Metabolic Response of RAW 264.7 Macrophages to Exposure to Crude Particulate Matter and a Reduced Content of Organic Matter
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
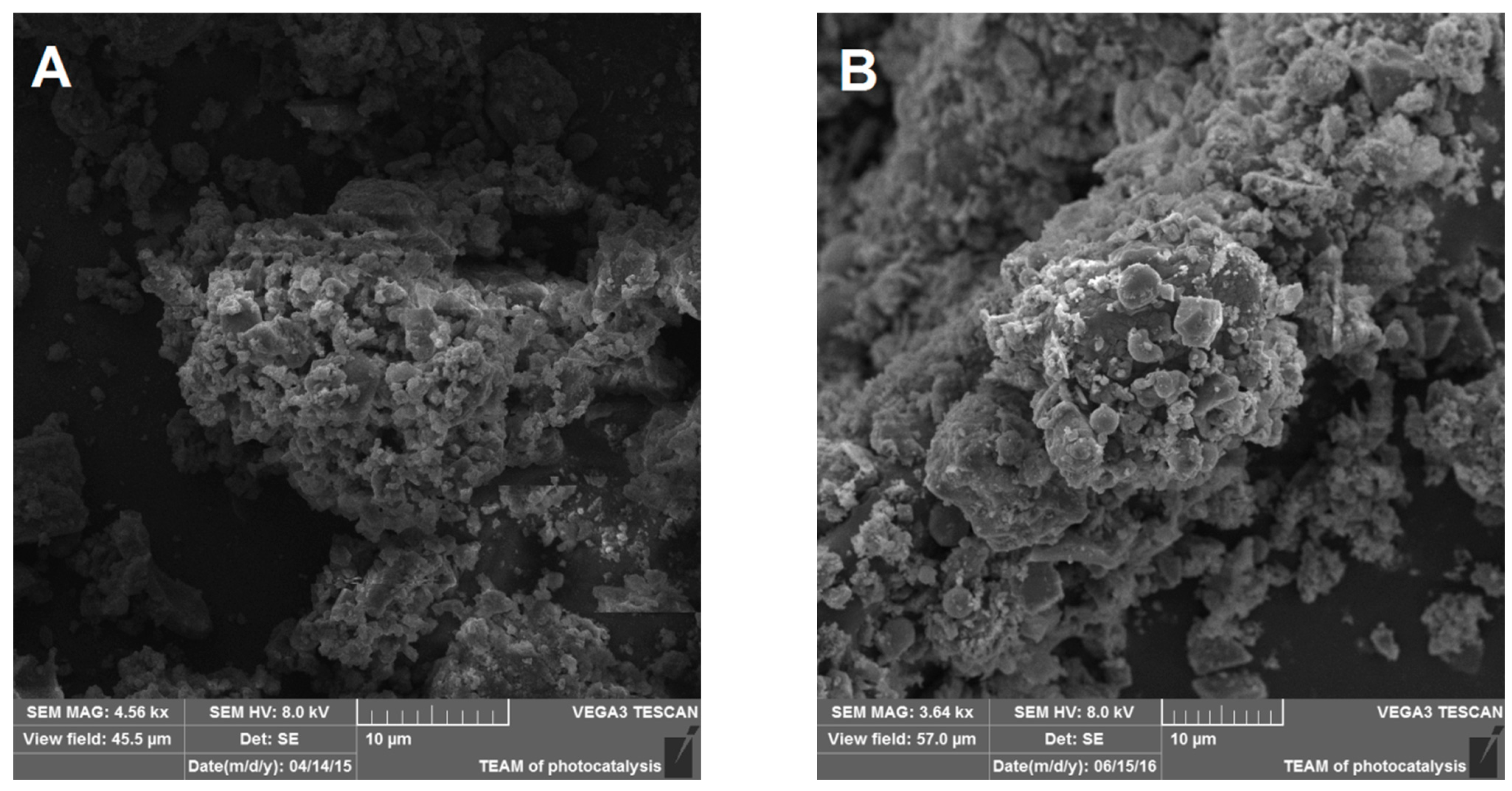
2.2. PM Treatment and Characterization
2.3. Cell Culture and Treatment
2.4. Viability Assays
2.5. Assessment of Metabolic Activity
2.6. Detection of Reactive Oxygen Species
2.7. Assessment of Cell Number
2.8. Assessment of Nitric Oxide Release
2.9. Assessment of Respiratory Burst
2.10. Statistical Analysis
3. Results
3.1. NIST1648A and LAp120 Composition
3.2. Effect of PM on Cell Viability
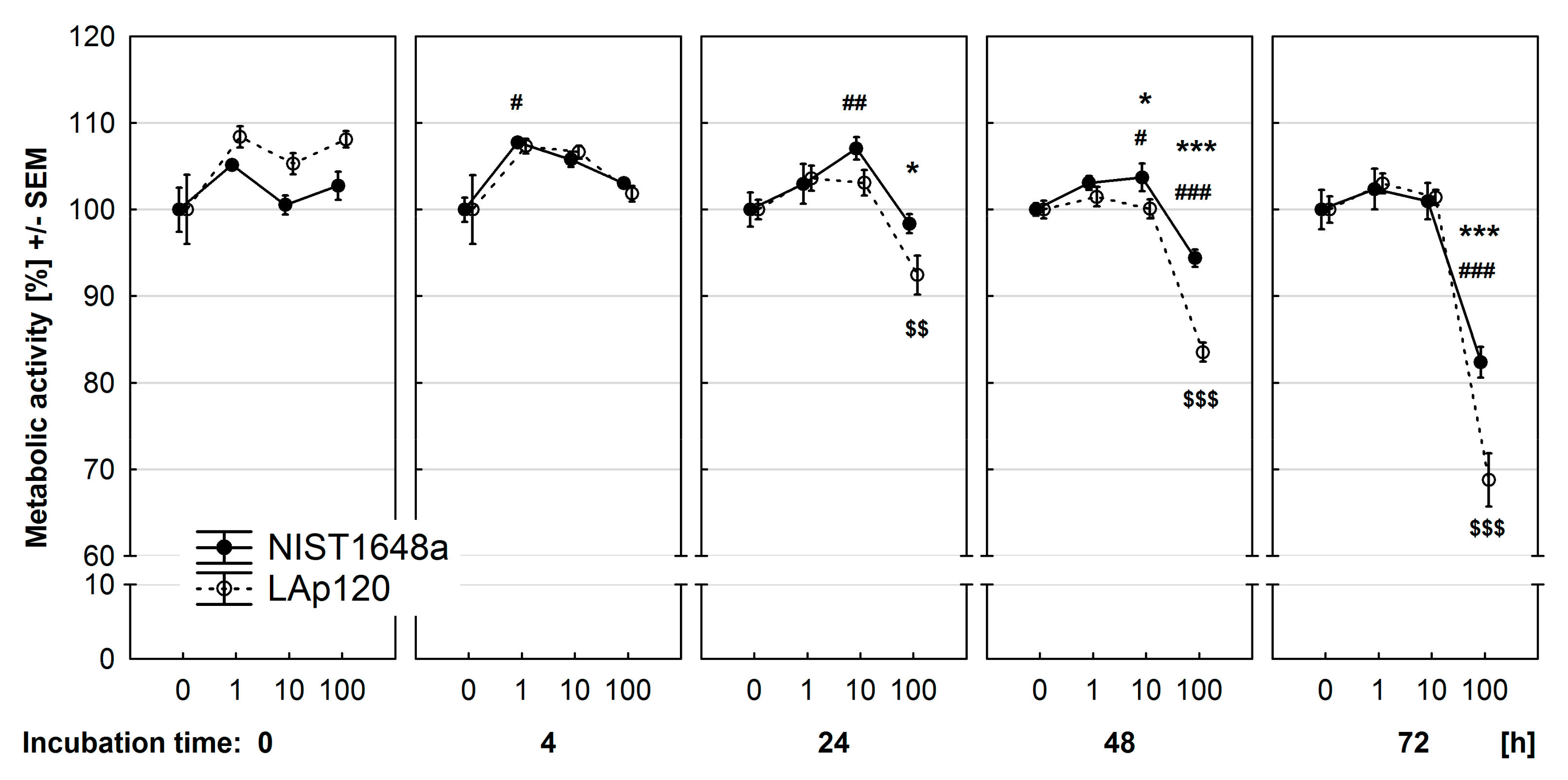
3.3. Effect of PM on Metabolic Activity
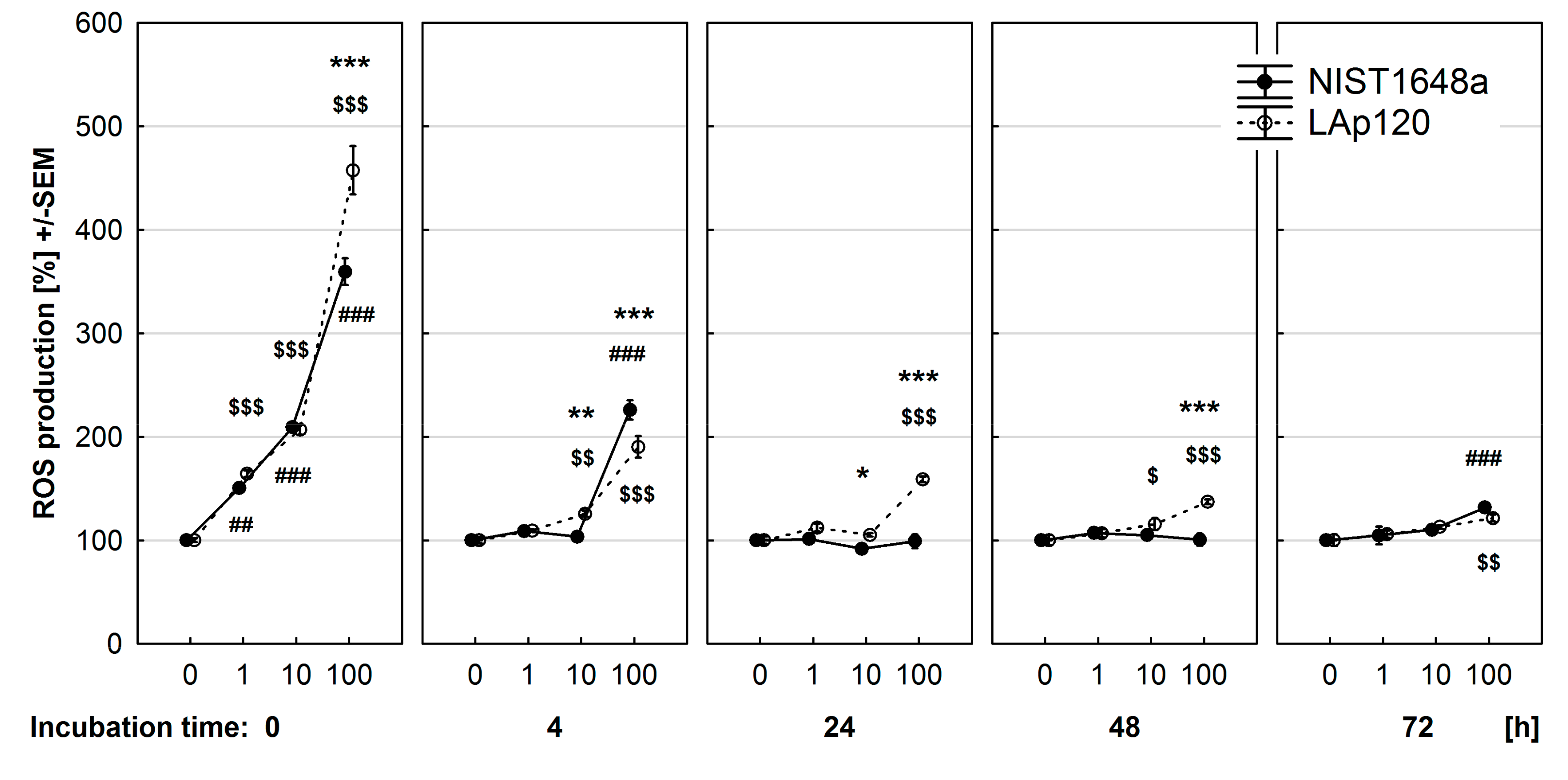
3.4. Effect of PM on ROS Synthesis
3.5. Effect of PM on Cell Number
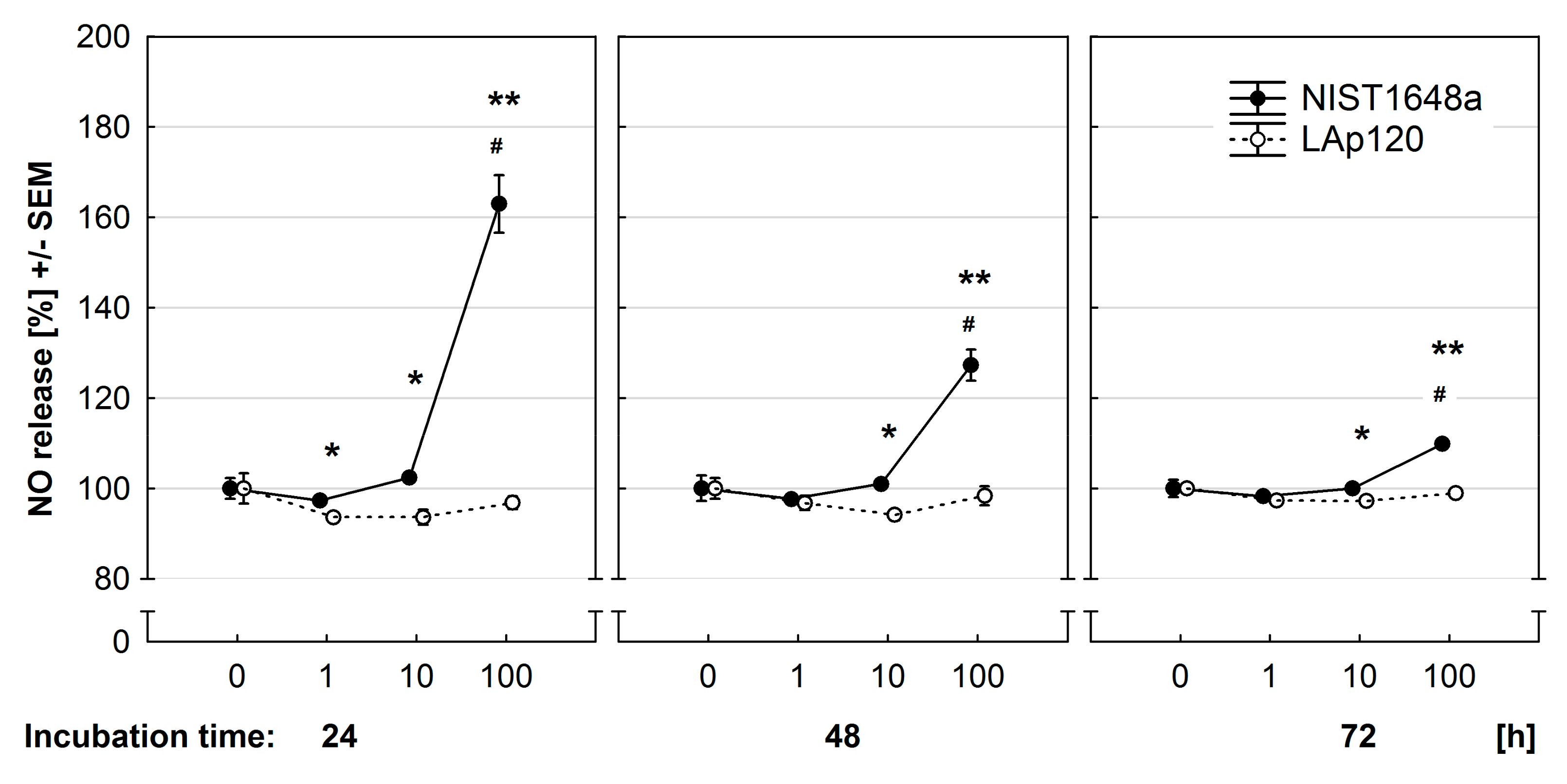
3.6. Effect of PM on NO Release
3.7. Effect of PM on Respiratory Burst
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morakinyo, O.M.; Mokgobu, M.I.; Mukhola, M.S.; Hunter, R.P. Health Outcomes of Exposure to Biological and Chemical Components of Inhalable and Respirable Particulate Matter. Int. J. Environ. Res. Public Health 2016, 13, 592. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-H.; Kabir, E.; Kabir, S. A Review on the Human Health Impact of Airborne Particulate Matter. Environ. Int. 2015, 74, 136–143. [Google Scholar] [CrossRef]
- Cheung, K.; Daher, N.; Kam, W.; Shafer, M.M.; Ning, Z.; Schauer, J.J.; Sioutas, C. Spatial and Temporal Variation of Chemical Composition and Mass Closure of Ambient Coarse Particulate Matter (PM10-2.5) in the Los Angeles Area. Atmos. Environ. 2011, 45, 2651–2662. [Google Scholar] [CrossRef]
- Samek, L.; Gdowik, A.; Ogarek, J.; Furman, L. Elemental Composition and Rough Source Apportionment of Fine Particulate Matter in Air in Cracow, Poland. Environ. Prot. Eng. 2016, 42, 71–83. [Google Scholar] [CrossRef]
- Karagulian, F.; Belis, C.A.; Dora, C.F.C.; Prüss-Ustün, A.M.; Bonjour, S.; Adair-Rohani, H.; Amann, M. Contributions to Cities’ Ambient Particulate Matter (PM): A Systematic Review of Local Source Contributions at Global Level. Atmos. Environ. 2015, 120, 475–483. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Size, Source and Chemical Composition as Determinants of Toxicity Attributable to Ambient Particulate Matter. Atmos. Environ. 2012, 60, 504–526. [Google Scholar] [CrossRef]
