Cadmium Accumulation and Regulation in the Freshwater Mussel Anodonta woodiana
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Metals
2.2. Stable Isotope Cd Spiking in Water and Diet
2.3. Simultaneous Exposure to Waterborne 112Cd and Dietary 113Cd
2.4. Transcriptome Analysis
2.5. Heavy Metal Analysis
2.6. Statistical Analysis
3. Results
3.1. Survival and Growth of Mussels
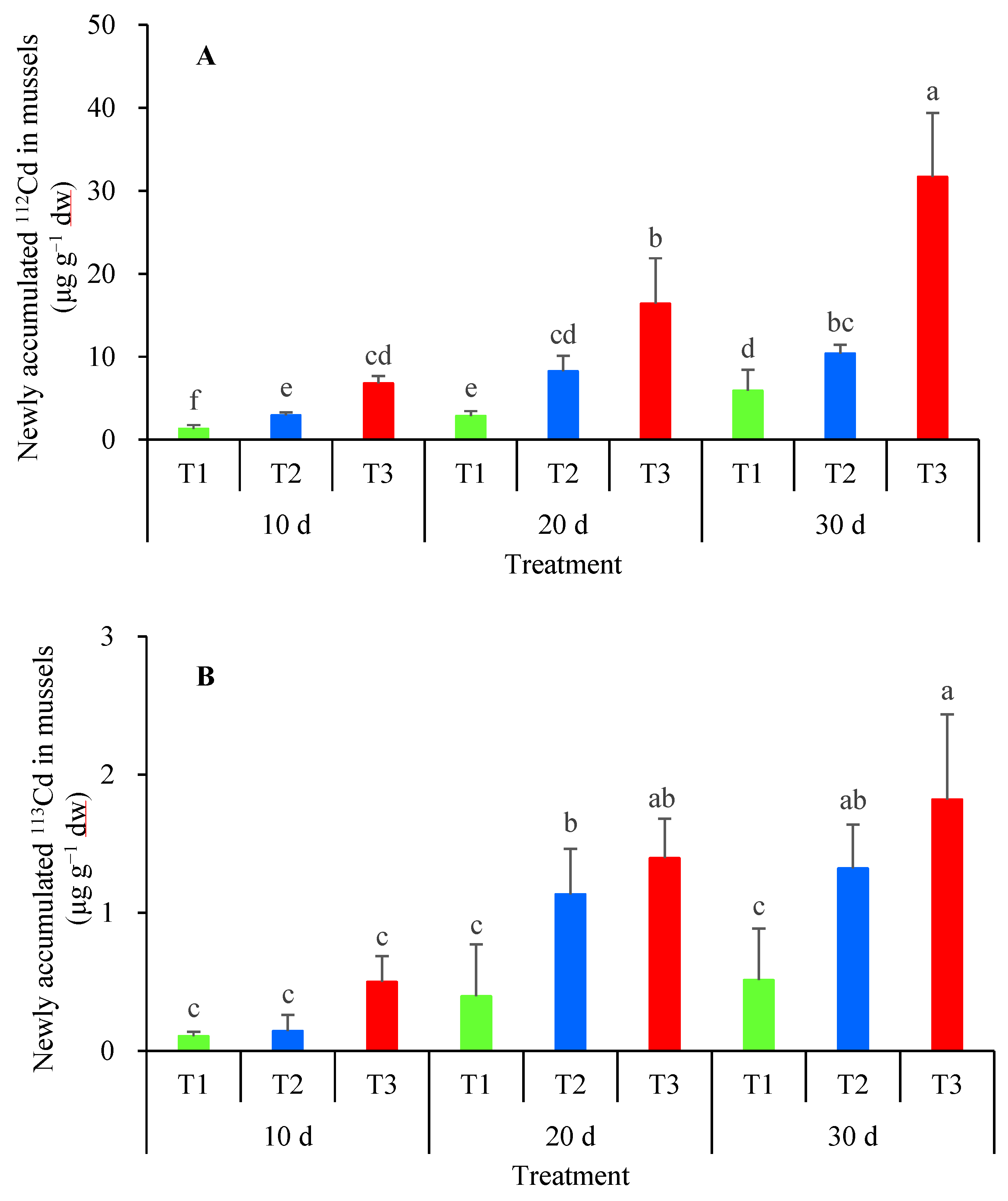
3.2. Cadmium Accumulation in Mussels from Waterborne and Dietary Exposure
3.3. Transcriptomic Response of Mussels to Cd Accumulation
4. Discussion
4.1. Effect of Cd Exposure on A. woodiana
4.2. Accumulation Characteristics of Waterborne and Dietary Cd in A. woodiana
4.3. Molecular Mechanisms of Cd Accumulation Regulation in A. woodiana
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Johnson, A.C.; Jin, X.; Nakada, N.; Sumpter, J.P. Learning from the past and considering the future of chemicals in the environment. Science 2020, 367, 384–387. [Google Scholar] [CrossRef]
- Xu, W.; Wang, S.; Ruan, W.; Hao, M.; Jiang, K.; Guo, H.; Geng, A.; Man, M.; Hu, Z.; Liu, Y.; et al. Cadmium exposure and health outcomes: An umbrella review of meta-analyses. Environ. Res. 2025, 276, 121547. [Google Scholar] [CrossRef]
- Yuan, Z.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing anthropogenic cadmium emissions: From sources to pollution. Sci. Total Environ. 2019, 676, 87–96. [Google Scholar] [CrossRef]
- Irfan, M.; Liu, X.; Hussain, K.; Mushtaq, S.; Cabrera, J.; Zhang, P. The global research trend on cadmium in freshwater: A bibliometric review. Environ. Sci. Pollut. Res. 2023, 30, 71585–71598. [Google Scholar] [CrossRef]
- Long, Y.; Wu, C.; Jiang, C.; Hu, S.; Liu, Y. Simulating the impacts of an upstream dam on pollutant transport: A case study on the Xiangjiang River, China. Water 2016, 8, 516. [Google Scholar] [CrossRef]
