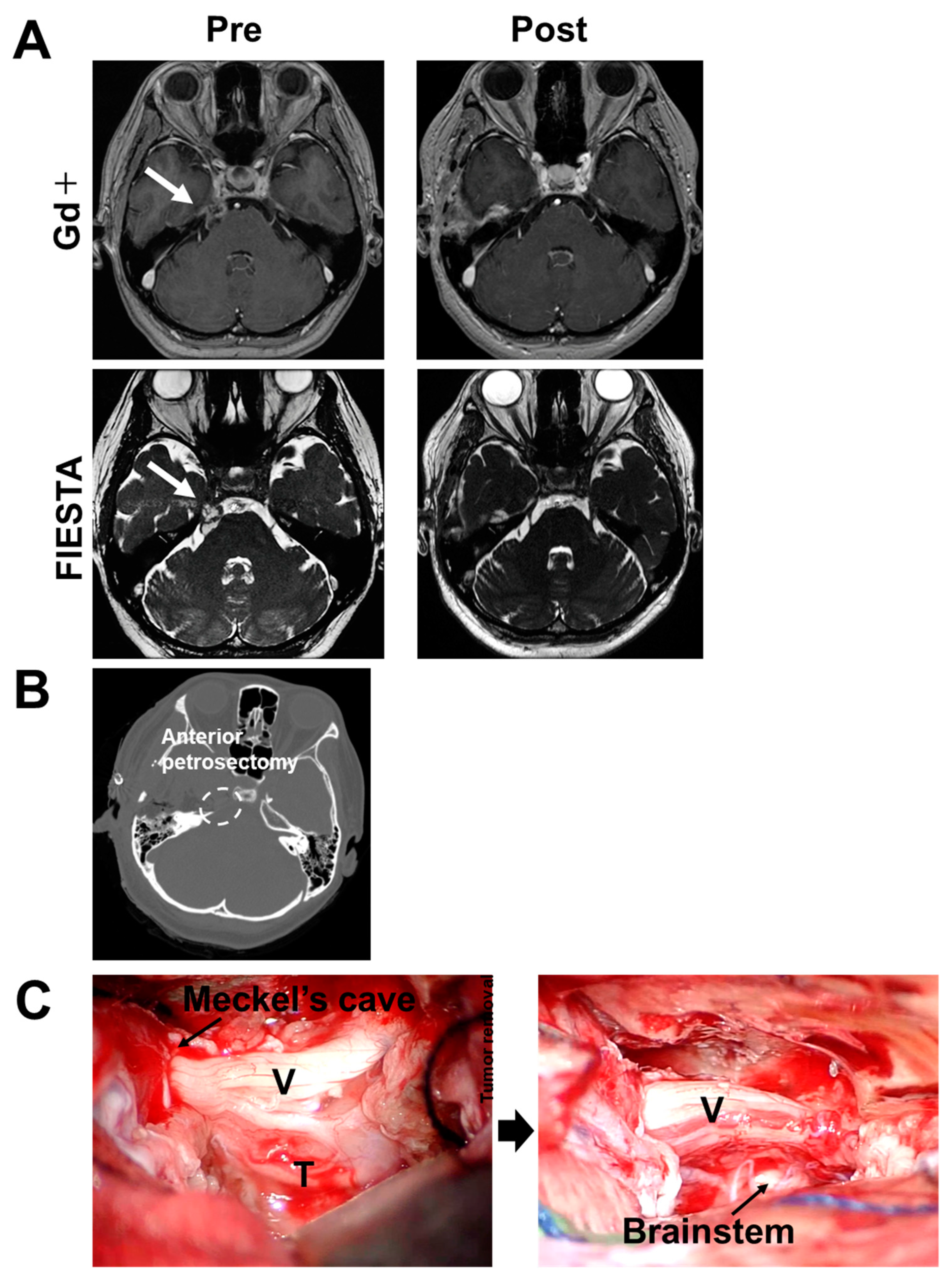
2.1. Case 1
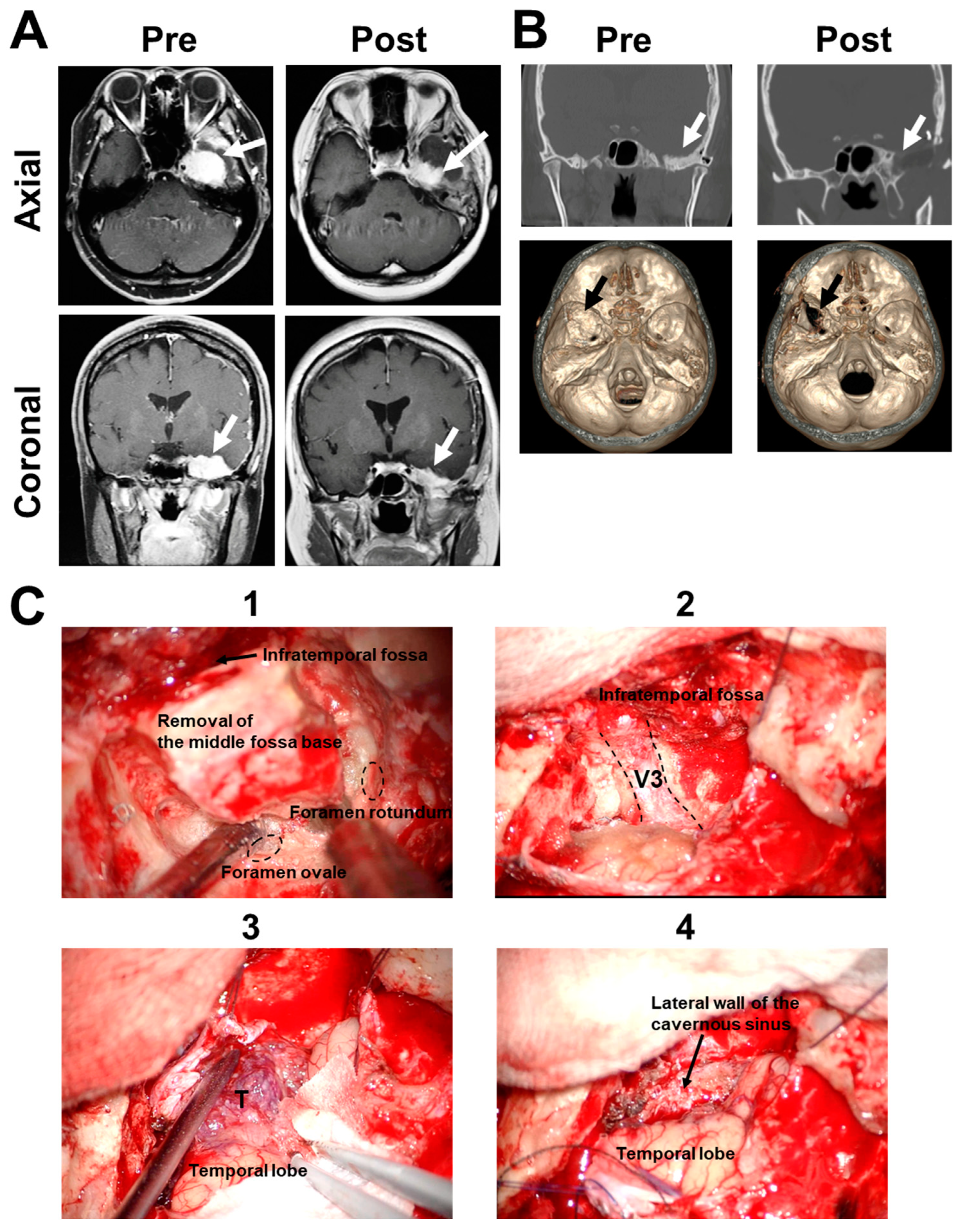
2.2. Case 2
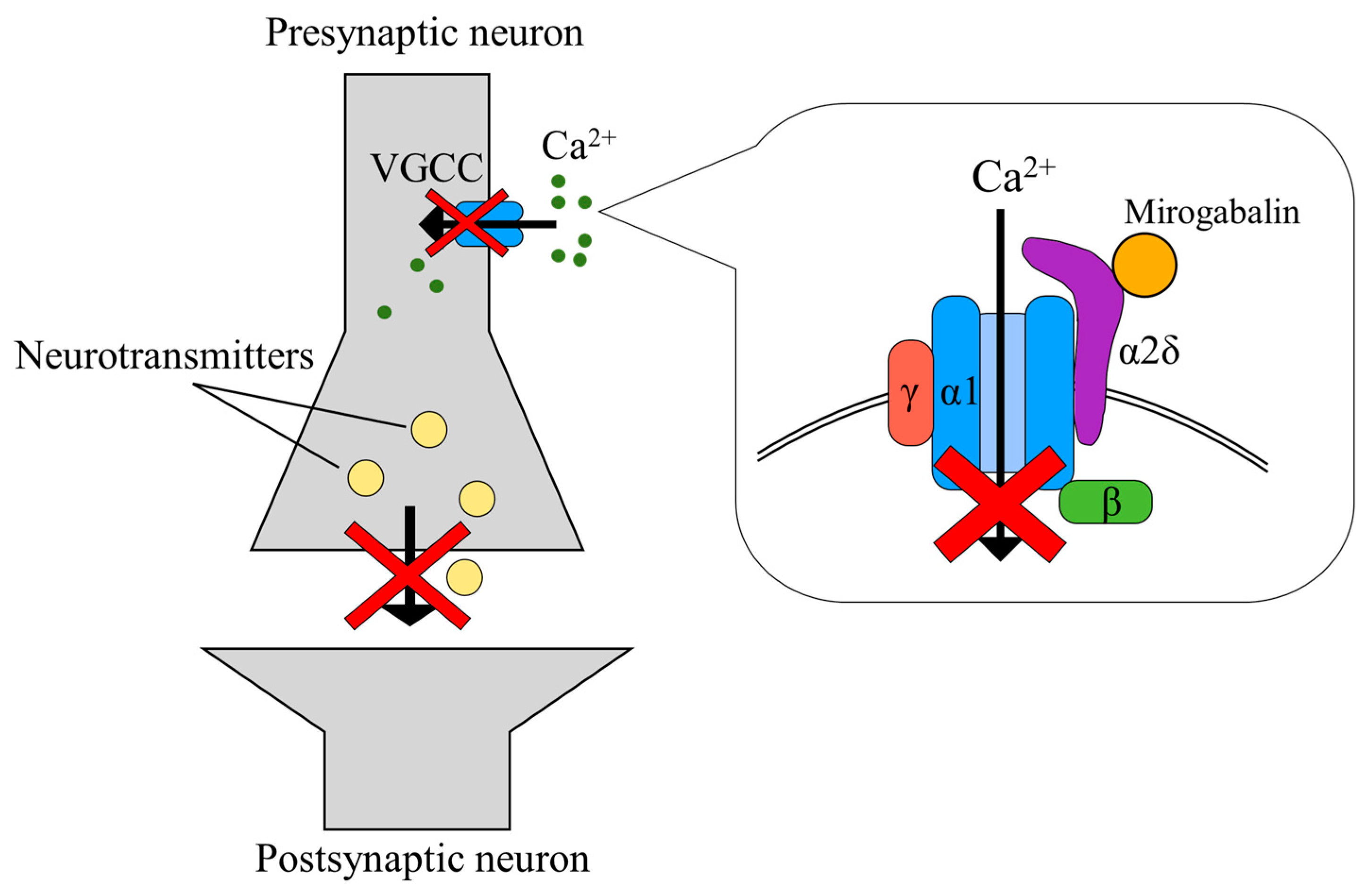
3. Discussion
4. Limitation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thirumala, P.D.; Kassasm, A.B.; Habeych, M.; Wichman, K.; Chang, Y.F.; Gardner, P.; Prevedello, D.; Snyderman, C.; Carrau, R.; Crammond, D.J.; et al. Somatosensory evoked potential monitoring during endoscopic endonasal approach to skull base surgery: Analysis of observed changes. Neurosurgery 2011, 69, ons64–ons76. [Google Scholar] [CrossRef]
- Maurer, J.; Pelster, H.; Ronald, G.; Amedee, A.M.; Wolf, J. lntraoperative monitoring of motor cranial nerves in skull base surgery. Skull Base Surg. 1995, 5, 169–175. [Google Scholar] [CrossRef][Green Version]
- Parthasarathy, D.; Thirumala, P.D.; Mohanraj, S.K.; Habeych, M.; Wichman, K.; Chang, Y.F.; Gardner, P.; Snyderman, C.; Crammond, D.J.; Balzer, J. Value of free-run electromyographic monitoring of lower cranial nerves in endoscopic endonasal approach to skull base surgeries. J. Neurol. Surg. B Skull Base 2012, 73, 236–244. [Google Scholar]
- Chong, M.S.; Bahra, A.; Zakrzewska, J.M. Guidelines for the management of trigeminal neuralgia. Clevel. Clin. J. Med. 2023, 90, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Pergolizzi, J.V., Jr.; Gharibo, C.; Magnusson, P.; Breve, F.; LeQuang, J.A.; Varrassi, G. Pharmacotherapeutic management of trigeminal neuropathic pain: An update. Expert Opin. Pharmacother. 2022, 23, 1155–1164. [Google Scholar] [CrossRef] [PubMed]
- Westerlund, U.; Linderoth, B.; Mathiesen, T. Trigeminal complications arising after surgery of cranial base meningiomas. Neurosurg. Rev. 2012, 35, 203–209. [Google Scholar] [CrossRef]