- Rohr, A.C.; Wyzga, R.E. Attributing Health Effects to Individual Particulate Matter Constituents. Atmos. Environ. 2012, 62, 130–152. [Google Scholar] [CrossRef]
- Wyzga, R.E.; Rohr, A.C. Long-Term Particulate Matter Exposure: Attributing Health Effects to Individual PM Components. J. Air Waste Manag. Assoc. 2015, 65, 523–543. [Google Scholar] [CrossRef]
- Jayaraj, R.L.; Rodriguez, E.A.; Wang, Y.; Block, M.L. Outdoor Ambient Air Pollution and Neurodegenerative Diseases: The Neuroinflammation Hypothesis. Curr. Environ. Health Rep. 2017, 4, 166–179. [Google Scholar] [CrossRef]
- Gawda, A.; Majka, G.; Nowak, B.; Marcinkiewicz, J. Air Pollution, Oxidative Stress, and Exacerbation of Autoimmune Diseases. Cent. Eur J. Immunol. 2017, 42, 305–312. [Google Scholar] [CrossRef]
- Hiraiwa, K.; van Eeden, S.F. Contribution of Lung Macrophages to the Inflammatory Responses Induced by Exposure to Air Pollutants. Mediat. Inflamm. 2013, 2013, 619523. [Google Scholar] [CrossRef] [Green Version]
- Mills, C.D.; Ley, K. M1 and M2 Macrophages: The Chicken and the Egg of Immunity. J. Innate Immun. 2014, 6, 716–726. [Google Scholar] [CrossRef]
- Ghio, A.J.; Carraway, M.S.; Madden, M.C. Composition of Air Pollution Particles and Oxidative Stress in Cells, Tissues, and Living Systems. J. Toxicol. Environ. Health B Crit. Rev. 2012, 15, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Abramov, A.Y. Role of Mitochondrial ROS in the Brain: From Physiology to Neurodegeneration. FEBS Lett. 2018, 592, 692–702. [Google Scholar] [CrossRef]
- Thomas, D.C. The Phagocyte Respiratory Burst: Historical Perspectives and Recent Advances. Immunol. Lett. 2017, 192, 88–96. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef] [Green Version]
- Happo, M.S.; Hirvonen, M.-R.; Uski, O.; Kasurinen, S.; Kelz, J.; Brunner, T.; Obernberger, I.; Jalava, P.I. Particulate Emissions from Modern and Old Technology Wood Combustion Induce Distinct Time-Dependent Patterns of Toxicological Responses in Vitro. Toxicol. Vitr. 2017, 44, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Pardo, M.; Qiu, X.; Zimmermann, R.; Rudich, Y. Particulate Matter Toxicity Is Nrf2 and Mitochondria Dependent: The Roles of Metals and Polycyclic Aromatic Hydrocarbons. Chem. Res. Toxicol. 2020, 33, 1110–1120. [Google Scholar] [CrossRef]
- Jia, Y.-Y.; Wang, Q.; Liu, T. Toxicity Research of PM2.5 Oompositions in Vitro. Int. J. Environ. Res. Public Health 2017, 14, 232. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Standards and Technology. Certificate of Analysis; Standard Reference Material® 1648a; Urban Particulate Matter: Gaithersburg, MD, USA, 2015. [Google Scholar]
