Bittersweet Sugars: How Unusual Glycan Structures May Connect Epithelial-to-Mesenchymal Transition and Multidrug Resistance in Cancer
Abstract
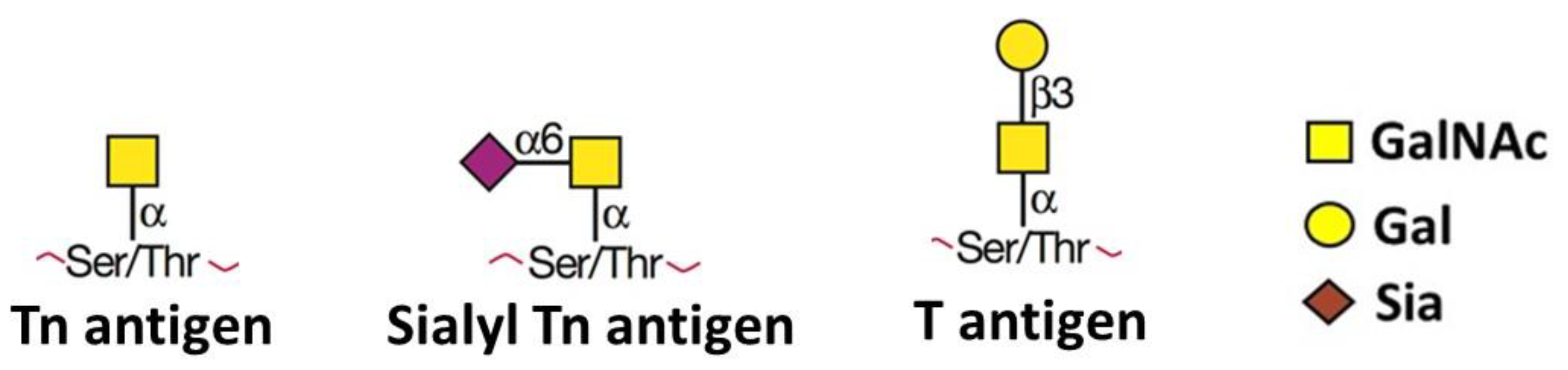
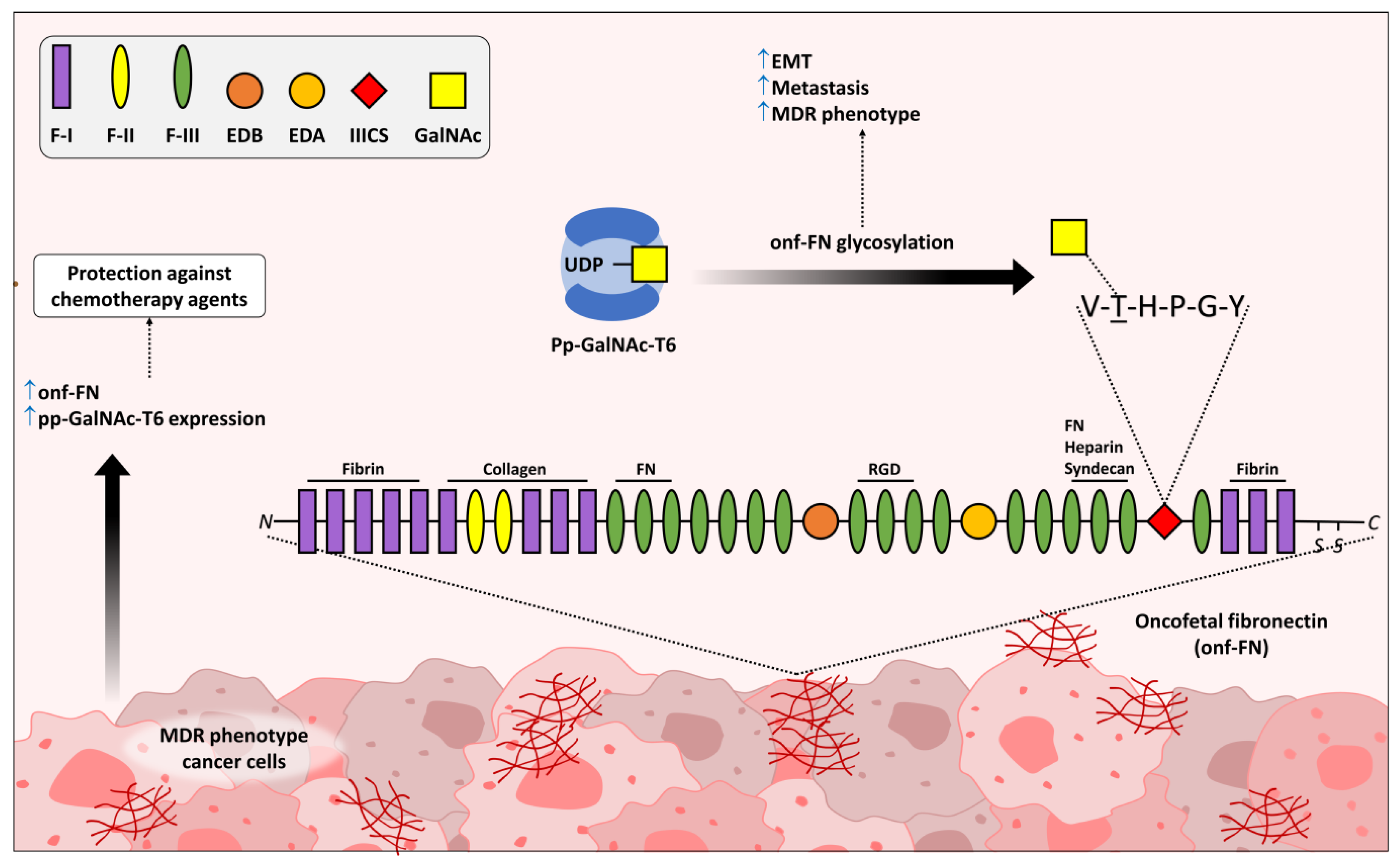
1. Unusual O-Linked Glycan Structures and Their Impact in Cancer Biology
2. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dos Reis, J.S.; Rodrigues da Costa Santos, M.A.; Mendonca, D.P.; Martins do Nascimento, S.I.; Barcelos, P.M.; Correia de Lima, R.G.; da Costa, K.M.; Freire-de-Lima, C.G.; Morrot, A.; Previato, J.O.; et al. Glycobiology of Cancer: Sugar Drives the Show. Medicines 2022, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.M.; Karlsson, N.G.; Khoo, K.H.; Thaysen-Andersen, M.; Wells, L.; Packer, N.H. Glycomics and Glycoproteomics. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor: New York, NY, USA, 2022; pp. 689–704. [Google Scholar]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Diniz-Lima, I.; da Fonseca, L.M.; Dos Reis, J.S.; Rodrigues da Costa Santos, M.A.; da Costa, K.M.; do Nascimento Santos, C.A.; Barcelos, P.M.; Guimaraes-Pinto, K.; Filardy, A.A.; Freire-de-Lima, M.E.; et al. The Sweet Side of Fungal Infections: Structural Glycan Diversity and Its Importance for Pathogenic Adaptation. Medicines 2022, 9, 37. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, S.; Liu, J.; Zhang, Y.; Liu, Y.; Shen, S.; Tian, F.; Yan, G.; Gao, Y.; Qin, X. Identification of serum glycobiomarkers for Hepatocellular Carcinoma using lectin microarrays. Front. Immunol. 2022, 13, 973993. [Google Scholar] [CrossRef]
- Llop, E.; Peracaula, R. Lectin Affinity Chromatography for the Discovery of Novel Cancer Glycobiomarkers: A Case Study with PSA Glycoforms and Prostate Cancer. Methods Mol. Biol. 2022, 2370, 301–313. [Google Scholar] [CrossRef]
- Azevedo, R.; Soares, J.; Gaiteiro, C.; Peixoto, A.; Lima, L.; Ferreira, D.; Relvas-Santos, M.; Fernandes, E.; Tavares, A.; Cotton, S.; et al. Glycan affinity magnetic nanoplatforms for urinary glycobiomarkers discovery in bladder cancer. Talanta 2018, 184, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Balmana, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef]
- Wang, Y.N.; Lee, H.H.; Hsu, J.L.; Yu, D.; Hung, M.C. The impact of PD-L1 N-linked glycosylation on cancer therapy and clinical diagnosis. J. Biomed. Sci. 2020, 27, 77. [Google Scholar] [CrossRef]
- Xu, M.; Yang, A.; Xia, J.; Jiang, J.; Liu, C.F.; Ye, Z.; Ma, J.; Yang, S. Protein glycosylation in urine as a biomarker of diseases. Transl. Res. J. Lab. Clin. Med. 2023, 253, 95–107. [Google Scholar] [CrossRef]