- Yang, Y.; Hassan, M.F.; Ali, W.; Zou, H.; Liu, Z.; Ma, Y. Effects of cadmium pollution on human health: A narrative review. Atmosphere 2025, 16, 225. [Google Scholar] [CrossRef]
- Allan, I.J.; Vrana, B.; Greenwood, R.; Mills, G.A.; Roig, B.; Gonzalez, C. A “toolbox” for biological and chemical monitoring requirements for the European Union’s Water Framework Directive. Talanta 2006, 69, 302–322. [Google Scholar] [CrossRef] [PubMed]
- Ogidi, O.I.; Onwuagba, C.G.; Richard-Nwachukwu, N. Biomonitoring tools, techniques and approaches for environmental assessments. In Biomonitoring of Pollutants in the Global South; Springer Nature: Singapore, 2024; pp. 243–273. [Google Scholar]
- Douda, K.; Zieritz, A.; Vodáková, B.; Urbańska, M.; Bolotov, I.N.; Marková, J.; Froufe, E.; Bogan, A.E.; Lopes-Lima, M. Review of the globally invasive freshwater mussels in the genus Sinanodonta Modell, 1945. Hydrobiologia 2025, 852, 1243–1273. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.; Gan, J. Trace element accumulation in bivalve mussels Anodonta woodiana from Taihu Lake, China. Arch. Environ. Contam. Toxicol. 2010, 59, 593–601. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Huang, H.; Liber, K.; Jiang, T.; Yang, J. Cadmium bioaccumulation and distribution in the freshwater bivalve Anodonta woodiana exposed to environmentally relevant Cd levels. Sci. Total Environ. 2021, 791, 148289. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, T.; Liu, H.; Yang, J. Bioaccumulation characteristics of 20 elements between Anodonta woodiana and Lamprotula leai. J. Agro-Environ. Sci. 2023, 42, 2190–2197, (In Chinese with English Abstract). [Google Scholar]
