Vitamin D Levels in Patients with Active and Remission Graves’ Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Study Design
2.3. Variables and Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
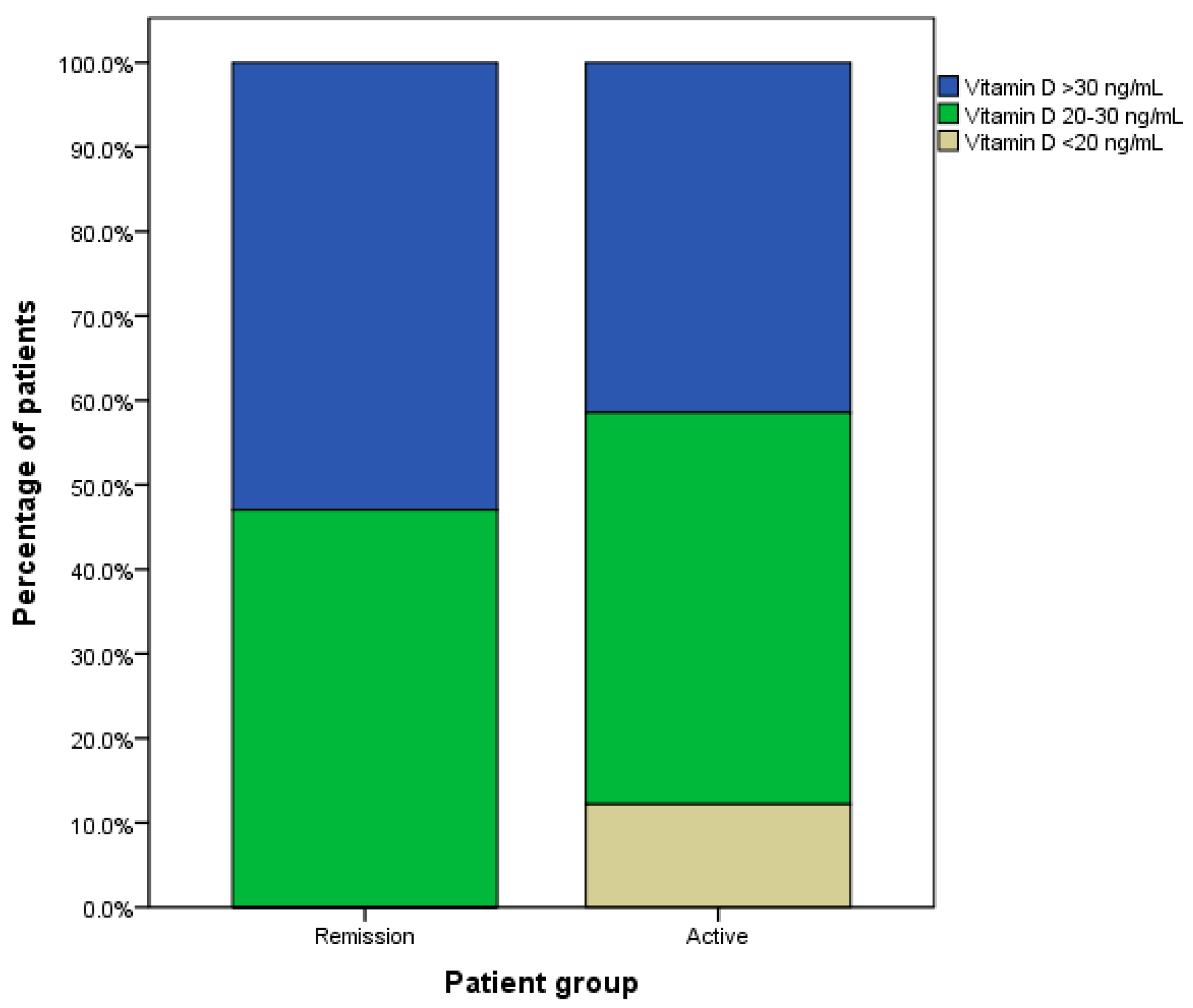
3.2. The Serum Vitamin D Levels
3.3. The Factors Affecting Vitamin D Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smith, T.J.; Hegedus, L. Graves’ Disease. N. Engl. J. Med. 2016, 375, 1552–1565. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Fallahi, P.; Elia, G.; Ragusa, F.; Paparo, S.R.; Ruffilli, I.; Patrizio, A.; Gonnella, D.; Giusti, C.; Virili, C.; et al. Graves’ disease: Clinical manifestations, immune pathogenesis (cytokines and chemokines) and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101388. [Google Scholar] [CrossRef] [PubMed]
- Weetman, A.P. Graves’ disease. N. Engl. J. Med. 2000, 343, 1236–1248. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Baeke, F.; Takiishi, T.; Korf, H.; Gysemans, C.; Mathieu, C. Vitamin D: Modulator of the immune system. Curr. Opin. Pharmacol. 2010, 10, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Leko, M.B.; Jureško, I.; Rozić, I.; Pleić, N.; Gunjača, I.; Zemunik, T. Vitamin D and the Thyroid: A Critical Review of the Current Evidence. Int. J. Mol. Sci. 2023, 24, 3586. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Song, E.; Oh, H.-S.; Park, S.; Kwon, H.; Jeon, M.J.; Kim, W.B.; Shong, Y.K.; Kim, T.Y. Vitamin D deficiency affects thyroid autoimmunity and dysfunction in iodine-replete area: Korea national health and nutrition examination survey. Endocrine 2017, 58, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-analysis of the association between vitamin D and autoimmune thyroid disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Chailurkit, L.-O.; Aekplakorn, W.; Ongphiphadhanakul, B. Regional variation and determinants of vitamin D status in sunshine-abundant Thailand. BMC Public Health 2011, 11, 853. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Okamoto, Y.; Hamada, N.; Miyashita, K.; Takahara, M.; Sakamoto, F.; Miyatsuka, T.; Kitamura, T.; Katakami, N.; Kawamori, D.; et al. Serum vitamin D levels are decreased in patients without remission of Graves’ disease. Endocrine 2013, 43, 230–232. [Google Scholar] [CrossRef] [PubMed]