- Mikrut, M.; Regiel-Futyra, A.; Samek, L.; Macyk, W.; Stochel, G.; van Eldik, R. Generation of Hydroxyl Radicals and Singlet Oxygen by Particulate Matter and Its Inorganic Components. Environ. Pollut. 2018, 238, 638–646. [Google Scholar] [CrossRef]
- Jantas, D.; Greda, A.; Golda, S.; Korostynski, M.; Grygier, B.; Roman, A.; Pilc, A.; Lason, W. Neuroprotective Effects of Metabotropic Glutamate Receptor Group II and III Activators against MPP(+)-Induced Cell Death in Human Neuroblastoma SH-SY5Y Cells: The Impact of Cell Differentiation State. Neuropharmacology 2014, 83, 36–53. [Google Scholar] [CrossRef]
- Zamai, L.; Canonico, B.; Luchetti, F.; Ferri, P.; Melloni, E.; Guidotti, L.; Cappellini, A.; Cutroneo, G.; Vitale, M.; Papa, S. Supravital Exposure to Propidium Iodide Identifies Apoptosis on Adherent Cells. Cytometry 2001, 44, 57–64. [Google Scholar] [CrossRef]
- Nicoletti, I.; Migliorati, G.; Pagliacci, M.C.; Grignani, F.; Riccardi, C. A Rapid and Simple Method for Measuring Thymocyte Apoptosis by Propidium Iodide Staining and Flow Cytometry. J. Immunol. Methods 1991, 139, 271–279. [Google Scholar] [CrossRef]
- Wersto, R.P.; Chrest, F.J.; Leary, J.F.; Morris, C.; Stetler-Stevenson, M.A.; Gabrielson, E. Doublet Discrimination in DNA Cell-Cycle Analysis. Cytometry 2001, 46, 296–306. [Google Scholar] [CrossRef]
- Roman, A.; Kuśmierczyk, J.; Kreiner, G.; Nalepa, I. Assessment of Leukocyte Activity in Mice Devoid of the Glucocorticoid Receptor in the Noradrenergic System (GRDBHCre). Immunobiology 2018, 223, 227–238. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (Resazurin) Fluorescent Dye for the Assessment of Mammalian Cell Cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, A.R.; Schmaldienst, S.; Stuhlmeier, K.M.; Chen, W.; Knapp, W.; Zlabinger, G.J. A Microplate Assay for the Detection of Oxidative Products Using 2′,7′-Dichlorofluorescin-Diacetate. J. Immunol. Methods 1992, 156, 39–45. [Google Scholar] [CrossRef]
- Landreman, A.P.; Shafer, M.M.; Hemming, J.C.; Hannigan, M.P.; Schauer, J.J. A Macrophage-Based Method for the Assessment of the Reactive Oxygen Species (ROS) Activity of Atmospheric Particulate Matter (PM) and Application to Routine (Daily-24 h) Aerosol Monitoring Studies. Aerosol. Sci. Technol. 2008, 42, 946–957. [Google Scholar] [CrossRef]