- Królak, E.; Zdanowski, B. The bioaccumulation of heavy metals by the mussels Anodonta woodiana (Lea, 1834) and Dreissena polymorpha (Pall.) in the Heated Konin Lakes. Arch. Pol. Fish. 2001, 9, 229–237. [Google Scholar]
- Gecheva, G.; Yancheva, V.; Velcheva, I.; Georgieva, E.; Stoyanova, S.; Arnaudova, D.; Stefanova, V.; Georgieva, D.; Genina, V.; Todorova, B.; et al. Integrated monitoring with moss-bag and mussel transplants in reservoirs. Water 2020, 12, 1800. [Google Scholar] [CrossRef]
- Georgieva, E.; Antal, L.; Stoyanova, S.; Arnaudova, D.; Velcheva, I.; Iliev, I.; Vasileva, T.; Bivolarski, V.; Mitkovska, V.; Chassovnikarova, T.; et al. Biomarkers for pollution in caged mussels from three reservoirs in Bulgaria: A pilot study. Heliyon 2022, 8, e09069. [Google Scholar] [CrossRef]
- Guo, Z.; Gao, N.; Wu, Y.; Zhang, L. The simultaneous uptake of dietary and waterborne Cd in gastrointestinal tracts of marine yellowstripe goby Mugilogobius chulae. Environ. Pollut. 2017, 223, 31–41. [Google Scholar] [CrossRef]
- Zhong, G.; Lin, Z.; Liu, F.; Xie, M.; Chen, R.; Tan, Q.G. Toxicokinetics and Mussel Watch: Addressing interspecies differences for coastal cadmium contamination assessment. Environ. Sci. Technol. 2024, 58, 14618–14628. [Google Scholar] [CrossRef]
- Zhao, Y.; Kang, X.; Shang, D.; Ning, J.; Ding, H.; Zhai, Y.; Sheng, X. Hyperaccumulation of cadmium by scallop Chlamys farreri revealed by comparative transcriptome analysis. Biometals 2020, 33, 397–413. [Google Scholar] [CrossRef] [PubMed]
- Zhan, J.; Wang, S.; Li, F.; Ji, C.; Wu, H. Global characterization of dose-dependent effects of cadmium in clam Ruditapes philippinarum. Environ. Pollut. 2021, 273, 116443. [Google Scholar] [CrossRef] [PubMed]
- ASTM-E2455-22; Standard Guide for Conducting Laboratory Toxicity Tests with Freshwater Mussels. ASTM International: West Conshohocken, PA, USA, 2022.
