Mycotoxin-Caused Intestinal Toxicity: Underlying Molecular Mechanisms and Further Directions
Abstract
1. Introduction
2. An Overview of Mycotoxin-Induced Intestinal Toxicity Effects
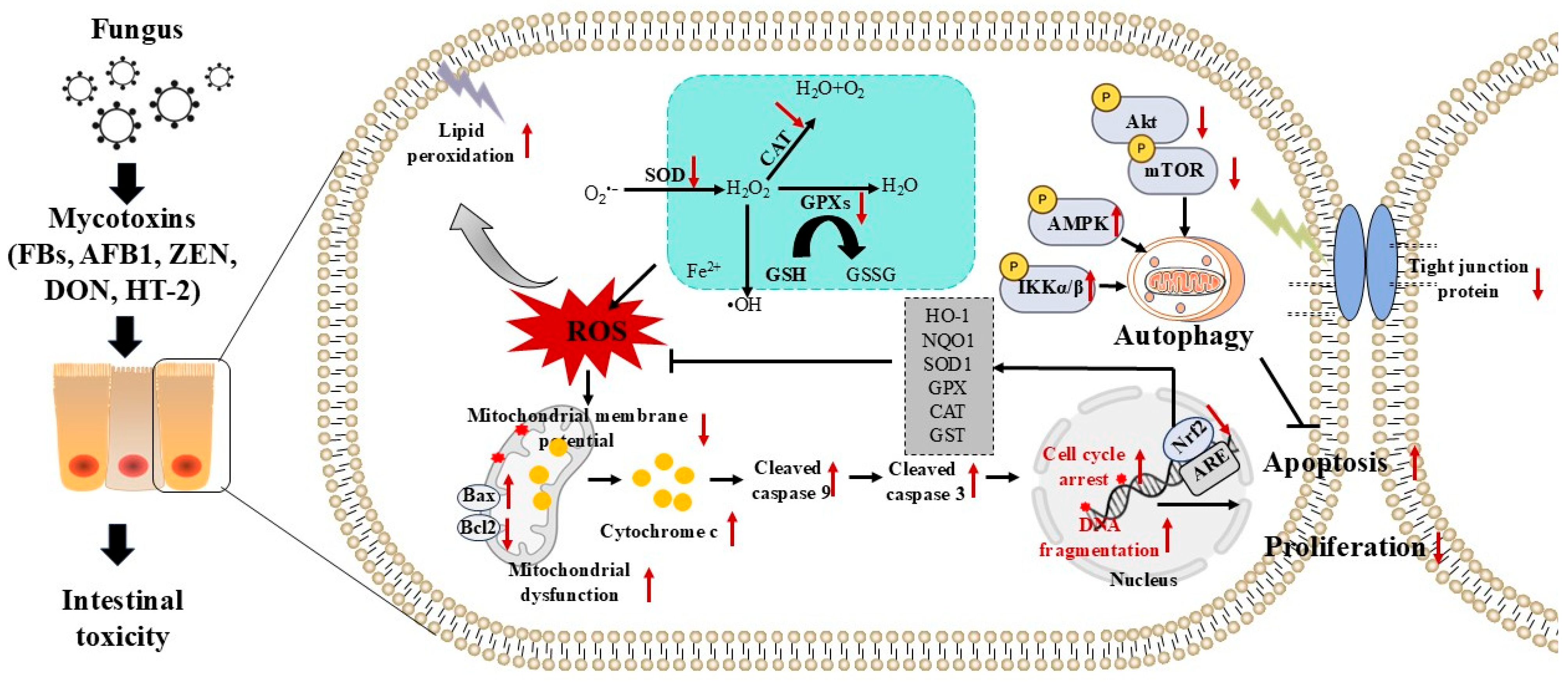
3. Mycotoxin Exposure Triggers Oxidative Stress and Cell Apoptosis
4. Mycotoxin Exposure Triggers the Intestinal Immune Dysfunction and Inflammatory Responses
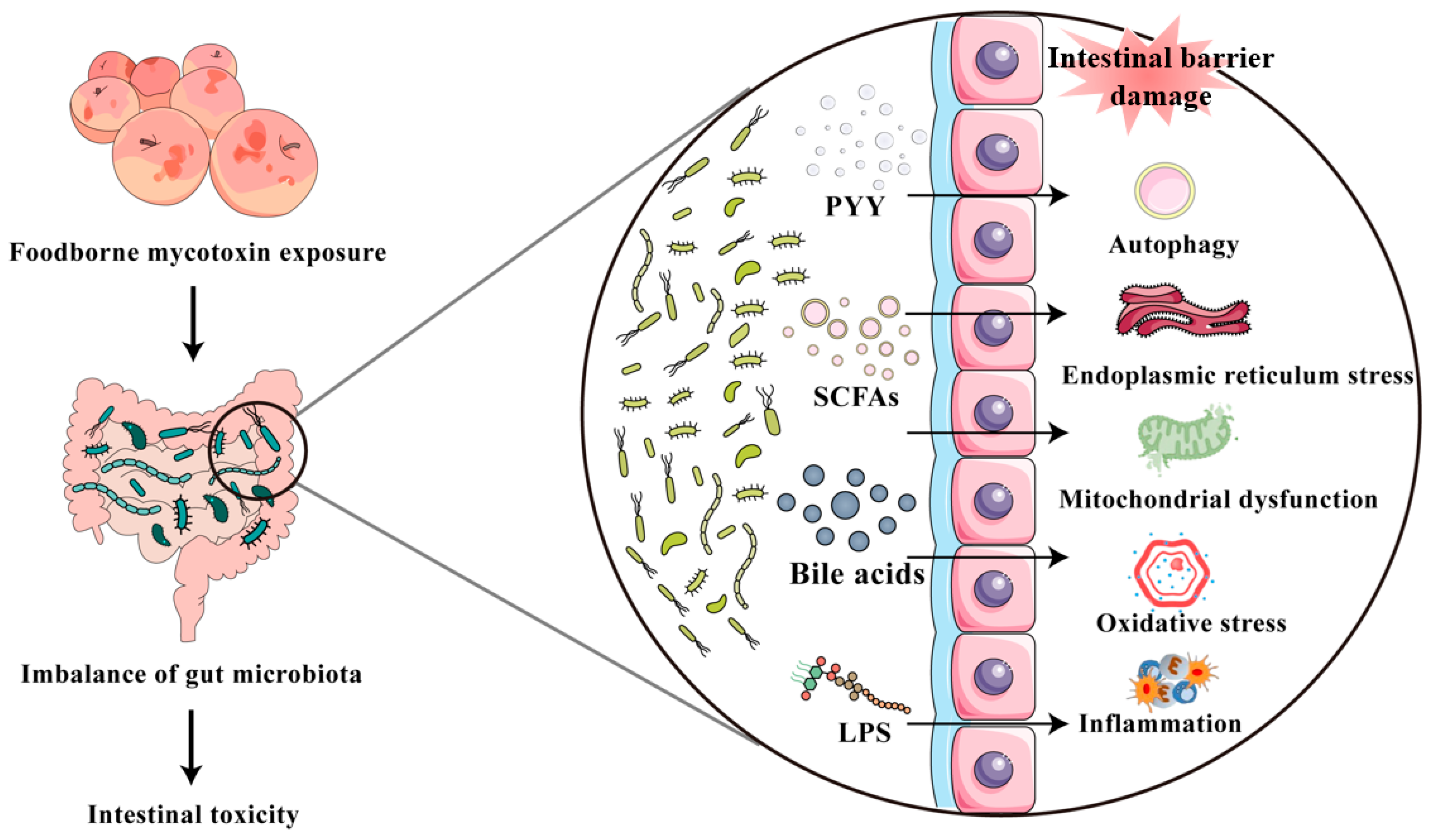
5. Mycotoxin Exposure Causes Disruption of Intestinal Microbiota
6. Role of MiRNAs in Mycotoxin-Induced Intestine Toxicity
7. Summary and Prospect
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Khan, R.; Anwar, F.; Ghazali, F.M. A comprehensive review of mycotoxins: Toxicology, detection, and effective mitigation approaches. Heliyon 2024, 10, e28361. [Google Scholar] [CrossRef]
- Li, M.; Tang, S.; Peng, X.; Sharma, G.; Yin, S.; Hao, Z.; Li, J.; Shen, J.; Dai, C. Lycopene as a therapeutic agent against aflatoxin B1-related toxicity: Mechanistic insights and future directions. Antioxidants 2024, 13, 452. [Google Scholar] [CrossRef]
- Dai, C.; Tian, E.; Hao, Z.; Tang, S.; Wang, Z.; Sharma, G.; Jiang, H.; Shen, J. Aflatoxin B1 toxicity and protective effects of curcumin: Molecular mechanisms and clinical implications. Antioxidants 2022, 11, 2031. [Google Scholar] [CrossRef]
- dsm-firmenich World Mycotoxin Survey. Available online: https://www.dsm.com/content/dam/dsm/anh/en/documents/REP_MTXsurvey_Q4_2023_EN_0124_AUE_doublePage.pdf (accessed on 23 July 2025).