- Grzesik, K.; Janik, M.; Hoja-Lukowicz, D. The hidden potential of glycomarkers: Glycosylation studies in the service of cancer diagnosis and treatment. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188889. [Google Scholar] [CrossRef]
- Jabine, T.B. Reporting chronic conditions in the National Health Interview Survey. A review of findings from evaluation studies and methodological test. Vital Health Stat. 2 1987, 105, 1–45. [Google Scholar]
- Vicente, M.M.; Alves, I.; Gaifem, J.; Rodrigues, C.S.; Fernandes, A.; Dias, A.M.; Stambuk, J.; Petrovic, T.; Oliveira, P.; Ferreira-da-Silva, F.; et al. Altered IgG glycosylation at COVID-19 diagnosis predicts disease severity. Eur. J. Immunol. 2022, 52, 946–957. [Google Scholar] [CrossRef]
- Freire-de-Lima, L. Sweet and sour: The impact of differential glycosylation in cancer cells undergoing epithelial-mesenchymal transition. Front. Oncol. 2014, 4, 59. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, L.; Peri, F.; Airoldi, C. Glycoconjugates in cancer therapy. Anti-Cancer Agents Med. Chem. 2008, 8, 92–121. [Google Scholar] [CrossRef] [PubMed]
- Ratan, C.; Cicily, K.D.D.; Nair, B.; Nath, L.R. MUC Glycoproteins: Potential Biomarkers and Molecular Targets for Cancer Therapy. Curr. Cancer Drug Targets 2021, 21, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef]
- da Fonseca, L.M.; Calvalhan, D.M.; Previato, J.O.; Mendonca Previato, L.; Freire-de-Lima, L. Resistance to paclitaxel induces glycophenotype changes and mesenchymal-to-epithelial transition activation in the human prostate cancer cell line PC-3. Tumour Biol. 2020, 42, 1010428320957506. [Google Scholar] [CrossRef]
- da Fonseca, L.M.; da Silva, V.A.; da Costa, K.M.; Dos Reis, J.S.; Previato, J.O.; Previato, L.M.; Freire-de-Lima, L. Resistance to cisplatin in human lung adenocarcinoma cells: Effects on the glycophenotype and epithelial to mesenchymal transition markers. Glycoconj. J. 2022, 39, 247–259. [Google Scholar] [CrossRef]
- Taniguchi, N.; Ohkawa, Y.; Maeda, K.; Harada, Y.; Nagae, M.; Kizuka, Y.; Ihara, H.; Ikeda, Y. True significance of N-acetylglucosaminyltransferases GnT-III, V and alpha1,6 fucosyltransferase in epithelial-mesenchymal transition and cancer. Mol. Aspects Med. 2021, 79, 100905. [Google Scholar] [CrossRef]
- Taniguchi, N.; Miyoshi, E.; Ko, J.H.; Ikeda, Y.; Ihara, Y. Implication of N-acetylglucosaminyltransferases III and V in cancer: Gene regulation and signaling mechanism. Biochim. Biophys. Acta 1999, 1455, 287–300. [Google Scholar] [CrossRef]
