Everyday Evaluation of Herb/Dietary Supplement–Drug Interaction: A Pilot Study
Abstract
1. Introduction
2. Results
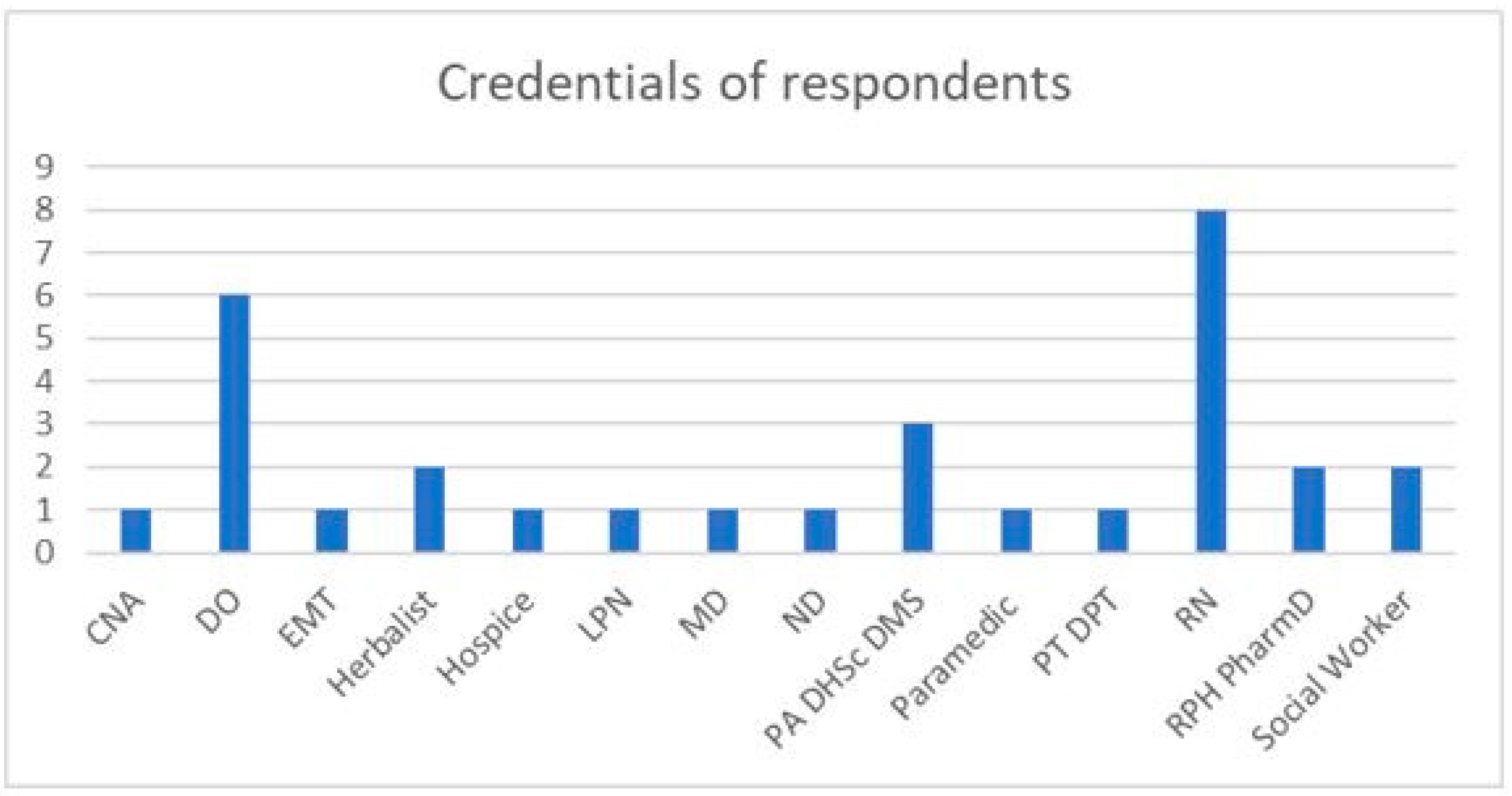
2.1. Summary Statistics from Survey
2.2. Reasons for Using HDS
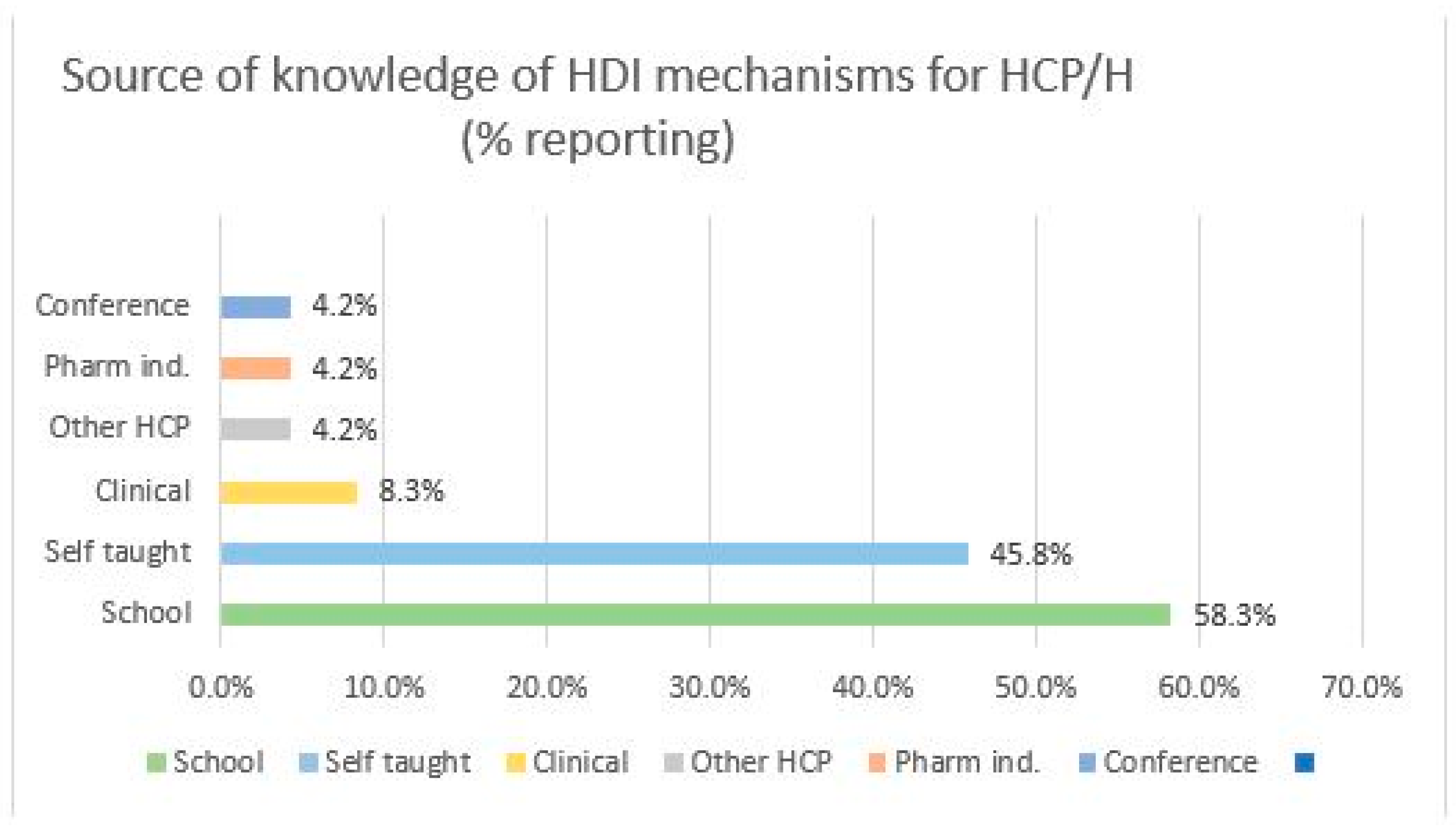
2.3. Source of Knowledge of HDI Mechanisms
