Abstract
Background: Adverse effects of antiseizure medications (ASMs) remain one of the major causes of non-adherence. Cosmetic side effects (CSEs) are among the most commonly reported side effects of ASMs. In this context, alopecia is one of the CSEs that has a high intolerance rate leading to poor therapeutical compliance. Methods: We performed a literature review concerning alopecia as a secondary effect of ASMs. Results: There are 1656 individuals reported with ASM-induced alopecia. Valproate (983), lamotrigine (355), and carbamazepine (225) have been extensively reported. Other ASMs associated with alopecia were cenobamate (18), levetiracetam (14), topiramate (13), lacosamide (7), vigabatrin (6), phenobarbital (5), gabapentin (5), phenytoin (4), pregabalin (4), eslicarbazepine (3), brivaracetam (2), clobazam (2), perampanel (2), trimethadione (2), rufinamide (2), zonisamide (2), primidone (1), and tiagabine (1). There were no reports of oxcarbazepine and felbamate with drug-induced alopecia. Hair loss seen with ASMs was diffuse and non-scarring. Telogen effluvium was the most common cause of alopecia. A characteristic feature was the reversibility of alopecia after ASM dose adjustment. Conclusions: Alopecia should be considered one important adverse effect of ASMs. Patients reporting hair loss with ASM therapy should be further investigated, and specialist consultation is recommended.
1. Introduction
The mainstay of treatment for epilepsy is antiseizure medications (ASMs). Approximately 70% of individuals with epilepsy obtain seizure freedom with adequate ASMs therapy [1]. In this context, low literacy levels, high cost, availability, low tolerability, and irregular medication use are some factors related to non-compliance [2]. However, the adverse effects of ASMs remain one of the leading causes of non-adherence. Furthermore, adverse effects can significantly impact the quality of life in people with epilepsy [3]. Cosmetic side effects (CSEs) are the fourth most commonly reported side effect associated with ASMs [4]. Increased body weight, acne vulgaris, hirsutism, and alopecia are some CSEs already reported with ASMs. In a study with 1903 individuals taking commonly used ASMs, weight gain and hair loss were the most common causes of ASMs discontinuation [5]. Additionally, CSEs are estimated to cost around USD 3500 per patient due to therapeutical modifications, consultation costs, and treatment of adverse drug reactions [6]. In addition to the economic burden, CSEs can negatively impact social functioning and emotional well-being and decrease self-esteem and quality of life in epilepsy patients [4].
Alopecia is generally differentiated into cicatricial or scarring alopecia, nonscarring alopecia, and structural hair disorders. In non-scarring alopecia, hair follicle damage is not permanent [7,8]. Non-scarring alopecia is further classified into focal, diffuse, and patterned hair loss [9]. This review will focus on diffuse non-scarring hair loss, the most common type of alopecia found in drug-induced hair loss [10]. The majority of the literature on drug-induced alopecia is related to chemotherapy. Studies assessing the quality of life in individuals undergoing chemotherapy revealed that alopecia ranked amongst the most troublesome side effects affecting body image and was described as distressing [11]. Interestingly, there are no differences between men and women concerning body image regarding the degree of alopecia. However, women’s psychological well-being was lower than men’s because the incidence of alopecia was higher in women [12].
Drug-induced alopecia usually presents as diffuse, non-scarring hair loss commonly reversible upon drug discontinuation [13,14]. Telogen effluvium and anagen effluvium are the two main types of hair loss secondary to medications [15,16]. Anagen effluvium is abrupt hair loss in the anagen phase secondary to impairment of the mitotic or metabolic activity of the hair follicle [17,18]. In this context, anti-neoplastic agents are the most commonly reported drugs associated with anagen effluvium [19,20]. Other medications (bismuth, levodopa, colchicine, and cyclosporine) were rarely reported to cause alopecia [21,22]. Some authors hypothesized that the hair loss probably occurred due to the abrupt cessation of mitotic activity, which can weaken the partially keratinized proximal portion of the hair shaft [23]. This may lead to narrowing and eventual breakage of the hair canal, which can cause complete failure of hair formation. Hair shedding usually begins within the first three weeks of the offending drug. Near complete hair loss is well-established after three months. Interestingly, the scalp is the most common location for hair loss due to its long anagen phase [24].
Telogen effluvium is a common cause of diffuse hair loss [25]. This condition is characterized by hair follicles entering their resting phase (telogen) and falling out too early. People with telogen effluvium can shed more than 100 hairs a day. It usually occurs three months after the introduction of the causative drug. Drugs related to telogen effluvium include anticonvulsants, oral retinoids, oral contraceptives, antithyroid drugs, hypolipidemic drugs, beta-blockers, captopril, and amphetamines [26].
It is believed that the most common antiseizure medications associated with hair loss are valproate and carbamazepine [27]. Additionally, therapies that might improve antiseizure medication-induced alopecia still need to be studied. This study aims to review the literature regarding alopecia and hair loss as secondary effects of ASMs.
2. Methods
2.1. Search Strategy
Primarily, we searched six databases to locate studies on ASM-induced alopecia published in electronic form. Excerpta Medica (Embase), Google Scholar, Latin American & Caribbean Health Sciences Literature (Lilacs), Medline, Scientific Electronic Library Online (Scielo), and Science Direct were searched. Search terms were “alopecia, hair loss, antiepileptic medication, antiseizure medication, epilepsy, and seizure”. During our secondary search [(alopecia) AND (ASM)], we searched for alopecia in 23 ASMs, of which 21 were associated with hair loss (Table 1).

Table 1.
FreeText and MeSH search terms in the US National Library of Medicine.
2.2. Inclusion and Exclusion Criteria
Original articles, case reports, case series, letters to editors, and bulletins were included in this review with no language restriction. Google Translate services were used when non-English literature was beyond the authors’ proficiency (Portuguese, English, Spanish, French, and German) or when the abstract in English could not provide enough information. The authors independently screened the titles and abstracts of all papers from the initial search. Disagreements between the authors were resolved through discussion.
All articles discussing at least one ASM causing hair loss were included. Cases where the cause of hair loss was already known and either alopecia did not worsen or was unrelated to ASMs were excluded. Furthermore, cases not accessible by electronic methods, including after a formal request to the authors (by email), were excluded. Patients with more than one contributing factor to alopecia were evaluated using the Naranjo algorithm to estimate the probability of the event occurring.
2.3. Data Extraction
We identified 1680 articles from a primary search. We excluded 1500 articles based on the title and abstract. During our secondary investigation, we identified 151 articles. We included 31 articles from the secondary search. This review included 115 studies containing 1656 individuals (Figure 1).
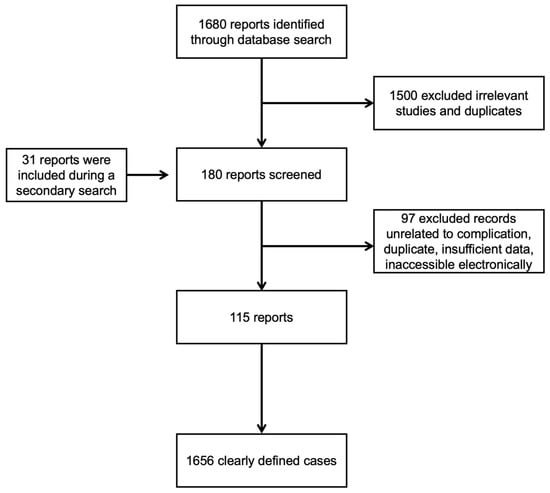
Figure 1.
Flowchart of the screening process.
3. Antiseizure Medications
3.1. Valproate (VPA)
VPA is one of the most frequently used ASMs for treating generalized and focal seizures. It is also indicated for managing bipolar disorders, neuropathic pain, and migraine prophylaxis [28]. VPA is associated with neurological and cosmetic side effects [29]. Alopecia is among the 10 most commonly reported adverse effects of VPA use [30]. The incidence of alopecia secondary to VPA greatly varies from 0.5 to 24% [31]. The diagnosis is based on the history of hair loss or abnormalities following VPA administration. Additionally, it can be confirmed by performing pull tests and modified wash tests.
Hair loss most commonly occurs after three to six months of VPA introduction [32]. Apparently, there is a direct relationship between the occurrence of hair loss and blood levels of VPA. High VPA blood levels (80–150 mcg/L) are associated with alopecia occurrence in 28% of the individuals taking VPA. On the other hand, adequate blood level concentrations (25–50 mcg/L) of VPA are related to a 4 percent occurrence of alopecia [33]. However, a meta-analysis found no significant correlation between the dose or duration of VPA therapy and alopecia [34].
There are five main hypotheses to explain hair loss associated with VPA use [35,36]. The pathophysiology of hair loss includes biotin deficiency, hyperandrogenism, mineral deficiency, telogen effluvium, and vitamin D deficiency (Figure 2) [37,38,39,40,41]. The hair loss associated with VPA is typically incomplete and usually reversible after discontinuing the causative drug [23]. Treatment will include general measures, such as reassurance and hair care techniques. Additionally, some authors reported specific treatment options that should be carefully evaluated case-by-case (Table 2) [35,36,42,43,44,45,46,47,48,49].
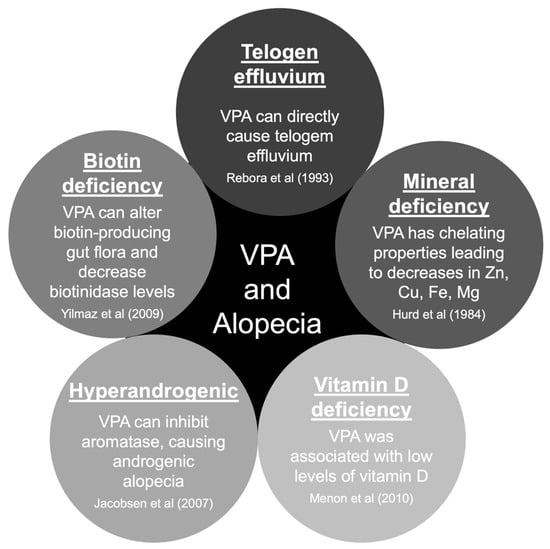
Figure 2.