- Taniguchi, N.; Ihara, S.; Saito, T.; Miyoshi, E.; Ikeda, Y.; Honke, K. Implication of GnT-V in cancer metastasis: A glycomic approach for identification of a target protein and its unique function as an angiogenic cofactor. Glycoconj. J. 2001, 18, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Zhao, H.; Wang, Y.; Cai, H.; Xiao, Y.; Zeng, Y.; Chen, H. Tumor-associated antigens: Tn antigen, sTn antigen, and T antigen. HLA 2016, 88, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Hakomori, S. Aberrant glycosylation in cancer cell membranes as focused on glycolipids: Overview and perspectives. Cancer Res. 1985, 45, 2405–2414. [Google Scholar] [PubMed]
- Miles, D.W.; Happerfield, L.C.; Smith, P.; Gillibrand, R.; Bobrow, L.G.; Gregory, W.M.; Rubens, R.D. Expression of sialyl-Tn predicts the effect of adjuvant chemotherapy in node-positive breast cancer. Br. J. Cancer 1994, 70, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J. The Role of Sialyl-Tn in Cancer. Int. J. Mol. Sci. 2016, 17, 275. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.; Campos, D.; Gomes, J.; Pinto, F.; Macedo, J.A.; Matos, R.; Mereiter, S.; Pinto, M.T.; Polonia, A.; Gartner, F.; et al. O-glycans truncation modulates gastric cancer cell signaling and transcription leading to a more aggressive phenotype. EBioMedicine 2019, 40, 349–362. [Google Scholar] [CrossRef]
- Ju, T.; Lanneau, G.S.; Gautam, T.; Wang, Y.; Xia, B.; Stowell, S.R.; Willard, M.T.; Wang, W.; Xia, J.Y.; Zuna, R.E.; et al. Human tumor antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer Res. 2008, 68, 1636–1646. [Google Scholar] [CrossRef]
- Guo, M.; Luo, B.; Pan, M.; Li, M.; Zhao, F.; Dou, J. MUC1 plays an essential role in tumor immunity of colorectal cancer stem cell vaccine. Int. Immunopharmacol. 2020, 85, 106631. [Google Scholar] [CrossRef]
- Freitas, R.; Peixoto, A.; Ferreira, E.; Miranda, A.; Santos, L.L.; Ferreira, J.A. Immunomodulatory glycomedicine: Introducing next generation cancer glycovaccines. Biotechnol. Adv. 2023, 65, 108144. [Google Scholar] [CrossRef]
- Hofmann, B.T.; Schluter, L.; Lange, P.; Mercanoglu, B.; Ewald, F.; Folster, A.; Picksak, A.S.; Harder, S.; El Gammal, A.T.; Grupp, K.; et al. COSMC knockdown mediated aberrant O-glycosylation promotes oncogenic properties in pancreatic cancer. Mol. Cancer 2015, 14, 109. [Google Scholar] [CrossRef]