- Yue, J.; Guo, D.; Gao, X.; Wang, J.; Nepovimova, E.; Wu, W.; Kuca, K. Deoxynivalenol (vomitoxin)-induced anorexia is induced by the release of intestinal hormones in mice. Toxins 2021, 13, 512. [Google Scholar] [CrossRef]
- Lahjouji, T.; Bertaccini, A.; Neves, M.; Puel, S.; Oswald, I.P.; Soler, L. Acute exposure to zearalenone disturbs intestinal homeostasis by modulating the Wnt/β-Catenin signaling pathway. Toxins 2020, 12, 113. [Google Scholar] [CrossRef]
- Yan, W.K.; Liu, Y.N.; Song, S.S.; Kang, J.W.; Zhang, Y.; Lu, L.; Wei, S.W.; Xu, Q.X.; Zhang, W.Q.; Liu, X.Z.; et al. Zearalenone affects the growth of endometriosis via estrogen signaling and inflammatory pathways. Ecotoxicol. Environ. Saf. 2022, 241, 113826. [Google Scholar] [CrossRef]
- Dilkin, P.; Direito, G.; Simas, M.M.; Mallmann, C.A.; Corrêa, B. Toxicokinetics and toxicological effects of single oral dose of fumonisin B1 containing Fusarium verticillioides culture material in weaned piglets. Chem. Biol. Interact. 2010, 185, 157–162. [Google Scholar] [CrossRef]
- Fouad, A.M.; Ruan, D.; El-Senousey, H.K.; Chen, W.; Jiang, S.; Zheng, C. Harmful effects and control strategies of aflatoxin b1 produced by Aspergillus flavus and Aspergillus parasiticus strains on poultry: Review. Toxins 2019, 11, 176. [Google Scholar] [CrossRef]
- Kumar, A.; Pathak, H.; Bhadauria, S.; Sudan, J. Aflatoxin contamination in food crops: Causes, detection, and management: A review. Food Prod. Process. Nutr. 2021, 3, 17. [Google Scholar] [CrossRef]
- Shanakhat, H.; Sorrentino, A.; Raiola, A.; Romano, A.; Masi, P.; Cavella, S. Current methods for mycotoxins analysis and innovative strategies for their reduction in cereals: An overview. J. Sci. Food Agric. 2018, 98, 4003–4013. [Google Scholar] [CrossRef]
- Li, M.; Yu, R.; Bai, X.; Wang, H.; Zhang, H. Fusarium: A treasure trove of bioactive secondary metabolites. Nat. Prod. Rep. 2020, 37, 1568–1588. [Google Scholar] [CrossRef]
- Rai, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, zearalenone. Crit. Rev. Food Sci. Nutr. 2020, 60, 2710–2729. [Google Scholar] [CrossRef]
- Ruan, H.; Huang, Y.; Yue, B.; Zhang, Y.; Lv, J.; Miao, K.; Zhang, D.; Luo, J.; Yang, M. Insights into the intestinal toxicity of foodborne mycotoxins through gut microbiota: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2023, 22, 4758–4785. [Google Scholar] [CrossRef]
- Dai, C.; Sharma, G.; Liu, G.; Shen, J.; Shao, B.; Hao, Z. Therapeutic detoxification of quercetin for aflatoxin B1-related toxicity: Roles of oxidative stress, inflammation, and metabolic enzymes. Environ. Pollut. (Barking Essex 1987) 2024, 345, 123474. [Google Scholar] [CrossRef]
- Adugna, C.; Wang, K.; Du, J.; Li, C. Deoxynivalenol mycotoxin dietary exposure on broiler performance and small intestine health: A comprehensive meta-analysis. Poult. Sci. 2024, 103, 104412. [Google Scholar] [CrossRef]
- Duarte, S.C.; Lino, C.M.; Pena, A. Ochratoxin A in feed of food-producing animals: An undesirable mycotoxin with health and performance effects. Vet. Microbiol. 2011, 154, 1–13. [Google Scholar] [CrossRef]
- Costamagna, D.; Gaggiotti, M.; Smulovitz, A.; Abdala, A.; Signorini, M. Mycotoxin sequestering agent: Impact on health and performance of dairy cows and efficacy in reducing AFM(1) residues in milk. Environ. Toxicol. Pharmacol. 2024, 105, 104349. [Google Scholar] [CrossRef]
- Kolawole, O.; Graham, A.; Donaldson, C.; Owens, B.; Abia, W.A.; Meneely, J.; Alcorn, M.J.; Connolly, L.; Elliott, C.T. Low doses of mycotoxin mixtures below eu regulatory limits can negatively affect the performance of broiler chickens: A longitudinal study. Toxins 2020, 12, 433. [Google Scholar] [CrossRef]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Kozieł, M.J.; Ziaja, M.; Piastowska-Ciesielska, A.W. Intestinal barrier, claudins and mycotoxins. Toxins 2021, 13, 758. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, L.; Liu, H.; Wang, J.; Zheng, N. The Compromised intestinal barrier induced by mycotoxins. Toxins 2020, 12, 619. [Google Scholar] [CrossRef]
- Song, C.; Chai, Z.; Chen, S.; Zhang, H.; Zhang, X.; Zhou, Y. Intestinal mucus components and secretion mechanisms: What we do and do not know. Exp. Mol. Med. 2023, 55, 681–691. [Google Scholar] [CrossRef]
- Gao, J.; Song, G.; Shen, H.; Wu, Y.; Zhao, C.; Zhang, Z.; Jiang, Q.; Li, X.; Ma, X.; Tan, B.; et al. Allicin Improves intestinal epithelial barrier function and prevents LPS-induced barrier damages of intestinal epithelial cell monolayers. Front. Immunol. 2022, 13, 847861. [Google Scholar] [CrossRef]
- Kang, R.; Li, S.; Perveen, A.; Shen, J.; Li, C. Effects of maternal T-2 toxin exposure on microorganisms and intestinal barrier function in young mice. Ecotoxicol. Environ. Saf. 2022, 247, 114252. [Google Scholar] [CrossRef]
- Bastos-Amador, P.; Duarte, E.L.; Torres, J.; Caldeira, A.T.; Silva, I.; Salvador, C.; Assunção, R.; Alvito, P.; Ferreira, M. Maternal dietary exposure to mycotoxin aflatoxin B(1) promotes intestinal immune alterations and microbiota modifications increasing infection susceptibility in mouse offspring. Food Chem. Toxicol. 2023, 173, 113596. [Google Scholar] [CrossRef]
- Xue, D.; Cheng, Y.; Pang, T.; Kuai, Y.; An, Y.; Wu, K.; Li, Y.; Lai, M.; Wang, B.; Wang, S. Sodium butyrate alleviates deoxynivalenol-induced porcine intestinal barrier disruption by promoting mitochondrial homeostasis via PCK2 signaling. J. Hazard. Mater. 2023, 459, 132013. [Google Scholar] [CrossRef]
- Du, K.; Wang, C.; Liu, P.; Li, Y.; Ma, X. Effects of dietary mycotoxins on gut microbiome. Protein Pept. Lett. 2017, 24, 397–405. [Google Scholar] [CrossRef]