- Sureda, F.X.; Gabriel, C.; Comas, J.; Pallàs, M.; Escubedo, E.; Camarasa, J.; Camins, A. Evaluation of Free Radical Production, Mitochondrial Membrane Potential and Cytoplasmic Calcium in Mammalian Ne l. urons by Flow Cytometry. Brain Res. Brain Res. Protoc. 1999, 4, 280–287. [Google Scholar] [CrossRef]
- Roman, A.; Kuśmierczyk, J.; Klimek, E.; Rogóż, Z.; Nalepa, I. Effects of Co-Administration of Fluoxetine and Risperidone on Properties of Peritoneal and Pleural Macrophages in Rats Subjected to the Forced Swimming Test. Pharm. Rep. 2012, 64, 1368–1380. [Google Scholar] [CrossRef]
- Kueng, W.; Silber, E.; Eppenberger, U. Quantification of Cells Cultured on 96-Well Plates. Anal. Biochem. 1989, 182, 16–19. [Google Scholar] [CrossRef]
- Marcinkiewicz, J.; Pater, M.; Grabowska, A. An Improved Experimental Model for the Study of in Vitro Release of Nitric Oxide by Murine Peritoneal Macrophages. Arch. Immunol. Ther. Exp. 1994, 42, 95–99. [Google Scholar]
- Choi, H.S.; Kim, J.W.; Cha, Y.-N.; Kim, C. A Quantitative Nitroblue Tetrazolium Assay for Determining Intracellular Superoxide Anion Production in Phagocytic Cells. J. Immunoass. Immunochem. 2006, 27, 31–44. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, F.; Wang, L.; Rui, W.; Long, F.; Zhao, Y.; Chen, D.; Ding, W. Airborne Fine Particulate Matter Induces Multiple Cell Death Pathways in Human Lung Epithelial Cells. Apoptosis 2014, 19, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Michael, S.; Montag, M.; Dott, W. Pro-Inflammatory Effects and Oxidative Stress in Lung Macrophages and Epithelial Cells Induced by Ambient Particulate Matter. Environ. Pollut. 2013, 183, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, F.; Rui, W.; Long, F.; Wang, L.; Feng, Z.; Chen, D.; Ding, W. PM2.5-Induced Oxidative Stress Triggers Autophagy in Human Lung Epithelial A549 Cells. Toxicol. Vitr. 2013, 27, 1762–1770. [Google Scholar] [CrossRef]
- Chan, F.K.-M.; Moriwaki, K.; De Rosa, M.J. Detection of Necrosis by Release of Lactate Dehydrogenase Activity. Methods Mol. Biol. 2013, 979, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Yoon, S.C.; Lee, T.Y.; Jeong, D. Discriminative Cytotoxicity Assessment Based on Various Cellular Damages. Toxicol. Lett. 2009, 184, 13–17. [Google Scholar] [CrossRef]