- Khiaowichit, J.; Talabnin, C.; Dechsukhum, C.; Silsirivanit, A.; Talabnin, K. Down-Regulation of C1GALT1 Enhances the Progression of Cholangiocarcinoma through Activation of AKT/ERK Signaling Pathways. Life 2022, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Beatson, R.; Tajadura-Ortega, V.; Achkova, D.; Picco, G.; Tsourouktsoglou, T.D.; Klausing, S.; Hillier, M.; Maher, J.; Noll, T.; Crocker, P.R.; et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat. Immunol. 2016, 17, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, E.; Boelaars, K.; Brown, K.; Eveline Li, R.J.; Kruijssen, L.; Bruijns, S.C.M.; van Ee, T.; Schetters, S.T.T.; Crommentuijn, M.H.W.; van der Horst, J.C.; et al. Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat. Commun. 2021, 12, 1270. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhao, H.; Li, J.; Jiang, H. Mucin-type sialyl-Tn antigen is associated with PD-L1 expression and predicts poor clinical prognosis in breast cancer. Gland. Surg. 2021, 10, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- da Fonseca, L.M.; da Silva, V.A.; Freire-de-Lima, L.; Previato, J.O.; Mendonca-Previato, L.; Capella, M.A. Glycosylation in Cancer: Interplay between Multidrug Resistance and Epithelial-to-Mesenchymal Transition? Front. Oncol. 2016, 6, 158. [Google Scholar] [CrossRef]
- Glavey, S.V.; Huynh, D.; Reagan, M.R.; Manier, S.; Moschetta, M.; Kawano, Y.; Roccaro, A.M.; Ghobrial, I.M.; Joshi, L.; O’Dwyer, M.E. The cancer glycome: Carbohydrates as mediators of metastasis. Blood Rev. 2015, 29, 269–279. [Google Scholar] [CrossRef]
- Thomas, D.; Sagar, S.; Caffrey, T.; Grandgenett, P.M.; Radhakrishnan, P. Truncated O-glycans promote epithelial-to-mesenchymal transition and stemness properties of pancreatic cancer cells. J. Cell Mol. Med. 2019, 23, 6885–6896. [Google Scholar] [CrossRef]
- Terashima, S.; Takano, Y.; Ohori, T.; Kanno, T.; Kimura, T.; Motoki, R.; Kawaguchi, T. Sialyl-Tn antigen as a useful predictor of poor prognosis in patients with advanced stomach cancer. Surg. Today 1998, 28, 682–686. [Google Scholar] [CrossRef]
- Takanami, I. Expression of Thomsen-Friedenreich antigen as a marker of poor prognosis in pulmonary adenocarcinoma. Oncol. Rep. 1999, 6, 341–344. [Google Scholar] [CrossRef]
- Dong, X.; Jiang, Y.; Liu, J.; Liu, Z.; Gao, T.; An, G.; Wen, T. T-Synthase Deficiency Enhances Oncogenic Features in Human Colorectal Cancer Cells via Activation of Epithelial-Mesenchymal Transition. Biomed. Res. Int. 2018, 2018, 9532389. [Google Scholar] [CrossRef]
- Pinho, S.; Marcos, N.T.; Ferreira, B.; Carvalho, A.S.; Oliveira, M.J.; Santos-Silva, F.; Harduin-Lepers, A.; Reis, C.A. Biological significance of cancer-associated sialyl-Tn antigen: Modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007, 249, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, K.; Shirakihara, T.; Nakano, A.; Imamura, T.; Miyazono, K.; Saitoh, M. Role of Ras