- Zhou, B.; Xiao, K.; Guo, J.; Xu, Q.; Xu, Q.; Lv, Q.; Zhu, H.; Zhao, J.; Liu, Y. Necroptosis contributes to the intestinal toxicity of deoxynivalenol and is mediated by methyltransferase SETDB1. J. Hazard. Mater. 2024, 474, 134601. [Google Scholar] [CrossRef]
- Lin, J.; Zuo, C.; Liang, T.; Huang, Y.; Kang, P.; Xiao, K.; Liu, Y. Lycopene alleviates multiple-mycotoxin-induced toxicity by inhibiting mitochondrial damage and ferroptosis in the mouse jejunum. Food Funct. 2022, 13, 11532–11542. [Google Scholar] [CrossRef]
- Singh, N.; Dev, I.; Pal, S.; Yadav, S.K.; Idris, M.M.; Ansari, K.M. Transcriptomic and proteomic insights into patulin mycotoxin-induced cancer-like phenotypes in normal intestinal epithelial cells. Mol. Cell. Biochem. 2022, 477, 1405–1416. [Google Scholar] [CrossRef]
- Miner-Williams, W.M.; Moughan, P.J. Intestinal barrier dysfunction: Implications for chronic inflammatory conditions of the bowel. Nutr. Res. Rev. 2016, 29, 40–59. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, W.; Xiao, B.; Wang, Y.; Li, Y.; Wu, A.; Zhang, Q.; Liu, X.; Liu, S.; Yuan, Z.; et al. Melatonin alleviates t-2 toxin-induced intestinal injury by enhancing gut barrier function and modulating microbiota in weaned piglets. J. Agric. Food Chem. 2025, 73, 6903–6916. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Dong, R.; Zhang, Z.; Jia, F.; Yu, H.; Wang, Y.; Zhang, Z. Protective effect of selenomethionine on intestinal injury induced by T- 2 toxin. Res. Vet. Sci. 2020, 132, 439–447. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, J.; Huang, W.; Yan, J.; Shan, A.; Gao, X. Curcumin mitigates deoxynivalenol-induced intestinal epithelial barrier disruption by regulating Nrf2/p53 and NF-κB/MLCK signaling in mice. Food Chem. Toxicol. 2022, 167, 113281. [Google Scholar] [CrossRef]
- Pierron, A.; Bracarense, A.; Cossalter, A.M.; Laffitte, J.; Schwartz-Zimmermann, H.E.; Schatzmayr, G.; Pinton, P.; Moll, W.D.; Oswald, I.P. Deepoxy-deoxynivalenol retains some immune-modulatory properties of the parent molecule deoxynivalenol in piglets. Arch. Toxicol. 2018, 92, 3381–3389. [Google Scholar] [CrossRef]
- Pierron, A.; Balbo, L.C.; Soler, L.; Pinton, P.; Puel, S.; Laffitte, J.; Albin, M.; Bracarense, A.; Rodriguez, M.A.; Oswald, I.P. Deoxynivalenol Induces local inflammation and lesions in tissues at doses recommended by the EU. Int. J. Mol. Sci. 2024, 25, 9790. [Google Scholar] [CrossRef]
- Pasternak, J.A.; Aiyer, V.I.A.; Hamonic, G.; Beaulieu, A.D.; Columbus, D.A.; Wilson, H.L. Molecular and physiological effects on the small intestine of weaner pigs following feeding with deoxynivalenol-contaminated feed. Toxins 2018, 10, 40. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Zhang, G. A proteomic study on the protective effect of kaempferol pretreatment against deoxynivalenol-induced intestinal barrier dysfunction in a Caco-2 cell model. Food Funct. 2020, 11, 7266–7279. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Wang, S.B.; Wang, R.G.; Zhang, W.; Wang, P.L.; Su, X.O. Phosphoproteome analysis reveals the molecular mechanisms underlying deoxynivalenol-induced intestinal toxicity in IPEC-J2 Cells. Toxins 2016, 8, 270. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Zan, G.X.; Zhu, Q.J.; Gao, C.Q.; Yan, H.C.; Wang, X.Q. Recombinant porcine r-spondin 1 facilitates intestinal stem cell expansion along the crypt-villus axis through potentiating Wnt/β-Catenin signaling in homeostasis and deoxynivalenol injury. J. Agric. Food Chem. 2022, 70, 10644–10653. [Google Scholar] [CrossRef]
- Xia, S.; Yan, C.; Gu, J.; Yuan, Y.; Zou, H.; Liu, Z.; Bian, J. Resveratrol alleviates zearalenone-induced intestinal dysfunction in mice through the NF-κB/Nrf2/HO-1 signalling pathway. Foods 2024, 13, 1217. [Google Scholar] [CrossRef]
- Huangfu, B.; Li, J.; Xu, T.; Ren, X.; Zhang, R.; Chen, Y.; Wang, J.; Huang, K.; He, X. Zearalenone induces intestinal damage and flora disturbance in rats by triggering ferroptosis via the system Xc(-)-GSH-GPX4 signaling pathway. Ecotoxicol. Environ. Saf. 2025, 302, 118600. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Rudyk, H.; Dobrowolski, P.; Donaldson, J.; Świetlicka, I.; Puzio, I.; Kamiński, D.; Wiącek, D.; Kushnir, V.; Brezvyn, O.; et al. Changes in the intestinal histomorphometry, the expression of intestinal tight junction proteins, and the bone structure and liver of pre-laying hens following oral administration of Fumonisins for 21 Days. Toxins 2021, 13, 375. [Google Scholar] [CrossRef]
- Antonissen, G.; Van Immerseel, F.; Pasmans, F.; Ducatelle, R.; Janssens, G.P.; De Baere, S.; Mountzouris, K.C.; Su, S.; Wong, E.A.; De Meulenaer, B.; et al. Mycotoxins deoxynivalenol and fumonisins alter the extrinsic component of intestinal barrier in broiler chickens. J. Agric. Food Chem. 2015, 63, 10846–10855. [Google Scholar] [CrossRef]
- Shanmugasundaram, R.; Kappari, L.; Pilewar, M.; Jones, M.K.; Olukosi, O.A.; Pokoo-Aikins, A.; Applegate, T.J.; Glenn, A.E. Exposure to subclinical doses of fumonisins, deoxynivalenol, and zearalenone affects immune response, amino acid digestibility, and intestinal morphology in broiler chickens. Toxins 2025, 17, 16. [Google Scholar] [CrossRef]
- da Silva, E.O.; Gerez, J.R.; Hohmann, M.S.N.; Verri, W.A., Jr.; Bracarense, A. Phytic acid decreases oxidative stress and intestinal lesions induced by Fumonisin B1 and deoxynivalenol in intestinal explants of pigs. Toxins 2019, 11, 18. [Google Scholar] [CrossRef]
- Ye, Y.; Yang, D.; Huang, H.; Li, Y.; Ji, J.; Wang, J.S.; Sun, X. Effect of fumonisin b1 and hydrolyzed fb1 exposure on intestinal and hepatic toxicity in BALB/c mice. J. Agric. Food Chem. 2025, 73, 10603–10614. [Google Scholar] [CrossRef]
