Risk and Burden of Preterm Birth Associated with Prenatal Exposure to Ambient PM2.5: National Birth Cohort Analysis in the Iranian Population
Abstract
1. Introduction
2. Methods
2.1. Data Collection
2.2. Data Analysis
2.2.1. Associations of PM2.5 Exposure with PTB Outcomes
2.2.2. Assessment of PTB Burden Attributable to PM2.5
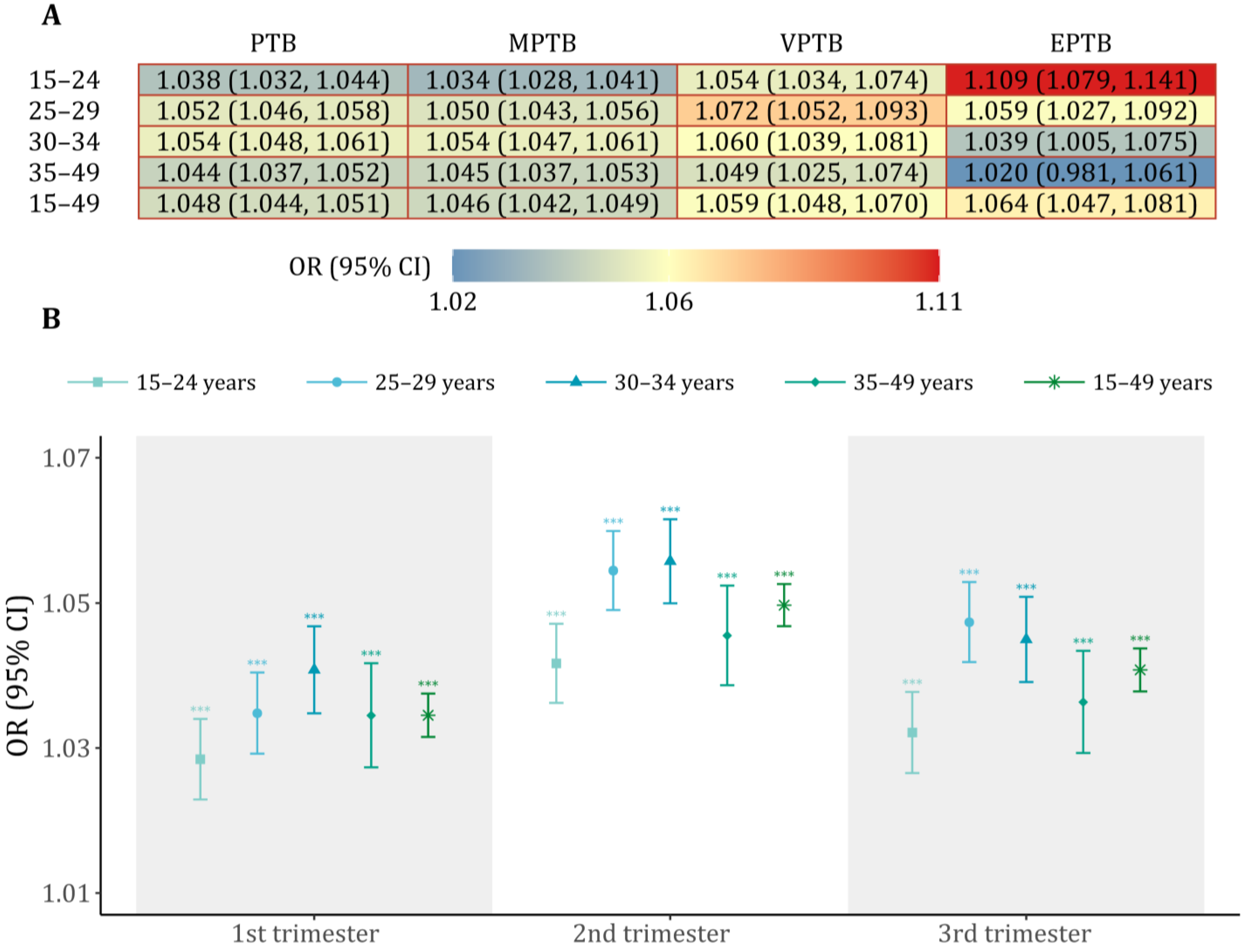
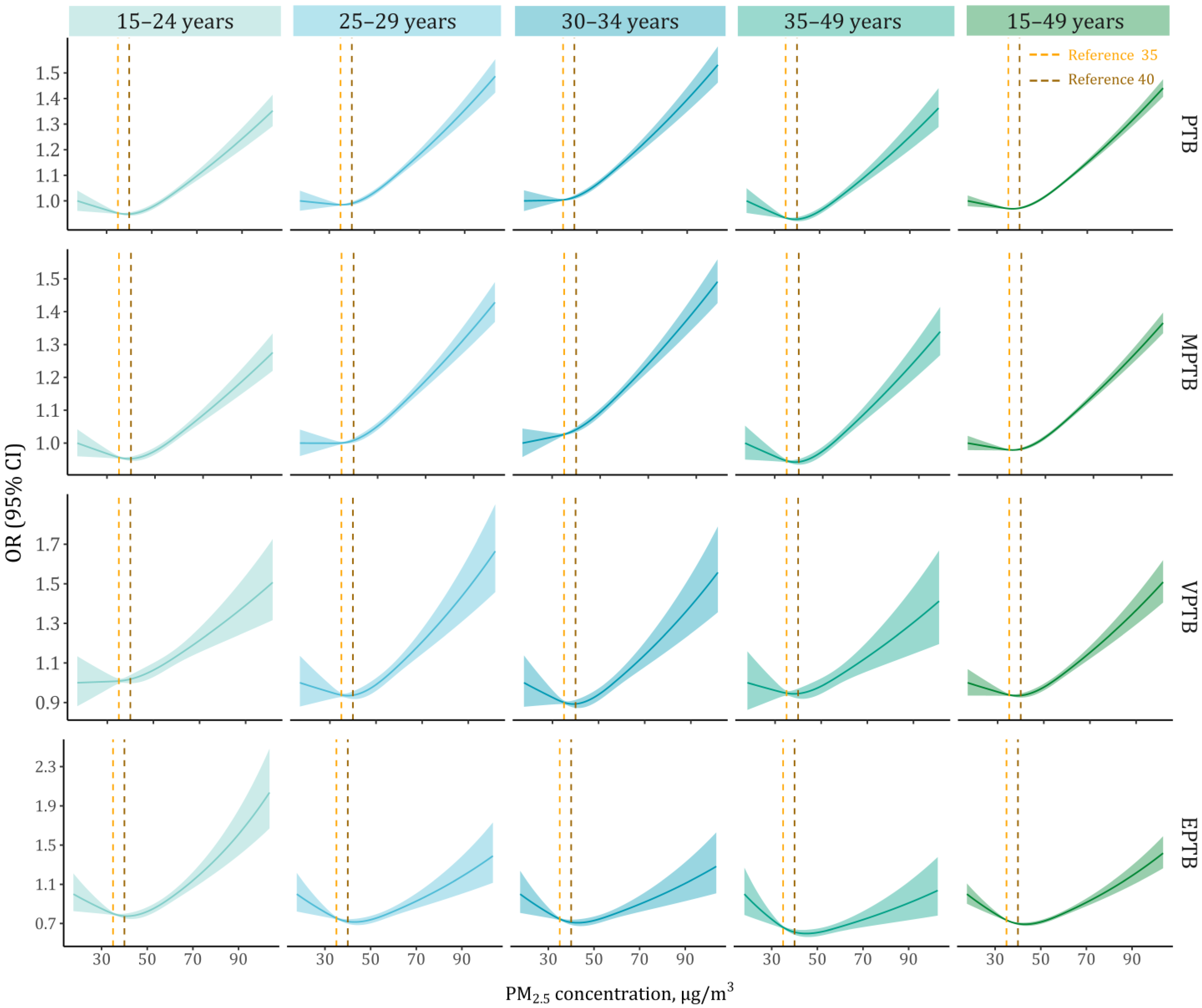
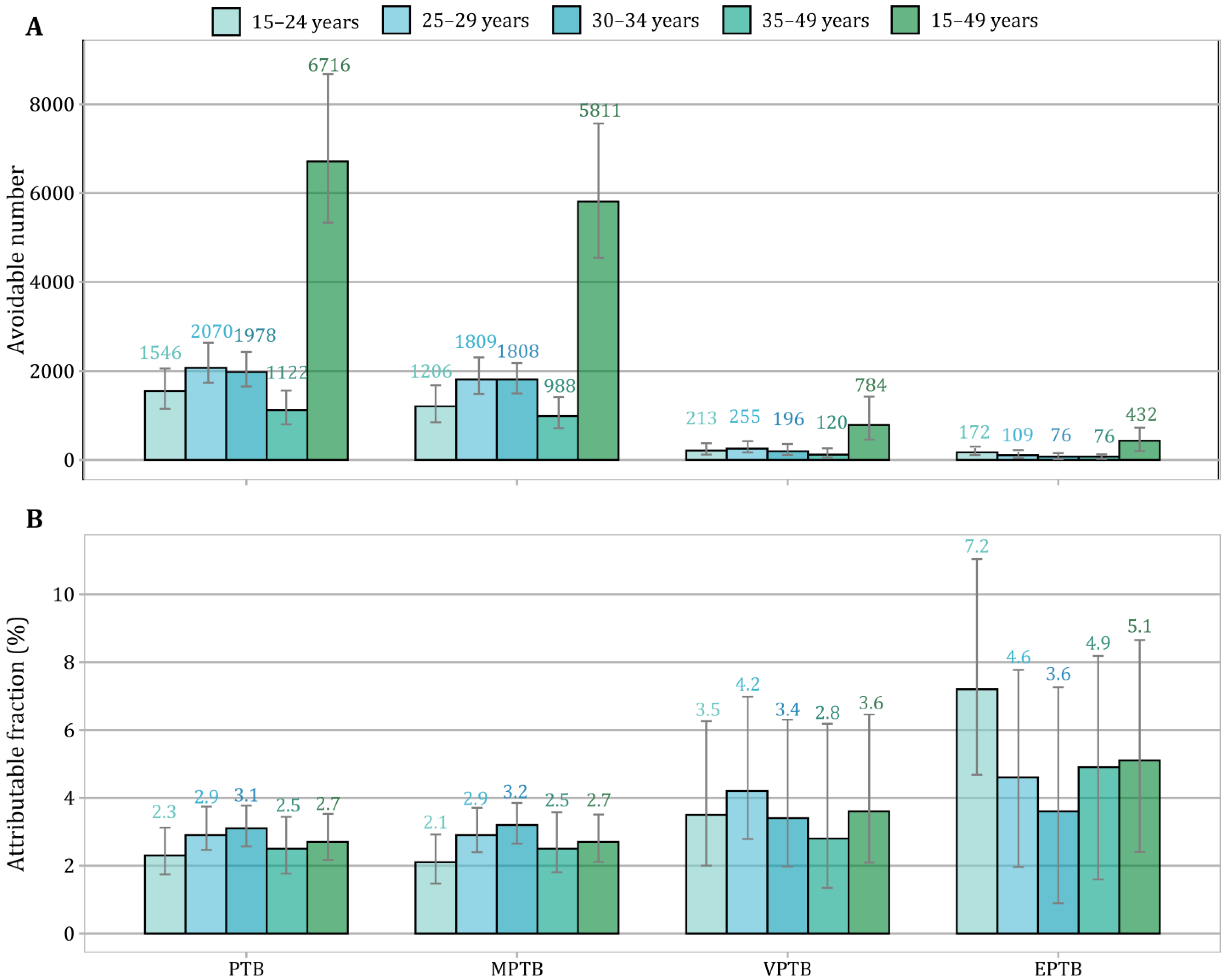
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perin, J.; Mulick, A.; Yeung, D.; Villavicencio, F.; Lopez, G.; Strong, K.L.; Prieto-Merino, D.; Cousens, S.; Black, R.E.; Liu, L. Global, regional, and national causes of under-5 mortality in 2000–19: An updated systematic analysis with implications for the Sustainable Development Goals. Lancet Child Adolesc. Health 2022, 6, 106–115. [Google Scholar] [CrossRef]
- Saigal, S.; Doyle, L.W. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet 2008, 371, 261–269. [Google Scholar] [CrossRef]
- Ohuma, E.O.; Moller, A.B.; Bradley, E.; Chakwera, S.; HussainAlkhateeb, L.; Lewin, A.; Okwaraji, Y.B.; Mahanani, W.R.; Johansson, E.W.; Lavin, T.; et al. National, regional, and global estimates of preterm birth in 2020, with trends from 2010: A systematic analysis. Lancet 2023, 402, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.B.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S.; Jampathong, N.; Kongwattanakul, K.; Laopaiboon, M.; et al. Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2019, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Rentschler, J.; Leonova, N. Global air pollution exposure and poverty. Nat. Commun. 2023, 14, 4432. [Google Scholar] [CrossRef]