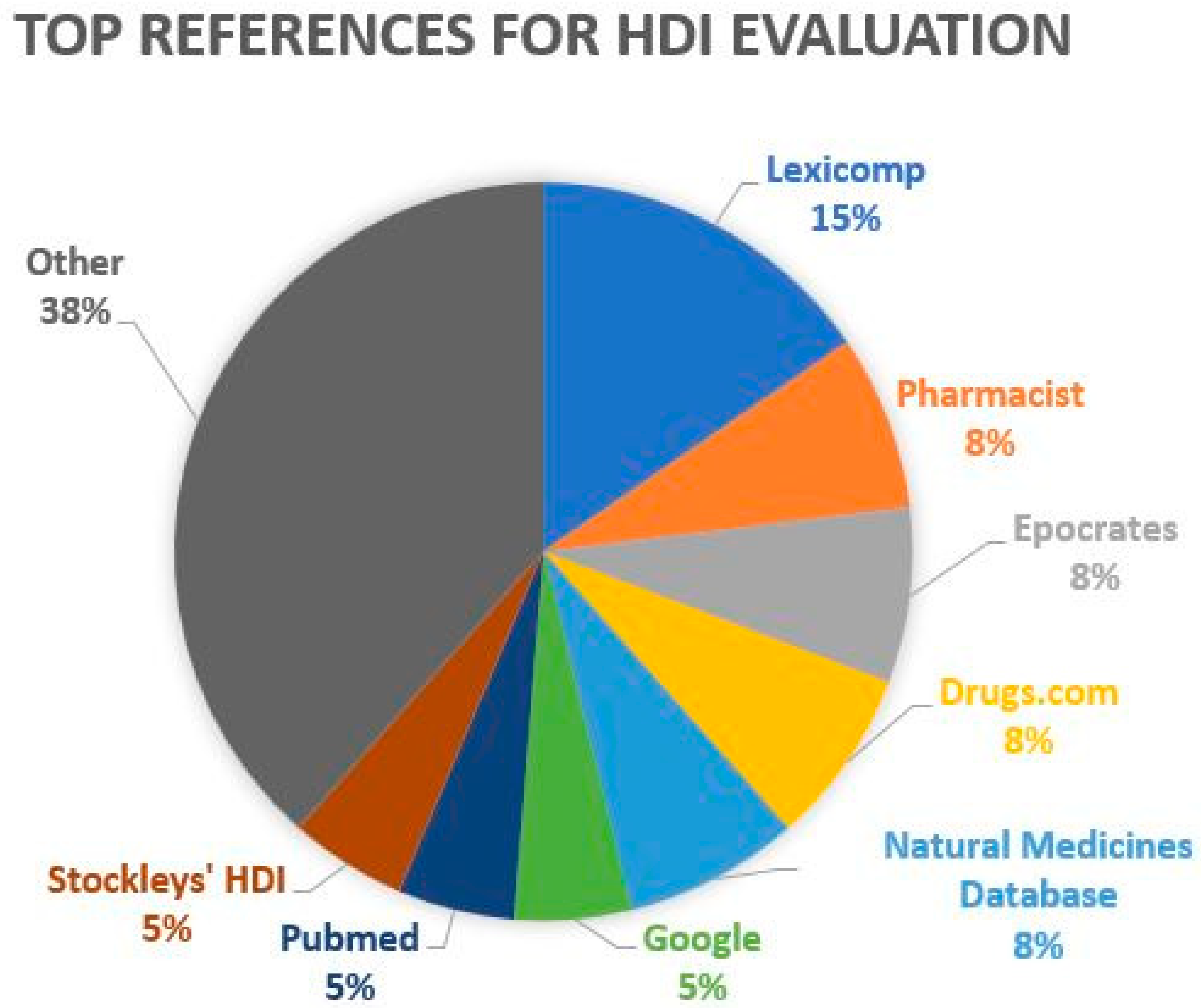
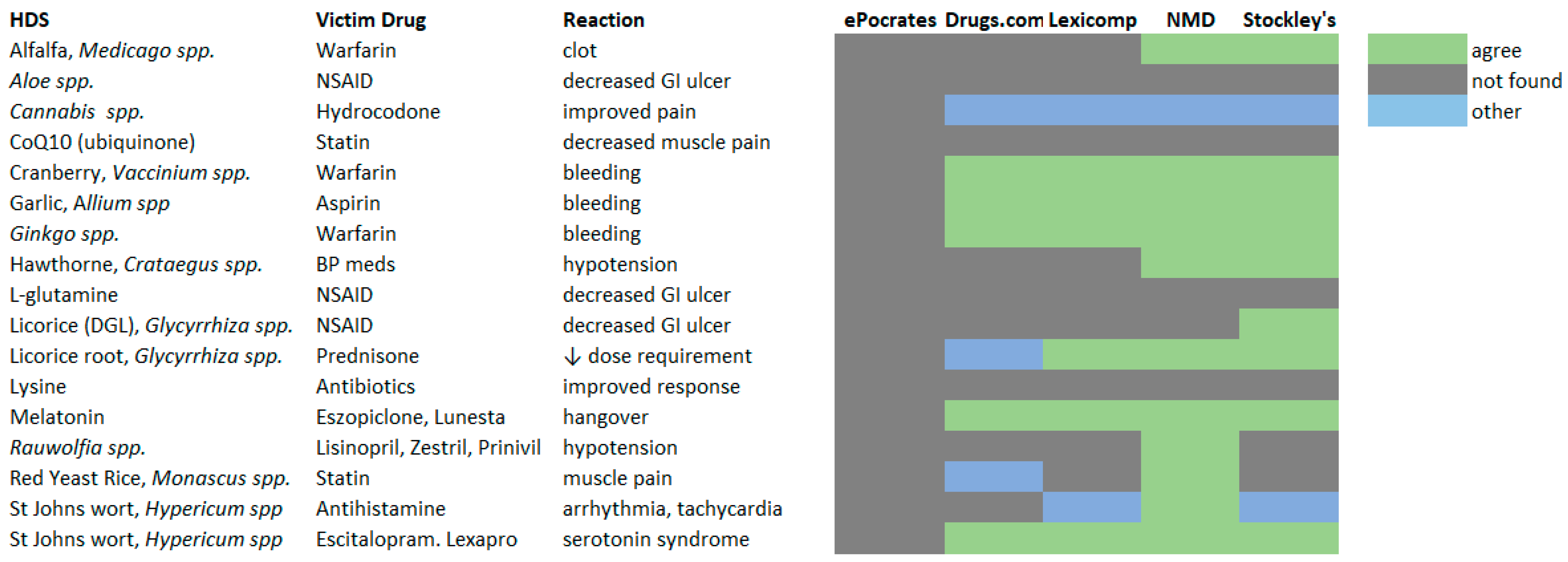
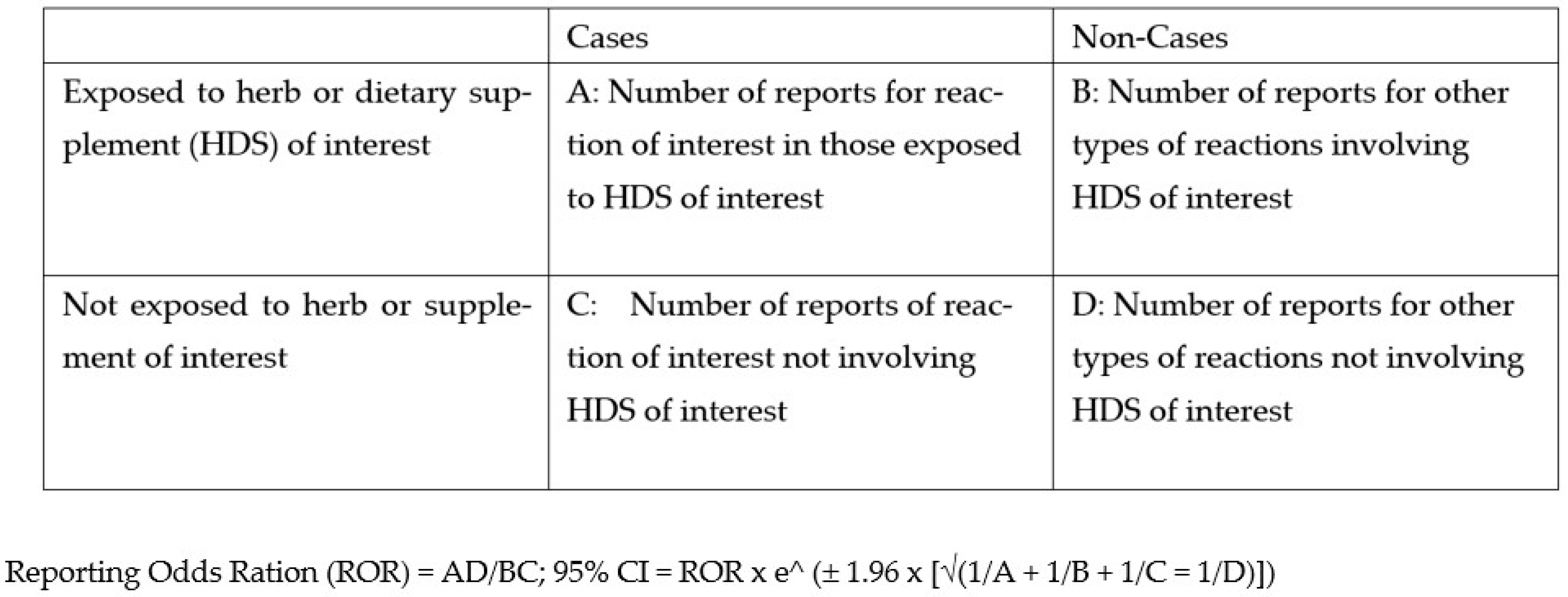
2.4. Herb–Drug Interaction Checkers and Disproportionality Analysis
3. Discussion
4. Materials and Methods
4.1. Online Questionnaire
4.2. Evaluation of Herb–Drug Interaction Checking Resources
4.3. Disproportionality Evaluation of Herbal Product and Reaction from CAERS
4.4. Disproportionality Analysis of Herbal Product with Drug with Reaction of Interest from FAERS
4.5. Analysis of Survey Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mishra, S.; Stierman, B.; Gahche, J.J.; Potischman, N. Dietary Supplement Use Among Adults: United States, 2017–2018. NCHS Data Brief 2021, 399, 1–8. [Google Scholar]
- Dietary Supplement Health and Education Act of 1994, Public Law 103–417. 103rd Congress. Public Law. 1994. Available online: https://www.congress.gov/bill/103rd-congress/senate-bill/784/text (accessed on 20 December 2022).