Pathophysiology of valproate-induced alopecia. Abbreviations: Cu, copper; Mg, magnesium; VPA, valproate/valproic acid; Zn, zinc [37,38,39,40,41].

Table 2.
Management of VPA-associated alopecia by Praharaj et al. [36] adapted by Rissardo et al.
3.2. Carbamazepine (CBZ)
CBZ has a broad use for the treatment of focal seizures. It is also used for treating bipolar disorder and trigeminal neuralgia. Although CBZ is not as notorious for causing alopecia as VPA, the incidence of alopecia with it ranges from 0.3% to 6% [4,50]. In 1985, Shuper et al. reported the first case of CBZ-induced alopecia. An 8.5-year-old girl with headaches and multifocal epilepsy was managed with CBZ. Hair loss stopped after CBZ discontinuation, and hair regrowth was observed [51].
Pillans and Woods reported 177 cases of CBZ-induced alopecia in 1995 [33]. In 1992, Mattson et al. conducted a double-blind trial in 480 individuals with focal seizures and generalized tonic-clonic seizures using VPA or CBZ. Hair loss or abnormal hair texture was observed in 12 percent of subjects taking VPA and 6 percent taking CBZ [52].
In 1997, Ikeda et al. published two individuals that developed hair loss secondary to CBZ therapy. In the first case, the patient was treated for focal epilepsy. Hair loss developed after three months of starting the drug. The blood concentration of CBZ was maintained at 5.0 mg/L. Hair regrowth after replacing the offending drug was observed. The second case reported by Ikeda et al. was an individual diagnosed with tuberous sclerosis with focal seizures. CBZ was started and maintained at a steady level with a blood concentration of 6.5 mg/L. Hair loss was observed after two months of starting CBZ therapy. After reducing the CBZ dose from 600 mg/day to 200 mg/day, hair shedding was reduced, and new hair began to grow [53]. Another case report from Oh et al. demonstrated hair loss four months after starting CBZ at 600 mg/day. The hair loss was reversed after decreasing the dose of CBZ to 200 mg/day [54].
3.3. Lamotrigine (LTG)
LTG is used to treat epilepsy as a monotherapy or as an adjunct to other antiseizure polytherapy. It is a first-line treatment for primary generalized tonic–clonic seizures, focal seizures, atypical absence seizures, myoclonic seizures, and atonic seizures [55]. Apart from using it as an ASM, it can also be used in mood disorders and depression management [56]. The incidence of hair loss associated with LTG is 0.8%. Interestingly, three in every four individuals who developed LTG-induced alopecia reported this side effect as a significant factor for LTG withdrawal [4].
LTG-induced epidermal necrolysis with some hair loss is well known. However, there are few reports of isolated hair loss without epidermal necrolysis associated with LTG in the literature [57]. In 2004, Patrizi et al. reported the first case of LTG-induced alopecia. A 24-year-old female with focal epilepsy was started on LTG and magnesium VPA. The magnesium VPA dose was decreased to 600 mg/day, and the LTG dosage was increased to 100 mg/day. After a few months, hair loss was observed. Trichogram ruled out androgenetic alopecia. LTG probably was the main cause for the development of alopecia, but the effect of VPA on hair structure cannot be excluded [58].
In 2006, Hillemacher et al. reported a case of a 63-year-old diagnosed with bipolar disorder. LTG was started and increased to 150 mg/day. Hair loss was noted in the third week of treatment. A trichogram showed hair with a prolonged telogen phase and dystrophic characteristics [59]. This strongly suggested telogen effluvium as the cause of LTG-induced alopecia. In 2017, Solmi et al. reported a patient affected by treatment-resistant major depressive disorder who was managed with LTG, and telogen effluvium was observed [60].
Tengstrand et al.’s study is one of the most significant articles in the literature regarding LTG-induced alopecia. The authors assessed 337 individuals from 19 countries who developed hair loss associated with LTG. In 291 reports, alopecia developed with LTG monotherapy. They found that alopecia should be considered a significant factor affecting adherence to LTG therapy. The time to onset of alopecia after LTG intake was variable, in which 17 individuals presented alopecia within one month, 48 presented between one and six months, and 31 presented after six months of LTG onset [61]. It is worth mentioning that Tengstrand et al.’s study assessed VigiBase reports, a database with limited information on patient demographic characteristics and clinical descriptions [61].
3.4. Levetiracetam (LEV)
LEV is considered a broad-spectrum ASM. This drug was approved by the FDA in 1999 for the management of epilepsy [62]. Interestingly, LEV differs in structure and mechanism of action from other marketed ASMs [63]. LEV has favorable pharmacokinetics and a low potential for drug interactions [64]. In a study with 1903 individuals, a 0.4% prevalence of hair loss due to LEV therapy was observed [4].
Hair loss is a rare adverse effect of LEV therapy. In 2014, Zou et al. reported five cases of LEV-induced alopecia. The doses of LEV ranged within 500–1000 mg/day. Hair loss secondary to LEV was observed to occur between three and eight weeks of LEV therapy. The individuals presented with diffuse non-scarring hair loss. Complete recovery from alopecia was seen in two out of five subjects. The other two patients noticed an improvement in hair loss after decreasing the dose from 1000 mg/day to 750 mg/day. One individual decided to continue with medication despite hair loss [65]. The authors concluded that the alopecia associated with LEV was due to telogen effluvium. Aghamollaii et al. published a case series of three patients that experienced hair loss with LEV [66].
3.5. Gabapentin (GBP)
GBP is associated with mild adverse events, such as somnolence, fatigue, ataxia, and dizziness, which are reported in about three in every four patients [67]. It is a first-line treatment for the management of neuropathic pain [68]. Eker et al. reported a case of alopecia with GBP therapy for neuropathic pain. The patient was started on GBP 1800 mg/day. Hair loss was noticed one week after the GBP therapy onset. Patchy areas of alopecia were noticed. When GBP was discontinued, hair regrowth was observed [69].
The first case of GBP-induced alopecia was reported in 1997 by Picard et al. A 15-year-old girl with seizures was taking CBZ and phenytoin. GBP 1800 mg/day was prescribed as add-on therapy. The patient experienced alopecia during the second month of treatment with GBP. Hair loss was diffuse without bald patches. GBP was discontinued, but CBZ and phenytoin were continued. Hair regrowth was observed three weeks after GBP discontinuation, and alopecia was completely reversed within one month [70].
3.6. Topiramate (TPM)
TPM may cause alopecia in approximately one percent of its users [8]. According to Chen et al., alopecia prevalence was 1.7% among 230 TPM users. TPM was discontinued in all the individuals who developed alopecia because the patients decided to stop the treatment [4]. Chuang et al. reported a 15-year-old girl with frontal lobe epilepsy who developed hair loss after two months of TPM adjunctive therapy. The hair loss was reversible upon discontinuation of the drug. There was a recurrence of alopecia after the TPM rechallenge [71]. Another case report demonstrated hair loss that started after three months of starting TPM at a dosage of 50 mg/day for migraine headaches. Hair started regrowing with a dose reduction to 25 mg/day, and alopecia recurred after the reintroduction of a 50 mg/day dosage of TPM [72].
Lagrand et al. described a case of an individual who developed alopecia with different medications for managing her tremor-dominant Parkinson’s disease. She presented hair loss after the introduction, in different moments, of levodopa/benserazide, propranolol, and TPM [73]. Their case is interesting because it suggests a possible genetic predisposition for the development of alopecia after the administration of determined groups of medications.
3.7. Phenytoin (PHT)
As part of the Columbia and Yale ASM Database Project, Chen et al. reviewed patient records, including demographics, medical history, ASM use, and side effects for 1903 adult patients (≥16 years of age) newly started on an ASM. Cosmetic side effects were determined by patient or physician reports in the medical record, including acne, gingival hyperplasia, hair loss, hirsutism, and weight gain. PHT was taken by 404 out of 1903 patients. In total, 0.3% attributed hair loss due to PHT and 0.3% intolerability due to hair loss [4]. Herranz et al. assessed the clinical side effects of long-term monotherapy of phenobarbital, primidone, PHT, CBZ, and VPA in 392 pediatric individuals. PHT was more commonly associated with cosmetic side effects than the other investigated drugs. However, there was no report of PHT-induced alopecia. Instead, hirsutism was observed in 9% of children taking PHT [74]. Interestingly, VPA was the drug most commonly associated with alopecia, which occurred in 0.8% of children [75].
Kuhne et al. described a case of Munchausen by proxy syndrome mimicking childhood-onset systemic lupus erythematosus. A patient presenting with malar rash, photosensitivity, alopecia, arthralgia, arterial hypertension, macroscopic hematuria, seizure, and positive antinuclear antibodies was reported. The pediatric patient received high doses of PHT, which led to drug-induced lupus erythematosus [76]. Thus, subjects presenting with alopecia during PHT therapy should be assessed for other clinical manifestations suggesting autoimmune diseases. There are two other cases in the literature with alopecia secondary to PHT-induced lupus erythematosus [77,78].
Hirsutism is more frequent than alopecia as an adverse effect of PHT [75]. In this context, a study assessed the effectiveness of PHT in suppressing chemotherapy-induced hair loss. The authors revealed that oral PHT could significantly suppress hair loss due to cyclophosphamide therapy in rats. PHT co-administration was related to improved hair growth, increased hair-shaft thickness, and reduced skin lipid peroxidation [79].
3.8. Pregabalin (PGB)
Isolated alopecia secondary to PGB was uncommonly described in the literature. Chen et al. found only one patient who developed alopecia among 143 PGB users [4]. In another study with the Netherlands Pharmacovigilance Centre Lareb data, the incidence of PGB-induced alopecia was 0.07% [80]. Noteworthily, PGB dose may be directly associated with hair loss. In Wistar rats, alopecia was more frequently observed with higher doses of PGB [81].
Turgut et al. reported an adult female diagnosed with fibromyalgia. PGB 75 mg/day was started, and the dose was increased to 150 mg/day after one week. Within three weeks of PGB therapy, significant hair loss was observed. PGB was discontinued. Complete hair regrowth was observed after two weeks of PGB withdrawal [82].
In clinical practice, PGB-induced alopecia is commonly seen as part of drug reactions with eosinophilia and systemic symptoms (DRESS). However, it was scarcely reported in the literature. Suh et al. described an individual presenting with alopecia and pruritic erythema after PGB use. DRESS diagnosis was made. After PGB discontinuation, hair regrowth was observed [83].
