Effect of Dexamethasone on Abiraterone Pharmacokinetics in Mice: Determined by LC/MS Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Compounds and Materials
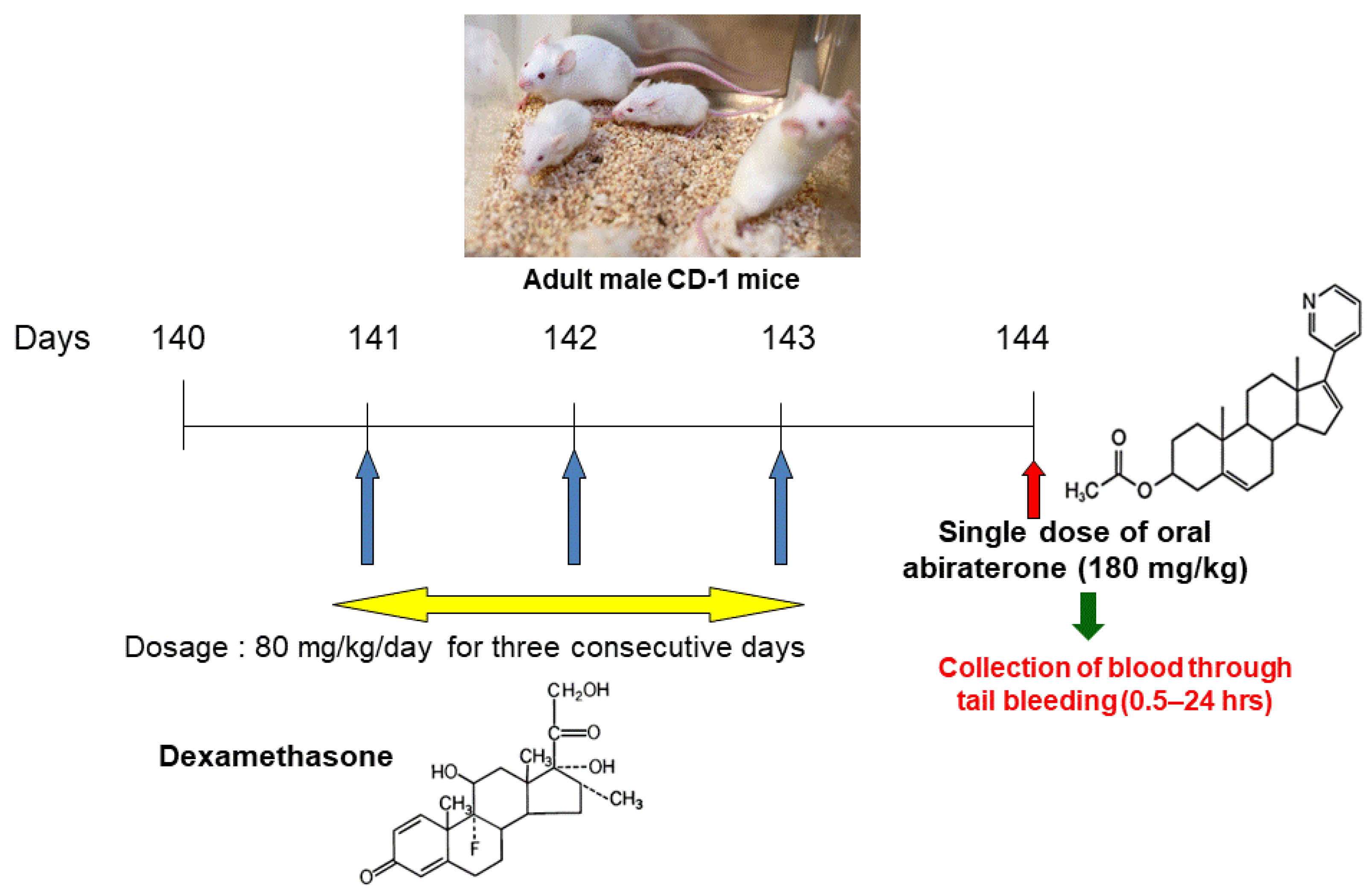
2.2. Single Dose Pharmacokinetic Study Design
2.3. Serum Extraction and Sample Preparation
