Monitoring of PCDD/Fs and PCBs in European Eels (Anguilla anguilla) from Lake Garda: A Persistent Environmental Concern
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Sample Collection
- A group of 30 eels with a length superior to 80 cm;
- A group of 30 eels with a length between 65 and 80 cm;
- A group of 30 eels with a length between 50 and 65 cm.
2.3. Chemical Analysis, Method Performance, and Quality Control
2.4. Statistical Analysis
2.4.1. Quantitative Analysis of TEQ Diox+PCB-DL and PCB-NDL Compounds
2.4.2. Qualitative Analysis on TEQ Diox+PCB-DL and PCB-NDL Compounds
3. Results
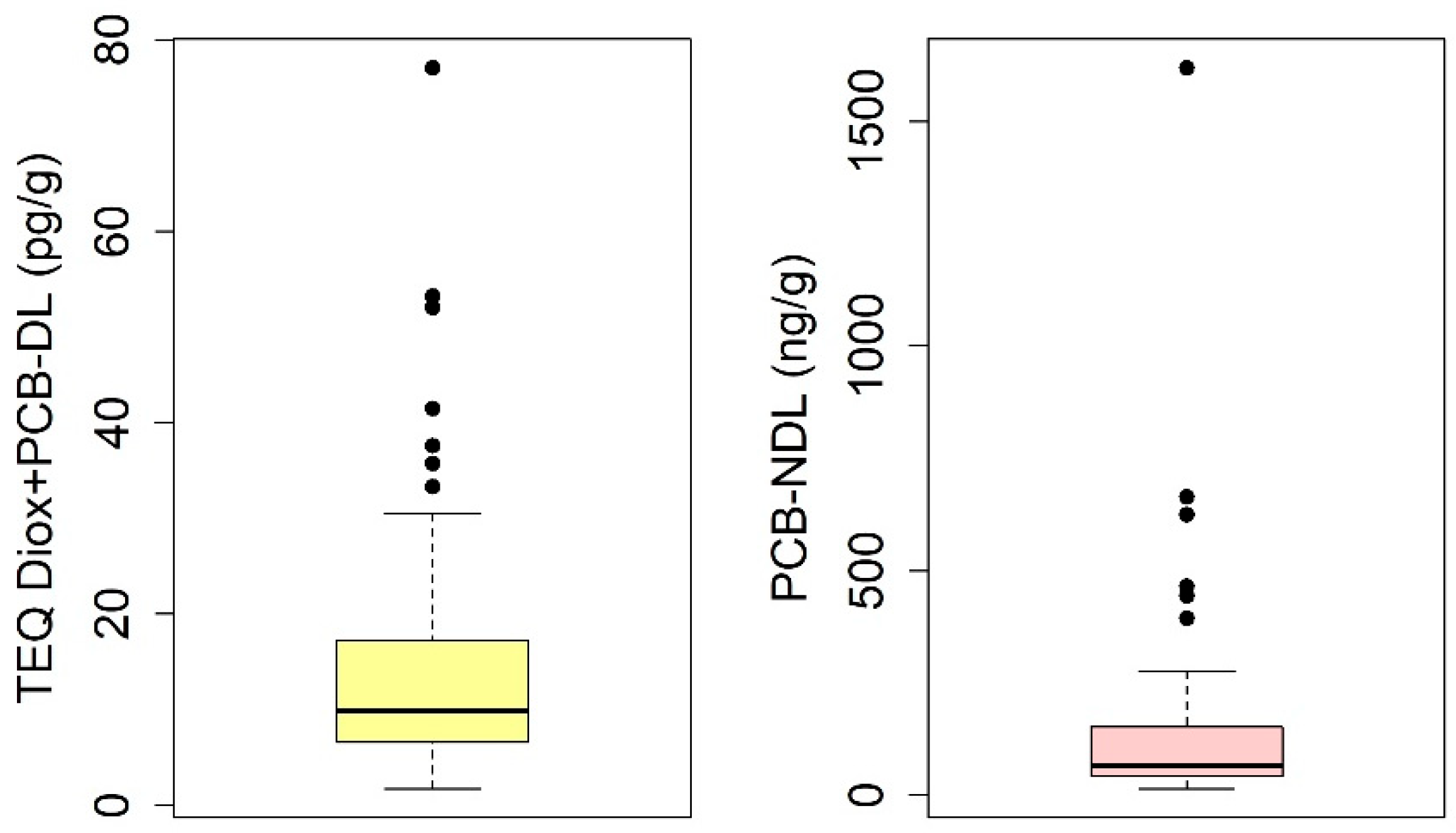
3.1. Chemical Analysis
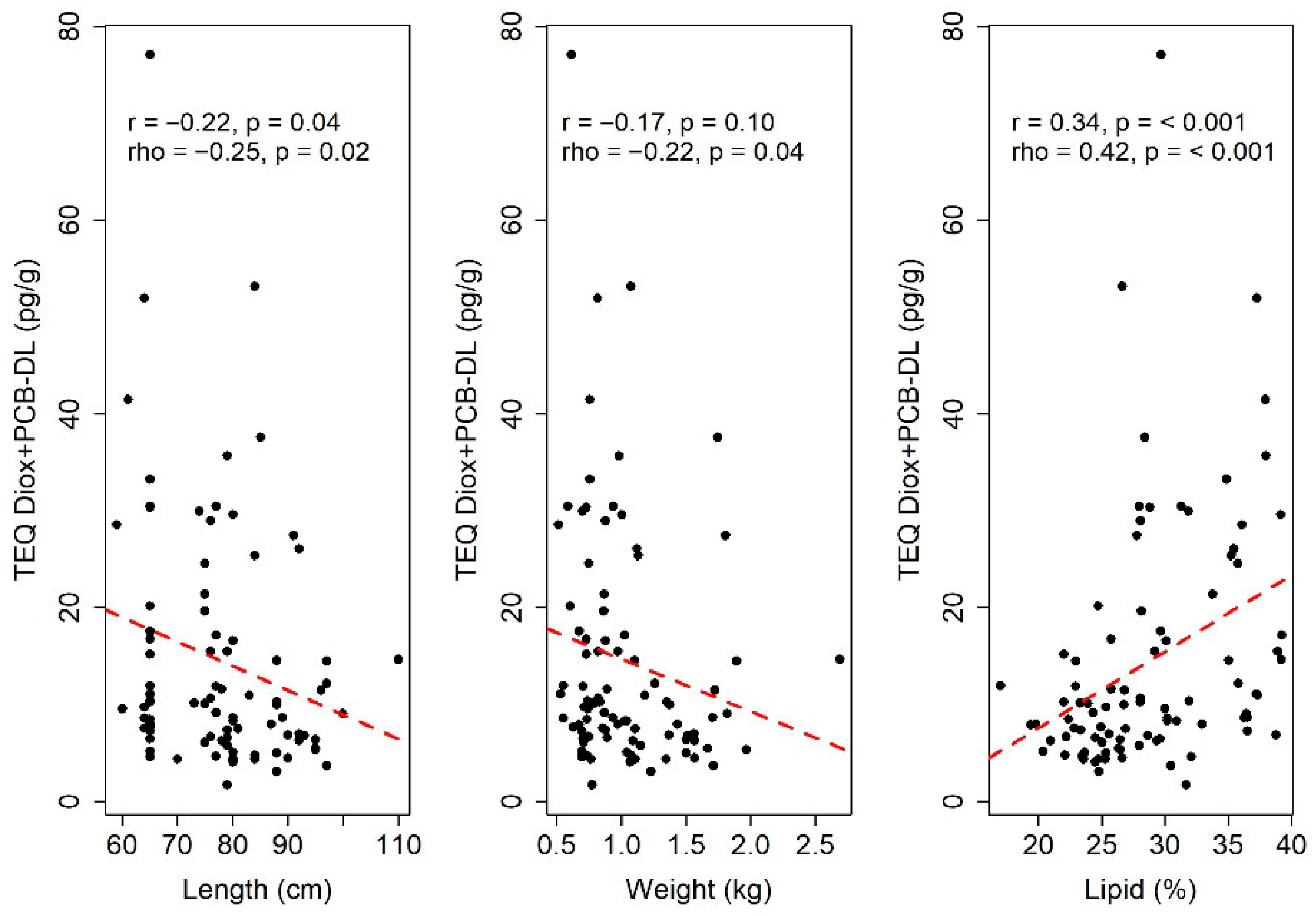
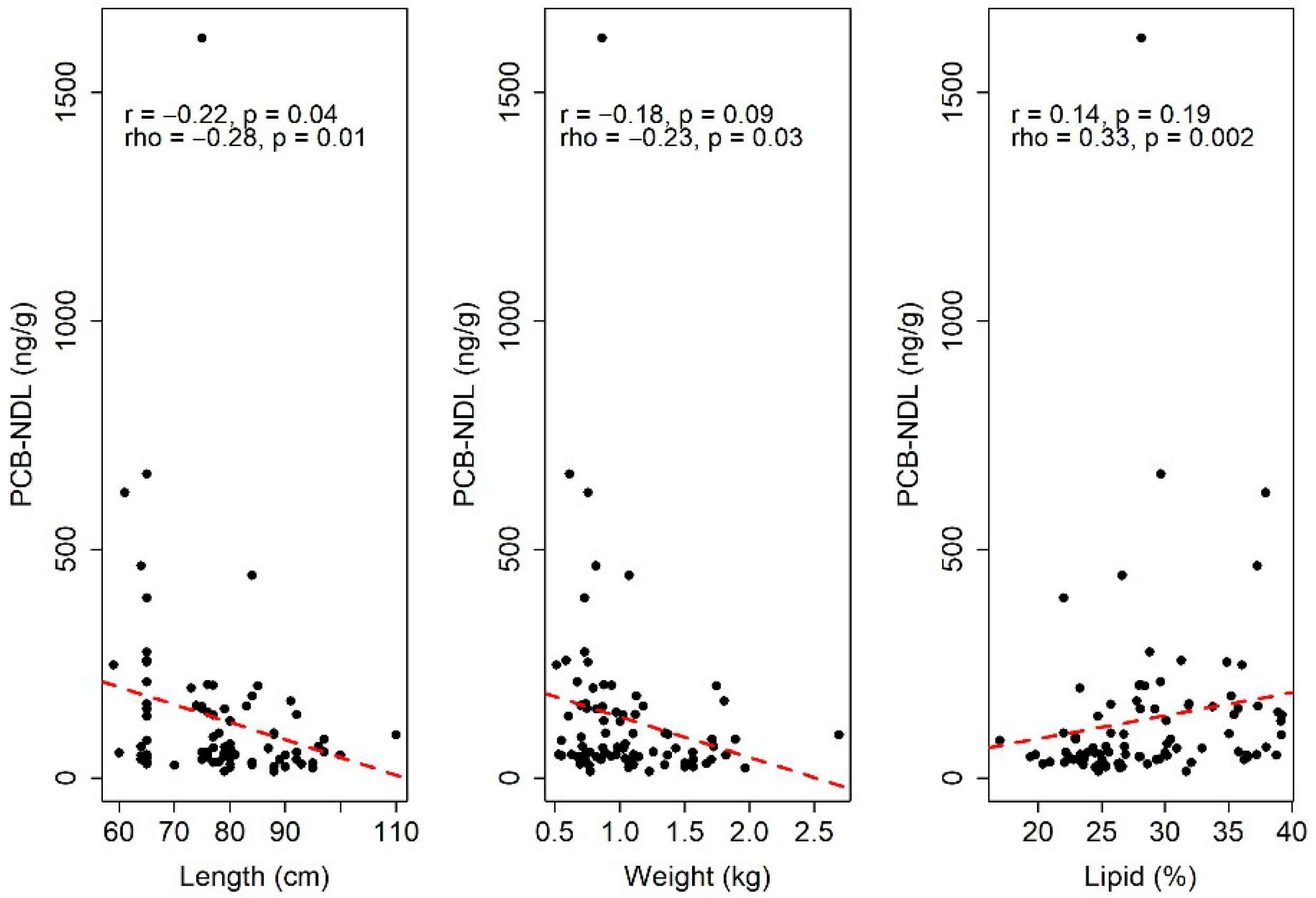
3.2. Statistical Analysis
3.2.1. Quantitative Analysis of the TEQ Diox+PCB-DL and PCB-NDL Compounds
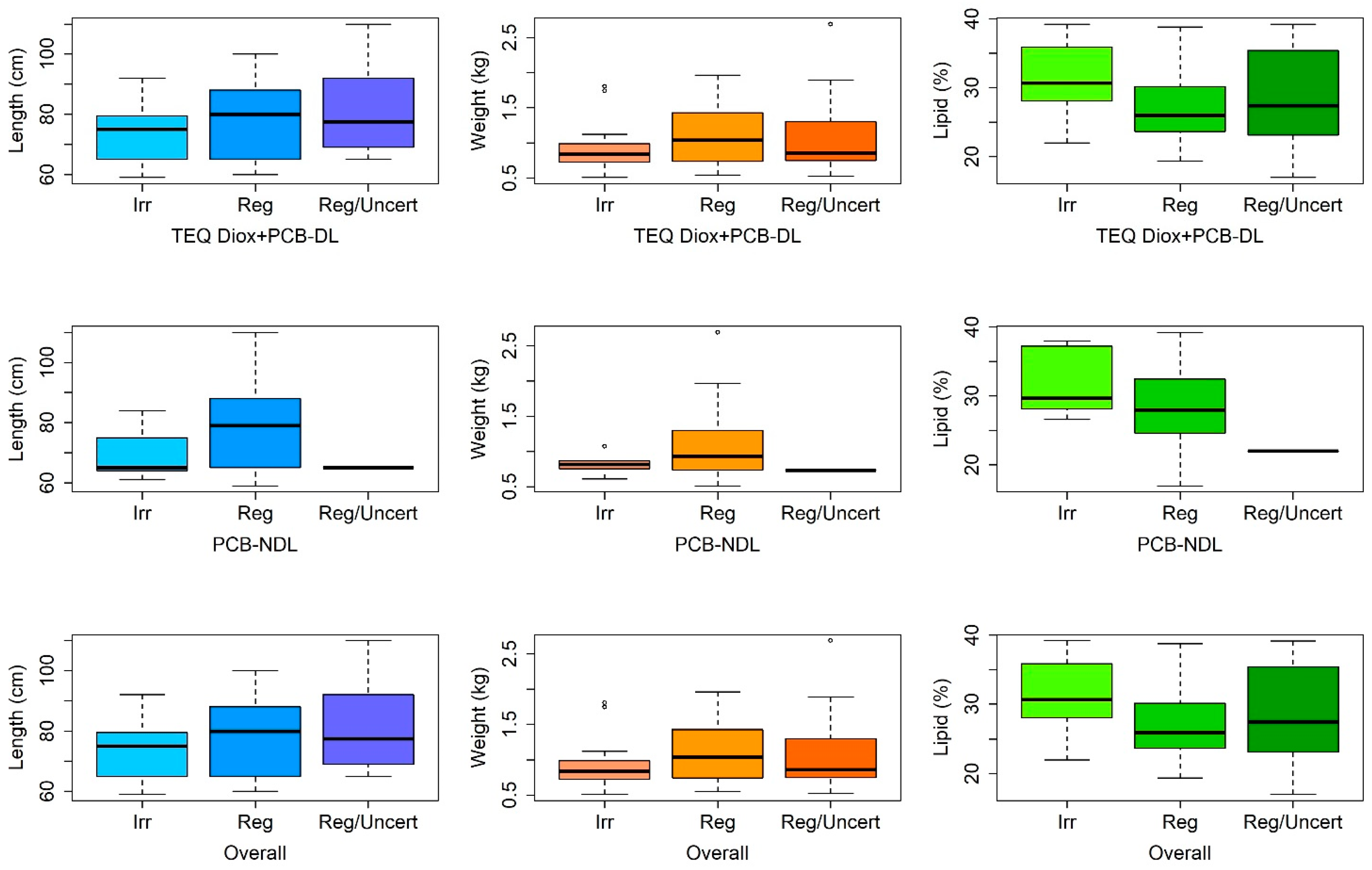
3.2.2. Qualitative Analysis of the Physical Variables According to the Classification of TEQ Diox+PCB-DL, PCB-NDL, and Overall