- Zaqout, M.S.K.; Sumizawa, T.; Igisu, H.; Wilson, D.; Myojo, T.; Ueno, S. Binding of Titanium Dioxide Nanoparticles to Lactate Dehydrogenase. Environ. Health Prev. Med. 2012, 17, 341–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, X.; Gelein, R.; Corson, N.; Wade-Mercer, P.; Jiang, J.; Biswas, P.; Finkelstein, J.N.; Elder, A.; Oberdörster, G. Validation of an LDH Assay for Assessing Nanoparticle Toxicity. Toxicology 2011, 287, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Montiel-Dávalos, A.; de Jesús Ibarra-Sánchez, M.; Ventura-Gallegos, J.L.; Alfaro-Moreno, E.; López-Marure, R. Oxidative Stress and Apoptosis Are Induced in Human Endothelial Cells Exposed to Urban Particulate Matter. Toxicol. Vitr. 2010, 24, 135–141. [Google Scholar] [CrossRef]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate Matter 2.5 Damages Skin Cells by Inducing Oxidative Stress, Subcellular Organelle Dysfunction, and Apoptosis. Arch. Toxicol. 2018. [Google Scholar] [CrossRef] [Green Version]
- Darzynkiewicz, Z.; Bruno, S.; Del Bino, G.; Gorczyca, W.; Hotz, M.A.; Lassota, P.; Traganos, F. Features of Apoptotic Cells Measured by Flow Cytometry. Cytometry 1992, 13, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kang, Z.; Jiang, S.; Zhao, J.; Yan, S.; Xu, F.; Xu, J. Effects of Ambient Fine Particles PM2.5 on Human HaCaT Cells. Int. J. Environ. Res. Public Health 2017, 14, 72. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Tian, D.; He, J.; Zhang, L.; Tang, X.; Zhang, L.; Wang, Y.; Li, L.; Zhao, J.; Yuan, X.; et al. Exposure Scenario: Another Important Factor Determining the Toxic Effects of PM2.5 and Possible Mechanisms Involved. Environ. Pollut. 2017, 226, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Magnani, N.D.; Marchini, T.; Tasat, D.R.; Alvarez, S.; Evelson, P.A. Lung Oxidative Metabolism after Exposure to Ambient Particles. Biochem. Biophys. Res. Commun. 2011, 412, 667–672. [Google Scholar] [CrossRef]
- Delgado-Buenrostro, N.L.; Freyre-Fonseca, V.; Cuéllar, C.M.G.; Sánchez-Pérez, Y.; Gutierrez-Cirlos, E.B.; Cabellos-Avelar, T.; Orozco-Ibarra, M.; Pedraza-Chaverri, J.; Chirino, Y.I. Decrease in Respiratory Function and Electron Transport Chain Induced by Airborne Particulate Matter (PM10) Exposure in Lung Mitochondria. Toxicol. Pathol. 2013, 41, 628–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peixoto, M.S.; de Oliveira Galvão, M.F.; Batistuzzo de Medeiros, S.R. Cell Death Pathways of Particulate Matter Toxicity. Chemosphere 2017, 188, 32–48. [Google Scholar] [CrossRef]