2.4. Quantification of Abiraterone by LC/MS Method
2.5. Data Analyses
3. Results
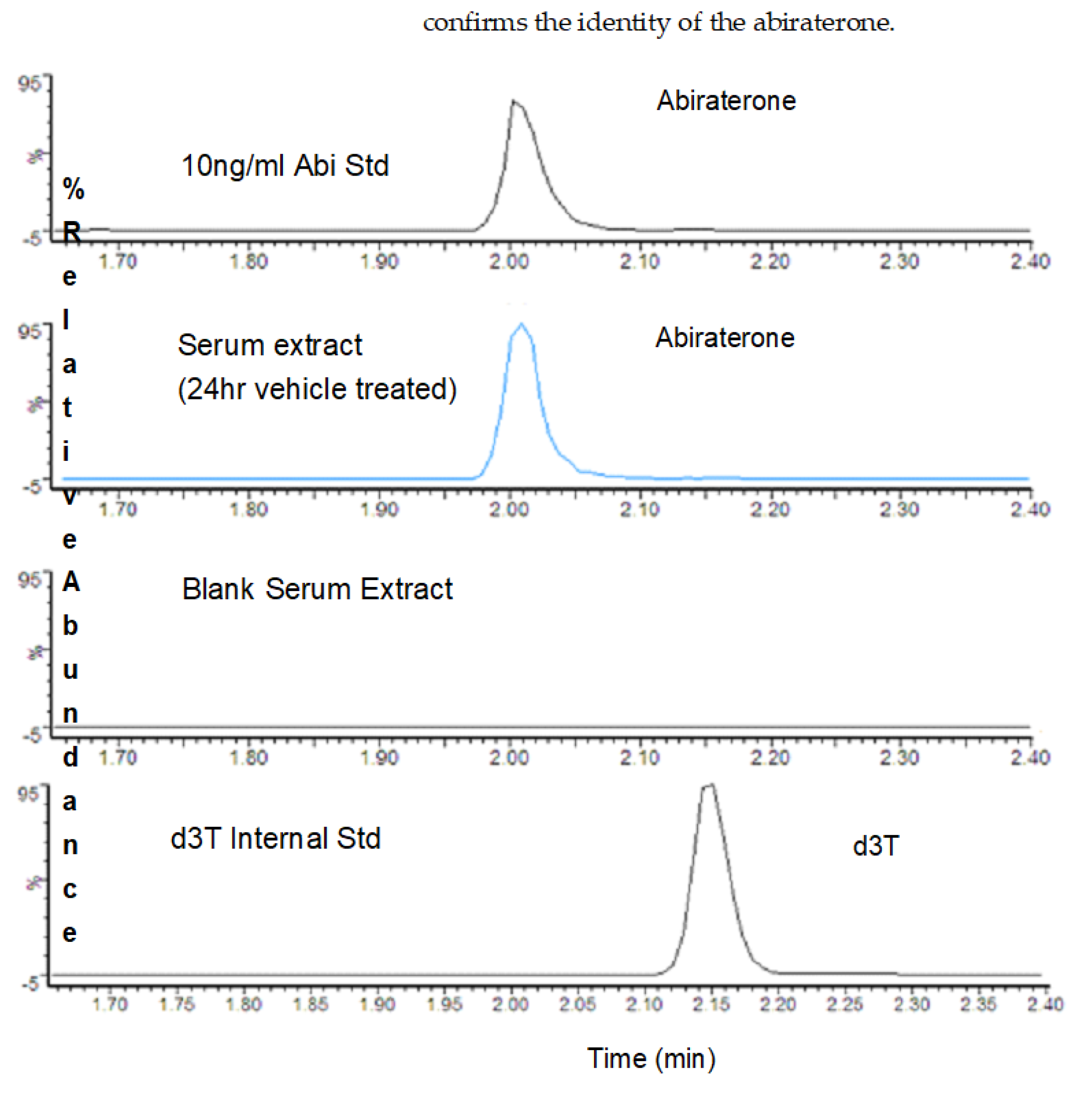
3.1. LC/MS Analysis
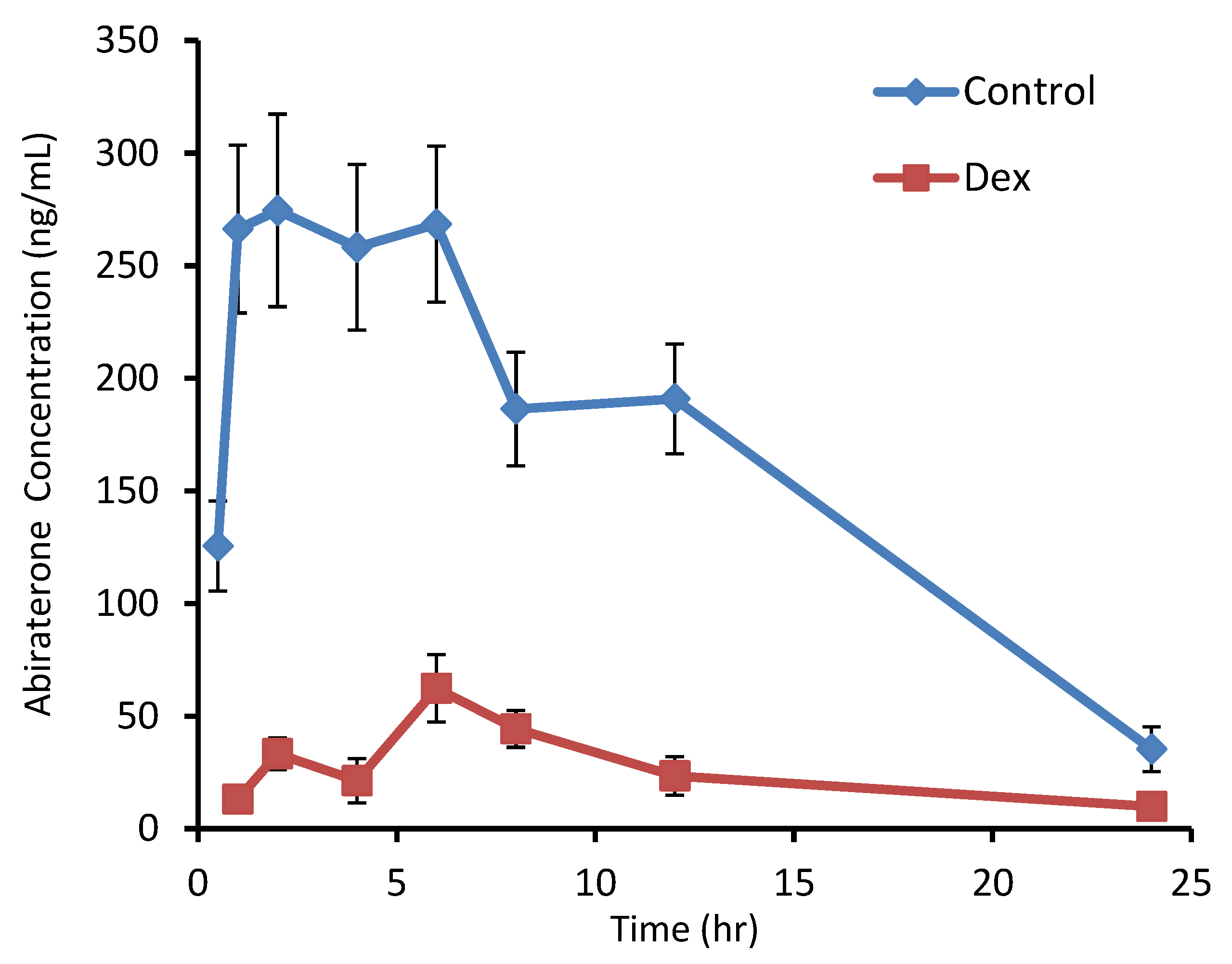
3.2. Effect of Dexamethasone on Abiraterone Pharmacokinetics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Worldwide Cancer Research Fund International. Worldwide Cancer Data. Available online: https://www.wcrf.org/cancer-trends/worldwide-cancer-data/ (accessed on 4 January 2023).
- Janssen Biotech Inc. ZYTIGA® (Abiraterone Acetate). Available online: https://www.zytiga.com/ (accessed on 4 January 2023).