- Deeks, E.D. Mirogabalin: First Global Approval. Drugs 2019, 79, 463–468. [Google Scholar] [CrossRef]
- Baba, M.; Matsui, N.; Kuroha, M.; Wasaki, Y.; Ohwada, S. Mirogabalin for the treatment of diabetic peripheral neuropathic pain: A randomized, double-blind, placebo-controlled phase III study in Asian patients. J. Diabetes Investig. 2019, 10, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Kato, J.; Matsui, N.; Kakehi, Y.; Murayama, E.; Ohwada, S.; Sugihara, M. Mirogabalin for the management of postherpetic neuralgia: A randomized, double-blind, placebo-controlled phase 3 study in Asian patients. Pain 2019, 160, 1175–1185. [Google Scholar] [CrossRef]
- Kawase, T.; Shiobara, R.; Toya, S. Anterior transpetrosal-transtentorial approach for sphenopetroclival meningiomas: Surgical method and results in 10 patients. Neurosurgery 1991, 28, 869–875. [Google Scholar] [CrossRef]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15, S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Broderick, J.P.; Adeoye, O.; Elm, J. Evolution of the Modified Rankin Scale and Its Use in Future Stroke Trials. Stroke 2017, 48, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.P.; Karoly, P.; Braver, S. The measurement of clinical pain intensity: A comparison of six methods. Pain 1986, 27, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.; De Donato, G.; Taibah, A.; Russo, A.; Falcioni, M.; Mancini, F. Infratemporal fossa approaches to the lateral skull base. Keio J. Med. 1999, 48, 189–200. [Google Scholar] [CrossRef]