3.9. Perampanel (PMP)
PMP has been approved as an add-on treatment for refractory focal seizures and primary generalized tonic–clonic seizures in idiopathic generalized epilepsy [84]. The most frequently reported side effects are dizziness and fatigue. In this context, the only described CSE were weight gain (7.4–19.2%) and skin rash (10.6%) [85,86,87]. It is worth mentioning that these CSEs were only observed in specific populations, such as Asian individuals [87].
Rohracher et al. pooled observational data of PMP comprising a full analysis set of 2396 individuals. Only one individual reported alopecia during the first year of PMP therapy [88]. Villanueva et al. assessed the safety and efficacy of long-term PMP in 464 subjects. The incidence of PMP-induced alopecia was 0.2% [89].
The mechanism of action of PMP involves a non-competitive antagonism of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor. The AMPA receptors are considered the major subtype of ionotropic glutamate receptors [90]. Noteworthy glutamate can promote hair growth in mice models. Additionally, it is believed that glutamic acid can control hair follicle proliferation, decreasing gene expression related to apoptosis in the skin and increasing cell viability and proliferation in human keratinocytes [91]. Therefore, the low levels of glutamate in PMP therapy may be associated with hair loss.
3.10. Phenobarbital, Vigabatrin, Tiagabine, and Trimethadione
Phenobarbital-induced isolated alopecia was rarely described. Alopecia was usually described as part of an anticonvulsant hypersensitivity syndrome induced by phenobarbital. Huang et al. reported an individual presenting with alopecia in the convalescent status of phenobarbital-induced anticonvulsant hypersensitivity syndrome. Skin histology revealed peri-follicular, peri-bulbar, and supra-bulbar lymphocyte infiltration [92]. In the literature, two other cases of alopecia were present in the convalescent period of phenobarbital hypersensitivity syndrome [93,94]. All individuals reported they had a favorable prognosis with complete hair regrowth within three months [92,93,94]. Huang et al. proposed that phenobarbital could promote lymphocyte infiltration into the peri-follicular, peri-bulbar, and supra-bulbar anatomical regions. Additionally, phenobarbital dose may be associated with the incidence of alopecia. Ghorani-Azam et al. systematically reviewed the literature concerning phenobarbital in neonates and pediatrics that were treated for seizures. Interestingly, alopecia was a reported sign of phenobarbital poisoning [95].
Vigabatrin has also been reported to cause hair loss. In Lampl et al., 5 out of 52 patients who received vigabatrin for focal seizures experienced moderate hair loss or changes in hair structure. The complaints began after three to seven weeks of initiating vigabatrin treatment. Hair loss recovery was seen in all patients after cessation of treatment [96]. Vigabatrin showed different results in rodent studies. It was observed that mice tolerated high doses of vigabatrin, but Sprague Dawley rats developed acute alopecia and body weight gain. These CSEs were improved within six months of vigabatrin discontinuation [97].
Tiagabine may increase the level of γ-aminobutyric acid. Vossler et al. assessed the adverse effects of long-term tiagabine use in 292 subjects. Only one patient developed alopecia secondary to tiagabine [98]. Another study by Mercke et al. found an incidence of 1% of tiagabine-induced alopecia [8].
Trimethadione is historically important for managing absence seizures and refractory temporal lobe epilepsy. There are some case reports in the literature about alopecia associated with this medication [99].
For further description of the cases reported in the literature about alopecia secondary to antiseizure medication, refer to Table 3. Table 4 shows the results from PubMed, cases encountered in the literature, and incidence of ASM-induced alopecia.

Table 3.
Literature review of antiseizure medication-induced alopecia.

Table 4.
Summary of antiseizure medication-induced alopecia.
4. Discussion
VPA, CBZ, and LTG were the most studied causes of ASM-induced alopecia. Additionally, LEV, TPM, and cenobamate were uncommonly associated with hair loss. However, the rest of the anticonvulsants were rarely reported with alopecia in the literature. To the author’s knowledge, there were no cases of oxcarbazepine and felbamate affecting hair growth. Interestingly, alopecia was usually seen with higher than the usual therapeutic doses of many ASMs [66]. However, there is no established dose-dependent relationship, and in a few cases, hair loss occurred at lower doses of ASM [106].
In most cases reported, hair loss stopped after dose adjustment of the ASM, which included discontinuing the drug or decreasing its dose [70]. In many cases, hair loss recured after ASM reintroduction or an increased dose of ASM back to the original strength that caused alopecia in the first place [72]. Nevertheless, the reversibility of alopecia has been seen as a characteristic feature in all ASMs [73]. Noteworthily, the optimal therapeutic dosage of antiseizure medications should be carefully assessed, especially in individuals with multiple comorbidities due to the modified pharmacokinetics and pharmacodynamics of drugs, which can lead to high percentages of adverse events [172].
The time duration has also been variable in the examples mentioned in this review. The most common duration from starting the drug or increasing the dosage to the onset of hair loss was between one and six months. However, it can vary with the different drugs and their doses. We have mentioned different timelines and associated doses at which hair loss was reported in Table 2. Moreover, hair loss etiology probably was related to telogen effluvium due to features such as non-scarring diffuse distribution, time of alopecia onset, and reversible nature of hair loss. Other than one case, anagen effluvium was not associated with the main cause of ASM-induced alopecia [116].
Telogen effluvium can be diagnosed by identifying known triggers from the history in the three to four months preceding the onset of hair shedding. Additionally, endocrine, nutritional, and autoimmune etiologies should be ruled out. Usually, the diagnosis of telogen effluvium was made by demonstrating a compatible chronology of ASM exposure and onset of hair loss. Hair regrowth within three months of ASM discontinuation can support diagnosis. A drug rechallenge was attempted in some individuals with a re-occurrence of hair loss [43].
Further tests to diagnose telogen effluvium are a hair pull test, 60-second timed hair count, trichogram, trichoscopy, and scalp biopsy [173]. A scalp biopsy can be conducted to identify the earliest stages of androgenetic alopecia, which was already reported with VPA [163]. However, in almost all the studies we analyzed, history was sufficient to label telogen effluvium [174]. A trichogram was performed in only a few reported cases [115,116].
The hair pull test is strongly positive for telogen effluvium [175]. It is carried out by grasping 40–60 closely grouped scalp hair with the thumb and index finger, and gentle traction is applied as the hair is pulled firmly and slowly from the scalp. Normally, only two to three hairs come out. In telogen effluvium, more than 10 percent of hair is easily pulled out from any part of the scalp [176].
In telogen effluvium, the average number of scalp hairs shed daily ranges from 30 to 100, although there is a seasonal fluctuation with the greatest loss around late summer [177,178]. Inspection of telogen hairs with the naked eye can distinguish anagen from telogen hairs [179]. Telogen hairs have depigmented hair bulbs and the absence of inner root sheaths, whereas anagen hairs have inner root sheaths [180]. The inner root sheath can be subdivided into three layers, the cuticle, Huxley’s, and outer Henle’s layers.
Trichogram can help in telogen effluvium diagnosis. Trichogram from a hair pluck sample usually shows more than 25 percent telogen hair in acute telogen effluvium [181]. A 60-s timed hair count can also be performed. Usually, more than 100 hairs will be observed with telogen effluvium (the normal value is 10 hairs) [182]. Another method, which involves combing the air forward for 60 s over a contrasting cloth before shampooing, can be used to assess the disease progression and resolution [183].
An interesting association between hair loss and ASMs is blood zinc concentrations. Low blood zinc levels have been reported in association with higher levels of LEV, especially in cases with reported hair loss [66]. In their letter to the editor, Calabro et al. discussed the relationship between LEV and blood zinc levels. They described a case of a 35-year-old female who was started on LEV and titrated up to 1500 mg/day for generalized tonic–clonic seizures. In the second month’s follow-up, she noticed patchy alopecia. Her zinc levels were below normal (45 μg/dL). Six weeks after LEV discontinuation, hair regrowth was noted. At a one-year follow-up, serum zinc levels were back to normal [106]. Another reported case showed borderline low normal zinc levels one year after stopping LEV due to LEV-induced alopecia [107]. Zou et al. reported that zinc supplementation effectively treated LEV-induced alopecia in five individuals [65]. In this context, Aghamollai et al. prescribed zinc sulfate supplementation for managing hair loss due to LEV. The authors described three patients with alopecia secondary to LEV for whom zinc sulfate 220 mg/day was started. In all three cases, hair loss improvement was seen [66].
VPA-induced alopecia has also been linked with abnormal concentrations of zinc. It has been suggested that low zinc levels may occur due to VPA-induced zinc chelation [184]. However, evidence has shown variable results of zinc blood levels in VPA-treated patients [8]. In animal models, VPA has been shown to induce zinc and selenium deficiencies [44,185,186]. Suzuki et al. showed that long-term ASM therapy, including VPA and CBZ, can decrease zinc levels in the male sex. Interestingly, increased copper concentrations were found in the female sex with long-term ASM therapy [187]. In this context, Kuzuya et al. demonstrated no change in serum zinc or copper levels in patients with epilepsy who had undergone one month of VPA therapy [188]. Therefore, we hypothesize that ASMs can lead to abnormal concentrations of some metals by influencing the absorption of these substances. This can partially explain the development of abnormal levels of zinc and copper with chronic instead of acute ASM therapy.
Zinc and selenium supplementations were already prescribed for VPA-induced alopecia. Fatemi et al. reported an individual with refractory bipolar disorder who developed alopecia secondary to VPA, leading to drug discontinuation. A VPA therapy rechallenge was attempted with zinc and selenium supplementation. There was no occurrence of any hair structural abnormality [44]. Asadi–Pooya systematically reviewed the literature regarding the cosmetic adverse effects of antiseizure medications. They found strong evidence of ASM associated with cosmetic adverse effects. PHT was extensively reported to cause gingival hyperplasia, hirsutism, and acne. VPA was related to causing hair loss and hirsutism. Additionally, they suggest that the evidence for zinc or biotin supplementation in VPA and LEV-associated alopecia needs to be further assessed [189].