signaling in the induction of snail by transforming growth factor-beta. J. Biol. Chem. 2009, 284, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, J.; Dong, X.; Hu, X.; Jiang, Y.; Li, L.; Du, T.; Yang, L.; Wen, T.; An, G.; et al. Tn antigen promotes human colorectal cancer metastasis via H-Ras mediated epithelial-mesenchymal transition activation. J. Cell Mol. Med. 2019, 23, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Beaman, E.M.; Carter, D.R.F.; Brooks, S.A. GALNTs: Master regulators of metastasis-associated epithelial-mesenchymal transition (EMT)? Glycobiology 2022, 32, 556–579. [Google Scholar] [CrossRef] [PubMed]
- Freire-de-Lima, L.; Gelfenbeyn, K.; Ding, Y.; Mandel, U.; Clausen, H.; Handa, K.; Hakomori, S.I. Involvement of O-glycosylation defining oncofetal fibronectin in epithelial-mesenchymal transition process. Proc. Natl. Acad. Sci. USA 2011, 108, 17690–17695. [Google Scholar] [CrossRef] [PubMed]
- Alisson-Silva, F.; Freire-de-Lima, L.; Donadio, J.L.; Lucena, M.C.; Penha, L.; Sa-Diniz, J.N.; Dias, W.B.; Todeschini, A.R. Increase of O-glycosylated oncofetal fibronectin in high glucose-induced epithelial-mesenchymal transition of cultured human epithelial cells. PLoS ONE 2013, 8, e60471. [Google Scholar] [CrossRef]
- Ding, Y.; Gelfenbeyn, K.; Freire-de-Lima, L.; Handa, K.; Hakomori, S.I. Induction of epithelial-mesenchymal transition with O-glycosylated oncofetal fibronectin. FEBS Lett. 2012, 586, 1813–1820. [Google Scholar] [CrossRef]
- Matsuura, H.; Hakomori, S. The oncofetal domain of fibronectin defined by monoclonal antibody FDC-6: Its presence in fibronectins from fetal and tumor tissues and its absence in those from normal adult tissues and plasma. Proc. Natl. Acad. Sci. USA 1985, 82, 6517–6521. [Google Scholar] [CrossRef]
- Matsuura, H.; Greene, T.; Hakomori, S. An alpha-N-acetylgalactosaminylation at the threonine residue of a defined peptide sequence creates the oncofetal peptide epitope in human fibronectin. J. Biol. Chem. 1989, 264, 10472–10476. [Google Scholar] [CrossRef]
- Loridon-Rosa, B.; Vielh, P.; Matsuura, H.; Clausen, H.; Cuadrado, C.; Burtin, P. Distribution of oncofetal fibronectin in human mammary tumors: Immunofluorescence study on histological sections. Cancer Res. 1990, 50, 1608–1612. [Google Scholar]
- Mandel, U.; Hamilton Therkildsen, M.; Reibel, J.; Sweeney, B.; Matsuura, H.; Hakomori, S.; Dabelsteen, E.; Clausen, H. Cancer-associated changes in glycosylation of fibronectin. Immunohistological localization of oncofetal fibronectin defined by monoclonal antibodies. APMIS 1992, 100, 817–826. [Google Scholar] [CrossRef] [PubMed]