- Simoncic, V.; Enaux, C.; Deguen, S.; Kihal-Talantikite, W. Adverse Birth Outcomes Related to NO2 and PM Exposure: European Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 8116. [Google Scholar] [CrossRef]
- Nyadanu, S.D.; Dunne, J.; Tessema, G.A.; Mullins, B.; Kumi-Boateng, B.; Bell, M.L.; Duko, B.; Pereira, G. Prenatal exposure to ambient air pollution and adverse birth outcomes: An umbrella review of 36 systematic reviews and meta-analyses. Environ. Pollut. 2022, 306, 119465. [Google Scholar] [CrossRef]
- Jacobs, M.; Zhang, G.; Chen, S.; Mullins, B.; Bell, M.; Jin, L.; Guo, Y.; Huxley, R.; Pereira, G. The association between ambient air pollution and selected adverse pregnancy outcomes in China: A systematic review. Sci. Total Environ. 2017, 579, 1179–1192. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Guo, Y.; Zhou, H.; Wang, X.; Wang, Q.; Shen, H.; Zhang, Y.; Yan, D.; Li, S.; et al. Folic Acid Supplementation and the Association between Maternal Airborne Particulate Matter Exposure and Preterm Delivery: A National Birth Cohort Study in China. Environ. Health Perspect. 2020, 128, 127010. [Google Scholar] [CrossRef]
- Qiu, Z.; Li, W.; Qiu, Y.; Chen, Z.; Yang, F.; Xu, W.; Gao, Y.; Liu, Z.; Li, Q.; Jiang, M.; et al. Third trimester as the susceptibility window for maternal PM2.5 exposure and preterm birth: A nationwide surveillance-based association study in China. Sci. Total Environ. 2023, 880, 163274. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef]