There are important limitations in the present study that should be discussed. First, the data in the literature regarding alopecia secondary to antiseizure medication are diverse and sometimes even contradictory. Second, different types of studies with different outcomes to provide an overall view of the literature were included. Third, a significant number of reports only observed alopecia as a secondary outcome, which can affect the incidence of alopecia throughout the studies. Fourth, the majority of the reports of management were about valproate-induced alopecia, but these reports were isolated and had a low level of evidence.
5. Future Perspectives
Future studies with ASMs should assess hair abnormalities with a trichogram. Observing hair structures is essential for diagnosing telogen effluvium. Special attention should be paid to those ASMs whose hair abnormalities were already noted in animal studies. Additionally, the complaints by the individuals in clinical trials with ASM should be further assessed. A mild report of hair loss with ASM should be investigated, and the patient should be recommended to a specialist. Moreover, clinical trials with the supplementation of metal and vitamins in ASM-induced alopecia are important. The development of strong evidence with different treatment options can improve the quality of life and management in people with epilepsy, mainly in those seizure-free individuals with specific types of medications, in which a small dose adjustment can lead to the development of new seizures.
Interestingly, most reports in the literature only described hair loss as a secondary objective of antiseizure medication. Noteworthily, only primary objectives are considered significant due to statistical power. Therefore, future studies need to investigate alopecia as a primary objective of hair loss for further epidemiological assessment and causality. Moreover, most studies describing the management of ASM-induced alopecia are case reports, which can significantly impact the evidence of therapeutic choice.
Understanding ASM’s interaction with genes to cause adverse effects is also important. Pharmacogenomic studies can provide crucial information regarding the potential adverse reactions that the patient may develop with the use of specific medication. These studies are mandatory in individuals with ASM-induced alopecia because there is a significantly higher incidence of VPA-induced alopecia in European and American populations when compared to Asian individuals.
6. Conclusions
Drug-related hair loss is challenging to diagnose. It requires an understanding of normal hair growth and the different causal factors that are involved in it. In this context, hair loss is an important adverse effect of ASM because it affects a significant percentage of the population. Additionally, it can negatively impact the quality of life of people with epilepsy leading to poor therapeutical adherence and a high economic burden. VPA, LEV, and LTG-associated alopecia were extensively reported in the literature. Management of alopecia includes reassurance, hair care techniques, and drug modification. The most frequent management was ASM discontinuation. In some cases, ASM dose reduction was attempted. Hair loss secondary to ASMs was reversible. Metals and vitamin supplementations in the management of ASM-induced alopecia should be investigated.
Author Contributions
J.P.R., A.L.F.C., M.C., H.J.S. and U.H. conceptualized and designed the methodology of the literature review; J.P.R. and U.H. extracted and collected the relevant information and drafted the manuscript; H.J.S. and H.J.S. supervised the article selection and reviewed and edited the manuscript; J.P.R., A.L.F.C., M.C., H.J.S. and U.H. reviewed and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Getnet, A.; Woldeyohannes, S.M.; Bekana, L.; Mekonen, T.; Fekadu, W.; Menberu, M.; Yimer, S.; Assaye, A.; Belete, A.; Belete, H. Antiepileptic Drug Nonadherence and Its Predictors among People with Epilepsy. Behav. Neurol. 2016, 2016, 3189108. [Google Scholar] [CrossRef] [PubMed]
- Buck, D.; Jacoby, A.; Baker, G.A.; Chadwick, D.W. Factors influencing compliance with antiepileptic drug regimes. Seizure 1997, 6, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kanner, A.M. To comply with AED therapy… what patients are not told! Epilepsy Curr. 2009, 9, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Choi, H.; Hirsch, L.J.; Moeller, J.; Javed, A.; Kato, K.; Legge, A.; Buchsbaum, R.; Detyniecki, K. Cosmetic side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav. 2015, 42, 129–137. [Google Scholar] [CrossRef]
- Perucca, P.; Gilliam, F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012, 11, 792–802. [Google Scholar] [CrossRef]
- Kinderen, R.J.; Evers, S.M.; Rinkens, R.; Postulart, D.; Vader, C.I.; Majoie, M.H.; Aldenkamp, A.P. Side-effects of antiepileptic drugs: The economic burden. Seizure 2014, 23, 184–190. [Google Scholar] [CrossRef]
- Stroud, J.D. Diagnosis and management of the hair loss patient. Cutis 1987, 40, 272–276. [Google Scholar]
- Mercke, Y.; Sheng, H.; Khan, T.; Lippmann, S. Hair loss in psychopharmacology. Ann. Clin. Psychiatry 2000, 12, 35–42. [Google Scholar] [CrossRef]
- Botega, A.R.; Amorim, C.V.; Teixeira, F.; Mello, C.D.; Stelini, R.F.; Velho, P.N.; Cintra, M.L. Scarring versus Non-Scarring Alopecia: An Interobserver Histopathological Reproducibility Study. Ski. Appendage Disord. 2023, 9, 34–41. [Google Scholar] [CrossRef]
- Llau, M.E.; Viraben, R.; Montastruc, J.L. Drug-induced alopecia: Review of the literature. Therapie 1995, 50, 145–150. [Google Scholar]
- Lemieux, J.; Maunsell, E.; Provencher, L. Chemotherapy-induced alopecia and effects on quality of life among women with breast cancer: A literature review. Psychooncology 2008, 17, 317–328. [Google Scholar] [CrossRef]
- Can, G.; Demir, M.; Erol, O.; Aydiner, A. A comparison of men and women’s experiences of chemotherapy-induced alopecia. Eur. J. Oncol. Nurs. 2013, 17, 255–260. [Google Scholar] [CrossRef]
- Paus, R.; Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef]
- Lindner, G.; Botchkarev, V.A.; Botchkareva, N.V.; Ling, G.; Veen, C.; Paus, R. Analysis of apoptosis during hair follicle regression (catagen). Am. J. Pathol. 1997, 151, 1601–1617. [Google Scholar]
- Lousada, M.B.; Lachnit, T.; Edelkamp, J.; Rouillé, T.; Ajdic, D.; Uchida, Y.; Nardo, A.; Bosch, T.C.; Paus, R. Exploring the human hair follicle microbiome. Br. J. Dermatol. 2021, 184, 802–815. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef]
- Popa, A.; Carsote, M.; Cretoiu, D.; Dumitrascu, M.C.; Nistor, C.E.; Sandru, F. Study of the Thyroid Profile of Patients with Alopecia. J. Clin. Med. 2023, 12, 1115. [Google Scholar] [CrossRef]
- Courtois, M.; Loussouarn, G.; Hourseau, C.; Grollier, J.F. Ageing and hair cycles. Br. J. Dermatol. 1995, 132, 86–93. [Google Scholar] [CrossRef]
- Bemt, P.M.; Brodie-Meijer, C.C.; Krijnen, R.M.; Nieboer, C. Drug induced alopecia. Ned. Tijdschr. Geneeskd. 1999, 143, 990–994. [Google Scholar]
- Murad, A.; Maguire, J.; Bergfeld, W. Drug-induced alopecia areata? Clin. Exp. Dermatol. 2021, 46, 363–366. [Google Scholar] [CrossRef]
- Starace, M.; Orlando, G.; Bruni, F.; Alessandrini, A.; Piraccini, B.M. Anagen effluvium and the role of trichoscopy. Clin. Exp. Dermatol. 2022, 47, 589–591. [Google Scholar] [CrossRef] [PubMed]
- Rossi, A.; Caro, G.; Fortuna, M.C.; Pigliacelli, F.; D’Arino, A.; Carlesimo, M. Prevention and Treatment of Chemotherapy-Induced Alopecia. Dermatol. Pract. Concept. 2020, 10, e2020074. [Google Scholar] [CrossRef] [PubMed]
- Moattari, C.R.; Jafferany, M. Psychological Aspects of Hair Disorders: Consideration for Dermatologists, Cosmetologists, Aesthetic, and Plastic Surgeons. Ski. Appendage Disord. 2022, 8, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Tosti, A.; Misciali, C.; Piraccini, B.M.; Peluso, A.M.; Bardazzi, F. Drug-induced hair loss and hair growth. Incidence, management and avoidance. Drug Saf. 1994, 10, 310–317. [Google Scholar] [CrossRef]
- Trüeb, R.M. Chemotherapy-induced hair loss. Ski. Ther. Lett. 2010, 15, 5–7. [Google Scholar]
- Malkud, S. Telogen Effluvium: A Review. J. Clin. Diagn. Res. 2015, 9, 1–3. [Google Scholar] [CrossRef]
- Sinclair, R. Diffuse hair loss. Int. J. Dermatol. 1999, 38, 8–18. [Google Scholar] [CrossRef]
- McKinney, P.A.; Finkenbine, R.D.; DeVane, C.L. Alopecia and mood stabilizer therapy. Ann. Clin. Psychiatry 1996, 8, 183–185. [Google Scholar] [CrossRef]
- Johannessen, C.U. Mechanisms of action of valproate: A commentatory. Neurochem. Int. 2000, 37, 103–110. [Google Scholar] [CrossRef]
- Rissardo, J.P.; Caprara, A.L.; Durante, Í. Valproate-associated Movement Disorder: A Literature Review. Prague Med. Rep. 2021, 122, 140–180. [Google Scholar] [CrossRef]
- Schmidt, D. Adverse effects of valproate. Epilepsia 1984, 25, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Völzke, E.; Doose, H. Dipropylacetate (Dépakine, Ergenyl) in the treatment of epilepsy. Epilepsia 1973, 14, 185–193. [Google Scholar] [CrossRef]