- da Costa Santos, M.A.R.; Dos Reis, J.S.; do Nascimento Santos, C.A.; da Costa, K.M.; Barcelos, P.M.; de Oliveira Francisco, K.Q.; Barbosa, P.; da Silva, E.D.S.; Freire-de-Lima, C.G.; Morrot, A.; et al. Expression of O-glycosylated oncofetal fibronectin in alternatively activated human macrophages. Immunol. Res. 2023, 71, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, Z.; Gao, S.; Li, C.; Feng, Y.; Zhou, X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front. Oncol. 2020, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Xu, J.Y.; Shi, X.Y.; Huang, W.; Ruan, T.Y.; Xie, P.; Ding, J.L. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab. Investig. 2013, 93, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Yang, C.; Wang, S.; Shi, D.; Zhang, C.; Lin, X.; Xiong, B. M2 macrophages confer resistance to 5-fluorouracil in colorectal cancer through the activation of CCL22/PI3K/AKT signaling. Onco. Targets Ther. 2019, 12, 3051–3063. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Nakayama, H.; Nagata, M.; Yoshida, R.; Kawahara, K.; Hirosue, A.; Tanaka, T.; Yuno, A.; Matsuoka, Y.; Kojima, T.; et al. Overexpression of fibronectin confers cell adhesion-mediated drug resistance (CAM-DR) against 5-FU in oral squamous cell carcinoma cells. Int. J. Oncol. 2014, 44, 1376–1384. [Google Scholar] [CrossRef]
- Xing, H.; Weng, D.; Chen, G.; Tao, W.; Zhu, T.; Yang, X.; Meng, L.; Wang, S.; Lu, Y.; Ma, D. Activation of fibronectin/PI-3K/Akt2 leads to chemoresistance to docetaxel by regulating survivin protein expression in ovarian and breast cancer cells. Cancer Lett. 2008, 261, 108–119. [Google Scholar] [CrossRef]
- Ye, Y.; Zhang, R.; Feng, H. Fibronectin promotes tumor cells growth and drugs resistance through a CDC42-YAP-dependent signaling pathway in colorectal cancer. Cell Biol. Int. 2020, 44, 1840–1849. [Google Scholar] [CrossRef]
- Saw, P.E.; Park, J.; Jon, S.; Farokhzad, O.C. A drug-delivery strategy for overcoming drug resistance in breast cancer through targeting of oncofetal fibronectin. Nanomedicine 2017, 13, 713–722. [Google Scholar] [CrossRef]
- Reis, J.S.D.; Santos, M.; da Costa, K.M.; Freire-de-Lima, C.G.; Morrot, A.; Previato, J.O.; Previato, L.M.; da Fonseca, L.M.; Freire-de-Lima, L. Increased expression of the pathological O-glycosylated form of oncofetal fibronectin in the multidrug resistance phenotype of cancer cells. Matrix Biol. J. Int. Soc. Matrix Biol. 2023, 118, 47–68. [Google Scholar] [CrossRef]