- Beckett, R.D.; Rodeffer, K.M.; Snodgrass, R. Abiraterone for the treatment of metastatic castrate-resistant prostate cancer. Ann. Pharmacother. 2012, 46, 1016–1024. [Google Scholar] [CrossRef] [PubMed]
- Fizazi, K.; Scher, H.I.; Molina, A.; Logothetis, C.J.; Chi, K.N.; Jones, R.J.; Staffurth, J.N.; North, S.; Vogelzang, N.J.; Saad, F.; et al. Abiraterone acetate for treatment of metastatic castration-resistant prostate cancer: Final overall survival analysis of the COU-AA-301 randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2012, 13, 983–992. [Google Scholar] [CrossRef]
- E Ang, J.; Olmos, D.; De Bono, J.S. CYP17 blockade by abiraterone: Further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br. J. Cancer 2009, 100, 671–675. [Google Scholar] [CrossRef]
- Thakur, A.; Roy, A.; Ghosh, A.; Chhabra, M.; Banerjee, S. Abiraterone acetate in the treatment of prostate cancer. Biomed. Pharmacother. 2018, 101, 211–218. [Google Scholar] [CrossRef]
- Mostaghel, E.A.; Marck, B.T.; Plymate, S.R.; Vessella, R.L.; Balk, S.; Matsumoto, A.M.; Nelson, P.S.; Montgomery, R.B. Resistance to CYP17A1 inhibition with abiraterone in castration-resistant prostate cancer: Induction of steroidogenesis and androgen receptor splice variants. Clin. Cancer Res. 2011, 17, 5913–5925. [Google Scholar] [CrossRef]
- Schultz, H.B.; Meola, T.R.; Thomas, N.; Prestidge, C.A. Oral formulation strategies to improve the bioavailability and mitigate the food effect of abiraterone acetate. Int. J. Pharm. 2020, 577, 119069. [Google Scholar] [CrossRef] [PubMed]
- Bernard, A.; Vaccaro, N.; Acharya, M.; Jiao, J.; Monbaliu, J.; De Vries, R.; Stieltjes, H.; Yu, M.; Tran, N.; Chien, C. Impact on abiraterone pharmacokinetics and safety: Open-label drug-drug interaction studies with ketoconazole and rifampicin. Clin. Pharmacol. Drug Dev. 2015, 4, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Chi, K.N.; Tolcher, A.; Lee, P.; Rosen, P.J.; Kollmannsberger, C.K.; Papadopoulos, K.P.; Patnaik, A.; Molina, A.; Jiao, J.; Pankras, C.; et al. Effect of abiraterone acetate plus prednisone on the pharmacokinetics of dextromethorphan and theophylline in patients with metastatic castration-resistant prostate cancer. Cancer Chemother. Pharmacol. 2013, 71, 237–244. [Google Scholar] [CrossRef]
- Deb, S.; Chin, M.Y.; Adomat, H.; Guns, E.S.T. Abiraterone inhibits 1alpha,25-dihydroxyvitamin D3 metabolism by CYP3A4 in human liver and intestine in vitro. J. Steroid Biochem. Mol. Biol. 2014, 144, 50–58. [Google Scholar] [CrossRef]
- Monbaliu, J.; Gonzalez, M.; Bernard, A.; Jiao, J.; Sensenhauser, C.; Snoeys, J.; Stieltjes, H.; Wynant, I.; Smit, J.W.; Chien, C. In Vitro and In Vivo Drug-Drug Interaction Studies to Assess the Effect of Abiraterone Acetate, Abiraterone, and Metabolites of Abiraterone on CYP2C8 Activity. Drug Metab. Dispos. 2016, 44, 1682–1691. [Google Scholar] [CrossRef]
- Cook, A.M.; McDonnell, A.M.; Lake, R.A.; Nowak, A.K. Dexamethasone co-medication in cancer patients undergoing chemotherapy causes substantial immunomodulatory effects with implications for chemo-immunotherapy strategies. Oncoimmunology 2016, 5, e1066062. [Google Scholar] [CrossRef] [PubMed]
