The TyG Index Mediates Air-Pollution-Associated Chronic Kidney Disease Incidence in HIV/AIDS Patients: A 20-Year Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Biomarker Measurements
2.3. Exposure Assessment
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Description of the Study Sample and Exposure
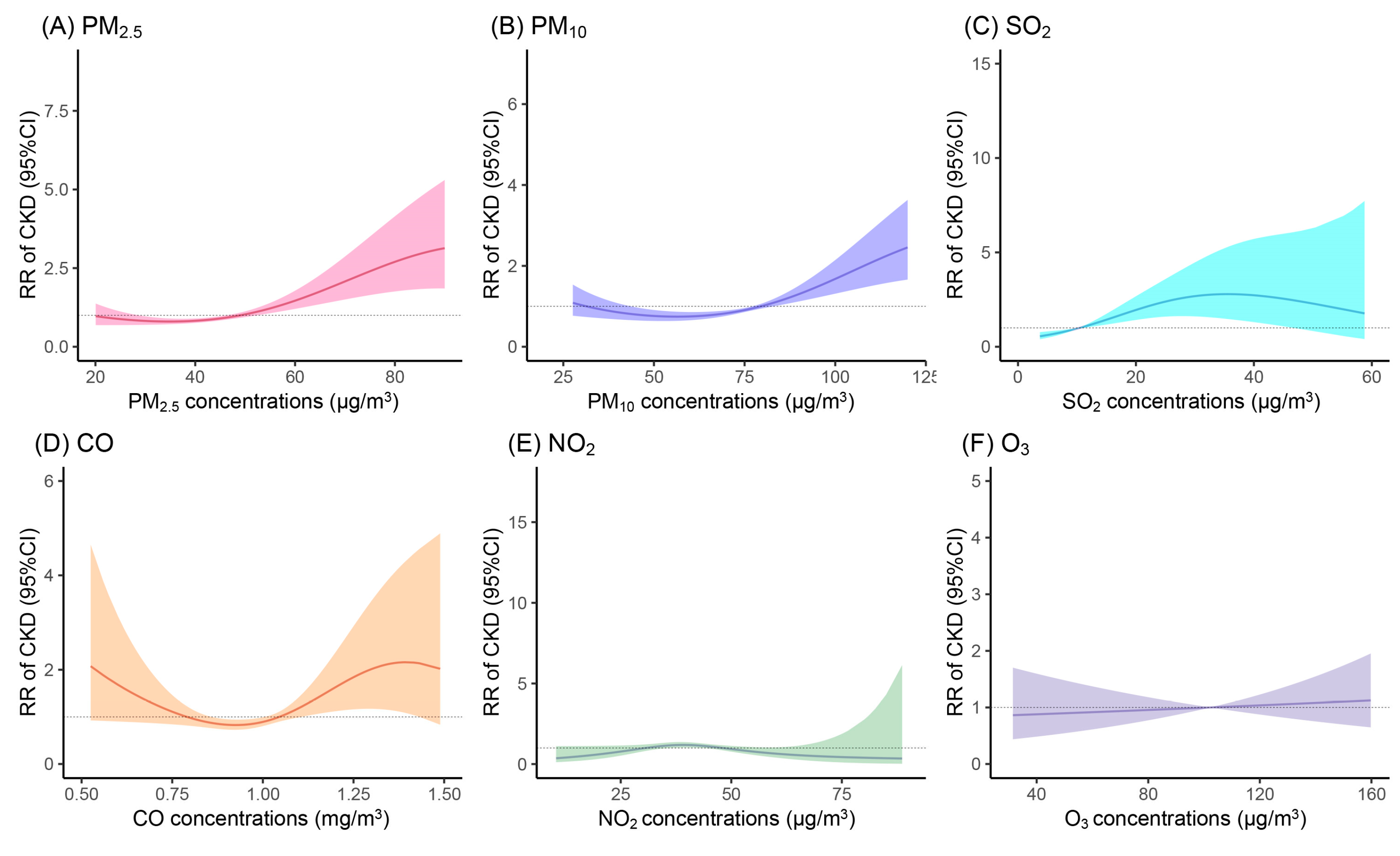
3.2. Associations Between AP Exposure and Incidence of CKD
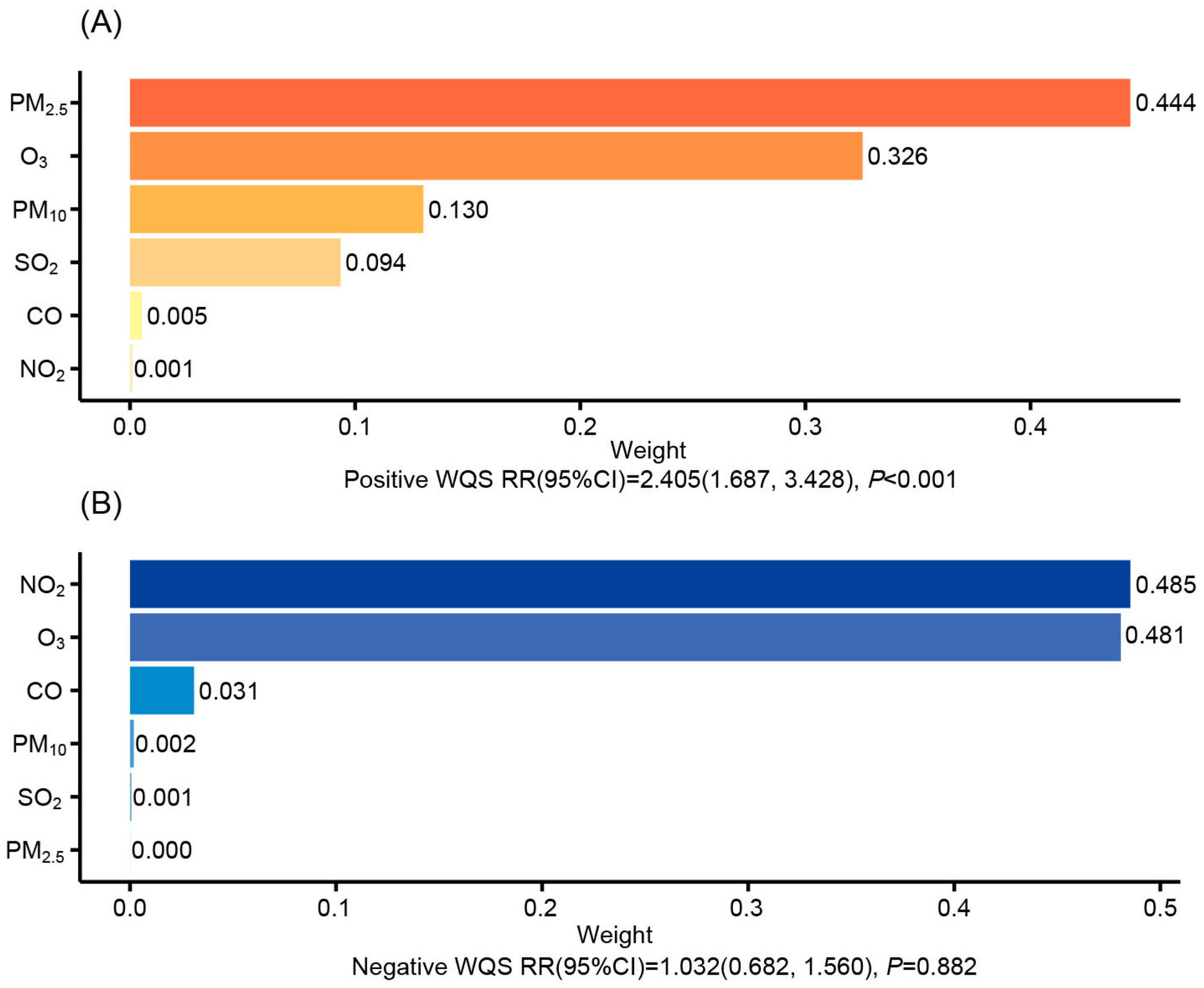
3.3. Mixed AP Exposure on CKD Incidence via Weighted Quantile Sum Modeling
3.4. Mediation Analyses and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PM2.5 | the aerodynamic diameter is less than 2.5 μg/m3; |
| PM10 | the aerodynamic diameter is less than 10 μg/m3 |
| SO2 | sulfur dioxide |
| CO | carbon monoxide |
| NO2 | nitrogen dioxide |
| O3 | ozone |
| CKD | chronic kidney disease |
| AP | air pollutant |
| TyG | triglyceride-glucose |
| CRIMS | Comprehensive Response Information Management System |
| CDC | Center for Disease Control and Prevention |
| TG | triglyceride |
| WQS | weighted quantile sum |
| FBG | fasting blood glucose |
| Scr | serum creatinine |
| eGFR | estimated glomerular filtration rate |
| TDF | tenofovir disoproxil fumarate |
| RRs | risk factors |
| CIs | confidence intervals |
| IQR | interquartile range |
| CKD | Epidemiology Collaboration (CKD-EPI) |
| BMI | body mass index |
References
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. Jama 2019, 322, 1294–1304. [Google Scholar] [CrossRef]
- Jung, J.; Park, J.Y.; Kim, Y.C.; Lee, H.; Kim, E.; Kim, Y.S.; Lee, J.P.; Kim, H. Effects of air pollution on mortality of patients with chronic kidney disease: A large observational cohort study. Sci. Total Environ. 2021, 786, 147471. [Google Scholar] [CrossRef]
- Li, Z.H.; Song, W.Q.; Qiu, C.S.; Li, H.-M.; Tang, X.-L.; Shen, D.; Zhang, P.-D.; Zhang, X.-R.; Ren, J.-J.; Gao, J.; et al. Long-term air pollution exposure, habitual physical activity, and incident chronic kidney disease. Ecotoxicol. Environ. Saf. 2023, 265, 115492. [Google Scholar] [CrossRef] [PubMed]
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [PubMed]