- Ghosh, R.; Causey, K.; Burkart, K.; Wozniak, S.; Cohen, A.; Brauer, M. Ambient and household PM2.5 pollution and adverse perinatal outcomes: A meta-regression and analysis of attributable global burden for 204 countries and territories. PLoS Med. 2021, 18, e1003718. [Google Scholar] [CrossRef]
- Luo, S.; Wang, Y.; Mayvaneh, F.; Relvas, H.; Baaghideh, M.; Wang, K.; Yuan, Y.; Yin, Z.; Zhang, Y. Surrounding greenness is associated with lower risk and burden of low birth weight in Iran. Nat. Commun. 2023, 14, 7595. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Guo, Y.; Zhou, H.; Wang, X.; Wang, Q.; Shen, H.; Zhang, Y.; Yan, D.; Zhang, Y.; et al. Association of Long-term Exposure to Airborne Particulate Matter of 1 μm or Less with Preterm Birth in China. JAMA Pediatr. 2018, 172, e174872. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, S.; Wu, S.; Yang, Y.; Xu, J.; Zhang, Y.; Wang, Q.; Shen, H.; Zhang, Y.; Yan, D.; et al. Effects of greenness on preterm birth: A national longitudinal study of 3.7 million singleton births. Innovation 2022, 3, 100241. [Google Scholar] [CrossRef] [PubMed]
- Hammer, M.S.; van Donkelaar, A.; Li, C.; Lyapustin, A.; Sayer, A.M.; Hsu, N.C.; Levy, R.C.; Garay, M.J.; Kalashnikova, O.V.; Kahn, R.A.; et al. Global Estimates and Long-Term Trends of Fine Particulate Matter Concentrations (1998–2018). Environ. Sci. Technol. 2020, 54, 7879–7890. [Google Scholar] [CrossRef] [PubMed]