- Yu, S.; Zou, L.; Zhao, J.; Zhu, Y. Resveratrol alleviates fumonisin-induced intestinal cytotoxicity by modulating apoptosis, tight junction, and inflammation in IPEC-J2 porcine intestinal epithelial cells. Environ. Toxicol. 2024, 39, 905–914. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, X.; Sharma, G.; Dai, C. Thymol as a potential neuroprotective agent: Mechanisms, efficacy, and future prospects. J. Agric. Food Chem. 2024, 72, 6803–6814. [Google Scholar] [CrossRef]
- Minervini, F.; Garbetta, A.; D’Antuono, I.; Cardinali, A.; Martino, N.A.; Debellis, L.; Visconti, A. Toxic mechanisms induced by fumonisin b1 mycotoxin on human intestinal cell line. Arch. Environ. Contam. Toxicol. 2014, 67, 115–123. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Choi, H.; Garavito-Duarte, Y.; Gormley, A.R.; Kim, S.W. Aflatoxin B1: Challenges and strategies for the intestinal microbiota and intestinal health of monogastric animals. Toxins 2025, 17, 43. [Google Scholar] [CrossRef]
- Pan, H.; Hu, T.; He, Y.; Zhong, G.; Wu, S.; Jiang, X.; Rao, G.; You, Y.; Ruan, Z.; Tang, Z.; et al. Curcumin attenuates aflatoxin B1-induced ileum injury in ducks by inhibiting NLRP3 inflammasome and regulating TLR4/NF-κB signaling pathway. Mycotoxin Res. 2024, 40, 255–268. [Google Scholar] [CrossRef]
- Li, J.; Shi, M.; Wang, Y.; Liu, J.; Liu, S.; Kang, W.; Liu, X.; Chen, X.; Huang, K.; Liu, Y. Probiotic-derived extracellular vesicles alleviate AFB1-induced intestinal injury by modulating the gut microbiota and AHR activation. J. Nanobiotechnol. 2024, 22, 697. [Google Scholar] [CrossRef]
- Ding, J.; Cheng, X.; Zeng, C.; Zhao, Q.; Xing, C.; Zhang, C.; Cao, H.; Guo, X.; Hu, G.; Zhuang, Y. Aflatoxin B1 promotes pyroptosis in ipec-j2 cells by disrupting mitochondrial dynamics through the AMPK/NLRP3 pathway. J. Agric. Food Chem. 2024, 72, 28093–28108. [Google Scholar] [CrossRef]
- He, W.; Wang, J.; Han, M.; Wang, L.; Li, L.; Zhang, J.; Chen, S.; Guo, J.; Zhai, X.; Yang, J. Potential toxicity and mechanisms of T-2 and HT-2 individually or in combination on the intestinal barrier function of porcine small intestinal epithelial cells. Toxins 2023, 15, 682. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Wei, J.T.; Huang, Y.X.; Khalil, M.M.; Wu, W.D.; Kuča, K.; Sun, L.H. T-2 toxin-induced intestinal damage with dysregulation of metabolism, redox homeostasis, inflammation, and apoptosis in chicks. Arch. Toxicol. 2023, 97, 805–817. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Yu, H.; Lu, Y.; Qian, Y.; Wang, M. Metabolomics and lipidomics reveal the metabolic disorders induced by single and combined exposure of Fusarium mycotoxins in IEC-6 cells. Foods 2025, 14, 230. [Google Scholar] [CrossRef]
- Basso, K.; Gomes, F.; Bracarense, A.P. Deoxynivanelol and fumonisin, alone or in combination, induce changes on intestinal junction complexes and in E-cadherin expression. Toxins 2013, 5, 2341–2352. [Google Scholar] [CrossRef]
- Peters, A.; Nawrot, T.S.; Baccarelli, A.A. Hallmarks of environmental insults. Cell 2021, 184, 1455–1468. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Li, M.; Tang, S.; Velkov, T.; Shen, J.; Dai, C. Copper exposure induces mitochondrial dysfunction and hepatotoxicity via the induction of oxidative stress and PERK/ATF4 -mediated endoplasmic reticulum stress. Environ. Pollut. (Barking Essex 1987) 2024, 352, 124145. [Google Scholar] [CrossRef]
- Ben Salah-Abbès, J.; Mannai, M.; Belgacem, H.; Zinedine, A.; Abbès, S. Efficacy of lactic acid bacteria supplementation against Fusarium graminearum growth in vitro and inhibition of Zearalenone causing inflammation and oxidative stress in vivo. Toxicon Off. J. Int. Soc. Toxinol. 2021, 202, 115–122. [Google Scholar] [CrossRef]
- Zhu, C.; Liang, S.; Zan, G.; Wang, X.; Gao, C.; Yan, H.; Wang, X.; Zhou, J. Selenomethionine alleviates don-induced oxidative stress via modulating Keap1/Nrf2 signaling in the small intestinal epithelium. J. Agric. Food Chem. 2023, 71, 895–904. [Google Scholar] [CrossRef]
- Liang, S.J.; Wang, X.Q. Deoxynivalenol induces intestinal injury: Insights from oxidative stress and intestinal stem cells. Environ. Sci. Pollut. Res. Int. 2023, 30, 48676–48685. [Google Scholar] [CrossRef]
- de Souza, M.; Baptista, A.A.S.; Valdiviezo, M.J.J.; Justino, L.; Menck-Costa, M.F.; Ferraz, C.R.; da Gloria, E.M.; Verri, W.A., Jr.; Bracarense, A. Lactobacillus spp. reduces morphological changes and oxidative stress induced by deoxynivalenol on the intestine and liver of broilers. Toxicon Off. J. Int. Soc. Toxinol. 2020, 185, 203–212. [Google Scholar] [CrossRef]
- Xu, X.; Yan, G.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Liu, S.; Zhu, Q.; Lu, F. Astilbin ameliorates deoxynivalenol-induced oxidative stress and apoptosis in intestinal porcine epithelial cells (IPEC-J2). J. Appl. Toxicol. JAT 2020, 40, 1362–1372. [Google Scholar] [CrossRef]
- Chen, F.; Wang, Y.; Chen, Y.; Fan, J.; Zhang, C.; He, X.; Yang, X. JNK molecule is a toxic target for IPEC-J2 cell barrier damage induced by T-2 toxin. Ecotoxicol. Environ. Saf. 2023, 263, 115247. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, M.; Li, J.; Shan, A. DL-Selenomethionine alleviates oxidative stress induced by zearalenone via Nrf2/Keap1 signaling pathway in IPEC-J2 Cells. Toxins 2021, 13, 557. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a transcription factor for stress response and beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- McCord, J.M.; Gao, B.; Hybertson, B.M. The complex genetic and epigenetic regulation of the Nrf2 pathways: A Review. Antioxidants 2023, 12, 366. [Google Scholar] [CrossRef]