- Yuan, H.; Liu, E.; Shen, J. The accumulation and potential ecological risk of heavy metals in microalgae from a eutrophic lake (Taihu Lake, China). Environ. Sci. Pollut. Res. 2015, 22, 17123–17134. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Tu, R.; Wang, W.X. Different responses of abalone Haliotis discushannai to waterborne and dietary-borne copper and zinc exposure. Ecotox. Environ. Safe. 2013, 91, 10–17. [Google Scholar] [CrossRef]
- Cooper, S.; Hare, L.; Campbell, P.G.C. Modeling cadmium uptake from water and food by the freshwater bivalve Pyganodon grandis. Can. J. Fish. Aquat. Sci. 2010, 67, 1874–1888. [Google Scholar] [CrossRef]
- Huang, X.; Ke, C.; Wang, W.X. Bioaccumulation of silver, cadmium and mercury in the abalone Haliotis diversicolor from water and food sources. Aquaculture 2008, 283, 194–202. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, J.C.; Liu, F.; He, S.; Zhou, W. Removal of selenium containing algae by the bivalve Sinanodonta woodiana and the potential risk to human health. Environ. Pollut. 2018, 242, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, H.; Liber, K.; Jiang, T.; Yang, J. Copper-induced ionoregulatory disturbance, histopathology, and transcriptome responses in freshwater mussel (Anodonta woodiana) gills. Fishes 2023, 8, 368. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Trinity: Reconstructing a full-length transcriptome without a genome from RNA-Seq data. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Chen, X.; Liu, H.; Su, Y.; Jiang, T.; Yang, J. Acute toxicity of cadmium and its effects on lipid peroxidation and DNA damage in “standardized” Anodonta woodiana. J. Agro-Environ. Sci. 2017, 36, 1960–1967, (In Chinese with English Abstract). [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real- time quantitative PCR and the 2–ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Xing, H.; Li, Y.; Yang, H.; Wang, L. Effects of cadmium on antioxidant enzyme activities and lipid peroxidation in the mantle and gill of the freshwater bivalve A. woodiana woodiana. Acta Sci. Circum. 2013, 33, 856–860, (In Chinese with English Abstract). [Google Scholar]
- Li, Y.; Yang, H.; Liu, N.; Luo, J.; Wang, Q.; Wang, L. Cadmium accumulation and metallothionein biosynthesis in cadmium-treated freshwater mussel Anodonta woodiana. PLoS ONE 2015, 10, e0117037. [Google Scholar] [CrossRef]
- Karimi, R.; Fisher, N.S.; Folt, C.L. Multielement stoichiometry in aquatic invertebrates: When growth dilution matters. Am. Nat. 2010, 176, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Wang, W.X. Uptake of aqueous and dietary metals by mussel Perna viridis with different Cd exposure histories. Environ. Sci. Technol. 2005, 39, 9363–9369. [Google Scholar] [CrossRef]
- de Laeter, J.R.; Böhlke, J.K.; De Bièvre, P.; Hidaka, H.; Peiser, H.S.; Rosman, K.J.R.; Taylor, P.D.P. Atomic weights of the elements. Review 2000 (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 683–800. [Google Scholar] [CrossRef]
- GB 2762-2022; National Food Safety Standards: Limits of Contaminants in Food. National Health Commission of the People’s Republic of China, State Administration for Market Regulation: Beijing, China, 2022.
- Jing, W.; Lang, L.; Lin, Z.; Liu, N.; Wang, L. Cadmium bioaccumulation and elimination in tissues of the freshwater mussel Anodonta woodiana. Chemosphere 2019, 219, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ye, H.; Xiao, J.; Hogstrand, C.; Zhang, L. Biokinetic modeling of Cd bioaccumulation from water, diet and sediment in a marine benthic goby: A triple stable isotope tracing technique. Environ. Sci. Technol. 2018, 52, 8429–8437. [Google Scholar] [CrossRef]