- van Donkelaar, A.; Martin, R.V.; Brauer, M.; Boys, B.L. Use of Satellite Observations for Long-Term Exposure Assessment of Global Concentrations of Fine Particulate Matter. Environ. Health Perspect. 2015, 123, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Yang, Y.; Qian, Z.; Ruan, Z.; Chang, J.; Vaughn, M.G.; Zhao, Q.; Lin, H. Ambient PM2.5 and birth outcomes: Estimating the association and attributable risk using a birth cohort study in nine Chinese cities. Environ. Int. 2019, 126, 329–335. [Google Scholar] [CrossRef]
- Cassidy-Bushrow, A.E.; Burmeister, C.; Lamerato, L.; Lemke, L.D.; Mathieu, M.; O’Leary, B.F.; Sperone, F.G.; Straughen, J.K.; Reiners, J.J. Prenatal airshed pollutants and preterm birth in an observational birth cohort study in Detroit, Michigan, USA. Environ. Res. 2020, 189, 109845. [Google Scholar] [CrossRef]
- Mohammadi, A.; Pishgar, E.; Salari, Z.; Kiani, B. Geospatial analysis of cesarean section in Iran (2016–2020): Exploring clustered patterns and measuring spatial interactions of available health services. BMC Pregnancy Childbirth 2022, 22, 582. [Google Scholar] [CrossRef]
- Tang, Z.; Long, X.; Wang, K.; Berger, K.; Zhang, Y.; Mayvaneh, F. Risk and burden of low birthweight related to maternal PM2.5 exposure in Iran: A national causal inference study. Ecotoxicol. Environ. Saf. 2024, 288, 117414. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, Y.; Mayvaneh, F.; Sun, H.; Zhang, Y.; Hu, C. Maternal ozone exposure lowers infant’s birthweight: A nationwide cohort of over 4 million livebirths in Iran. Ecotoxicol. Environ. Saf. 2024, 283, 116840. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Adolescent Pregnancy. Available online: https://www.who.int/news-room/fact-sheets/detail/adolescent-pregnancy (accessed on 8 May 2025).