- Detarya, M.; Sawanyawisuth, K.; Aphivatanasiri, C.; Chuangchaiya, S.; Saranaruk, P.; Sukprasert, L.; Silsirivanit, A.; Araki, N.; Wongkham, S.; Wongkham, C. The O-GalNAcylating enzyme GALNT5 mediates carcinogenesis and progression of cholangiocarcinoma via activation of AKT/ERK signaling. Glycobiology 2020, 30, 312–324. [Google Scholar] [CrossRef] [PubMed]
- Huanna, T.; Tao, Z.; Xiangfei, W.; Longfei, A.; Yuanyuan, X.; Jianhua, W.; Cuifang, Z.; Manjing, J.; Wenjing, C.; Shaochuan, Q.; et al. GALNT14 mediates tumor invasion and migration in breast cancer cell MCF-7. Mol. Carcinog. 2015, 54, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- Shan, J.; Liu, Y.; Wang, Y.; Li, Y.; Yu, X.; Wu, C. GALNT14 Involves the Regulation of Multidrug Resistance in Breast Cancer Cells. Transl. Oncol. 2018, 11, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Stanley, P. What Have We Learned from Glycosyltransferase Knockouts in Mice? J. Mol. Biol. 2016, 428, 3166–3182. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, L.M.d.; Diniz-Lima, I.; da Costa Santos, M.A.R.; Franklim, T.N.; da Costa, K.M.; Santos, A.C.d.; Morrot, A.; Decote-Ricardo, D.; Valente, R.d.C.; Freire-de-Lima, C.G.; et al. Bittersweet Sugars: How Unusual Glycan Structures May Connect Epithelial-to-Mesenchymal Transition and Multidrug Resistance in Cancer. Medicines 2023, 10, 36. https://doi.org/10.3390/medicines10060036
Fonseca LMd, Diniz-Lima I, da Costa Santos MAR, Franklim TN, da Costa KM, Santos ACd, Morrot A, Decote-Ricardo D, Valente RdC, Freire-de-Lima CG, et al. Bittersweet Sugars: How Unusual Glycan Structures May Connect Epithelial-to-Mesenchymal Transition and Multidrug Resistance in Cancer. Medicines. 2023; 10(6):36. https://doi.org/10.3390/medicines10060036
Chicago/Turabian StyleFonseca, Leonardo Marques da, Israel Diniz-Lima, Marcos André Rodrigues da Costa Santos, Tatiany Nunes Franklim, Kelli Monteiro da Costa, Ariely Costa dos Santos, Alexandre Morrot, Debora Decote-Ricardo, Raphael do Carmo Valente, Celio Geraldo Freire-de-Lima, and et al. 2023. "Bittersweet Sugars: How Unusual Glycan Structures May Connect Epithelial-to-Mesenchymal Transition and Multidrug Resistance in Cancer" Medicines 10, no. 6: 36. https://doi.org/10.3390/medicines10060036
APA StyleFonseca, L. M. d., Diniz-Lima, I., da Costa Santos, M. A. R., Franklim, T. N., da Costa, K. M., Santos, A. C. d., Morrot, A., Decote-Ricardo, D., Valente, R. d. C., Freire-de-Lima, C. G., dos Reis, J. S., & Freire-de-Lima, L. (2023). Bittersweet Sugars: How Unusual Glycan Structures May Connect Epithelial-to-Mesenchymal Transition and Multidrug Resistance in Cancer. Medicines, 10(6), 36. https://doi.org/10.3390/medicines10060036