- Yu, T.; Deng, X.; Yang, X.; Yin, Y.; Liu, Y.; Xu, S. New insights into evodiamine attenuates IPEC-J2 cells pyroptosis induced by T-2 toxin—Activating Keap1-Nrf2/NF-κB signaling pathway through binding with Keap1. J. Environ. Manag. 2024, 370, 122605. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, H.; You, Y.; Zhong, G.; Ruan, Z.; Liao, J.; Zhang, H.; Pan, J.; Tang, Z.; Hu, L. Multi-omics reveals the protective effects of curcumin against AFB1-induced oxidative stress and inflammatory damage in duckling intestines. Comp. Biochem. physiology. Toxicol. Pharmacol. CBP 2024, 276, 109815. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, J.; Wang, J.; Xie, H.; Zhang, Z.; Shi, L.; Zhu, X.; Lv, Q.; Chen, X.; Liu, Y. Selenomethionine attenuates ochratoxin A-induced small intestinal injury in rabbits by activating the Nrf2 pathway and inhibiting NF-κB activation. Ecotoxicol. Environ. Saf. 2023, 256, 114837. [Google Scholar] [CrossRef]
- Li, R.; Tan, B.; Jiang, Q.; Chen, F.; Liu, K.; Liao, P. Eucommia ulmoides flavonoids alleviate intestinal oxidative stress damage in weaned piglets by regulating the Nrf2/Keap1 signaling pathway. Ecotoxicol. Environ. Saf. 2024, 288, 117373. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Q.; Li, M.; Xu, J.; Wang, J.; Li, M.; Wei, L.; Lv, Q.; Chen, X.; Wang, Y.; et al. SeMet attenuates AFB1-induced intestinal injury in rabbits by activating the Nrf2 pathway. Ecotoxicol. Environ. Saf. 2022, 239, 113640. [Google Scholar] [CrossRef]
- Taguchi, K.; Takaku, M.; Egner, P.A.; Morita, M.; Kaneko, T.; Mashimo, T.; Kensler, T.W.; Yamamoto, M. Generation of a new model rat: Nrf2 knockout rats are sensitive to aflatoxin B1 Toxicity. Toxicol. Sci. Off. J. Soc. Toxicol. 2016, 152, 40–52. [Google Scholar] [CrossRef]
- Pang, Y.; Zhang, L.; Liu, Q.; Peng, H.; He, J.; Jin, H.; Su, X.; Zhao, J.; Guo, J. NRF2/PGC-1α-mediated mitochondrial biogenesis contributes to T-2 toxin-induced toxicity in human neuroblastoma SH-SY5Y cells. Toxicol. Appl. Pharmacol. 2022, 451, 116167. [Google Scholar] [CrossRef]
- Zhao, L.; Deng, J.; Xu, Z.J.; Zhang, W.P.; Khalil, M.M.; Karrow, N.A.; Sun, L.H. Mitigation of Aflatoxin B(1) hepatoxicity by dietary hedyotis diffusa is associated with activation of NRF2/ARE signaling in chicks. Antioxidants 2021, 10, 878. [Google Scholar] [CrossRef]
- Rajput, S.A.; Shaukat, A.; Wu, K.; Rajput, I.R.; Baloch, D.M.; Akhtar, R.W.; Raza, M.A.; Najda, A.; Rafał, P.; Albrakati, A.; et al. Luteolin alleviates AflatoxinB(1)-induced apoptosis and oxidative stress in the liver of mice through activation of Nrf2 signaling pathway. Antioxidants 2021, 10, 1268. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, F.; Zhou, X.; Liu, M.; Zang, H.; Liu, X.; Shan, A.; Feng, X. Alleviation of oral exposure to aflatoxin B1-induced renal dysfunction, oxidative stress, and cell apoptosis in mice kidney by curcumin. Antioxidants 2022, 11, 1082. [Google Scholar] [CrossRef]
- Chen, X.; Mu, P.; Zhu, L.; Mao, X.; Chen, S.; Zhong, H.; Deng, Y. T-2 toxin induces oxidative stress at low doses via Atf3ΔZip2a/2b-mediated ubiquitination and degradation of Nrf2. Int. J. Mol. Sci. 2021, 22, 7936. [Google Scholar] [CrossRef]
- Xu, Q.; Shi, W.; Lv, P.; Meng, W.; Mao, G.; Gong, C.; Chen, Y.; Wei, Y.; He, X.; Zhao, J.; et al. Critical role of caveolin-1 in aflatoxin B1-induced hepatotoxicity via the regulation of oxidation and autophagy. Cell Death Dis. 2020, 11, 6. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—sources, functions, oxidative damage. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2020, 48, 124–127. [Google Scholar]
- Dos Santos, L.; Bertoli, S.R.; Ávila, R.A.; Marques, V.B. Iron overload, oxidative stress and vascular dysfunction: Evidences from clinical studies and animal models. Biochim. Biophys. Acta Gen. Subj. 2022, 1866, 130172. [Google Scholar] [CrossRef]
- Wan, D.; Wu, Q.; Qu, W.; Liu, G.; Wang, X. Pyrrolidine dithiocarbamate (pdtc) inhibits DON-induced mitochondrial dysfunction and apoptosis via the NF-κB/iNOS pathway. Oxidative Med. Cell. Longev. 2018, 2018, 1324173. [Google Scholar] [CrossRef]
- Brunelle, J.K.; Letai, A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009, 122, 437–441. [Google Scholar] [CrossRef]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Q.; Wang, J.; Sun, J.; Xiang, Y.; Jin, X. Aflatoxin B1 disrupts the intestinal barrier integrity by reducing junction protein and promoting apoptosis in pigs and mice. Ecotoxicol. Environ. Saf. 2022, 247, 114250. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, Y.; Wu, Z.; Wang, H.; Chen, Z.; Wang, J.; Bao, W. Protective effects of melatonin on deoxynivalenol-induced oxidative stress and autophagy in IPEC-J2 cells. Food Chem. Toxicol. 2023, 177, 113803. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.; Cao, X.; Chen, X.; Zou, T.; You, J. Plant-derived polyphenols as Nrf2 activators to counteract oxidative stress and intestinal toxicity induced by deoxynivalenol in swine: An Emerging Research Direction. Antioxidants 2022, 11, 2379. [Google Scholar] [CrossRef]
- Shen, T.; Miao, Y.; Ding, C.; Fan, W.; Liu, S.; Lv, Y.; Gao, X.; De Boevre, M.; Yan, L.; Okoth, S.; et al. Activation of the p38/MAPK pathway regulates autophagy in response to the CYPOR-dependent oxidative stress induced by zearalenone in porcine intestinal epithelial cells. Food Chem. Toxicol. 2019, 131, 110527. [Google Scholar] [CrossRef]
- Tang, Y.; Li, J.; Li, F.; Hu, C.A.; Liao, P.; Tan, K.; Tan, B.; Xiong, X.; Liu, G.; Li, T.; et al. Autophagy protects intestinal epithelial cells against deoxynivalenol toxicity by alleviating oxidative stress via IKK signaling pathway. Free Radic. Biol. Med. 2015, 89, 944–951. [Google Scholar] [CrossRef]