- Kazancioğlu, R. Risk factors for chronic kidney disease: An update. Kidney Int. Suppl. 2013, 3, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Kataria, A.; Trasande, L.; Trachtman, H. The effects of environmental chemicals on renal function. Nat. Rev. Nephrol. 2015, 11, 610–625. [Google Scholar] [CrossRef]
- Blum, M.F.; Surapaneni, A.; Stewart, J.D.; Liao, D.; Yanosky, J.D.; Whitsel, E.A.; Power, M.C.; Grams, M.E. Particulate Matter and Albuminuria, Glomerular Filtration Rate, and Incident CKD. Clin. J. Am. Soc. Nephrol. 2020, 15, 311–319. [Google Scholar] [CrossRef]
- Bowe, B.; Xie, Y.; Li, T.; Yan, Y.; Xian, H.; Al-Al, Z. Associations of ambient coarse particulate matter, nitrogen dioxide, and carbon monoxide with the risk of kidney disease: A cohort study. Lancet Planet. Health 2017, 1, e267–e276. [Google Scholar] [CrossRef]
- Bowe, B.; Artimovich, E.; Xie, Y.; Yan, Y.; Cai, M.; Al-Aly, Z. The global and national burden of chronic kidney disease attributable to ambient fine particulate matter air pollution: A modelling study. BMJ Glob. Health 2020, 5, e002063. [Google Scholar] [CrossRef]
- Lin, Y.T.; Lo, Y.C.; Chiang, H.Y.; Jung, C.-R.; Wang, C.-M.; Chan, T.-C.; Kuo, C.-C.; Hwang, B.-F. Particulate Air Pollution and Progression to Kidney Failure with Replacement Therapy: An Advanced CKD Registry-Based Cohort Study in Taiwan. Am. J. Kidney Dis. 2020, 76, 645–657.e1. [Google Scholar] [CrossRef]
- Li, G.; Huang, J.; Wang, J.; Zhao, M.; Liu, Y.; Guo, X.; Wu, S.; Zhang, L. Long-Term Exposure to Ambient PM2.5 and Increased Risk of CKD Prevalence in China. J. Am. Soc. Nephrol. 2021, 32, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Wang, X.; Xie, N.; Yan, H.; Ma, H.; Liu, M.; Kong, W.; Zhu, Z.; Bai, W.; Xiang, H. Short-term associations of PM2.5 and PM2.5 constituents with immune biomarkers: A panel study in people living with HIV/AIDS. Environ. Pollut. 2023, 317, 120743. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, Q.; Liang, W.; Han, A.; Xie, N.; Xiang, H.; Wang, X. Short-Term Exposure to PM2.5 and O3 Impairs Liver Function in HIV/AIDS Patients: Evidence from a Repeated Measurements Study. Toxics 2023, 11, 729. [Google Scholar] [CrossRef]