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Food Safety Authority. Results of the monitoring of non dioxin-like PCBs in food and feed. EFSA J. 2010, 8, 1701. [Google Scholar] [CrossRef]
- European Food Safety Authority. Update of the monitoring of levels of dioxins and PCBs in food and feed. EFSA J. 2012, 10, 2832. [Google Scholar] [CrossRef]
- Blanchet-Letrouvé, I.; Zalouk-Vergnoux, A.; Vénisseau, A.; Couderc, M.; Le Bizec, B.; Elie, P.; Herrenknecht, C.; Mouneyrac, C.; Poirier, L. Dioxin-like, non-dioxin like PCB and PCDD/F contamination in European eel (Anguilla anguilla) from the Loire estuarine continuum: Spatial and biological variabilities. Sci. Total Environ. 2014, 472, 562–571. [Google Scholar] [CrossRef]
- Reddy, A.V.B.; Moniruzzaman, M.; Aminabhavi, T.M. Polychlorinated biphenyls (PCBs) in the environment: Recent updates on sampling, pretreatment, cleanup technologies and their analysis. Chem. Eng. J. 2019, 358, 1186–1207. [Google Scholar] [CrossRef]
- Byer, J.D.; Alaee, M.; Brown, R.S.; Lebeuf, M.; Backus, S.; Keir, M.; Pacepavicius, G.; Casselman, J.; Belpaire, C.; Oliveira, K.; et al. Spatial trends of dioxin-like compounds in Atlantic anguillid eels. Chemosphere 2013, 91, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Gallocchio, F.; Moressa, A.; Pascoli, F.; Vetri, A.; Toffan, A.; Pretto, T.; Arcangeli, G.; Angeletti, R.; Ricci, A. Effect of TiO2 Nanoparticle on Bioaccumulation of ndl-PCBs in Mediterranean Mussels (Mitilus galloprovincialis). Animals 2023, 13, 1208. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; Hardy, A.; Benford, D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, e05327. [Google Scholar] [CrossRef]
- Freese, M.; Sühring, R.; Pohlmann, J.; Wolschke, H.; Magath, V.; Ebinghaus, R.; Hanel, R. A question of origin: Dioxin-like PCBs and their relevance in stock management of European eels. Ecotoxicology 2016, 25, 41–55. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, C.; Belpaire, C. The effects of contaminants in European eel: A review. Ecotoxicology 2010, 19, 239–266. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, C.; Focant, J.-F.; Eppe, G.; De Pauw, E.; Belpaire, C. Reproduction of European eel jeopardised by high levels of dioxins and dioxin-like PCBs? Sci. Total Environ. 2011, 409, 4039–4047. [Google Scholar] [CrossRef]
- Palstra, A.P.; Van Ginneken, V.; Murk, A.J.; Van Den Thillart, G. Are dioxin-like contaminants responsible for the eel (Anguilla anguilla) drama? Naturwissenschaften 2006, 93, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gómez, C.; Fernández, B.; Barcala, E.; García-Aparicio, V.; Jumilla, E.; Gea-Pacheco, Á.; León, V.M. The impact of chemical pollution on the European eel (Anguilla anguilla) from a Mediterranean hypersaline coastal lagoon. Environ. Sci. Pollut. Res. 2023, 30, 80106–80122. [Google Scholar] [CrossRef]
- Chiesa, L.M.; Zanardi, E.; Nobile, M.; Panseri, S.; Ferretti, E.; Ghidini, S.; Foschini, S.; Ianieri, A.; Arioli, F. Food risk characterization from exposure to persistent organic pollutants and metals contaminating eels from an Italian lake. Food Addit. Contam. Part A 2019, 36, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Zucchetta, M.; Maio, G.; Pranovi, F.; Piero, F. Ricostruzione storica delle catture della pesca nel lago di garda historical reconstruction of fishery catches for the lake garda. Ital. J. 2019, 5, 1. [Google Scholar]
- European Commission. European Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Revision, B.; Van den Berg, M.; Birnbaum, L.; Bosveld, A.T.; Brunstrm, B.; Cook, P. Tetra-through Octa-Chlorinated Dioxins and Furans by Isotope Dilution HRGC/HRMS; Environmental Protection Agency, Office of Water Engineering and Analysis Division (4303): Washington, DC, USA, 1994. [Google Scholar]
- United States Environmental Protection Agency (USEPA). Method 1668, Revision A Chlorinated Biphenyl Congeners in Water, Soil, Sediment, Biosolids, and Tissue by HRGC/HRMS; USEPA: Washington, DC, USA, 2003.
- Van den Berg, M.; Birnbaum, L.S.; Denison, M.; De Vito, M.; Farland, W.; Feeley, M.; Fiedler, H.; Hakansson, H.; Hanberg, A.; Haws, L. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci. 2006, 93, 223–241. [Google Scholar] [CrossRef]
- Mukaka, M.M. A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar]
- Hand, D.J.; Till, R.J. A simple generalisation of the area under the ROC curve for multiple class classification problems. Mach. Learn. 2001, 45, 171–186. [Google Scholar] [CrossRef]
- Hosmer, D.W., Jr.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2010. [Google Scholar]
- Ripley, B.; Venables, W. Nnet: Feed-Forward Neural Networks and Multinomial Log-Linear Models 2023. Available online: https://CRAN.R-project.org/package=nnet (accessed on 1 September 2024).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.; Müller, M.; Siegert, S.; Doering, M. pROC: Display and Analyze ROC Curves. R Package Version 01. 2021. Available online: https://cran.r-project.org/web/packages/pROC/pROC.pdf (accessed on 10 August 2025).