- Matsuda, K.M.; Tanaka-Mizutsugu, H.; Kishi, Y.; Hino, H.; Kagami, S. A case of trigeminal trophic syndrome responding to mirogabalin. Eur. J. Dermatol. 2020, 30, 206–207. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Lu, J.; Duan, Y.; Li, D. The Clinical Application and Progress of Mirogabalin on Neuropathic Pain as a Novel Selective Gabapentinoids. Mediat. Inflamm. 2023, 2023, 4893436. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Abdi, S.; Huh, B.; Kim, K.H. Mirogabalin: Could it be the next generation gabapentin or pregabalin? Korean J. Pain 2021, 34, 4–18. [Google Scholar] [CrossRef]
- Noma, N.; Ozasa, K.; Young, A. Altered somatosensory processing in secondary trigeminal neuralgia: A case report. J. Indian Prosthodont. Soc. 2021, 21, 308–310. [Google Scholar] [CrossRef]
- Kikuchi, K.; Tagawa, Y.; Murata, M.; Ishida, S. Effects of Mirogabalin on Hyperalgesia and Chronic Ocular Pain in Tear-Deficient Dry-Eye Rats. Investig. Ophthalmol. Vis. Sci. 2023, 64, 27. [Google Scholar] [CrossRef]
- Vadivelu, N.; Kai, A.M.; Tran, D.; Kodumudi, G.; Legler, A.; Ayrian, E. Options for perioperative pain management in neurosurgery. J. Pain Res. 2016, 9, 37–47. [Google Scholar] [CrossRef]
- Doi, R.; Miyazaki, T.; Tsuchiya, T.; Matsumoto, K.; Tomoshige, K.; Machino, R.; Mizoguchi, S.; Matsumoto, T.; Yamaguchi, K.; Takatsuna, H.; et al. Mirogabalin treatment of postoperative neuropathic pain after thoracic surgery: Study protocol for a multicenter, randomized, open-label, parallel-group, interventional trial. J. Thorac. Dis. 2021, 13, 6062–6070. [Google Scholar] [CrossRef] [PubMed]