- Ma, H.; Liang, W.; Han, A.; Zhang, Q.; Gong, S.; Bai, Y.; Gao, D.; Xiang, H.; Wang, X. Ambient particulate matter and renal function decline in people with HIV/AIDS. Aids 2024, 38, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Q.; Yan, X. Association between triglyceride-glucose index and hypertension: A cohort study based on the China Health and Nutrition Survey (2009–2015). BMC Cardiovasc. Disord. 2024, 24, 168. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, K.; Hu, X.; Pei, J. The impact of sex-related disparities on the association between triglyceride-glucose index and renal function decline in patients with type 2 diabetes: Insights from the ACCORD trial. Diabetes Res. Clin. Pr. 2025, 224, 112163. [Google Scholar] [CrossRef]
- Lu, Y.; Qiu, W.; Liao, R.; Cao, W.; Huang, F.; Wang, X.; Li, M.; Li, Y. Subacute PM2.5 Exposure Induces Hepatic Insulin Resistance Through Inflammation and Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 812. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.; Guo, J.; Sun, L.; Huang, W.; Xue, W.; Fan, T.; Cribb, M. Satellite-Derived 1-km-Resolution PM1 Concentrations from 2014 to 2018 across China. Environ. Sci. Technol. 2019, 53, 13265–13274. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.; Xue, W.; Sun, L.; Fan, T.; Liu, L.; Su, T.; Cribb, M. The ChinaHighPM10 dataset: Generation, validation, and spatiotemporal variations from 2015 to 2019 across China. Environ. Int. 2021, 146, 106290. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.Q.; Lyapustin, A.; Sun, L.; Peng, Y.; Xue, W.; Su, T.; Cribb, M. Reconstructing 1-km-resolution high-quality PM2.5 data records from 2000 to 2018 in China: Spatiotemporal variations and policy implications. Remote Sens. Environ. 2021, 252, 112136. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.Q.; Cribb, M.; Huang, W.; Xue, W.; Sun, L.; Guo, J.; Peng, Y.; Li, J.; Lyapustin, A.; et al. Improved 1 km resolution PM2.5 estimates across China using enhanced space-time extremely randomized trees. Atmos. Chem. Phys. 2020, 20, 3273–3289. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.Q.; Li, K.; Dickerson, R.R.; Pinker, R.T.; Wang, J.; Liu, X.; Sun, L.; Xue, W.; Cribb, M. Full-coverage mapping and spatiotemporal variations of ground-level ozone (O3) pollution from 2013 to 2020 across China. Remote Sens. Environ. 2022, 270, 112775. [Google Scholar] [CrossRef]