- Gurley, B.J.; McGill, M.; Koturbash, I. Hepatotoxicity due to herbal dietary supplements: Past, present and the future. Food Chem. Toxicol. 2022, 169, 113445. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Majid, F.; Eckl, V.; Reynolds, C.M. Herbal supplement sales in US increase by record breaking 17.3% in 2020. HerbalGram 2021, 131, 52–65. [Google Scholar]
- Gurley, B.J.; Markowitz, J.S.; Williams, D.K.; Barone, G.W. Practical considerations when designing and conducting clinical pharmacokinetic herb–drug interaction studies. Int. J. Pharmacokinet. 2017, 2, 57–69. [Google Scholar] [CrossRef]
- Rashrash, M.; Schommer, J.C.; Brown, L.M. Prevalence and Predictors of Herbal Medicine Use Among Adults in the United States. J. Patient Exp. 2017, 4, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Adane, F.; Seyoum, G.; Alamneh, Y.M.; Abie, W.; Desta, M.; Sisay, B. Herbal medicine use and predictors among pregnant women attending antenatal care in Ethiopia: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2020, 20, 1–11. [Google Scholar] [CrossRef]
- Lam, C.S.; Koon, H.K.; Ma, C.T.; Au, K.Y.; Zuo, Z.; Chung, V.C.-H.; Cheung, Y.T. Real-world data on herb-drug interactions in oncology: A scoping review of pharmacoepidemiological studies. Phytomedicine 2022, 103. [Google Scholar] [CrossRef]
- Prely, H.; Herledan, C.; Caffin, A.G.; Baudouin, A.; Larbre, V.; Maire, M.; Schwiertz, V.; Vantard, N.; Ranchon, F.; Rioufol, C. Real-life drug–drug and herb–drug interactions in outpatients taking oral anticancer drugs: Comparison with databases. J. Cancer Res. Clin. Oncol. 2021, 148, 707–718. [Google Scholar] [CrossRef]
- Tripković, K.; Nešković, A.; Janković, J.; Odalović, M. Predictors of self-medication in Serbian adult population: Cross-sectional study. Int. J. Clin. Pharm. 2018, 40, 627–634. [Google Scholar] [CrossRef]
- Alsanad, S.M.; Williamson, E.M.; Howard, R.L. Cancer Patients at Risk of Herb/Food Supplement-Drug Interactions: A Systematic Review. Phytother. Res. 2014, 28, 1749–1755. [Google Scholar] [CrossRef]
- Pilla Reddy, V.; Jo, H.; Neuhoff, S. Food constituent–and herb–drug interactions in oncology: Influence of quantitative modelling on Drug labelling. Br. J. Clin. Pharmacol. 2021, 87, 3988–4000. [Google Scholar] [CrossRef]
- US Food and Drug Administration (US FDA). Mixing Medications and Dietary Supplements can Endanger Your Health. 2022. Available online: https://www.fda.gov/consumers/consumer-updates/mixing-medications-and-dietary-supplements-can-endanger-your-health (accessed on 22 December 2022).