- Xue, T.; Tong, M.; Li, J.; Wang, R.; Guan, T.; Li, J.; Li, P.; Liu, H.; Lu, H.; Li, Y.; et al. Estimation of stillbirths attributable to ambient fine particles in 137 countries. Nat. Commun. 2022, 13, 6950. [Google Scholar] [CrossRef] [PubMed]
- American College of Obstetricians and Gynecologists. Pregnancy at Age 35 Years or Older. Available online: https://www.acog.org/clinical/clinical-guidance/obstetric-care-consensus/articles/2022/08/pregnancy-at-age-35-years-or-older (accessed on 8 May 2025).
- Ye, T.; Xu, R.; Yue, X.; Chen, G.; Yu, P.; Coêlho, M.S.Z.S.; Saldiva, P.H.N.; Abramson, M.J.; Guo, Y.; Li, S. Short-term exposure to wildfire-related PM2.5 increases mortality risks and burdens in Brazil. Nat. Commun. 2022, 13, 7651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. All-Cause Mortality Risk and Attributable Deaths Associated with Long-Term Exposure to Ambient PM2.5 in Chinese Adults. Environ. Sci. Technol. 2021, 55, 6116–6127. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, K.F. What’s the Relative Risk? A Method of Correcting the Odds Ratio in Cohort Studies of Common Outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef]
- Nachman, R.M.; Mao, G.; Zhang, X.; Hong, X.; Chen, Z.; Soria, C.S.; He, H.; Wang, G.; Caruso, D.; Pearson, C.; et al. Intrauterine Inflammation and Maternal Exposure to Ambient PM2.5 during Preconception and Specific Periods of Pregnancy: The Boston Birth Cohort. Environ. Health Perspect. 2016, 124, 1608–1615. [Google Scholar] [CrossRef]
- Fussell, J.C.; Jauniaux, E.; Smith, R.B.; Burton, G.J. Ambient air pollution and adverse birth outcomes: A review of underlying mechanisms. BJOG Int. J. Obstet. Gynaecol. 2024, 131, 538–550. [Google Scholar] [CrossRef]
- You, Y.A.; Park, S.; Kwon, E.; Kim, Y.A.; Hur, Y.M.; Lee, G.I.; Kim, S.M.; Song, J.M.; Kim, M.S.; Kim, Y.J.; et al. Maternal PM2.5 exposure is associated with preterm birth and gestational diabetes mellitus, and mitochondrial OXPHOS dysfunction in cord blood. Environ. Sci. Pollut. Res. 2024, 31, 10565–10578. [Google Scholar] [CrossRef]
- Li, Z.; Tang, Y.; Song, X.; Lazar, L.; Li, Z.; Zhao, J. Impact of ambient PM2.5 on adverse birth outcome and potential molecular mechanism. Ecotoxicol. Environ. Saf. 2019, 169, 248–254. [Google Scholar] [CrossRef]
- Sheridan, P.; Ilango, S.; Bruckner, T.A.; Wang, Q.; Basu, R.; Benmarhnia, T. Ambient Fine Particulate Matter and Preterm Birth in California: Identification of Critical Exposure Windows. Am. J. Epidemiol. 2019, 188, 1608–1615. [Google Scholar] [CrossRef]
- Ottone, M.; Broccoli, S.; Parmagnani, F.; Giannini, S.; Scotto, F.; Bonvicini, L.; Luberto, F.; Bacco, D.; Trentini, A.; Poluzzi, V.; et al. Source-related components of fine particulate matter and risk of adverse birth outcomes in Northern Italy. Environ. Res. 2020, 186, 109564. [Google Scholar] [CrossRef] [PubMed]