- Wei, J.; Li, Z.Q.; Wang, J.; Li, C.; Gupta, P.; Cribb, M. Ground-level gaseous pollutants (NO2, SO2, and CO) in China: Daily seamless mapping and spatiotemporal variations. Atmos. Chem. Phys. 2023, 23, 1511–1532. [Google Scholar] [CrossRef]
- Oh, J.; Ye, S.; Kang, D.H.; Ha, E. Association between exposure to fine particulate matter and kidney function: Results from the Korea National Health and Nutrition Examination Survey. Environ. Res. 2022, 212, 113080. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.W.; Li, Y.L.; Li, S.X.; Yang, Y.-P.; Li, F.; Li, Y.; Wang, J.; Deng, P.-Z.; Wu, J.-J.; Wang, W.; et al. Association of Long-term Ambient Fine Particulate Matter (PM2.5) and Incident CKD: A Prospective Cohort Study in China. Am. J. Kidney Dis. 2022, 80, 638–647.e1. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jia, L.; Lin, Y.; Zhang, C.; Sun, X.; Jiang, L.; Yao, X.; Wang, N.; Deng, H.; Wang, S.; et al. Association of air pollution and risk of chronic kidney disease: A systematic review and meta-analysis. J. Biochem. Mol. Toxicol. 2024, 38, e23610. [Google Scholar] [CrossRef]
- Yang, Y.R.; Chen, Y.M.; Chen, S.Y.; Chan, C.C. Associations between Long-Term Particulate Matter Exposure and Adult Renal Function in the Taipei Metropolis. Environ. Health Perspect. 2017, 125, 602–607. [Google Scholar] [CrossRef]
- Lin, H.C.; Hung, P.H.; Hsieh, Y.Y.; Lai, T.-J.; Hsu, H.-T.; Chung, M.-C.; Chung, C.-J. Long-term exposure to air pollutants and increased risk of chronic kidney disease in a community-based population using a fuzzy logic inference model. Clin. Kidney J. 2022, 15, 1872–1880. [Google Scholar] [CrossRef]
- Liang, K.H.; Colombijn, J.M.T.; Verhaar, M.C.; Ghannoum, M.; Timmermans, E.J.; Vernooij, R.W.M. The general external exposome and the development or progression of chronic kidney disease: A systematic review and meta-analyses. Environ. Pollut. 2024, 358, 124509. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Nguyen, L.T.; Feng, M.; Wang, B.; Xu, B.; Yarak, R.A.; Chan, Y.L.; Viswanathan, S.; Komala, M.G.; Pollock, C.A.; et al. Cross-Generational Impact of Maternal Exposure to Low Level of PM2.5 on Kidney Health. Am. J. Nephrol. 2025, 56, 222–235. [Google Scholar] [CrossRef]