- Kivity, S.; Agmon-Levin, N.; Zisappl, M.; Shapira, Y.; Nagy, E.V.; Dankó, K.; Szekanecz, Z.; Langevitz, P.; Shoenfeld, Y. Vitamin D and autoimmune thyroid diseases. Cell. Mol. Immunol. 2011, 8, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, H.; Noguchi, S.; Takatsu, K.; Koike, E.; Murakami, T.; Watanabe, S.; Uchino, S.; Yamashita, H.; Kawamoto, H. High Prevalence of Vitamin D Deficiency in Japanese Female Patients with Graves’ Disease. Endocr. J. 2001, 48, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Planck, T.; Shahida, B.; Malm, J.; Manjer, J. Vitamin D in Graves Disease: Levels, Correlation with Laboratory and Clinical Parameters, and Genetics. Eur. Thyroid. J. 2017, 7, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.-Y.; Cao, B.; Yin, J.; Wang, D.-F.; Chen, K.-L.; Lu, Q.-B. Vitamin D and Graves’ Disease: A Meta-Analysis Update. Nutrients 2015, 7, 3813–3827. [Google Scholar] [CrossRef] [PubMed]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and thyroid disorders: A systematic review and Meta-analysis of observational studies. BMC Endocr. Disord. 2021, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- Shafer, R.B.; Gregory, D.H. Calcium malabsorption in hyperthyroidism. Gastroenterology 1972, 63, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Okamoto, Y.; Hamada, N.; Miyashita, K.; Takahara, M.; Sakamoto, F.; Miyatsuka, T.; Kitamura, T.; Katakami, N.; Kawamori, D.; et al. Serum vitamin D levels are decreased and associated with thyroid volume in female patients with newly onset Graves’ disease. Endocrine 2012, 42, 739–741. [Google Scholar] [CrossRef] [PubMed]
- Koehler, V.F.; Filmann, N.; Mann, W.A. Vitamin D Status and Thyroid Autoantibodies in Autoimmune Thyroiditis. Horm. Metab. Res. 2019, 51, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Ragusa, F.; Elia, G.; Paparo, S.R.; Ruffilli, I.; Patrizio, A.; Giusti, C.; Gonnella, D.; Cristaudo, A.; et al. Graves’ disease: Epidemiology, genetic and environmental risk factors and viruses. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101387. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | Active GD | Remission GD | p-Value |
|---|---|---|---|
| (n = 41) | (n = 34) | ||
| Age (year), mean (95% CI) | 41.53 (31.46–51.60) | 39.63 (32.29–46.96) | 0.647 |
| Male, n (%) | 13 (31.71) | 8 (23.53) | 0.432 |
| Weight (kg), mean (95% CI) | 58.63 (55.12–62.15) | 57.38 (51.18–63.57) | 0.962 |
| Height (cm), mean (95% CI) | 163.07 (159.03–167.10) | 158.88 (155.08–162.67) | 0.072 |
| Body mass index (kg/m2), mean (95%CI) | 22.09 (20.75–23.44) | 22.85 (20.13–25.56) | 0.456 |
| Goiter size (g), mean (95%CI) | 55 (36.66–73.34) | 31.56 (26.44–36.68) | <0.001 |
| Current smoking, n (%) | 8 (19.51) | 4 (11.76) | 0.362 |