- Cheshire, W.P., Jr. Defining the role for gabapentin in the treatment of trigeminal neuralgia: A retrospective study. J. Pain 2002, 3, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Obermann, M.; Yoon, M.S.; Sensen, K.; Maschke, M.; Diener, H.C.; Katsarava, Z. Efficacy of pregabalin in the treatment of trigeminal neuralgia. Cephalalgia 2008, 28, 174–181. [Google Scholar] [CrossRef]
- Al-Quliti, K.W. Update on neuropathic pain treatment for trigeminal neuralgia. The pharmacological and surgical options. Neurosciences 2015, 20, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Burgess, J.; Javed, S.; Frank, B.; Malik, R.A.; Alam, U. Mirogabalin besylate in the treatment of neuropathic pain. Drugs Today 2020, 56, 135–149. [Google Scholar] [CrossRef]
- Zajączkowska, R.; Mika, J.; Leppert, W.; Kocot-Kępska, M.; Malec-Milewska, M.; Wordliczek, J. Mirogabalin-A Novel Selective Ligand for the α2δ Calcium Channel Subunit. Pharmaceuticals 2021, 14, 112. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Yamaguchi, S.; Suzuki, T.; Kato, J.; Chiba, S.; Hirakawa, N.; Yamaguchi, K.; Tanabe, Y.; Takatsuna, H.; Kenyoshi, Y.; et al. Switching from Pregabalin to Mirogabalin in Patients with Peripheral Neuropathic Pain: A Multi-Center, Prospective, Single-Arm, Open-Label Study (MIROP Study). Pain Ther. 2021, 10, 711–727. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for neuropathic pain in adults: A systematic review and meta-analysis and updated NeuPSIG recommendations. Lancet Neurol. 2015, 142, 162–173. [Google Scholar] [CrossRef]
- Hange, N.; Poudel, S.; Ozair, S.; Paul, T.; Nambakkam, M.; Shrestha, R.; Greye, F.; Shah, S.; Raj Adhikari, Y.; Thapa, S.; et al. Managing Chronic Neuropathic Pain: Recent Advances and New Challenges. Neurol. Res. Int. 2022, 2022, 8336561. [Google Scholar] [CrossRef]