- Pillans, P.I.; Woods, D.J. Drug-associated alopecia. Int. J. Dermatol. 1995, 34, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, A.; Sackellares, J.C.; Shu, V. Safety and efficacy of divalproex sodium monotherapy in partial epilepsy: A double-blind, concentration-response design clinical trial. Depakote Monotherapy for Partial Seizures Study Group. Neurology 1997, 48, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, H.; Xu, D.; Zhu, L.; Liu, L. Risk of valproic acid-related alopecia: A systematic review and meta-analysis. Seizure 2019, 69, 61–69. [Google Scholar] [CrossRef]
- Praharaj, S.K.; Munoli, R.N.; Udupa, S.T.; Vaidyanathan, S. Valproate-associated hair abnormalities: Pathophysiology and management strategies. Hum. Psychopharmacol. 2022, 37, e2814. [Google Scholar] [CrossRef]
- Rebora, A. Telogen effluvium: An etiopathogenetic theory. Int. J. Dermatol. 1993, 32, 339–340. [Google Scholar] [CrossRef]
- Yilmaz, Y.; Tasdemir, H.A.; Paksu, M.S. The influence of valproic acid treatment on hair and serum zinc levels and serum biotinidase activity. Eur. J. Paediatr. Neurol. 2009, 13, 439–443. [Google Scholar] [CrossRef]
- Hurd, R.W.; Rinsvelt, H.A.; Wilder, B.J.; Karas, B.; Maenhaut, W.; Reu, L. Selenium, zinc, and copper changes with valproic acid: Possible relation to drug side effects. Neurology 1984, 34, 1393–1395. [Google Scholar] [CrossRef]
- Jacobsen, N.W.; Halling-Sorensen, B.; Birkved, F.K. Inhibition of human aromatase complex (CYP19) by antiepileptic drugs. Toxicol. In Vitro 2008, 22, 146–153. [Google Scholar] [CrossRef]
- Menon, B.; Harinarayan, C.V. The effect of anti epileptic drug therapy on serum 25-hydroxyvitamin D and parameters of calcium and bone metabolism--a longitudinal study. Seizure 2010, 19, 153–158. [Google Scholar] [CrossRef]
- Uehlinger, C.; Barrelet, L.; Touabi, M.; Baumann, P. Alopecia and mood stabilizers: Two case reports. Eur. Arch. Psychiatry Clin. Neurosci. 1992, 242, 85–88. [Google Scholar] [CrossRef]
- Henriksen, O.; Johannessen, S.I. Clinical and pharmacokinetic observations on sodium valproate—A 5-year follow-up study in 100 children with epilepsy. Acta Neurol. Scand. 1982, 65, 504–523. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Calabrese, J.R. Treatment of valproate-induced alopecia. Ann. Pharmacother. 1995, 29, 1302. [Google Scholar] [CrossRef]
- Trost, L.B.; Bergfeld, W.F.; Calogeras, E. The diagnosis and treatment of iron deficiency and its potential relationship to hair loss. J. Am. Acad. Dermatol. 2006, 54, 824–844. [Google Scholar] [CrossRef]
- Castro-Gago, M.; Gómez-Lado, C.; Eirís-Puñal, J.; Díaz-Mayo, I.; Castiñeiras-Ramos, D.E. Serum biotinidase activity in children treated with valproic acid and carbamazepine. J. Child. Neurol. 2010, 25, 32–35. [Google Scholar] [CrossRef]
- Sahin, E.K.; Can, S.S.; Caykoylu, A.; Atagun, M.I. Agomelatine may alleviate valproate induced hair loss. J. Psych. Neurol. Sci. 2017, 30, 269–270. [Google Scholar]
- Thomson, S.R.; Mamulpet, V.; Adiga, S. Sodium Valproate Induced Alopecia: A Case Series. J. Clin. Diagn. Res. 2017, 11, 1–2. [Google Scholar] [CrossRef]
- Kakunje, A.; Prabhu, A.; Priya, E.S.; Karkal, R.; Kumar, P.; Gupta, N.; Rahyanath, P.K. Valproate: It’s Effects on Hair. Int. J. Trichol. 2018, 10, 150–153. [Google Scholar] [CrossRef]
- Pellock, J.M. Carbamazepine side effects in children and adults. Epilepsia 1987, 28, 64–70. [Google Scholar] [CrossRef]
- Shuper, A.; Stahl, B.; Weitz, R. Carbamazepine-induced hair loss. Drug Intell. Clin. Pharm. 1985, 19, 924–925. [Google Scholar] [CrossRef] [PubMed]
- Mattson, R.H.; Cramer, J.A.; Collins, J.F. A comparison of valproate with carbamazepine for the treatment of complex partial seizures and secondarily generalized tonic-clonic seizures in adults. N. Engl. J. Med. 1992, 327, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, A.; Shibasaki, H.; Shiozaki, A.; Kimura, J. Alopecia with carbamazepine in two patients with focal seizures. J. Neurol. Neurosurg. Psychiatry 1997, 63, 549–550. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Kim, D.S.; Kwon, Y.S.; Lee, J.H.; Lee, K.H. Concurrence of palmoplantar psoriasiform eruptions and hair loss during carbamazepine treatment. Acta Derm. Venereol. 2008, 88, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, M.M. Overview of drugs used for epilepsy and seizures: Etiology, diagnosis, and treatment. Pharm. Ther. 2010, 35, 392–415. [Google Scholar]
- Rybakowski, J.K. Mood Stabilizers of First and Second Generation. Brain Sci. 2023, 13, 741. [Google Scholar] [CrossRef]
- Shirazi, Z.; Inaloo, S. Intravenous immunoglobulin in the treatment of lamotrigine- induced toxic epidermal necrolysis. Iran. J. Allergy Asthma Immunol. 2008, 7, 239–241. [Google Scholar]
- Patrizi, A.; Savoia, F.; Negosanti, F.; Posar, A.; Santucci, M.; Neri, I. Telogen effluvium caused by magnesium valproate and lamotrigine. Acta Derm. Venereol. 2005, 85, 77–78. [Google Scholar] [CrossRef]
- Hillemacher, T.; Bleich, S.; Kornhuber, J.; Frieling, H. Hair loss as a side effect of lamotrigine treatment. Am. J. Psychiatry 2006, 163, 1451. [Google Scholar] [CrossRef]
- Solmi, M.; Tamiello, G.I.; Manuli, G. Lamotrigine Induces Hair Loss in a Patient with Treatment-Resistant Major Depressive Disorder. Am. J. Ther. 2017, 24, 611–612. [Google Scholar] [CrossRef]
- Tengstrand, M.; Star, K.; Puijenbroek, E.P.; Hill, R. Alopecia in association with lamotrigine use: An analysis of individual case safety reports in a global database. Drug Saf. 2010, 33, 653–658. [Google Scholar] [CrossRef]
- Stephen, L.J.; Kelly, K.; Parker, P.; Brodie, M.J. Levetiracetam monotherapy--outcomes from an epilepsy clinic. Seizure 2011, 20, 554–557. [Google Scholar] [CrossRef]
- Hovinga, C.A. Levetiracetam: A novel antiepileptic drug. Pharmacotherapy 2001, 21, 1375–1388. [Google Scholar] [CrossRef]
- Neyens, L.G.; Alpherts, W.C.; Aldenkamp, A.P. Cognitive effects of a new pyrrolidine derivative (levetiracetam) in patients with epilepsy. Prog. Neuropsychopharmacol. Biol. Psychiatry 1995, 19, 411–419. [Google Scholar] [CrossRef]
- Zou, X.; Hong, Z.; Zhou, D. Hair loss with levetiracetam in five patients with epilepsy. Seizure 2014, 23, 158–160. [Google Scholar] [CrossRef]
- Aghamollaii, V.; Khan, Z.G.; Maneshi, A.; Ghaeli, P. Role of Zinc Supplementation in the Treatment of Levetiracetam-Induced Hair Loss: A Case Series. J. Pharm. Care. 2017, 4, 44–45. [Google Scholar]
- Goa, K.L.; Sorkin, E.M. Gabapentin. A review of its pharmacological properties and clinical potential in epilepsy. Drugs 1993, 46, 409–427. [Google Scholar] [CrossRef]
- Rose, M.A.; Kam, P.C. Gabapentin: Pharmacology and its use in pain management. Anaesthesia 2002, 57, 451–462. [Google Scholar] [CrossRef]
- Eker, H.E.; Cok, O.Y.; Aribogan, A. Alopecia associated with gabapentin in the treatment of neuropathic pain. J. Pain Symptom Manag. 2009, 37, 5–6. [Google Scholar] [CrossRef]
- Picard, C.; Jonville-Bera, A.P.; Billard, C.; Autret, E. Alopecia associated with gabapentin: First case. Ann. Pharmacother. 1997, 31, 1260. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Chang, W.N.; Chen, I.L.; Yang, J.Y.; Ho, J.C.; Kuo, H.W. Topiramate-induced hair loss: Case report. Dermatol. Psychosom. 2002, 3, 183–184. [Google Scholar] [CrossRef]
- Ghafoor, I.; Hosseini, H. Hair Loss Following The Topiramate Treatment. J. Babol. Univ. Med. Sci. 2017, 19, 71–74. [Google Scholar]
- Lagrand, T.J.; Lehn, A.C. Tremor Drugs in the Crosshairs. Tremor Other Hyperkinetic Mov. 2021, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Herranz, J.L.; Armijo, J.A.; Arteaga, R. Clinical side effects of phenobarbital, primidone, phenytoin, carbamazepine, and valproate during monotherapy in children. Epilepsia 1988, 29, 794–804. [Google Scholar] [CrossRef]
- Wallace, S.J. A comparative review of the adverse effects of anticonvulsants in children with epilepsy. Drug Saf. 1996, 15, 378–393. [Google Scholar] [CrossRef]
- Kuhne, A.C.; Pitta, A.C.; Galassi, S.C.; Gonçalves, A.M.; Cardoso, A.C.; Paz, J.A.; Campos, L.M.; Silva, C.A. Munchausen by proxy syndrome mimicking childhood-onset systemic lupus erythematosus. Lupus 2019, 28, 249–252. [Google Scholar] [CrossRef]
- Mangalvedhekar, S.S.; Gogtay, N.J.; Manjula, S.; Kadam, V.S.; Dalvi, S.S.; Shah, P.U.; Badakere, S.S.; Pradhan, V.D.; Kshirsagar, N.A. Phenytoin associated alopecia: Drug induced lupus. J. Assoc. Physicians India 2001, 49, 929–930. [Google Scholar]
- Neki, N.S.; Shah, D.M. Phenytoin induced alopecia & Lupus: A case report. RGUHS J. Med. Sci. 2015, 5, 188–189. [Google Scholar]
- Onaolapo, A.Y.; Adebayo, A.A.; Onaolapo, O.J. Oral phenytoin protects against experimental cyclophosphamide-chemotherapy induced hair loss. Pathophysiology 2018, 25, 31–39. [Google Scholar] [CrossRef]
- Harmark, L.; Puijenbroek, E.; Straus, S.; Grootheest, K. Intensive monitoring of pregabalin: Results from an observational, Web-based, prospective cohort study in the Netherlands using patients as a source of information. Drug Saf. 2011, 34, 221–231. [Google Scholar] [CrossRef]
- Morse, D.C.; Henck, J.W.; Bailey, S.A. Developmental Toxicity Studies with Pregabalin in Rats: Significance of Alterations in Skull Bone Morphology. Birth Defects Res. B Dev. Reprod. Toxicol. 2016, 107, 94–107. [Google Scholar] [CrossRef]
- Turgut, C.; İzki, A.A. Hair loss due to pregabaline: A case report. Med. Res. Rep. 2020, 3, 41–45. [Google Scholar]