- Bachwenkizi, J.; Liu, C.; Meng, X.; Zhang, L.; Wang, W.; van Donkelaar, A.; Martin, R.V.; Hammer, M.S.; Chen, R.; Kan, H. Maternal exposure to fine particulate matter and preterm birth and low birth weight in Africa. Environ. Int. 2022, 160, 107053. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiang, Y.; Yang, Y.; Xu, J.; Zhang, Y.; Wang, Q.; Shen, H.; Zhang, Y.; Yan, D.; Peng, Z.; et al. Composition of fine particulate matter and risk of preterm birth: A nationwide birth cohort study in 336 Chinese cities. J. Hazard. Mater. 2022, 425, 127645. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.Y.; Guo, Y.; Zhou, H.; Wang, X.; Wang, Q.; Shen, H.; Zhang, Y.; Yan, D.; Zhang, Y.; et al. Effect of airborne particulate matter of 2.5 μm or less on preterm birth: A national birth cohort study in China. Environ. Int. 2018, 121, 1128–1136. [Google Scholar] [CrossRef]
- Padula, A.M.; Mortimer, K.M.; Tager, I.B.; Hammond, S.K.; Lurmann, F.W.; Yang, W.; Stevenson, D.K.; Shaw, G.M. Traffic-related air pollution and risk of preterm birth in the San Joaquin Valley of California. Ann. Epidemiol. 2014, 24, 888–895. [Google Scholar] [CrossRef]
- Yu, Y.; Lin, H.; Liu, Q.; Ma, Y.; Zhao, L.; Li, W.; Zhou, Y.; Byun, H.-M.; Li, P.; Li, C.; et al. Association of residential greenness, air pollution with adverse birth outcomes: Results from 61,762 mother-neonatal pairs in project ELEFANT (2011–2021). Sci. Total Environ. 2024, 912, 169549. [Google Scholar] [CrossRef]
- Meng, Q.; Liu, J.; Shen, J.; Del Rosario, I.; Lakey, P.S.J.; Shiraiwa, M.; Su, J.; Weichenthal, S.; Zhu, Y.; Oroumiyeh, F.; et al. Fine Particulate Matter Metal Composition, Oxidative Potential, and Adverse Birth Outcomes in Los Angeles. Environ. Health Perspect. 2023, 131, 107012. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Brauer, M.; Bhatnagar, A.; Bhatt, D.L.; Brook, J.R.; Huang, W.; Munzel, T.; Newby, D.; Siegel, J.; Brook, R.D.; et al. Personal-Level Protective Actions Against Particulate Matter Air Pollution Exposure: A Scientific Statement from the American Heart Association. Circulation 2020, 142, e411–e431. [Google Scholar] [CrossRef]
- Liu, A.; Qian, N.; Yu, H.; Chen, R.; Kan, H. Estimation of disease burdens on preterm births and low birth weights attributable to maternal fine particulate matter exposure in Shanghai, China. Sci. Total Environ. 2017, 609, 815–821. [Google Scholar] [CrossRef]
- Liu, X.; Fan, S.; Luo, Y.; Hu, L.; Li, C.; Zhang, Y.; Li, J.; Qiu, H.; Dong, G.; Yang, B. Global, regional, and national burden of preterm birth attributable to ambient and household PM2.5 from 1990 to 2019: Worsening or improving? Sci. Total Environ. 2023, 871, 161975. [Google Scholar] [CrossRef]
- Malley, C.S.; Kuylenstierna, J.C.I.; Vallack, H.W.; Henze, D.K.; Blencowe, H.; Ashmore, M.R. Preterm birth associated with maternal fine particulate matter exposure: A global, regional and national assessment. Environ. Int. 2017, 101, 173–182. [Google Scholar] [CrossRef]
- Trasande, L.; Malecha, P.; Attina, T.M. Particulate Matter Exposure and Preterm Birth: Estimates of U.S. Attributable Burden and Economic Costs. Environ. Health Perspect. 2016, 124, 1913–1918. [Google Scholar] [CrossRef] [PubMed]