- Garcia-Borreguero, D.; Larrosa, O.; Williams, A.M.; Albares, J.; Pascual, M.; Palacios, J.C.; Fernandez, C. Treatment of restless legs syndrome with pregabalin: A double-blind, placebo-controlled study. Neurology 2010, 74, 1897–1904. [Google Scholar] [CrossRef]
- Williamson, A.; Hoggart, B. Pain: A review of three commonly used pain rating scales. J. Clin. Nurs. 2005, 14, 798–804. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karatsu, K.; Tamura, R.; Miyauchi, T.; Sogano, J.; Hino, U.; Iwama, T.; Toda, M. Clinical Effectiveness of Mirogabalin Besylate for Trigeminal Neuropathy after Skull Base Surgery: Illustrative Cases. Medicines 2023, 10, 48. https://doi.org/10.3390/medicines10080048
Karatsu K, Tamura R, Miyauchi T, Sogano J, Hino U, Iwama T, Toda M. Clinical Effectiveness of Mirogabalin Besylate for Trigeminal Neuropathy after Skull Base Surgery: Illustrative Cases. Medicines. 2023; 10(8):48. https://doi.org/10.3390/medicines10080048
Chicago/Turabian StyleKaratsu, Kosuke, Ryota Tamura, Tsubasa Miyauchi, Junki Sogano, Utaro Hino, Takashi Iwama, and Masahiro Toda. 2023. "Clinical Effectiveness of Mirogabalin Besylate for Trigeminal Neuropathy after Skull Base Surgery: Illustrative Cases" Medicines 10, no. 8: 48. https://doi.org/10.3390/medicines10080048
APA StyleKaratsu, K., Tamura, R., Miyauchi, T., Sogano, J., Hino, U., Iwama, T., & Toda, M. (2023). Clinical Effectiveness of Mirogabalin Besylate for Trigeminal Neuropathy after Skull Base Surgery: Illustrative Cases. Medicines, 10(8), 48. https://doi.org/10.3390/medicines10080048