- Liu, S.; Mao, X.; Ge, L.; Hou, L.; Le, G.; Gan, F.; Wen, L.; Huang, K. Phenethyl isothiocyanate as an anti-nutritional factor attenuates deoxynivalenol-induced IPEC-J2 cell injury through inhibiting ROS-mediated autophagy. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2022, 8, 300–309. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef]
- Fessler, M.B. The intracellular cholesterol landscape: Dynamic integrator of the immune response. Trends Immunol. 2016, 37, 819–830. [Google Scholar] [CrossRef]
- Watson, S.; Gong, Y.Y.; Routledge, M. Interventions targeting child undernutrition in developing countries may be undermined by dietary exposure to aflatoxin. Crit. Rev. Food Sci. Nutr. 2017, 57, 1963–1975. [Google Scholar] [CrossRef]
- Gu, M.J.; Song, S.K.; Lee, I.K.; Ko, S.; Han, S.E.; Bae, S.; Ji, S.Y.; Park, B.C.; Song, K.D.; Lee, H.K.; et al. Barrier protection via Toll-like receptor 2 signaling in porcine intestinal epithelial cells damaged by deoxynivalnol. Vet. Res. 2016, 47, 25. [Google Scholar] [CrossRef]
- Liu, M.; Gao, R.; Meng, Q.; Zhang, Y.; Bi, C.; Shan, A. Toxic effects of maternal zearalenone exposure on intestinal oxidative stress, barrier function, immunological and morphological changes in rats. PLoS ONE 2014, 9, e106412. [Google Scholar] [CrossRef]
- Gu, M.J.; Han, S.E.; Hwang, K.; Mayer, E.; Reisinger, N.; Schatzmayr, D.; Park, B.C.; Han, S.H.; Yun, C.H. Hydrolyzed fumonisin B(1) induces less inflammatory responses than fumonisin B(1) in the co-culture model of porcine intestinal epithelial and immune cells. Toxicol. Lett. 2019, 305, 110–116. [Google Scholar] [CrossRef]
- Hao, S.; Hu, J.; Song, S.; Huang, D.; Xu, H.; Qian, G.; Gan, F.; Huang, K. Selenium alleviates aflatoxin B1-induced immune toxicity through improving glutathione peroxidase 1 and selenoprotein S expression in primary porcine splenocytes. J. Agric. Food Chem. 2016, 64, 1385–1393. [Google Scholar] [CrossRef]
- Sharma, R.P. Immunotoxicity of mycotoxins. J. Dairy Sci. 1993, 76, 892–897. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of mycotoxin on immune response and consequences for pig health. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2016, 2, 63–68. [Google Scholar] [CrossRef]
- Burel, C.; Tanguy, M.; Guerre, P.; Boilletot, E.; Cariolet, R.; Queguiner, M.; Postollec, G.; Pinton, P.; Salvat, G.; Oswald, I.P.; et al. Effect of low dose of fumonisins on pig health: Immune status, intestinal microbiota and sensitivity to Salmonella. Toxins 2013, 5, 841–864. [Google Scholar] [CrossRef]
- Bulgaru, C.V.; Marin, D.E.; Pistol, G.C.; Taranu, I. Zearalenone and the immune response. Toxins 2021, 13, 248. [Google Scholar] [CrossRef]
- Wang, S.; Wu, K.; Xue, D.; Zhang, C.; Rajput, S.A.; Qi, D. Mechanism of deoxynivalenol mediated gastrointestinal toxicity: Insights from mitochondrial dysfunction. Food Chem. Toxicol. 2021, 153, 112214. [Google Scholar] [CrossRef]
- Sauter, K.A.; Magun, E.A.; Iordanov, M.S.; Magun, B.E. ZAK is required for doxorubicin, a novel ribotoxic stressor, to induce SAPK activation and apoptosis in HaCaT cells. Cancer Biol. Ther. 2010, 10, 258–266. [Google Scholar] [CrossRef]
- Hu, P.; Zong, Q.; Zhao, Y.; Gu, H.; Liu, Y.; Gu, F.; Liu, H.Y.; Ahmed, A.A.; Bao, W.; Cai, D. Lactoferrin attenuates intestinal barrier dysfunction and inflammation by modulating the MAPK pathway and gut microbes in mice. J. Nutr. 2022, 152, 2451–2460. [Google Scholar] [CrossRef]
- Zhang, H.; Deng, X.; Zhou, C.; Wu, W.; Zhang, H. Deoxynivalenol induces inflammation in IPEC-J2 cells by activating P38 Mapk And Erk1/2. Toxins 2020, 12, 180. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; He, W.; Chen, X.; Wei, Z.; Huang, K. Two-way immune effects of deoxynivalenol in weaned piglets and porcine alveolar macrophages: Due mainly to its exposure dosage. Chemosphere 2020, 249, 126464. [Google Scholar] [CrossRef]
- Graziani, F.; Pujol, A.; Nicoletti, C.; Pinton, P.; Armand, L.; Di Pasquale, E.; Oswald, I.P.; Perrier, J.; Maresca, M. The food-associated ribotoxin deoxynivalenol modulates inducible NO synthase in human intestinal cell model. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 145, 372–382. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, X.; He, Z.; Xiong, D.; Long, M. Damage on intestinal barrier function and microbial detoxification of deoxynivalenol: A review. J. Integr. Agric. 2024, 23, 2507–2524. [Google Scholar] [CrossRef]
- Van De Walle, J.; Romier, B.; Larondelle, Y.; Schneider, Y.-J. Influence of deoxynivalenol on NF-κB activation and IL-8 secretion in human intestinal Caco-2 cells. Toxicol. Lett. 2008, 177, 205–214. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Yang, S.J.; Chang, Y.H.; Wang, X.Q.; Liu, R.Q.; Jiang, F.W.; Chen, M.S.; Wang, J.X.; Liu, S.; Zhu, H.M.; et al. AHR activation relieves deoxynivalenol-induced disruption of porcine intestinal epithelial barrier functions. J. Hazard. Mater. 2024, 480, 136095. [Google Scholar] [CrossRef]
- Jiang, M.; Peng, X.; Fang, J.; Cui, H.; Yu, Z.; Chen, Z. Effects of aflatoxin b1 on T-cell subsets and mRNA expression of cytokines in the intestine of broilers. Int. J. Mol. Sci. 2015, 16, 6945–6959. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, D.; Zhang, J.; Tang, H.; Li, F.; Peng, Y.; Duan, X.; Meng, E.; Zhang, C.; Zeng, T.; et al. Role of macrophage AHR/TLR4/STAT3 signaling axis in the colitis induced by non-canonical AHR ligand aflatoxin B1. J. Hazard. Mater. 2023, 452, 131262. [Google Scholar] [CrossRef]