- Glass, H.C.; Costarino, A.T.; Stayer, S.A.; Brett, C.M.; Cladis, F.; Davis, P.J. Outcomes for extremely premature infants. Anesth. Analg. 2015, 120, 1337–1351. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Wang, Y.; Zhang, H.; Zhang, Y.; Zhao, J.; Wang, Q.; Shen, H.; Wang, Y.; Xie, X.; Wang, L.; et al. The association between ambient PM2.5 exposure and the risk of preterm birth in China: A retrospective cohort study. Sci. Total Environ. 2018, 633, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Li, C.; Yang, M.; Sun, S.; Zhang, Q.; Cao, J.; Ding, R. Maternal air pollution exposure increases the risk of preterm birth: Evidence from the meta-analysis of cohort studies. Environ. Res. 2021, 202, 111654. [Google Scholar] [CrossRef]
- Wang, Q.; Benmarhnia, T.; Zhang, H.; Knibbs, L.D.; Sheridan, P.; Li, C.; Bao, J.; Ren, M.; Wang, S.; He, Y.; et al. Identifying windows of susceptibility for maternal exposure to ambient air pollution and preterm birth. Environ. Int. 2018, 121, 317–324. [Google Scholar] [CrossRef]
- Cai, J.; Zhao, Y.; Kan, J.; Chen, R.; Martin, R.; van Donkelaar, A.; Ao, J.; Zhang, J.; Kan, H.; Hua, J. Prenatal Exposure to Specific PM2.5 Chemical Constituents and Preterm Birth in China: A Nationwide Cohort Study. Environ. Sci. Technol. 2020, 54, 14494–14501. [Google Scholar] [CrossRef]
- Younger, A.; Alkon, A.; Harknett, K.; Louis, R.J.; Thompson, L.M. Adverse birth outcomes associated with household air pollution from unclean cooking fuels in low- and middle-income countries: A systematic review. Environ. Res. 2022, 204, 112274. [Google Scholar] [CrossRef]

| Characteristics | 15–24 Years (n = 1,112,264) | 25–29 Years (n = 1,207,306) | 30–34 Years (n = 969,491) | 35–49 Years (n = 550,470) | 15–49 Years (n = 3,839,531) |
|---|---|---|---|---|---|
| Fetal information, n (%) | |||||
| PTB | 65,873 (5.9) | 70,550 (5.8) | 64,333 (6.6) | 45,299 (8.2) | 246,055 (6.4) |
| MPTB | 57,449 (5.2) | 62,134 (5.1) | 56,519 (5.8) | 39,522 (7.2) | 215,624 (5.6) |
| VPTB | 6034 (0.5) | 6063 (0.5) | 5718 (0.6) | 4216 (0.8) | 22,031 (0.6) |
| EPTB | 2390 (0.2) | 2353 (0.2) | 2096 (0.2) | 1561 (0.3) | 8400 (0.2) |
| GA (week), mean (SD) | 38.7 (1.6) | 38.6 (1.6) | 38.4 (1.6) | 38.3 (1.7) | 38.5 (1.6) |
| Male sex | 572,789 (51.5) | 622,174 (51.5) | 500,741 (51.6) | 285,058 (51.8) | 1,980,762 (51.6) |
| Season of birth | |||||
| Spring | 244,769 (22.0) | 259,654 (21.5) | 205,911 (21.2) | 119,781 (21.8) | 830,115 (21.6) |
| Summer | 276,764 (24.9) | 303,758 (25.2) | 237,333 (24.5) | 131,831 (23.9) | 949,686 (24.7) |
| Fall | 322,125 (29.0) | 354,198 (29.3) | 288,285 (29.7) | 161,275 (29.3) | 1,125,883 (29.3) |
| Winter | 268,606 (24.1) | 289,696 (24.0) | 237,962 (24.5) | 137,583 (25.0) | 933,847 (24.3) |
| Cesarean section | 425,461 (38.3) | 621,794 (51.5) | 565,792 (58.4) | 328,091 (59.6) | 1,941,138 (50.6) |
| Maternal characteristics, n (%) | |||||
| Education attainment | |||||
| Below high school | 575,172 (51.7) | 514,211 (42.6) | 423,149 (43.6) | 309,704 (56.3) | 1,822,236 (47.5) |
| High school | 394,381 (35.5) | 379,139 (31.4) | 280,765 (29.0) | 121,277 (22.0) | 1,175,562 (30.6) |
| College and above | 142,711 (12.8) | 313,956 (26.0) | 265,577 (27.4) | 119,489 (21.7) | 841,733 (21.9) |
| City residence | 792,323 (71.2) | 947,613 (78.5) | 778,635 (80.3) | 421,242 (76.5) | 2,939,813 (76.6) |
| Iranian nationality | 1,050,770 (94.5) | 1,167,419 (96.7) | 942,983 (97.3) | 534,466 (97.1) | 3,695,638 (96.3) |