- Bai, R.; Guan, L.; Zhang, W.; Xu, J.; Rui, W.; Zhang, F.; Ding, W. Comparative Study of the Effects of PM1-Induced Oxidative Stress on Autophagy and Surfactant Protein B and C Expressions in Lung Alveolar Type II Epithelial MLE-12 Cells. Biochim. Biophys. Acta 2016, 1860, 2782–2792. [Google Scholar] [CrossRef]
- Zou, Y.; Jin, C.; Su, Y.; Li, J.; Zhu, B. Water Soluble and Insoluble Components of Urban PM2.5 and Their Cytotoxic Effects on Epithelial Cells (A549) in Vitro. Environ. Pollut. 2016, 212, 627–635. [Google Scholar] [CrossRef]
- Yu, H.; Wei, J.; Cheng, Y.; Subedi, K.; Verma, V. Synergistic and Antagonistic Interactions among the Particulate Matter Components in Generating Reactive Oxygen Species Based on the Dithiothreitol Assay. Environ. Sci. Technol. 2018, 52, 2261–2270. [Google Scholar] [CrossRef]
- Samake, A.; Uzu, G.; Martins, J.M.F.; Calas, A.; Vince, E.; Parat, S.; Jaffrezo, J.L. The Unexpected Role of Bioaerosols in the Oxidative Potential of PM. Sci. Rep. 2017, 7, 10978. [Google Scholar] [CrossRef]
- Li, N.; Sioutas, C.; Cho, A.; Schmitz, D.; Misra, C.; Sempf, J.; Wang, M.; Oberley, T.; Froines, J.; Nel, A. Ultrafine Particulate Pollutants Induce Oxidative Stress and Mitochondrial Damage. Environ. Health Perspect. 2003, 111, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Shafer, M.M.; Hemming, J.D.C.; Antkiewicz, D.S.; Schauer, J.J. Oxidative Potential of Size-Fractionated Atmospheric Aerosol in Urban and Rural Sites across Europe. Faraday Discuss. 2016, 189, 381–405. [Google Scholar] [CrossRef]
- Segal, A.W.; Coade, S.B. Kinetics of Oxygen Consumption by Phagocytosing Human Neutrophils. Biochem. Biophys. Res. Commun. 1978, 84, 611–617. [Google Scholar] [CrossRef]
- Milano, M.; Dongiovanni, P.; Artoni, A.; Gatti, S.; Rosso, L.; Colombo, F.; Bollati, V.; Maggioni, M.; Mannucci, P.M.; Bertazzi, P.A.; et al. Particulate Matter Phagocytosis Induces Tissue Factor in Differentiating Macrophages. J. Appl. Toxicol. 2016, 36, 151–160. [Google Scholar] [CrossRef] [Green Version]
- Breznan, D.; Goegan, P.; Chauhan, V.; Karthikeyan, S.; Kumarathasan, P.; Cakmak, S.; Nadeau, D.; Brook, J.R.; Vincent, R. Respiratory Burst in Alveolar Macrophages Exposed to Urban Particles Is Not a Predictor of Cytotoxicity. Toxicol. In Vitro 2013, 27, 1287–1297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tollefson, A.K.; Oberley-Deegan, R.E.; Butterfield, K.T.; Nicks, M.E.; Weaver, M.R.; Remigio, L.K.; Decsesznak, J.; Chu, H.W.; Bratton, D.L.; Riches, D.W.; et al. Endogenous Enzymes (NOX and ECSOD) Regulate Smoke-Induced Oxidative Stress. Free Radic. Biol. Med. 2010, 49, 1937–1946. [Google Scholar] [CrossRef] [Green Version]