- Suh, J.H.; Oh, W.J.; Park, K.Y.; Seo, S.J.; Hong, C.K. DRESS syndrome induced by pregabalin in postherpetic neuralgia. Korean J. Derm. 2016, 68, 475–476. [Google Scholar]
- Franco, V.; Crema, F.; Iudice, A.; Zaccara, G.; Grillo, E. Novel treatment options for epilepsy: Focus on perampanel. Pharmacol. Res. 2013, 70, 35–40. [Google Scholar] [CrossRef]
- French, J.A.; Krauss, G.L.; Biton, V.; Squillacote, D.; Yang, H.; Laurenza, A.; Kumar, D.; Rogawski, M.A. Adjunctive perampanel for refractory partial-onset seizures: Randomized phase III study 304. Neurology 2012, 79, 589–596. [Google Scholar] [CrossRef]
- French, J.A.; Krauss, G.L.; Wechsler, R.T.; Wang, X.F.; DiVentura, B.; Brandt, C.; Trinka, E.; O’Brien, T.J.; Laurenza, A.; Patten, A.; et al. Perampanel for tonic-clonic seizures in idiopathic generalized epilepsy A randomized trial. Neurology 2015, 85, 950–957. [Google Scholar] [CrossRef]
- Lin, K.L.; Lin, J.J.; Chou, M.L.; Hung, P.C.; Hsieh, M.Y.; Chou, I.J.; Lim, S.N.; Wu, T.; Wang, H.S. Efficacy and tolerability of perampanel in children and adolescents with pharmacoresistant epilepsy: The first real-world evaluation in Asian pediatric neurology clinics. Epilepsy Behav. 2018, 85, 188–194. [Google Scholar] [CrossRef]
- Rohracher, A.; Zimmermann, G.; Villanueva, V.; Garamendi, I.; Sander, J.W.; Wehner, T.; Shankar, R.; Ben-Menachem, E.; Brodie, M.J.; Pensel, M.C.; et al. Perampanel in routine clinical use across Europe: Pooled, multicenter, observational data. Epilepsia 2018, 59, 1727–1739. [Google Scholar] [CrossRef]
- Villanueva, V.; Garcés, M.; López-González, F.J.; Rodriguez-Osorio, X.; Toledo, M.; Salas-Puig, J.; González-Cuevas, M.; Campos, D.; Serratosa, J.M.; González-Giráldez, B.; et al. Safety, efficacy and outcome-related factors of perampanel over 12 months in a real-world setting: The FYDATA study. Epilepsy Res. 2016, 126, 201–210. [Google Scholar] [CrossRef]
- Johansen, T.N.; Greenwood, J.R.; Frydenvang, K.; Madsen, U.; Krogsgaard-Larsen, P. Stereostructure-activity studies on agonists at the AMPA and kainate subtypes of ionotropic glutamate receptors. Chirality 2003, 15, 167–179. [Google Scholar] [CrossRef]
- Jara, C.P.; Berti, B.A.; Mendes, N.F.; Engel, D.F.; Zanesco, A.M.; Souza, G.F.; Bezerra, R.M.; Bagatin, J.T.; Maria-Engler, S.S.; Morari, J.; et al. Glutamic acid promotes hair growth in mice. Sci. Rep. 2021, 11, 15453. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Hsieh, M.Y.; Hsiao, P.F.; Sheen, J.M.; Yu, H.R.; Kuo, H.C.; Chen, S.T.; Huang, J.L.; Yang, K.D.; Lee, W.I. Alopecia areata universalis after phenobarbital-induced anti-convulsant hypersensitivity syndrome. Immunol. Investig. 2009, 38, 383–397. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, A.P.; Shah, M.; Schwarz, K.B.; Tsai, C.C. Graft versus host-like illness in a child with phenobarbital hypersensitivity. Pediatrics 1986, 78, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Bavdekar, S.B.; Muranjan, M.N.; Gogtay, N.J.; Kantharia, V.; Kshirsagar, N.A. Anticonvulsant hypersensitivity syndrome: Lymphocyte toxicity assay for the confirmation of diagnosis and risk assessment. Ann. Pharmacother. 2004, 38, 1648–1650. [Google Scholar] [CrossRef]
- Ghorani-Azam, A.; Balali-Mood, M.; Riahi-Zanjani, B.; Darchini-Maragheh, E.; Sadeghi, M. Acute Phenobarbital Poisoning for the Management of Seizures in Newborns and Children; A Systematic Literature Review. CNS Neurol. Disord. Drug Targets 2021, 20, 174–180. [Google Scholar] [CrossRef]
- Lampl, Y.; Gilad, R.; Sarova-Pinchas, I.; Barak, Y. Hair loss-an adverse reaction to treatment with vigabatrin. Acta Therap. 1996, 22, 51–55. [Google Scholar]
- Graham, D. Neuropathology of vigabatrin. Br. J. Clin. Pharmacol. 1989, 27, 43–45. [Google Scholar] [CrossRef]
- Vossler, D.G.; Morris, G.L.; Harden, C.L.; Montouris, G.; Faught, E.; Kanner, A.M.; Fix, A.; French, J.A. Tiagabine in clinical practice: Effects on seizure control and behavior. Epilepsy Behav. 2013, 8, 211–216. [Google Scholar] [CrossRef]
- Holowach, J.; Sanden, H.V. Alopecia as a side effect of treatment of epilepsy with trimethadione: Report of two cases. N. Engl. J. Med. 1960, 263, 1187. [Google Scholar] [CrossRef]
- Wadhwa, S. Carbamazepine Induced Hypersensitivity Syndrome with Alopecia. J. Assoc. Physic. Ind. 1997, 45, 1. [Google Scholar]
- Kohno, Y.; Ishii, A.; Shoji, S. A case of hair loss induced by carbamazepine. Rinsho Shinkeigaku 2004, 44, 379–381. [Google Scholar]
- Zenkov, L.R. Alopecia associated with treatment of symptomatic focal epilepsy with carbamazepine. Neurol. J. 2008, 13, 31–32. [Google Scholar]
- Kenyon, K.; Mintzer, S.; Nei, M. Carbamazepine treatment of generalized tonic-clonic seizures in idiopathic generalized epilepsy. Seizure 2014, 23, 234–236. [Google Scholar] [CrossRef]
- Rathore, C.; Rawat, K.S.; Prakash, S.; Rana, K. Carbamazepine-Induced Acute Alopecia Areata. Neurology 2021, 97, 501–502. [Google Scholar] [CrossRef]
- Chen, C.M.; Chen, J.Y.; Chen, K.T.; Chu, C.C.; Tzeng, J.I.; Lan, K.M. A Case Report-Alopecia as a Rare but Possible Side Effect of Gabapentin. Chin. J. Pain 2010, 20, 40–44. [Google Scholar]
- Calabro, R.S.; Bramanti, P.; Spina, E.; Italiano, D. Can zinc depletion play a role in LEV-induced hair loss? Considerations from a case study. Epilepsy Behav. 2013, 29, 254–255. [Google Scholar] [CrossRef]
- Hamd, R.S.; Hasbini, D.A. Adolescent’s Hair Loss due to Levetiracetam. J. Pediatr. Epilepsy 2018, 7, 152–153. [Google Scholar] [CrossRef]
- Missori, P.; Currà, A. Reversible subacute hair loss induced by levetiracetam. Neurol. Sci. 2023, 44, 2207–2208. [Google Scholar] [CrossRef]
- Krivda, L.K.; Campagna, L.J.; Mignano, M.S.; Cho, C.S. Prolonged Drug-Induced Hypersensitivity Syndrome/DRESS with Alopecia Areata and Autoimmune Thyroiditis. Fed. Pract. 2022, 39, 350–354. [Google Scholar] [CrossRef]
- Laljee, H.C.; Parsonage, M.J. Unwanted effects of sodium valproate (Epilim) in the treatment of adult patients with epilepsy. R. Soc. Med 1980, 1, 141–158. [Google Scholar]
- Khan, T.A.; Sheng, H.; Mercke, Y.K.; Lippmann, S.B. Divalproex-induced alopecia: A case report. Psychiatr. Serv. 1999, 50, 1500. [Google Scholar] [CrossRef] [PubMed]
- Cinbis, M.; Parlaz, N. A case of alopecia areata associated with low dosage VPA treatment. Eur. J. Paediatr. Neurol. 2007, 11, 121. [Google Scholar] [CrossRef]
- Wilting, I.; Laarhoven, J.H.; Koning-Verest, I.F.; Egberts, A.C. Valproic acid-induced hair-texture changes in a white woman. Epilepsia 2007, 48, 400–401. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Beste, B. Valproate-induced hair loss: What to tell patients. Curr. Psychiatry 2011, 10, 74–75. [Google Scholar]
- Ramakrishnappa, S.K.; Belhekar, M.N. Serum drug level-related sodium valproate-induced hair loss. Indian J. Pharmacol. 2013, 45, 187–188. [Google Scholar] [CrossRef]
- Panwar, J.B.; Ishwar, C.; Bhardwaj, B.L.; Tilakraj, R.; Bansal, R. Sodium valproate induced alopecia in a patient of epilepsy. J. Dent. Med. Sci. 2016, 15, 112–114. [Google Scholar]
- Grootens, K.P.; Hartong, E.G. A Case Report of Biotin Treatment for Valproate-Induced Hair Loss. J. Clin. Psychiatry 2017, 78, e838. [Google Scholar] [CrossRef]
- Uygur, Ö.F.; Uygur, H. Valproate Induced Hair Loss and Curly Hair in Bipolar Disorder. Clin. Psychopharmacol. Neurosci. 2019, 17, 566–567. [Google Scholar] [CrossRef]
- Govindan, K.; Mandadi, G.D. Alopecia in Breastfed Infant Possibly Due to Mother Getting Valproate. Indian J. Pediatr. 2021, 88, 519–520. [Google Scholar] [CrossRef]
- Breathnach, S.M.; McGibbon, D.H.; Ive, F.A.; Black, M.M. Carbamazepine (‘Tegretol’) and toxic epidermal necrolysis: Report of three cases with histopathological observations. Clin. Exp. Dermatol. 1982, 7, 585–591. [Google Scholar] [CrossRef]
- Jeavons, P.M.; Clark, J.E.; Harding, G.F. Valproate and curly hair. Lancet 1977, 1, 359. [Google Scholar] [CrossRef]
- Tomita, T.; Goto, H.; Yoshida, T.; Tanaka, K.; Sumiya, K.; Kohda, Y. Dose-dependent valproate-induced alopecia in patients with mental disorders. Indian J. Pharmacol. 2015, 47, 690–692. [Google Scholar] [CrossRef]
- Cooper-Mahkorn, D.; Bauer, J. Alopecia areata during treatment with zonisamide—Two case reports. Aktuelle Neurol. 2007, 34, 354–355. [Google Scholar] [CrossRef]
- Hirsch, M.; Hintz, M.; Specht, A.; Schulze-Bonhage, A. Tolerability, efficacy and retention rate of Brivaracetam in patients previously treated with Levetiracetam: A monocenter retrospective outcome analysis. Seizure 2018, 61, 98–103. [Google Scholar] [CrossRef]
- Ryvlin, P.; Dimova, S.; Elmoufti, S.; Floricel, F.; Laloyaux, C.; Nondonfaz, X.; Biton, V. Tolerability and efficacy of adjunctive brivaracetam in adults with focal seizures by concomitant antiseizure medication use: Pooled results from three phase 3 trials. Epilepsia 2022, 63, 2024–2036. [Google Scholar] [CrossRef]
- Talati, R.; Scholle, J.M.; Phung, O.J. Effectiveness and Safety of Antiepileptic Medications in Patients with Epilepsy [Internet]; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2011; (Comparative Effectiveness Reviews, No. 40.) Results. Available online: https://www.ncbi.nlm.nih.gov/books/NBK83942/ (accessed on 28 May 2023).