- Gurley, B.J.; Yates, C.R.; Markowitz, J.S. “… Not intended to diagnose, treat, cure or prevent any disease.” 25 years of botanical dietary supplement research and the lessons learned. Clin. Pharmacol. Thera-Peutics 2018, 104, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Graham, R.E.; Gandhi, T.K.; Borus, J.; Seger, A.C.; Burdick, E.; Bates, D.W.; Weingart, S.N. Risk of concurrent use of prescription drugs with herbal and dietary supplements in ambulatory care. In Advances in Patient Safety: New Directions and Alternative Approaches (Vol. 4: Technology and Medication Safety); Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2008. [Google Scholar]
- Arora, G.; Arora, A.; Chaudary, V.; Kamlija, M.; Kamlija, H. Possible herbal-drug interactions an evidenced base review. Altern. Ther. Health Med. 2020, 28, 70–77. [Google Scholar]
- Enioutina, E.Y.; Job, K.M.; Krepkova, L.V.; Reed, M.D.; Sherwin, C.M. How can we improve the safe use of herbal medicine and other natural products? A clinical pharmacologist mission. Expert Rev. Clin. Pharmacol. 2020, 13, 935–944. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Fang, F.; Kidwell, K.M.; Marcath, L.; Hertz, D.L. Comparison of eight screening tools to detect interactions between herbal supplements and oncology agents. J. Oncol. Pharm. Pr. 2020, 26, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ip, C.M.; Lai, Y.S.; Zuo, Z. Overview of Current Herb–Drug Interaction Databases. Drug Metab. Dispos. 2021, 50, 86–94. [Google Scholar] [CrossRef]
- Pochet, S.; Lechon, A.-S.; Lescrainier, C.; De Vriese, C.; Mathieu, V.; Hamdani, J.; Souard, F. Herb-anticancer drug interactions in real life based on VigiBase, the WHO global database. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef]
- US Food and Drug Administration (US FDA). Information for Consumers on Using Dietary Supplements. Available online: https://www.fda.gov/food/dietary-supplements/information-consumers-using-dietary-supplements (accessed on 22 December 2022).
- Knapik, J.J.; Trone, D.W.; Steelman, R.A.; Farina, E.K.; Lieberman, H.R. Adverse Effects Associated with Multiple Categories of Dietary Supplements: The Military Dietary Supplement Use Study. J. Acad. Nutr. Diet. 2022, 122, 1851–1863. [Google Scholar] [CrossRef]
- Starr, R.R. Too Little, Too Late: Ineffective Regulation of Dietary Supplements in the United States. Am. J. Public Heal. 2015, 105, 478–485. [Google Scholar] [CrossRef]
- Newmaster, S.G.; Grguric, M.; Shanmughanandhan, D.; Ramalingam, S.; Ragupathy, S. DNA bar-coding detects contamination and substitution in North American herbal products. BMC Med. 2013, 11, 1–13. [Google Scholar] [CrossRef]
- Babos, M.; Heinan, M.; Redmond, L.; Moiz, F.; Souza-Peres, J.; Samuels, V.; Masimukku, T.; Hamilton, D.; Khalid, M.; Herscu, P. Herb–Drug Interactions: Worlds Intersect with the Patient at the Center. Medicines 2021, 8, 44. [Google Scholar] [CrossRef]
- Lee, K.-M.; Jeon, J.-Y.; Lee, B.-J.; Lee, H.; Choi, H.-K. Application of Metabolomics to Quality Control of Natural Product Derived Medicines. Biomol. Ther. 2017, 25, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, H.; Hao, Y.; Du, K.; Du, H.; Ma, C.; Tu, H.; He, Y. A systematic review on botany, processing, application, phytochemistry and pharmacological action of Radix Rehmnniae. J. Ethnopharmacol. 2021, 285, 114820. [Google Scholar] [CrossRef] [PubMed]
- Rizo, J.; Guillén, D.; Farrés, A.; Díaz-Ruiz, G.; Sánchez, S.; Wacher, C.; Rodríguez-Sanoja, R. Omics in traditional vegetable fermented foods and beverages. Crit. Rev. Food Sci. Nutr. 2018, 60, 791–809. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.V.; Sreedhara, S.K.; Schneeweiss, S.; Franklin, J.M.; Gagne, J.J.; Huybrechts, K.F.; Patorno, E.; Jin, Y.; Lee, M.; Mahesri, M.; et al. Reproducibility of real-world evidence studies using clinical practice data to inform regulatory and coverage decisions. Nat. Commun. 2022, 13, 1–11. [Google Scholar] [CrossRef]
- Rombolà, L.; Scuteri, D.; Marilisa, S.; Watanabe, C.; Morrone, L.A.; Bagetta, G.; Corasaniti, M.T. Pharmacokinetic Interactions between Herbal Medicines and Drugs: Their Mechanisms and Clinical Relevance. Life 2020, 10, 106. [Google Scholar] [CrossRef]
- Evans, L.W.; Athukorala, M.; Martinez-Guryn, K.; Ferguson, B.S. The Role of Histone Acetylation and the Microbiome in Phytochemical Efficacy for Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 4006. [Google Scholar] [CrossRef]
- Bhamra, S.K.; Slater, A.; Howard, C.; Heinrich, M.; Johnson, M.R. Health care professionals’ personal and professional views of herbal medicines in the United Kingdom. Phytother. Res. 2019, 33, 2360–2368. [Google Scholar] [CrossRef]
- Ford, I.; Norrie, J. Pragmatic trials. New Engl. J. Med. 2016, 375, 454–463. [Google Scholar] [CrossRef]
- Drugs.com. Available online: https://www.drugs.com (accessed on 3 April 2020).