- Guo, H.; Wang, P.; Liu, C.; Zhou, T.; Chang, J.; Yin, Q.; Wang, L.; Jin, S.; Zhu, Q.; Lu, F. Effects of compound mycotoxin detoxifier on alleviating aflatoxin B(1)-induced inflammatory responses in intestine, liver and kidney of broilers. Toxins 2022, 14, 665. [Google Scholar] [CrossRef]
- Gao, Y.N.; Wang, Z.W.; Su, C.Y.; Wang, J.Q.; Zheng, N. Omics analysis revealed the intestinal toxicity induced by aflatoxin B1 and aflatoxin M1. Ecotoxicol. Environ. Saf. 2024, 278, 116336. [Google Scholar] [CrossRef]
- Wang, X.; Yu, H.; Shan, A.; Jin, Y.; Fang, H.; Zhao, Y.; Shen, J.; Zhou, C.; Zhou, Y.; Fu, Y.; et al. Toxic effects of Zearalenone on intestinal microflora and intestinal mucosal immunity in mice. Food Agric. Immunol. 2018, 29, 1002–1011. [Google Scholar] [CrossRef]
- Girish, C.K.; Smith, T.K.; Boermans, H.J.; Anil Kumar, P.; Girgis, G.N. Effects of dietary Fusarium mycotoxins on intestinal lymphocyte subset populations, cell proliferation and histological changes in avian lymphoid organs. Food Chem. Toxicol. 2010, 48, 3000–3007. [Google Scholar] [CrossRef]
- Stewart, A.S.; Pratt-Phillips, S.; Gonzalez, L.M. Alterations in intestinal permeability: The role of the “leaky gut” in health and disease. J. Equine Vet. Sci. 2017, 52, 10–22. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, D.; Kim, J.; Moon, Y. Effects of Mycotoxins on mucosal microbial infection and related pathogenesis. Toxins 2015, 7, 4484–4502. [Google Scholar] [CrossRef]
- Awad, W.A.; Ruhnau, D.; Hess, C.; Doupovec, B.; Schatzmayr, D.; Hess, M. Feeding of deoxynivalenol increases the intestinal paracellular permeability of broiler chickens. Arch. Toxicol. 2019, 93, 2057–2064. [Google Scholar] [CrossRef]
- Verbrugghe, E.; Vandenbroucke, V.; Dhaenens, M.; Shearer, N.; Goossens, J.; De Saeger, S.; Eeckhout, M.; D’Herde, K.; Thompson, A.; Deforce, D.; et al. T-2 toxin induced Salmonella Typhimurium intoxication results in decreased Salmonella numbers in the cecum contents of pigs, despite marked effects on Salmonella-host cell interactions. Vet. Res. 2012, 43, 22. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H.; Wang, J.; Tsim, K.W.K.; Wang, Y.; Shen, X.; Lei, H.; Liu, Y. Aflatoxin B(1)-induced liver pyroptosis is mediated by disturbing the gut microbial metabolites: The roles of pipecolic acid and norepinephrine. J. Hazard. Mater. 2024, 474, 134822. [Google Scholar] [CrossRef]
- Neish, A.S. The gut microflora and intestinal epithelial cells: A continuing dialogue. Microbes Infect. 2002, 4, 309–317. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Xu, L.; Zhang, X. Maintaining the Balance of Intestinal Flora through the Diet: Effective Prevention of Illness. Foods 2021, 10, 2312. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Tian, Y.; Huang, C.; Li, D.; Zhong, Q.; Ma, X. Interaction between Microbes and Host Intestinal Health: Modulation by Dietary Nutrients and Gut-Brain-Endocrine-Immune Axis. Curr. Protein Pept. Sci. 2015, 16, 592–603. [Google Scholar] [CrossRef]
- Cai, J.; Sun, L.; Gonzalez, F.J. Gut microbiota-derived bile acids in intestinal immunity, inflammation, and tumorigenesis. Cell Host Microbe 2022, 30, 289–300. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Dang, A.T.; Marsland, B.J. Microbes, metabolites, and the gut-lung axis. Mucosal Immunol. 2019, 12, 843–850. [Google Scholar] [CrossRef]
- Xia, D.; Mo, Q.; Yang, L.; Wang, W. Crosstalk between mycotoxins and intestinal microbiota and the alleviation approach via microorganisms. Toxins 2022, 14, 859. [Google Scholar] [CrossRef]
- Jin, J.; Beekmann, K.; Ringø, E.; Rietjens, I.M.C.M.; Xing, F. Interaction between food-borne mycotoxins and gut microbiota: A review. Food Control 2021, 126, 107998. [Google Scholar] [CrossRef]
- Vignal, C.; Djouina, M.; Pichavant, M.; Caboche, S.; Waxin, C.; Beury, D.; Hot, D.; Gower-Rousseau, C.; Body-Malapel, M. Chronic ingestion of deoxynivalenol at human dietary levels impairs intestinal homeostasis and gut microbiota in mice. Arch. Toxicol. 2018, 92, 2327–2338. [Google Scholar] [CrossRef]
- Charlet, R.; Bortolus, C.; Sendid, B.; Jawhara, S. Bacteroides thetaiotaomicron and Lactobacillus johnsonii modulate intestinal inflammation and eliminate fungi via enzymatic hydrolysis of the fungal cell wall. Sci. Rep. 2020, 10, 11510. [Google Scholar] [CrossRef]
- Ragoubi, C.; Quintieri, L.; Greco, D.; Mehrez, A.; Maatouk, I.; D’Ascanio, V.; Landoulsi, A.; Avantaggiato, G. Mycotoxin Removal by Lactobacillus spp. Their Application in Animal Liquid Feed. Toxins 2021, 13, 185. [Google Scholar] [CrossRef]
- Piotrowska, M.; Sliżewska, K.; Nowak, A.; Zielonka, L.; Zakowska, Z.; Gajęcka, M.; Gajęcki, M. The effect of experimental fusarium mycotoxicosis on microbiota diversity in porcine ascending colon contents. Toxins 2014, 6, 2064–2081. [Google Scholar] [CrossRef]
- Deng, F.; Zhao, L.; Wei, P.; Mai, E.; Chen, M.; Yang, H.; Mu, P.; Wu, J.; Wen, J.; Deng, Y. Role and mechanism of the outer membrane porin LamB in T-2 mycotoxin-mediated extensive drug resistance in Escherichia coli. J. Hazard. Mater. 2024, 480, 136437. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Zhang, K.; Xue, W. The role of gut microbiota and its metabolites short-chain fatty acids in food allergy. Food Sci. Hum. Wellness 2023, 12, 702–710. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Wei, B.; Xiao, H.; Xu, B.; Kuca, K.; Qin, Z.; Guo, X.; Wu, W.; Wu, Q. Emesis to trichothecene deoxynivalenol and its congeners correspond to secretion of peptide YY and 5-HT. Food Chem. Toxicol. 2023, 178, 113874. [Google Scholar] [CrossRef]
- Larraufie, P.; Doré, J.; Lapaque, N.; Blottière, H.M. TLR ligands and butyrate increase Pyy expression through two distinct but inter-regulated pathways. Cell Microbiol. 2017, 19, e12648. [Google Scholar] [CrossRef]