- Richens, A.; Davidson, D.L.; Cartlidge, N.E.; Easter, D.J. A multicentre comparative trial of sodium valproate and carbamazepine in adult onset epilepsy. J. Neurol. Neurosurg. Psychiatry 1994, 57, 682–687. [Google Scholar] [CrossRef]
- Verity, C.M.; Hosking, G.; Easter, D.J. A multicentre comparative trial of sodium valproate and carbamazepine in paediatric epilepsy. Dev. Med. Child. Neurol. 1995, 37, 97–108. [Google Scholar] [CrossRef]
- Steinhoff, B.J.; Ueberall, M.A.; Siemes, H.; Kurlemann, G.; Schmitz, B.; Bergmann, L. The LAM-SAFE Study: Lamotrigine versus carbamazepine or valproic acid in newly diagnosed focal and generalised epilepsies in adolescents and adults. Seizure 2005, 14, 597–605. [Google Scholar] [CrossRef]
- Privitera, M.D.; Brodie, M.J.; Mattson, R.H.; Chadwick, D.W.; Neto, W.; Wang, S. Topiramate, carbamazepine and valproate monotherapy: Double-blind comparison in newly diagnosed epilepsy. Acta Neurol. Scand. 2003, 107, 165–175. [Google Scholar] [CrossRef]
- Wheless, J.W.; Neto, W.; Wang, S. Topiramate, carbamazepine, and valproate monotherapy: Double-blind comparison in children with newly diagnosed epilepsy. J. Child Neurol. 2004, 19, 135–141. [Google Scholar] [CrossRef]
- Donati, F.; Gobbi, G.; Campistol, J.; Rapatz, G.; Daehler, M.; Sturm, Y.; Aldenkamp, A.P. The cognitive effects of oxcarbazepine versus carbamazepine or valproate in newly diagnosed children with partial seizures. Seizure 2007, 16, 670–679. [Google Scholar] [CrossRef]
- Koeppen, D.; Baruzzi, A.; Capozza, M.; Chauvel, P.; Courjon, J.; Favel, P.; Harmant, J.; Lorenz, H.; Oller, F.V.; Procaccianti, G.; et al. Clobazam in therapy-resistant patients with partial epilepsy: A double-blind placebo-controlled crossover study. Epilepsia 1987, 28, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Satishchandra, P.; Rathore, C.; Apte, A.; Kumar, A.; Mandal, A.; Chauhan, D.; Agadi, J.; Gurumukhani, J.; Asokan, K.; Venkateshwarlu, K.; et al. Evaluation of one-year effectiveness of clobazam as an add-on therapy to anticonvulsant monotherapy in participants with epilepsy having uncontrolled seizure episodes: An Indian experience. Epilepsy Behav. 2022, 130, 108671. [Google Scholar] [CrossRef] [PubMed]
- Klein, P.; Aboumatar, S.; Brandt, C.; Dong, F.; Krauss, G.L.; Mizne, S.; Sanchez-Alvarez, J.C.; Steinhoff, B.J.; Villanueva, V. Long-Term Efficacy and Safety From an Open-Label Extension of Adjunctive Cenobamate in Patients with Uncontrolled Focal Seizures. Neurology 2022, 99, e989–e998. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.R.; Klein, P.; Aboumatar, S.; Gelfand, M.; Halford, J.J.; Krauss, G.L.; Rosenfeld, W.E.; Vossler, D.G.; Wechsler, R.; Borchert, L.; et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia 2020, 61, 1099–1108. [Google Scholar] [CrossRef]
- Villanueva, V.; Santos-Carrasco, D.; Cabezudo-García, P.; Gómez-Ibáñez, A.; Garcés, M.; Serrano-Castro, P.; Castro-Vilanova, M.D.; Sayas, D.; Lopez-Gonzalez, F.J.; Rodríguez-Osorio, X.; et al. Real-world safety and effectiveness of cenobamate in patients with focal onset seizures: Outcomes from an Expanded Access Program. Epilepsia Open 2023. epub ahead of print. [Google Scholar] [CrossRef]
- Chaves, J.; Breia, P.; Pimentel, J.; Pelejão, R.; Carvalho, M.; Mateus, P.; Grebe, H.; Mestre, A.; Fernandes, H.; Sousa, R.; et al. Eslicarbazepine acetate as adjunctive therapy in clinical practice: ESLADOBA study. Acta Neurol. Scand. 2017, 136, 407–413. [Google Scholar] [CrossRef]
- Galiana, G.L.; Gauthier, A.C.; Mattson, R.H. Eslicarbazepine Acetate: A New Improvement on a Classic Drug Family for the Treatment of Partial-Onset Seizures. Drugs R D 2017, 17, 329–339. [Google Scholar] [CrossRef]
- Hixson, J.; Gidal, B.; Pikalov, A.; Zhang, Y.; Mehta, D.; Blum, D.; Cantu, D.; Grinnell, T. Efficacy and safety of eslicarbazepine acetate as a first or later adjunctive therapy in patients with focal seizures. Epilepsy Res. 2021, 171, 106561. [Google Scholar] [CrossRef]
- Knoll, J.; Stegman, K.; Suppes, T. Clinical experience using gabapentin adjunctively in patients with a history of mania or hypomania. J. Affect. Disord. 1998, 49, 229–233. [Google Scholar] [CrossRef]
- Collins, A.; Mannion, R.; Broderick, A.; Hussey, S.; Devins, M.; Bourke, B. Gabapentin for the treatment of pain manifestations in children with severe neurological impairment: A single-centre retrospective review. BMJ Paediatr. Open 2019, 3, e000467. [Google Scholar] [CrossRef] [PubMed]
- Runge, U.; Arnold, S.; Brandt, C.; Reinhardt, F.; Kühn, F.; Isensee, K.; Ramirez, F.; Dedeken, P.; Lauterbach, T.; Noack-Rink, M.; et al. A noninterventional study evaluating the effectiveness and safety of lacosamide added to monotherapy in patients with epilepsy with partial-onset seizures in daily clinical practice: The VITOBA study. Epilepsia 2015, 56, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Biton, V.; Levisohn, P.; Hoyler, S.; Vuong, A.; Hammer, A.E. Lamotrigine versus valproate monotherapy-associated weight change in adolescents with epilepsy: Results from a post hoc analysis of a randomized, double-blind clinical trial. J. Child Neurol. 2003, 18, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Morrell, M.J.; Hayes, F.J.; Sluss, P.M.; Adams, J.M.; Bhatt, M.; Ozkara, C.; Warnock, C.R.; Isojärvi, J. Hyperandrogenism, ovulatory dysfunction, and polycystic ovary syndrome with valproate versus lamotrigine. Ann. Neurol. 2008, 64, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Vining, E.P.; Mellitis, E.D.; Dorsen, M.M.; Cataldo, M.F.; Quaskey, S.A.; Spielberg, S.P.; Freeman, J.M. Psychologic and behavioral effects of antiepileptic drugs in children: A double-blind comparison between phenobarbital and valproic acid. Pediatrics 1987, 80, 165–174. [Google Scholar] [CrossRef]
- Kluger, G.; Kurlemann, G.; Haberlandt, E.; Ernst, J.P.; Runge, U.; Schneider, F.; Makowski, C.; Boor, R.; Bast, T. Effectiveness and tolerability of rufinamide in children and adults with refractory epilepsy: First European experience. Epilepsy Behav. 2009, 14, 491–495. [Google Scholar] [CrossRef]
- Tan, H.J.; Awadh, M.; O’Regan, M.; Martland, T.R.; Kneen, R. Effectiveness and Tolerability of Rufinamide in Children and Young People: A Survey of Experience from the United Kingdom. J. Pediatr. Epilepsy 2017, 6, 103–110. [Google Scholar]
- Krymchantowski, A.; Tavares, C. Weight variations in patients receiving topiramate migraine prophylaxis in a tertiary care setting. MedGenMed 2004, 6, 48. [Google Scholar]
- Turanli, G.; Celebi, A.; Yalnizoğlu, D.; Topçu, M.; Topaloğlu, H.; Banu, A.; Aysun, S. Vigabatrin in pediatric patients with refractory epilepsy. Turk. J. Pediatr. 2006, 48, 25–30. [Google Scholar]
- Christe, W.; Krämer, G.; Vigonius, U.; Pohlmann, H.; Steinhoff, B.J.; Brodie, M.J.; Moore, A. A double-blind controlled clinical trial: Oxcarbazepine versus sodium valproate in adults with newly diagnosed epilepsy. Epilepsy Res. 1997, 26, 451–460. [Google Scholar] [CrossRef]
- Jeavons, P.M.; Clark, J.E. Sodium valproate in treatment of epilepsy. Br. Med. J. 1974, 2, 584–586. [Google Scholar] [CrossRef]
- Gram, L.; Wulff, K.; Rasmussen, K.E.; Flachs, H.; Würtz-Jorgensen, A.; Sommerbeck, K.W.; Lohren, V. Valproate sodium: A controlled clinical trial including monitoring of drug levels. Epilepsia 1977, 18, 141–148. [Google Scholar] [CrossRef]