- Couderc, M.; Poirier, L.; Zalouk-Vergnoux, A.; Kamari, A.; Blanchet-Letrouvé, I.; Marchand, P.; Vénisseau, A.; Veyrand, B.; Mouneyrac, C.; Le Bizec, B. Occurrence of POPs and other persistent organic contaminants in the European eel (Anguilla anguilla) from the Loire estuary, France. Sci. Total Environ. 2015, 505, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Tapie, N.; Le Menach, K.; Pasquaud, S.; Elie, P.; Devier, M.H.; Budzinski, H. PBDE and PCB contamination of eels from the Gironde estuary: From glass eels to silver eels. Chemosphere 2011, 83, 175–185. [Google Scholar] [CrossRef]
- Jürgens, M.D.; Chaemfa, C.; Hughes, D.; Johnson, A.C.; Jones, K.C. PCB and organochlorine pesticide burden in eels in the lower Thames River (UK). Chemosphere 2015, 118, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Stachel, B.; Christoph, E.; Götz, R.; Herrmann, T.; Krüger, F.; Kühn, T.; Lay, J.; Löffler, J.; Päpke, O.; Reincke, H. Dioxins and dioxin-like PCBs in different fish from the river Elbe and its tributaries, Germany. J. Hazard. Mater. 2007, 148, 199–209. [Google Scholar] [CrossRef] [PubMed]

| Variable | Coefficient | Standard Error | p-Value | |
|---|---|---|---|---|
| TEQ Diox+PCB-DL (pg/g) | Intercept | 7.625 | 7.174 | 0.291 |
| Length (cm) | −0.214 | 0.079 | 0.008 | |
| Lipid (%) | 0.756 | 0.160 | <0.001 | |
| PCB-NDL (ng/g) | Intercept | 229.85 | 96.031 | 0.019 |
| Length (cm) | −3.854 | 1.038 | <0.001 | |
| Lipid (%) | 6.382 | 2.113 | 0.003 |
| TEQ Diox+PCB-DL | ||
| Irregular | Regular | Regular/uncertain |
| 28 | 46 | 16 |
| PCB-NDL | ||
| Irregular | Regular | Regular/uncertain |
| 5 | 84 | 1 |
| Overall | ||
| Irregular | Regular | Regular/uncertain |
| 28 | 46 | 16 |
| Variable | Coefficient | Standard Error | p-Value | Exp (Coefficient) | |
|---|---|---|---|---|---|
| Irregular | Intercept | −1.088 | 2.222 | 0.625 | 0.337 |
| Length (cm) | −0.0658 | 0.026 | 0.012 | 0.936 | |
| Lipid (%) | 0.1932 | 0.053 | <0.001 | 1.21 | |
| Regular/ Uncertain | Intercept | −3.134 | 2.378 | 0.187 | 0.043 |
| Length (cm) | 0.0078 | 0.027 | 0.775 | 1.01 | |
| Lipid (%) | 0.0522 | 0.0589 | 0.376 | 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallocchio, F.; Mancin, M.; Boscolo Anzoletti, A.; Angeletti, R.; Biancotto, G.; Fedrizzi, G.; Gasparini, M.; Angelone, B.; Bontacchio, S.; Di Millo, S.; et al. Monitoring of PCDD/Fs and PCBs in European Eels (Anguilla anguilla) from Lake Garda: A Persistent Environmental Concern. Toxics 2025, 13, 690. https://doi.org/10.3390/toxics13080690
Gallocchio F, Mancin M, Boscolo Anzoletti A, Angeletti R, Biancotto G, Fedrizzi G, Gasparini M, Angelone B, Bontacchio S, Di Millo S, et al. Monitoring of PCDD/Fs and PCBs in European Eels (Anguilla anguilla) from Lake Garda: A Persistent Environmental Concern. Toxics. 2025; 13(8):690. https://doi.org/10.3390/toxics13080690
Chicago/Turabian StyleGallocchio, Federica, Marzia Mancin, Aurora Boscolo Anzoletti, Roberto Angeletti, Giancarlo Biancotto, Giorgio Fedrizzi, Mara Gasparini, Barbara Angelone, Silvana Bontacchio, Sabrina Di Millo, and et al. 2025. "Monitoring of PCDD/Fs and PCBs in European Eels (Anguilla anguilla) from Lake Garda: A Persistent Environmental Concern" Toxics 13, no. 8: 690. https://doi.org/10.3390/toxics13080690
APA StyleGallocchio, F., Mancin, M., Boscolo Anzoletti, A., Angeletti, R., Biancotto, G., Fedrizzi, G., Gasparini, M., Angelone, B., Bontacchio, S., Di Millo, S., Cito, F., Diletti, G., & Arcangeli, G. (2025). Monitoring of PCDD/Fs and PCBs in European Eels (Anguilla anguilla) from Lake Garda: A Persistent Environmental Concern. Toxics, 13(8), 690. https://doi.org/10.3390/toxics13080690