- Zhou, L.; Ni, C.; Liao, R.; Tang, X.; Yi, T.; Ran, M.; Huang, M.; Liao, R.; Zhou, X.; Qin, D.; et al. Activating SRC/MAPK signaling via 5-HT1A receptor contributes to the effect of vilazodone on improving thrombocytopenia. eLife 2024, 13, RP94765. [Google Scholar] [CrossRef]
- Wang, J.; Bakker, W.; Zheng, W.; de Haan, L.; Rietjens, I.; Bouwmeester, H. Exposure to the mycotoxin deoxynivalenol reduces the transport of conjugated bile acids by intestinal Caco-2 cells. Arch. Toxicol. 2022, 96, 1473–1482. [Google Scholar] [CrossRef]
- Fang, Y.; Han, S.I.; Mitchell, C.; Gupta, S.; Studer, E.; Grant, S.; Hylemon, P.B.; Dent, P. Bile acids induce mitochondrial ROS, which promote activation of receptor tyrosine kinases and signaling pathways in rat hepatocytes. Hepatology 2004, 40, 961–971. [Google Scholar] [CrossRef]
- Zhang, F.L.; Ma, H.H.; Dong, P.Y.; Yan, Y.C.; Chen, Y.; Yang, G.M.; Shen, W.; Zhang, X.F. Bacillus licheniformis ameliorates Aflatoxin B1-induced testicular damage by improving the gut-metabolism-testis axis. J. Hazard. Mater. 2024, 468, 133836. [Google Scholar] [CrossRef]
- Guo, H.; Liu, T.; Li, J.; Li, E.; Wen, X.; Chen, F.; Li, S.; Li, Y.; Yin, Q.; Zhu, Q. Compound probiotics regulate the NRF2 antioxidant pathway to inhibit aflatoxin B(1)-induced autophagy in mouse Sertoli TM4 cells. Ecotoxicol. Environ. Saf. 2024, 281, 116619. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, Y.; Zuo, M.; Ding, S.; Li, J.; Feng, S.; Xiao, Y.; Tao, S. Novel mechanism by which extracellular vesicles derived from Lactobacillus murinus alleviates deoxynivalenol-induced intestinal barrier disruption. Environ. Int. 2024, 185, 108525. [Google Scholar] [CrossRef]
- Jiang, S.; Du, L.; Zhao, Q.; Su, S.; Huang, S.; Zhang, J. Tropical postbiotics alleviate the disorders in the gut microbiota and kidney damage induced by ochratoxin A exposure. Food Funct. 2024, 15, 3980–3992. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Chen, J.; Yang, S.; Li, P.; Wu, A.; Nepovimova, E.; Long, M.; Wu, W.; Kuca, K. MicroRNA regulates the toxicological mechanism of four mycotoxins in vivo and in vitro. J. Anim. Sci. Biotechnol. 2022, 13, 37. [Google Scholar] [CrossRef]
- Inui, M.; Martello, G.; Piccolo, S. MicroRNA control of signal transduction. Nat. Rev. Mol. Cell Biol. 2010, 11, 252–263. [Google Scholar] [CrossRef]
- Zuo, Y.B.; Zhang, Y.F.; Zhang, R.; Tian, J.W.; Lv, X.B.; Li, R.; Li, S.P.; Cheng, M.D.; Shan, J.; Zhao, Z.; et al. Ferroptosis in cancer progression: Role of noncoding RNAs. Int. J. Biol. Sci. 2022, 18, 1829–1843. [Google Scholar] [CrossRef]
- Rong, X.; Sun-Waterhouse, D.; Wang, D.; Jiang, Y.; Li, F.; Chen, Y.; Zhao, S.; Li, D. The significance of regulatory micrornas: Their roles in toxicodynamics of mycotoxins and in the protection offered by dietary therapeutics against mycotoxin-induced toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 48–66. [Google Scholar] [CrossRef]
- Grenier, B.; Hackl, M.; Skalicky, S.; Thamhesl, M.; Moll, W.D.; Berrios, R.; Schatzmayr, G.; Nagl, V. MicroRNAs in porcine uterus and serum are affected by zearalenone and represent a new target for mycotoxin biomarker discovery. Sci. Rep. 2019, 9, 9408. [Google Scholar] [CrossRef]
- Chuturgoon, A.A.; Phulukdaree, A.; Moodley, D. Fumonisin B1 modulates expression of human cytochrome P450 1b1 in human hepatoma (Hepg2) cells by repressing Mir-27b. Toxicol. Lett. 2014, 227, 50–55. [Google Scholar] [CrossRef]
- Rieswijk, L.; Brauers, K.J.; Coonen, M.L.; van Breda, S.G.; Jennen, D.G.; Kleinjans, J.C. Evaluating microRNA profiles reveals discriminative responses following genotoxic or non-genotoxic carcinogen exposure in primary mouse hepatocytes. Mutagenesis 2015, 30, 771–784. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Wang, H.; Zhang, Y.; Ji, M.; Xu, H.; Wang, C.; Sun, Z.; Gao, W.; Wang, S.L. miR-138-1* regulates aflatoxin B1-induced malignant transformation of BEAS-2B cells by targeting PDK1. Arch. Toxicol. 2016, 90, 1239–1249. [Google Scholar] [CrossRef]
- Liu, Y.X.; Long, X.D.; Xi, Z.F.; Ma, Y.; Huang, X.Y.; Yao, J.G.; Wang, C.; Xing, T.Y.; Xia, Q. MicroRNA-24 modulates aflatoxin B1-related hepatocellular carcinoma prognosis and tumorigenesis. BioMed Res. Int. 2014, 2014, 482926. [Google Scholar] [CrossRef]
- Marrone, A.K.; Tryndyak, V.; Beland, F.A.; Pogribny, I.P. Microrna responses to the genotoxic carcinogens aflatoxin B1 and benzo[a]pyrene in human HepaRG Cells. Toxicol. Sci. Off. J. Soc. Toxicol. 2016, 149, 496–502. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Qiao, W.; Zhou, J.; Hao, Z.; Oliveri Conti, G.; Velkov, T.; Tang, S.; Shen, J.; Dai, C. Mycotoxin-Caused Intestinal Toxicity: Underlying Molecular Mechanisms and Further Directions. Toxics 2025, 13, 625. https://doi.org/10.3390/toxics13080625
Li T, Qiao W, Zhou J, Hao Z, Oliveri Conti G, Velkov T, Tang S, Shen J, Dai C. Mycotoxin-Caused Intestinal Toxicity: Underlying Molecular Mechanisms and Further Directions. Toxics. 2025; 13(8):625. https://doi.org/10.3390/toxics13080625
Chicago/Turabian StyleLi, Tian, Weidong Qiao, Jiehong Zhou, Zhihui Hao, Gea Oliveri Conti, Tony Velkov, Shusheng Tang, Jianzhong Shen, and Chongshan Dai. 2025. "Mycotoxin-Caused Intestinal Toxicity: Underlying Molecular Mechanisms and Further Directions" Toxics 13, no. 8: 625. https://doi.org/10.3390/toxics13080625
APA StyleLi, T., Qiao, W., Zhou, J., Hao, Z., Oliveri Conti, G., Velkov, T., Tang, S., Shen, J., & Dai, C. (2025). Mycotoxin-Caused Intestinal Toxicity: Underlying Molecular Mechanisms and Further Directions. Toxics, 13(8), 625. https://doi.org/10.3390/toxics13080625