- Epocrates. [Database on the Internet]. Epocrates, Inc. 2022. Available online: https://online.epocrates.com/interaction-check (accessed on 18 December 2022).
- Lexicomp Online Database [Database on the Internet]. Hudson (OH). Subscription Required to View. 2022. Available online: http://online.lexi.com. (accessed on 22 December 2022).
- Natural Medicines Online Database [Database on the Internet]. San Francisco (CA): Therapeutic Re-search Center. Subscription Required to View. 2022. Available online: https://naturalmedicines.therapeuticresearch.com (accessed on 23 December 2022).
- Williamson, E.; Driver, S.; Baxter, K. (Eds.) Stockley’s Herbal Medicines Interactions; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- US Food and Drug Administration (US FDA). [Database on the Internet]. 2022. Available online: https://www.fda.gov/food/compliance-enforcement-food/cfsan-adverse-event-reporting-system-caers (accessed on 14 December 2022).
- US Food and Drug Administration (US FDA). [Database on the Internet]. Available online: https://fis.fda.gov/sense/app/95239e26-e0be-42d9-a960-9a5f7f1c25ee/sheet/7a47a261-d58b-4203-a8aa-6d3021737452/state/analysis (accessed on 23 December 2022).
- Lippi, G.; Mattiuzzi, C.; Cervellin, G. Statins popularity: A global picture. Br. J. Clin. Pharmacol. 2019, 85, 1614–1615. [Google Scholar] [CrossRef]
- Bate, A.; Evans, S. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol. Drug Saf. 2009, 18, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Faillie, J.L. Case–non-case studies: Principle, methods, bias, and interpretation. Therapies 2019, 74, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Social Science Statistics. [Internet Calculator]. 2022. Available online: https://www.socscistatistics.com/tests/fisher/default2.aspx. (accessed on 23 December 2022).
| Herb/Supplement Drug Interactions Experienced by Respondents | |||
| HDS | Drug | Reaction | Credential (response ID) |
| Cannabis | Hydrocodone | Improved pain relief * | Layperson (107) |
| Cannabis | Painkillers | Improved pain relief * | Layperson (104) |
| Coenzyme Q 10 | Statin | Reduced muscle pain * | Layperson (118) |
| Lysine | Antibiotic | Improved response * | Layperson (118) |
| Melatonin | Lunesta® | Hang-over | MD (112) |
| NS | NS | NS | Layperson (131) |
| NS | NS | NS | ND (2) |
| Red yeast rice | Statin | Muscle pain | Layperson (113) |
| St John’s wort | Antihistamine | Tachycardia | Layperson (117) |
| Herb/Supplement Drug Interactions Observed by Respondents | |||
| HDS | Drug | Reaction | Credential (ID), frequency |
| Alfalfa | Warfarin | NS | DO (30) |
| Aloe | NSAID | Reduced gastric ulcer * | ND (2) |
| Cranberry | Warfarin | Bleeding | RPh/PharmD (77), × 2 |
| Crataegus | BP meds | Hypotension | RPh/PharmD (77), several |
| Fish oil | Psych meds | NS | DO (3) |
| Garlic | Aspirin | NS | DO (3) |
| Ginger | Chemotherapy | Reduced nausea * | ND (2) |
| Ginkgo | Warfarin | Bleeding | RPh/PharmD (77), many |
| Leeks | Warfarin | Decreased INR | RPh/PharmD (77), × 5 |
| L-glutamine | NSAID | Reduced gastric ulcer * | ND (2) |
| Licorice (DGL) | NSAID | Reduced gastric ulcer * | ND (2) |
| Licorice root | Prednisone | Reduced dosing need * | Herbalist (34) |
| NS | NS | Elevated INR | RN (78) |
| Rauwolfia | Lisinopril | Additive effects | ND (2) |
| Salt substitute | ACE inhibitors | Hyperkalemia | RPh/PharmD (77) × 2 |
| Saw palmetto | Antibiotics | NS | DO (25) |
| St John’s wort | NS | NS | DO (25) |