- Joshi, K.S.; Jadhao, V.F.; Gujarathi, R.; Churiwala, W.; Natu, A.A. Human Immunodeficiency Virus-positive Patients on Highly Active Antiretroviral Therapy Continue to Have a Decline in Renal Function Irrespective of Tenofovir Usage. J. Glob. Infect. Dis. 2024, 16, 111–116. [Google Scholar] [CrossRef]
- Ribble, A.; Hellmann, J.; Conklin, D.J.; Bhatnagar, A.; Haberzettl, P. Fine particulate matter (PM2.5)-induced pulmonary oxidative stress contributes to increases in glucose intolerance and insulin resistance in a mouse model of circadian dyssynchrony. Sci. Total Environ. 2023, 877, 162934. [Google Scholar] [CrossRef]
- Zhao, L.; Fang, J.; Tang, S.; Deng, F.; Liu, X.; Shen, Y.; Liu, Y.; Kong, F.; Du, Y.; Cui, L.; et al. PM2.5 and Serum Metabolome and Insulin Resistance, Potential Mediation by the Gut Microbiome: A Population-Based Panel Study of Older Adults in China. Environ. Health Perspect. 2022, 130, 27007. [Google Scholar] [CrossRef]
- Ren, X.; Jiang, M.; Han, L.; Zheng, X. Association between triglyceride-glucose index and chronic kidney disease: A cohort study and meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2023, 33, 1121–1128. [Google Scholar] [CrossRef]
- Low, S.; Pek, S.; Moh, A.; Ang, K.; Khoo, J.; Shao, Y.-M.; Tang, W.E.; Lim, Z.; Subramaniam, T.; Sum, C.F.; et al. Triglyceride-glucose index is prospectively associated with chronic kidney disease progression in Type 2 diabetes—Mediation by pigment epithelium-derived factor. Diabetes Vasc. Dis. Res. 2022, 19, 14791641221113784. [Google Scholar] [CrossRef]
- Brook, R.D.; Rajagopalan, S.; Pope, C.A., 3rd; Brook, J.R.; Bhatnagar, A.; Diez-Roux, A.V.; Holguin, F.; Hong, Y.; Luepker, R.V.; Mittleman, M.A.; et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 2010, 121, 2331–2378. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Nie, S.; Ding, H.; Hou, F.F. Environmental pollution and kidney diseases. Nat. Rev. Nephrol. 