- Li, Y.Q.; Chen, C.M.; Liu, N.; Wang, L. Cadmium-induced ultrastructural changes and apoptosis in the gill of freshwater mussel Anodonta woodiana. Environ. Sci. Pollut. Res. 2022, 29, 23338–23351. [Google Scholar] [CrossRef]
- Zhou, L.; Li, M.; Zhong, Z.; Chen, H.; Wang, X.; Wang, M.; Xu, Z.; Cao, L.; Lian, C.; Zhang, H.; et al. Biochemical and metabolic responses of the deep-sea mussel Bathymodiolus platifrons to cadmium and copper exposure. Aquat. Toxicol. 2021, 236, 105845. [Google Scholar] [CrossRef]
- Jiao, T.; Chu, X.H.; Gao, Z.Q.; Yang, T.T.; Liu, Y.; Yang, L.; Zhang, D.Z.; Wang, J.L.; Tang, B.P.; Wu, K.; et al. New insight into the molecular basis of Fe (III) stress responses of Procambarus clarkii by transcriptome analysis. Ecotox. Environ. Safe. 2019, 182, 109388. [Google Scholar] [CrossRef]
- Jungmann, J.; Reins, H.A.; Schobert, C.; Jentsch, S. Resistance to cadmium mediated by ubiquitin-dependent proteolysis. Nature 1993, 361, 369–371. [Google Scholar] [CrossRef]
- Brunet, M.; Vargas, C.; Larrieu, D.; Torrisani, J.; Dufresne, M. E3 ubiquitin ligase TRIP12: Regulation, structure, and physiopathological functions. Int. J. Mol. Sci. 2020, 21, 8515. [Google Scholar] [CrossRef]

| Gene | Description | Primer Sequence |
|---|---|---|
| TubB | Tubulin beta chain | F-AGGCAAATATGTACCCAG |
| R-TTTCCAGCTCCACTCTGT | ||
| PhoA | Phospholipase A1 magnifin | F-AGCACCCTTATTCCAGTC |
| R-CAGATAGAAACGTCGTATTG | ||
| PheT | Phenylalanine-tRNA ligase | F-TGCAATATGATGAAAGATAC |
| R-ACTTGCTGTACGAAGTCA | ||
| ProH | Protein henna | F-TTTGACACGGTGAAGGAC |
| R-ACGGGATTCAATGTGGAG | ||
| β-actin | Beta-actin | F-ACGGATAACACAAGGAAAGGAAAC |
| R-ATGGATGGAAACACGGCTCT |
| Time (d) | Treatment | Shell Length (cm) | Dry Weight of Soft Tissue (g) |
|---|---|---|---|
| 10 | T0 | 7.43 ± 0.21 | 1.29 ± 0.20 |
| T1 | 7.08 ± 0.07 | 1.18 ± 0.36 | |
| T2 | 6.94 ± 0.29 | 1.23 ± 0.32 | |
| T3 | 6.90 ± 0.10 | 1.06 ± 0.17 | |
| 20 | T0 | 7.02 ± 0.16 | 1.22 ± 0.29 |
| T1 | 7.08 ± 0.20 | 1.14 ± 0.31 | |
| T2 | 6.93 ± 0.52 | 0.92 ± 0.04 | |
| T3 | 6.81 ± 0.23 | 0.86 ± 0.10 | |
| 30 | T0 | 7.18 ± 0.45 | 1.00 ± 0.16 |
| T1 | 6.98 ± 0.54 | 0.73 ± 0.07 | |
| T2 | 6.91 ± 0.08 | 0.82 ± 0.07 | |
| T3 | 7.02 ± 0.27 | 1.00 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Song, C.; Jiang, J.; Jiang, T.; Xue, J.; Bah, I.; Gu, M.; Wang, M.; Meng, S. Cadmium Accumulation and Regulation in the Freshwater Mussel Anodonta woodiana. Toxics 2025, 13, 646. https://doi.org/10.3390/toxics13080646
Chen X, Song C, Jiang J, Jiang T, Xue J, Bah I, Gu M, Wang M, Meng S. Cadmium Accumulation and Regulation in the Freshwater Mussel Anodonta woodiana. Toxics. 2025; 13(8):646. https://doi.org/10.3390/toxics13080646
Chicago/Turabian StyleChen, Xiubao, Chao Song, Jiazhen Jiang, Tao Jiang, Junren Xue, Ibrahim Bah, Mengying Gu, Meiyi Wang, and Shunlong Meng. 2025. "Cadmium Accumulation and Regulation in the Freshwater Mussel Anodonta woodiana" Toxics 13, no. 8: 646. https://doi.org/10.3390/toxics13080646
APA StyleChen, X., Song, C., Jiang, J., Jiang, T., Xue, J., Bah, I., Gu, M., Wang, M., & Meng, S. (2025). Cadmium Accumulation and Regulation in the Freshwater Mussel Anodonta woodiana. Toxics, 13(8), 646. https://doi.org/10.3390/toxics13080646