| Duration of disease (day), mean (95%CI) | 834 (527–1142) | 1454 (1086–1822) | 0.003 |
| Sun exposure (h/day), mean (95%CI) | 3.53 (2.10–4.97) | 3.08 (1.77–4.39) | 0.781 |
| Free T3 (pg/mL), mean (95%CI) | 10.32 (6.77–13.88) | 2.87 (2.45–3.28) | <0.001 |
| Free T4 (ng/dL), mean (95%CI) | 3.21 (2.39–4.02) | 1.52 (1.13–1.91) | <0.001 |
| TSH (mIU/L), mean (95% CI) | 0.022 (0.008–0.035) | 2.425 (0.756–4.094) | <0.001 |
| iPTH (pg/mL), mean (95% CI) | 45.81 (26.33–65.28) | 38.97 (28.89–49.05) | 0.543 |
| Calcium (mg/mL), mean (95% CI) | 9.25 (9.04–9.45) | 9.35 (9.15–9.55) | 0.417 |
| Albumin (mg/mL), mean (95% CI) | 4.19 (4.02–4.36) | 4.48 (4.28–4.67) | <0.001 |
| Thyroid Size | Free T3 | Free T4 | TSH | iPTH | Calcium | Vitamin D Level | |
|---|---|---|---|---|---|---|---|
| Daily sun exposure | r = 0.127 | r = −0.092 | r = −0.141 | r = −0.194 | r = 0.062 | r = 0.062 | r = 0.206 |
| p = 0.298 | p = 0.493 | p = 0.236 | p = 0.172 | p = 0.605 | p = 0.599 | p = 0.079 | |
| Thyroid size | r = 0.163 | r = 0.131 | r = −0.175 | r = 0.102 | r = 0.184 | r = −0.073 | |
| p = 0.245 | p = 0.291 | p = 0.239 | p = 0.408 | p = 0.131 | p = 0.551 | ||
| Free T3 | r = 0.727 * | r = −0.717 * | r = −0.045 | r = 0.037 | r = −0.056 | ||
| p < 0.001 | p < 0.001 | p = 0.733 | p = 0.783 | p = 0.672 | |||
| Free T4 | r = −0.594 * | r = −0.020 | r = −0.055 | r = −0.254 * | |||
| p < 0.001 | p = 0.866 | p = 0.648 | p = 0.031 | ||||
| TSH | r = −0.078 | r = 0.143 | r = 0.159 | ||||
| p = 0.586 | p = 0.312 | p = 0.260 | |||||
| iPTH | r = −0.281 * | r = −0.189 | |||||
| p = 0.015 | p = 0.108 | ||||||
| Calcium | r = 0.182 | ||||||
| p = 0.118 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rattanamusik, N.; Uitrakul, S.; Charoenpiriya, A. Vitamin D Levels in Patients with Active and Remission Graves’ Disease. Medicines 2023, 10, 41. https://doi.org/10.3390/medicines10070041
Rattanamusik N, Uitrakul S, Charoenpiriya A. Vitamin D Levels in Patients with Active and Remission Graves’ Disease. Medicines. 2023; 10(7):41. https://doi.org/10.3390/medicines10070041
Chicago/Turabian StyleRattanamusik, Natapon, Suriyon Uitrakul, and Atchara Charoenpiriya. 2023. "Vitamin D Levels in Patients with Active and Remission Graves’ Disease" Medicines 10, no. 7: 41. https://doi.org/10.3390/medicines10070041
APA StyleRattanamusik, N., Uitrakul, S., & Charoenpiriya, A. (2023). Vitamin D Levels in Patients with Active and Remission Graves’ Disease. Medicines, 10(7), 41. https://doi.org/10.3390/medicines10070041