2018, 14, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Chenxu, G.; Minxuan, X.; Yuting, Q.; Tingting, G.; Jinxiao, L.; Mingxing, W.; Sujun, W.; Yongjie, M.; Deshuai, L.; Qiang, L.; et al. iRhom2 loss alleviates renal injury in long-term PM2.5-exposed mice by suppression of inflammation and oxidative stress. Redox Biol. 2018, 19, 147–157. [Google Scholar] [CrossRef]
- Decrion, A.Z.; Dichamp, I.; Varin, A.; Herbein, G. HIV and inflammation. Curr. HIV Res. 2005, 3, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.E.; Baker, J.V. Assessing inflammation and its role in comorbidities among persons living with HIV. Curr. Opin. Infect. Dis. 2019, 32, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, H.; Zhang, Z.; Fu, Y.; Han, X.; Zhang, Y.; Xu, J.; Ding, H.; Cui, H.; Dong, T.; et al. Elevated CD54 Expression Renders CD4+ T Cells Susceptible to Natural Killer Cell-Mediated Killing. J. Infect. Dis. 2019, 220, 1892–1903. [Google Scholar] [CrossRef] [PubMed]
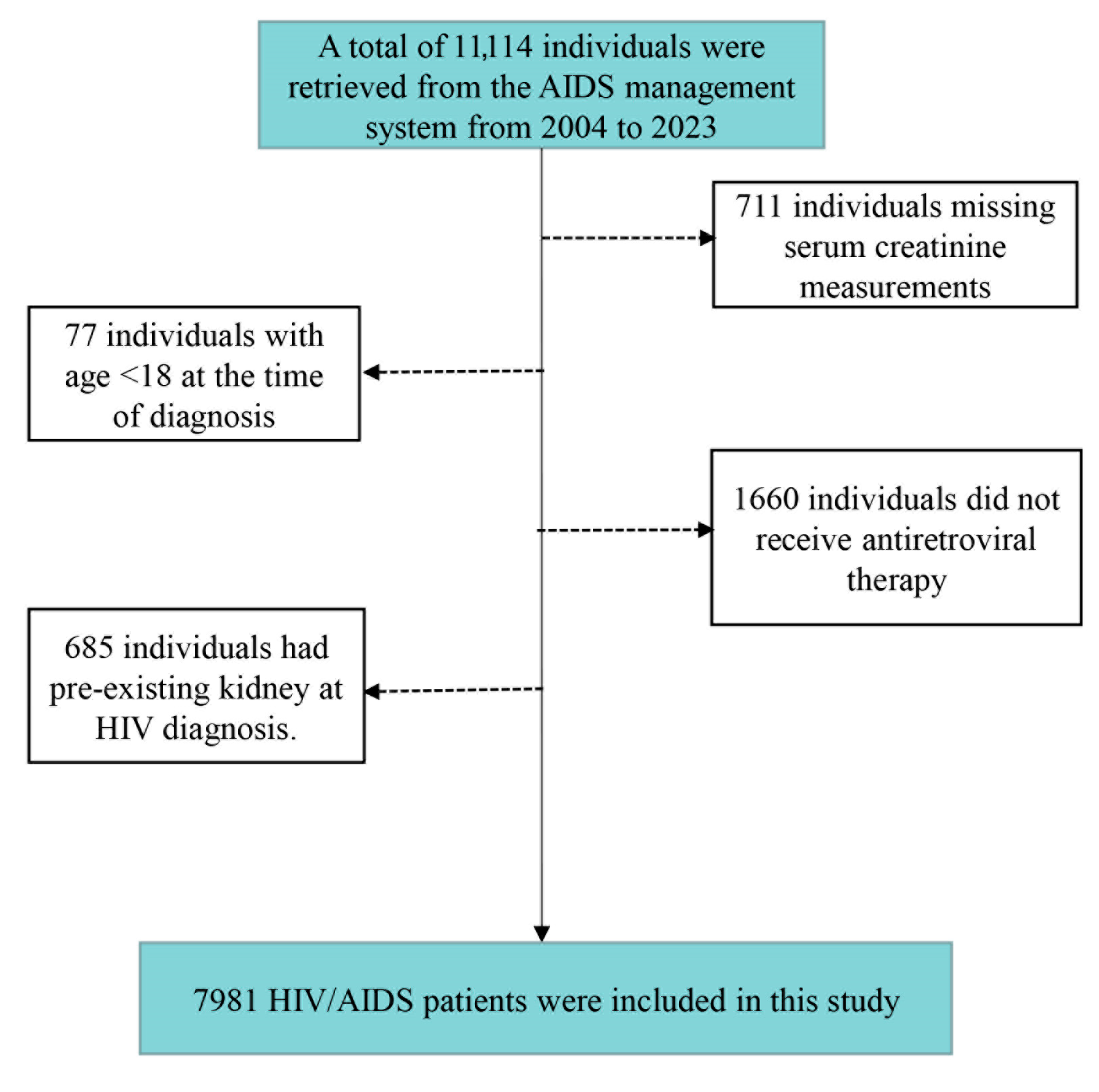
| Variables | Total (N = 7981) |
|---|---|
| Age, years, (Mean ± SD) | 38.7 ± 14.6 |
| BMI, kg/m2 (Mean ± SD) | 22.0 ± 3.81 |
| Sex, n (%) | |
| Male | 7152 (89.6) |
| Female | 829 (10.4) |
| Infection status | |
| HIV | 5033 (63.1) |
| AIDS | 2948 (36.9) |
| Education level, n (%) | |
| Junior secondary education | 2341 (29.3) |
| Senior secondary education | 2078 (26.0) |
| Higher education | 3562 (44.6) |
| Work types, n (%) | |
| Outdoor worker | 3358 (42.1) |
| Indoor worker | 4042 (50.6) |
| Unknown | 581 (7.3) |
| Marital status, n (%) | |
| Unmarried | 4165 (52.2) |
| Married | 2219 (27.8) |
| Divorced | 1597 (20.0) |
| Smoking status, n (%) | |
| Never | 5486 (68.7) |
| Former | 566 (7.1) |
| Current | 1929 (24.2) |
| Alcohol consumption, n (%) | |
| Never | 5670 (71.0) |
| Former | 549 (6.9) |
| Current | 1762 (22.1) |
| Use of TDF, n (%) | |
| No | 3116 (39.0) |
| Yes | 4865 (61.0) |
| Serum creatinine, μmol/L (Mean ± SD) | 71.2 ± 14.0 |
| Cholesterol, mmol/L (Mean ± SD) | 4.04 ± 0.881 |
| Triglyceride, mmol/L (Mean ± SD) | 1.82 ± 2.00 |
| Fasting blood glucose, mmol/L (Mean ± SD) | 5.74 ± 1.41 |
| Outcomes | Pollutants | Model 1 a | Model 2 b |