| MIP (%), mean (SD) | 43.2 (11.2) | 44.6 (11.1) | 45.1 (11.1) | 44.2 (11.2) | 44.3 (11.2) |
| UR (%), mean (SD) | 10.7 (2.5) | 10.7 (2.5) | 10.7 (2.5) | 10.7 (2.5) | 10.7 (2.5) |
| Diabetes | 11,752 (1.1) | 24,735 (2.0) | 32,116 (3.3) | 27,935 (5.1) | 96,538 (2.5) |
| Hypertension | 10,971 (1.0) | 14,067 (1.2) | 14,746 (1.5) | 13,887 (2.5) | 53,671 (1.4) |
| Primipara | 768,776 (69.1) | 502,512 (41.6) | 239,292 (24.7) | 75,397 (13.7) | 1,585,977 (41.3) |
| History of abortion | 107,761 (9.7) | 185,410 (15.4) | 199,887 (20.6) | 149,625 (27.2) | 642,683 (16.7) |
| PM2.5 (μg/m3), mean (SD) | |||||
| Trimester 1 | 41.3 (15.0) | 40.8 (14.4) | 40.3 (14.2) | 40.5 (14.3) | 40.8 (14.5) |
| Trimester 2 | 41.6 (15.2) | 41.1 (14.8) | 40.7 (14.7) | 41.0 (15.0) | 41.1 (14.9) |
| Trimester 3 | 42.1 (14.8) | 41.7 (14.4) | 41.3 (14.3) | 41.4 (14.4) | 41.7 (14.5) |
| Entire pregnancy | 41.7 (13.0) | 41.2 (12.7) | 40.8 (12.5) | 41.0 (12.7) | 41.2 (12.7) |
| Model | OR (95% CI) | p-Value for Effect Difference |
|---|---|---|
| PTB | ||
| Main analysis | 1.048 (1.044, 1.051) | Reference group |
| Main analysis + MIP | 1.047 (1.044, 1.051) | 0.891 |
| Main analysis + UR | 1.048 (1.044, 1.051) | 0.985 |
| Main analysis + MIP + UR | 1.047 (1.044, 1.050) | 0.838 |
| MPTB | ||
| Main analysis | 1.046 (1.042, 1.049) | Reference group |
| Main analysis + MIP | 1.045 (1.041, 1.048) | 0.680 |
| Main analysis + UR | 1.046 (1.042, 1.049) | 0.963 |
| Main analysis + MIP + UR | 1.044 (1.041, 1.048) | 0.549 |
| VPTB | ||
| Main analysis | 1.059 (1.048, 1.070) | Reference group |
| Main analysis + MIP | 1.066 (1.055, 1.077) | 0.356 |
| Main analysis + UR | 1.060 (1.049, 1.070) | 0.927 |
| Main analysis + MIP + UR | 1.069 (1.058, 1.080) | 0.192 |
| EPTB | ||
| Main analysis | 1.064 (1.047, 1.081) | Reference group |
| Main analysis + MIP | 1.064 (1.047, 1.082) | 0.952 |
| Main analysis + UR | 1.064 (1.047, 1.081) | 0.986 |
| Main analysis + MIP + UR | 1.065 (1.048, 1.082) | 0.918 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, L.; Zhang, Y.; Yuan, Y.; Mayvaneh, F.; Zhang, Y. Risk and Burden of Preterm Birth Associated with Prenatal Exposure to Ambient PM2.5: National Birth Cohort Analysis in the Iranian Population. Toxics 2025, 13, 680. https://doi.org/10.3390/toxics13080680
Tong L, Zhang Y, Yuan Y, Mayvaneh F, Zhang Y. Risk and Burden of Preterm Birth Associated with Prenatal Exposure to Ambient PM2.5: National Birth Cohort Analysis in the Iranian Population. Toxics. 2025; 13(8):680. https://doi.org/10.3390/toxics13080680
Chicago/Turabian StyleTong, Ling, Yalin Zhang, Yang Yuan, Fatemeh Mayvaneh, and Yunquan Zhang. 2025. "Risk and Burden of Preterm Birth Associated with Prenatal Exposure to Ambient PM2.5: National Birth Cohort Analysis in the Iranian Population" Toxics 13, no. 8: 680. https://doi.org/10.3390/toxics13080680
APA StyleTong, L., Zhang, Y., Yuan, Y., Mayvaneh, F., & Zhang, Y. (2025). Risk and Burden of Preterm Birth Associated with Prenatal Exposure to Ambient PM2.5: National Birth Cohort Analysis in the Iranian Population. Toxics, 13(8), 680. https://doi.org/10.3390/toxics13080680