- Gusev, V.A.; Danilovskaja, Y.V.; Vatolkina, O.Y.; Lomonosova, O.S.; Velichkovsky, B.T. Effect of Quartz and Alumina Dust on Generation of Superoxide Radicals and Hydrogen Peroxide by Alveolar Macrophages, Granulocytes, and Monocytes. Br. J. Ind. Med. 1993, 50, 732–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Predonzani, A.; Calì, B.; Agnellini, A.H.; Molon, B. Spotlights on Immunological Effects of Reactive Nitrogen Species: When Inflammation Says Nitric Oxide. World J. Exp. Med. 2015, 5, 64–76. [Google Scholar] [CrossRef]
- Imrich, A.; Ning, Y.; Kobzik, L. Insoluble Components of Concentrated Air Particles Mediate Alveolar Macrophage Responses in Vitro. Toxicol. Appl. Pharmacol. 2000, 167, 140–150. [Google Scholar] [CrossRef]
- Gawda, A.; Majka, G.; Nowak, B.; Śróttek, M.; Walczewska, M.; Marcinkiewicz, J. Air Particulate Matter SRM 1648a Primes Macrophages to Hyperinflammatory Response after LPS Stimulation. Inflamm. Res. 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camatini, M.; Corvaja, V.; Pezzolato, E.; Mantecca, P.; Gualtieri, M. PM10-Biogenic Fraction Drives the Seasonal Variation of Proinflammatory Response in A549 Cells. Environ. Toxicol. 2012, 27, 63–73. [Google Scholar] [CrossRef]
- Totlandsdal, A.I.; Låg, M.; Lilleaas, E.; Cassee, F.; Schwarze, P. Differential Proinflammatory Responses Induced by Diesel Exhaust Particles with Contrasting PAH and Metal Content. Environ. Toxicol. 2015, 30, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Breznan, D.; Goegan, P.; Nadeau, D.; Karthikeyan, S.; Brook, J.R.; Vincent, R. Effects of Ambient Air Particles on Nitric Oxide Production in Macrophage Cell Lines. Cell Biol. Toxicol. 2004, 20, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Shirmohammadi, F.; Hasheminassab, S.; Wang, D.; Saffari, A.; Schauer, J.J.; Shafer, M.M.; Delfino, R.J.; Sioutas, C. Oxidative Potential of Coarse Particulate Matter (PM(10-2.5)) and Its Relation to Water Solubility and Sources of Trace Elements and Metals in the Los Angeles Basin. Environ. Sci. Process. Impacts 2015, 17, 2110–2121. [Google Scholar] [CrossRef] [Green Version]
- Van Den Heuvel, R.; Den Hond, E.; Govarts, E.; Colles, A.; Koppen, G.; Staelens, J.; Mampaey, M.; Janssen, N.; Schoeters, G. Identification of PM10 Characteristics Involved in Cellular Responses in Human Bronchial Epithelial Cells (Beas-2B). Environ. Res. 2016, 149, 48–56. [Google Scholar] [CrossRef]