- Hassan, M.N.; Laljee, H.C.; Parsonage, M.J. Sodium valproate in the treatment of resistant epilepsy. Acta Neurol. Scand. 1976, 54, 209–218. [Google Scholar] [CrossRef]
- Coulter, D.L.; Wu, H.; Allen, R.J. Valproic acid therapy in childhood epilepsy. JAMA 1980, 244, 785–788. [Google Scholar] [CrossRef]
- Egger, J.; Brett, E.M. Effects of sodium valproate in 100 children with special reference to weight. Br. Med. J. 1981, 283, 577–581. [Google Scholar] [CrossRef]
- Turnbull, D.M.; Rawlins, M.D.; Weightman, D.; Chadwick, D.W. Plasma concentrations of sodium valproate: Their clinical value. Ann. Neurol. 1983, 14, 38–42. [Google Scholar] [CrossRef]
- Spitz, M.C.; Deasy, D.N. Conversion to valproate monotheraphy in nonretarded adults with primary Generalized tonic-clonic seizures. J. Epilepsy 1991, 4, 33–38. [Google Scholar] [CrossRef]
- Macritchie, K.A.; Geddes, J.R.; Scott, J.; Haslam, D.R.; Goodwin, G.M. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst. Rev. 2001, 2001, CD003196. [Google Scholar]
- Schulpis, K.H.; Karikas, G.A.; Tjamouranis, J.; Regoutas, S.; Tsakiris, S. Low serum biotinidase activity in children with valproic acid monotherapy. Epilepsia 2001, 42, 1359–1362. [Google Scholar] [CrossRef]
- Ebrahimi, H.; Shamsadini, S.; Eshkavari, S.E. Frequency of Sodium Valproate-Induced Hair Loss and Curly Hair. Iran. J. Pharmacol. Ther. 2005, 4, 143–145. [Google Scholar]
- Kocer, A.; Sasmaz, S.; Ince, N.; Kutlar, M.; Cagirici, S. Skin findings related to chronic usage of anti-epileptic drugs. Neurosci. (Riyadh) 2005, 10, 268–271. [Google Scholar]
- Joffe, H.; Cohen, L.S.; Suppes, T.; McLaughlin, W.L.; Lavori, P.; Adams, J.M.; Hwang, C.H.; Hall, J.E.; Sachs, G.S. Valproate is associated with new-onset oligoamenorrhea with hyperandrogenism in women with bipolar disorder. Biol. Psychiatry 2006, 59, 1078–1086. [Google Scholar] [CrossRef]
- McCabe, P.H.; Michel, N.C.; McNew, C.D.; Lehman, E.B. Conversion from delayed-release sodium valproate to extended-release sodium valproate: Initial results and long-term follow-up. Epilepsy Behav. 2006, 8, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak, J.; Kuncíková, M.; Magureanu, S. An observational study of first-line valproate monotherapy in focal epilepsy. Eur. J. Neurol. 2008, 15, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Castro-Gago, M.; Pérez-Gay, L.; Gómez-Lado, C.; Castiñeiras-Ramos, D.E.; Otero-Martínez, S.; Rodríguez-Segade, S. The influence of valproic acid and carbamazepine treatment on serum biotin and zinc levels and on biotinidase activity. J. Child Neurol. 2011, 26, 1522–1524. [Google Scholar] [CrossRef]
- Han, X.N.; Ma, L.; Zhang, H.L.; You, H.S. Analysis of 59 cases of adverse drug reactions induced by valproate sodium. Chin. Hosp. Pharm. J. 2015, 39, 57–60. [Google Scholar]
- Kompally, D.V.; Ananthula, K.; Adla, N.; Rajesh, V. Prospective Observational Study of Sodium Valproate in Seizure Control and Associated Adverse Drug Reactions in Pediatric Population. J. Dent. Med. Sci. 2015, 14, 28–34. [Google Scholar]
- Yamak, W.R.; Hmaimess, G.; Makke, Y.; Sabbagh, S.; Arabi, M.; Beydoun, A.; Nasreddine, W. Valproate-induced enuresis: A prospective study. Dev. Med. Child Neurol. 2015, 57, 737–741. [Google Scholar] [CrossRef]
- Druschky, K.; Bleich, S.; Grohmann, R.; Burda, K.; Frieling, H.; Hillemacher, T.; Neyazi, A.; Stübner, S.; Toto, S. Severe hair loss associated with psychotropic drugs in psychiatric inpatients-Data from an observational pharmacovigilance program in German-speaking countries. Eur. Psychiatry 2018, 54, 117–123. [Google Scholar] [CrossRef]
- Pruccoli, J.; Parmeggiani, A. The Role of Mood Stabilizers in Children and Adolescents with Anorexia Nervosa: A 1-year Follow-Up, Propensity Score-Matched Study. Pharmacopsychiatry 2023, 56, 118–125. [Google Scholar] [CrossRef]
- Liparoti, G.; Burchiani, B.; Mencaroni, E.; Tripodi, D.; Cara, G.; Verrotti, A. Individualizing doses of antiepileptic drugs. Expert Opin. Drug Metab. Toxicol. 2022, 18, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Asghar, F.; Shamim, N.; Farooque, U.; Sheikh, H.; Aqeel, R. Telogen Effluvium: A Review of the Literature. Cureus 2020, 12, e8320. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.O.; Siong-See, J.L.; Wang, E.C. Telogen Effluvium—A review of the science and current obstacles. J. Dermatol. Sci. 2021, 101, 156–163. [Google Scholar]
- Park, S.H.; Seol, J.E.; Kim, D.H.; Kim, H. Analysis of Microscopic Examination of Pulled Out Hair in Telogen Effluvium Patients. Ann. Dermatol. 2020, 32, 141–145. [Google Scholar] [CrossRef]
- McDonald, K.A.; Shelley, A.J.; Colantonio, S.; Beecker, J. Hair pull test: Evidence-based update and revision of guidelines. J. Am. Acad. Dermatol. 2017, 76, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Poonia, K.; Thami, G.P.; Bhalla, M.; Jaiswal, S.; Sandhu, J. NonScarring Diffuse Hair Loss in Women: A Clinico-Etiological Study from tertiary care center in North-West India. J. Cosmet. Dermatol. 2019, 18, 401–407. [Google Scholar] [CrossRef]
- Grover, C.; Khurana, A. Telogen effluvium. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 591–603. [Google Scholar] [CrossRef]
- Harrison, S.; Sinclair, R. Telogen effluvium. Clin. Exp. Dermatol. 2002, 27, 389–395. [Google Scholar] [CrossRef]
- Kanwar, A.J.; Narang, T. Anagen effluvium. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 604–612. [Google Scholar] [CrossRef]
- Liyanage, D.; Sinclair, R. Telogen Effluvium. Cosmetics 2016, 3, 13. [Google Scholar] [CrossRef]
- Dhurat, R.; Saraogi, P. Hair evaluation methods: Merits and demerits. Int. J. Trichol. 2009, 1, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Springer, K.; Brown, M.; Stulberg, D.L. Common hair loss disorders. Am. Fam. Physician 2003, 68, 93–102. [Google Scholar] [PubMed]
- Hoffmann, A.; Waśkiel-Burnat, A.; Żółkiewicz, J.; Blicharz, L.; Rakowska, A.; Goldust, M.; Olszewska, M.; Rudnicka, L. Pili Torti: A Feature of Numerous Congenital and Acquired Conditions. J. Clin. Med. 2021, 10, 3901. [Google Scholar] [CrossRef] [PubMed]
- Almohanna, H.M.; Ahmed, A.A.; Tsatalis, J.P.; Tosti, A. The Role of Vitamins and Minerals in Hair Loss: A Review. Dermatol. Ther. 2019, 9, 51–70. [Google Scholar] [CrossRef]
- Slonim, A.E.; Sadick, N.; Pugliese, M.; Meyers-Seifer, C.H. Clinical response of alopecia, trichorrhexis nodosa, and dry, scaly skin to zinc supplementation. J. Pediatr. 1992, 121, 890–895. [Google Scholar] [CrossRef]
- Suzuki, T.; Koizumi, J.; Moroji, T.; Shiraishi, H.; Hori, T.; Baba, A.; Kawai, N.; Tada, K. Effects of long-term anticonvulsant therapy on copper, zinc, and magnesium in hair and serum of epileptics. Biol. Psychiatry 1992, 31, 571–581. [Google Scholar] [CrossRef]
- Kuzuya, T.; Hasegawa, T.; Shimizu, K.; Nabeshima, T. Effect of anti-epileptic drugs on serum zinc and copper concentrations in epileptic patients. Int. J. Clin. Pharmacol. Ther. Toxicol. 1993, 31, 61–65. [Google Scholar]
- Asadi-Pooya, A.A.; Rostaminejad, M.; Zeraatpisheh, Z.; Damabi, N.M. Cosmetic adverse effects of antiseizure medications; A systematic review. Seizure 2021, 91, 9–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).