- Rhen, T.; Cidlowski, J.A. Antiinflammatory action of glucocorticoids--new mechanisms for old drugs. N. Engl. J. Med. 2005, 353, 1711–1723. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Sobhani, N.; Corona, S.P.; D’Angelo, A. Corticosteroid switch after progression on abiraterone acetate plus prednisone. Int. J. Clin. Oncol. 2020, 25, 240–246. [Google Scholar] [CrossRef]
- Van Praet, C.; Fonteyne, V.; Lumen, N. Dexamethasone use in metastatic castration-resistant prostate cancer patients treated with abiraterone acetate: This “cort” is not out of order! Asian J. Androl. 2022, 24, 225. [Google Scholar] [CrossRef]
- Kassi, E.; Moutsatsou, P. Glucocorticoid receptor signaling and prostate cancer. Cancer Lett. 2011, 302, 1–10. [Google Scholar] [CrossRef]
- Rushmore, T.H.; Kong, A.N. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr. Drug Metab. 2002, 3, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Li, C.Y.-T.; Kong, A.-N.T. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch. Pharm. Res. 2005, 28, 249–268. [Google Scholar] [CrossRef]
- Jamani, R.; Lee, E.K.; Berry, S.R.; Saluja, R.; DeAngelis, C.; Giotis, A.; Emmenegger, U. High prevalence of potential drug-drug interactions in patients with castration-resistant prostate cancer treated with abiraterone acetate. Eur. J. Clin. Pharmacol. 2016, 72, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Pandey, M.; Adomat, H.; Guns, E.S.T. Cytochrome P450 3A-mediated microsomal biotransformation of 1alpha,25-dihydroxyvitamin D3 in mouse and human liver: Drug-related induction and inhibition of catabolism. Drug Metab. Dispos. 2012, 40, 907–918. [Google Scholar] [CrossRef]
- Solymosi, T.; Ötvös, Z.; Angi, R.; Ordasi, B.; Jordán, T.; Molnár, L.; McDermott, J.; Zann, V.; Church, A.; Mair, S.; et al. Novel formulation of abiraterone acetate might allow significant dose reduction and eliminates substantial positive food effect. Cancer Chemother. Pharmacol. 2017, 80, 723–728. [Google Scholar] [CrossRef]
- Dizdar, O. Is dexamethasone a better partner for abiraterone than prednisolone? Oncologist 2015, 20, e13. [Google Scholar] [CrossRef] [PubMed]
| PK Parameters | Control | Dexamethasone |
|---|---|---|
| Cmax (ng/mL) | 271.4 | 53.3 * |
| Tmax (h) | 1.9 | 4.2 * |
| AUC0–24 h (ng.h/mL) | 3769.9 | 383.1 * |
| T1/2 (h) | 13.7 | 10.2 * |
| Oral CL (h/L) | 2.2 | 21.7 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deb, S.; Ben-Eltriki, M.; Adomat, H.; Chin, M.Y.; Tomlinson Guns, E.S. Effect of Dexamethasone on Abiraterone Pharmacokinetics in Mice: Determined by LC/MS Analysis. Medicines 2023, 10, 21. https://doi.org/10.3390/medicines10030021
Deb S, Ben-Eltriki M, Adomat H, Chin MY, Tomlinson Guns ES. Effect of Dexamethasone on Abiraterone Pharmacokinetics in Mice: Determined by LC/MS Analysis. Medicines. 2023; 10(3):21. https://doi.org/10.3390/medicines10030021
Chicago/Turabian StyleDeb, Subrata, Mohamed Ben-Eltriki, Hans Adomat, Mei Y. Chin, and Emma S. Tomlinson Guns. 2023. "Effect of Dexamethasone on Abiraterone Pharmacokinetics in Mice: Determined by LC/MS Analysis" Medicines 10, no. 3: 21. https://doi.org/10.3390/medicines10030021
APA StyleDeb, S., Ben-Eltriki, M., Adomat, H., Chin, M. Y., & Tomlinson Guns, E. S. (2023). Effect of Dexamethasone on Abiraterone Pharmacokinetics in Mice: Determined by LC/MS Analysis. Medicines, 10(3), 21. https://doi.org/10.3390/medicines10030021