| St John’s wort | Escitalopram | Serotonin syndrome | RPh/PharmD (77) |
| Thyroid support formula | Levothyroxine | NS | DO (30) |
| Thematic Reason | LP | HCP/H | Total | (%) | p |
|---|---|---|---|---|---|
| Less Expensive | 14 | 4 | 18 | 16.8 | 0.19 |
| Natural, less exposure to synthetics | 10 | 4 | 14 | 13.1 | 0.57 |
| Safer, gentler, fewer adverse effects | 5 | 5 | 10 | 9.3 | 0.49 |
| Evidence, experience, logical choice | 4 | 4 | 8 | 7.5 | 0.46 |
| Lack of trust in medical establishment | 6 | 2 | 8 | 7.5 | 0.71 |
| Benefit, to treat specific condition | 4 | 3 | 7 | 6.5 | 0.71 |
| Self-reliance, choice, autonomy | 1 | 6 | 7 | 6.5 | 0.01 * |
| Global effects, effects not seen from medications | 2 | 4 | 6 | 5.6 | 0.19 |
| Culture, family | 4 | 1 | 5 | 4.7 | 0.65 |
| Recommended by other | 5 | 0 | 5 | 4.7 | 0.16 |
| Accessibility | 3 | 1 | 4 | 3.7 | 1 |
| Efficacy | 4 | 0 | 4 | 3.7 | 0.29 |
| Avoid/reduce number of prescription medications | 2 | 2 | 4 | 3.7 | 0.62 |
| Curiosity, open-mindedness | 1 | 2 | 3 | 2.8 | 0.55 |
| Other | 2 | 1 | 3 | 2.8 | 1 |
| HDS | Reaction | A | B | C | D | ROR | 95% CI |
|---|---|---|---|---|---|---|---|
| Vaccinium macrocarpon (Cranberry) | Bleeding | 34 | 243 | 164 | 69,675 | 59.4 | 40.2–87.8 * |
| Crataegus spp. (Hawthorne) | Hypotension | 2 | 15 | 400 | 69,699 | 23.2 | 5.3–101.9 * |
| Allium sativa (Garlic) | Bleeding | 40 | 259 | 193 | 69,624 | 55.7 | 38.8–80.0 * |
| Ginkgo biloba (Ginkgo) | Bleeding | 30 | 115 | 179 | 69,792 | 101.7 | 66.3–156.0 * |
| Melatonin | Hangover | 13 | 79 | 260 | 69,764 | 44.1 | 24.2–80.4 * |
| Monascus purpureus (Red Yeast Rice) | Muscle pain | 35 | 120 | 424 | 69,537 | 47.8 | 32.4–70.5 * |
| Hypericum spp. (St John’s wort) | Tachycardia | 5 | 49 | 2387 | 67,675 | 2.9 | 1.2–7.3 * |
| Hypericum spp. (St John’s wort) | Serotonin syndrome | 5 | 49 | 1465 | 68,597 | 4.8 | 1.9–12.0 * |
| HDS and Drug | Reaction | A | B | C | D | ROR | 95% CI |
|---|---|---|---|---|---|---|---|
| Allium sativum (garlic) and aspirin | Bleeding | 4 | 1 | 21,712 | 26,474 | 4.9 | 0.6–43.6 |
| Ginkgo biloba and warfarin | Bleeding | 212 | 8 | 559 | 23,872 | 1131.7 | 555.9–2303.9 * |
| Melatonin and eszopiclone | Hangover | 18 | 81 | 676 | 26,235 | 8.6 | 5.1–14.4 * |
| M. purpureus (Red yeast rice) and atorvastatin | Muscle pain | 3 | 3 | 5821 | 30,641 | 5.3 | 1.1–26.1 * |
| V. macrocarpon and warfarin | Bleeding | 3 | 4 | 7885 | 12,684 | 1.2 | 0.27–5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza-Peres, J.V.; Flores, K.; Umloff, B.; Heinan, M.; Herscu, P.; Babos, M.B. Everyday Evaluation of Herb/Dietary Supplement–Drug Interaction: A Pilot Study. Medicines 2023, 10, 20. https://doi.org/10.3390/medicines10030020
Souza-Peres JV, Flores K, Umloff B, Heinan M, Herscu P, Babos MB. Everyday Evaluation of Herb/Dietary Supplement–Drug Interaction: A Pilot Study. Medicines. 2023; 10(3):20. https://doi.org/10.3390/medicines10030020
Chicago/Turabian StyleSouza-Peres, Joao Victor, Kimberly Flores, Bethany Umloff, Michelle Heinan, Paul Herscu, and Mary Beth Babos. 2023. "Everyday Evaluation of Herb/Dietary Supplement–Drug Interaction: A Pilot Study" Medicines 10, no. 3: 20. https://doi.org/10.3390/medicines10030020
APA StyleSouza-Peres, J. V., Flores, K., Umloff, B., Heinan, M., Herscu, P., & Babos, M. B. (2023). Everyday Evaluation of Herb/Dietary Supplement–Drug Interaction: A Pilot Study. Medicines, 10(3), 20. https://doi.org/10.3390/medicines10030020