|---|---|---|---|
| RR of CKD (95%CI) | PM2.5 | 1.212 (1.078, 1.363) | 1.165 (1.030, 1.317) |
| PM10 | 1.244 (1.069, 1.448) | 1.189 (1.016, 1.390) | |
| SO2 | 1.128 (1.074, 1.185) | 1.097 (1.039, 1.159) | |
| CO | 1.090 (0.916, 1.297) | 1.040 (0.876, 1.234) | |
| NO2 | 0.975 (0.811, 1.172) | 0.950 (0.788, 1.146) | |
| O3 | 1.024 (0.886, 1.184) | 1.035 (0.889, 1.205) |
| Exposure | Direct Effect (95% CI) | Indirect Effect (95% CI) | Proportion Mediated (95% CI) |
|---|---|---|---|
| PM2.5 | 1.0021 (1.0008, 1.0028) | 1.0003 (1.0000, 1.0005) | 10.21% (1.70%, 32.06%) |
| PM10 | 1.0020 (1.0008, 1.0027) | 1.0002 (1.000, 1.0005) | 9.16% (1.54%, 30.94%) |
| SO2 | 1.0018 (1.0010, 1.0024) | 1.0001 (1.0000, 1.0002) | 5.14% (0.75%, 13.93%) |
| CO | 0.9996 (0.9942, 1.0015) | 1.0008 (1.0001, 1.0019) | 32.93% (−574.65%, 519.90%) |
| NO2 | 0.9974 (0.9888, 1.0021) | 1.0008 (1.0002, 1.0016) | −17.41% (−383.13%, 433.09%) |
| O3 | 1.0005 (0.9960, 1.0019) | 0.9996 (0.9990, 0.9999) | −14.6% (−296.35%, 310.96%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Zhao, X.; Ye, L.; Hu, C.; Xie, Z.; Ma, J.; Wang, X.; Liang, W. The TyG Index Mediates Air-Pollution-Associated Chronic Kidney Disease Incidence in HIV/AIDS Patients: A 20-Year Cohort Study. Toxics 2025, 13, 669. https://doi.org/10.3390/toxics13080669
Liu X, Zhao X, Ye L, Hu C, Xie Z, Ma J, Wang X, Liang W. The TyG Index Mediates Air-Pollution-Associated Chronic Kidney Disease Incidence in HIV/AIDS Patients: A 20-Year Cohort Study. Toxics. 2025; 13(8):669. https://doi.org/10.3390/toxics13080669
Chicago/Turabian StyleLiu, Xiaoxia, Xiuli Zhao, Lu Ye, Chengfeng Hu, Zhihao Xie, Jianan Ma, Xia Wang, and Wei Liang. 2025. "The TyG Index Mediates Air-Pollution-Associated Chronic Kidney Disease Incidence in HIV/AIDS Patients: A 20-Year Cohort Study" Toxics 13, no. 8: 669. https://doi.org/10.3390/toxics13080669
APA StyleLiu, X., Zhao, X., Ye, L., Hu, C., Xie, Z., Ma, J., Wang, X., & Liang, W. (2025). The TyG Index Mediates Air-Pollution-Associated Chronic Kidney Disease Incidence in HIV/AIDS Patients: A 20-Year Cohort Study. Toxics, 13(8), 669. https://doi.org/10.3390/toxics13080669