- Mirowsky, J.E.; Jin, L.; Thurston, G.; Lighthall, D.; Tyner, T.; Horton, L.; Galdanes, K.; Chillrud, S.; Ross, J.; Pinkerton, K.E.; et al. In Vitro and in Vivo Toxicity of Urban and Rural Particulate Matter from California. Atmos. Environ. 2015, 103, 256–262. [Google Scholar] [CrossRef] [Green Version]
- Price, H.D.; Jones, T.P.; BéruBé, K.A. Resolution of the Mediators of in Vitro Oxidative Reactivity in Size-Segregated Fractions That May Be Masked in the Urban PM(10) Cocktail. Sci. Total Environ. 2014, 485–486, 588–595. [Google Scholar] [CrossRef] [Green Version]
- Samek, L.; Furman, L.; Mikrut, M.; Regiel-Futyra, A.; Macyk, W.; Stochel, G.; van Eldik, R. Chemical Composition of Submicron and Fine Particulate Matter Collected in Krakow, Poland. Consequences for the APARIC Project. Chemosphere 2017, 187, 430–439. [Google Scholar] [CrossRef]

| Material | Elemental Analysis | Total Organic Component Analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| C | H | N | S | TC 1 | IC 1 | TOC 1 | TN 1 | |
| NIST1648a | 14.10 | 2.41 | 3.12 | 4.96 | 9.21 | 0.09 | 9.12 | 2.75 |
| LAp120 | 1.87 | 1.20 | 0.83 | 6.18 | 1.75 | 0.07 | 1.68 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankowska-Kieltyka, M.; Roman, A.; Mikrut, M.; Kowalska, M.; van Eldik, R.; Nalepa, I. Metabolic Response of RAW 264.7 Macrophages to Exposure to Crude Particulate Matter and a Reduced Content of Organic Matter. Toxics 2021, 9, 205. https://doi.org/10.3390/toxics9090205
Jankowska-Kieltyka M, Roman A, Mikrut M, Kowalska M, van Eldik R, Nalepa I. Metabolic Response of RAW 264.7 Macrophages to Exposure to Crude Particulate Matter and a Reduced Content of Organic Matter. Toxics. 2021; 9(9):205. https://doi.org/10.3390/toxics9090205
Chicago/Turabian StyleJankowska-Kieltyka, Monika, Adam Roman, Magdalena Mikrut, Marta Kowalska, Rudi van Eldik, and Irena Nalepa. 2021. "Metabolic Response of RAW 264.7 Macrophages to Exposure to Crude Particulate Matter and a Reduced Content of Organic Matter" Toxics 9, no. 9: 205. https://doi.org/10.3390/toxics9090205
APA StyleJankowska-Kieltyka, M., Roman, A., Mikrut, M., Kowalska, M., van Eldik, R., & Nalepa, I. (2021). Metabolic Response of RAW 264.7 Macrophages to Exposure to Crude Particulate Matter and a Reduced Content of Organic Matter. Toxics, 9(9), 205. https://doi.org/10.3390/toxics9090205







