Prenatal and Childhood Exposure to Humidifier Disinfectants and Attention-Deficit/Hyperactivity Disorder (ADHD): Insights from a Retrospective Cohort Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Populations
2.2. Outcome of Interest
2.3. Incidence of ADHD
2.4. Assessment of Exposure Characteristics
2.5. Covariates
2.6. Statistical Analysis
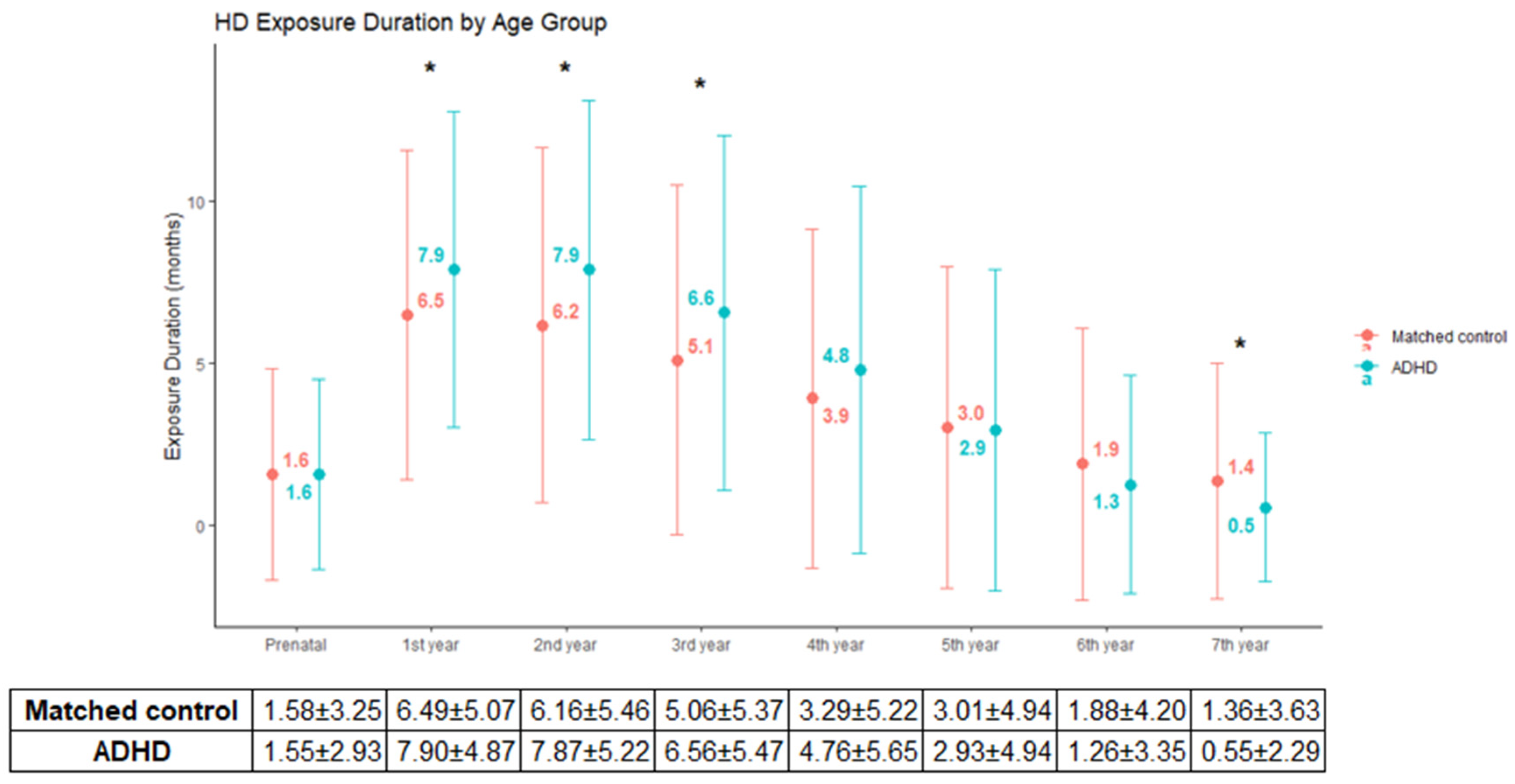
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grandjean, P.; Landrigan, P.J. Developmental neurotoxicity of industrial chemicals. Lancet 2006, 368, 2167–2178. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Kim, H.J. Epidemiologic research on lung damage caused by humidifier disinfectants. Epidemiol. Health 2016, 38, e2016031. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoon, J.; Cho, H.-J.; Lee, E.; Choi, Y.J.; Kim, Y.-H.; Lee, J.L.; Lee, Y.J.; Hong, S.-J. Rate of humidifier and humidifier disinfectant usage in Korean children: A nationwide epidemiologic study. Environ. Res. 2017, 155, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Austerman, J. ADHD and behavioral disorders: Assessment, management, and an update from DSM-5. Cleve. Clin. J. Med. 2015, 82, S2–S7. [Google Scholar] [CrossRef]
- Ilic, I.; Ilic, M. Global incidence of attention deficit/hyperactivity disorder among children. Biol. Life Sci. Forum 2022, 19, 6. [Google Scholar] [CrossRef]
- Williams, O.C.; Prasad, S.; McCrary, A.; Jordan, E.; Sachdeva, V.; Deva, S.; Kumar, H.; Mehta, J.; Neupane, P.; Gupta, A. Adult attention deficit hyperactivity disorder: A comprehensive review. Ann. Med. Surg. 2023, 85, 1802–1810. [Google Scholar] [CrossRef]
- Millichap, J.G. Etiologic classification of attention-deficit/hyperactivity disorder. Pediatrics 2008, 121, e358–e365. [Google Scholar] [CrossRef]
- Braun, J.M.; Kahn, R.S.; Froehlich, T.; Auinger, P.; Lanphear, B.P. Exposures to environmental toxicants and attention deficit hyperactivity disorder in U.S. children. Environ. Health Perspect. 2006, 114, 1904–1909. [Google Scholar] [CrossRef]
- Zhao, J.; He, T.; Wang, F.; Liu, W. Association of prenatal and postnatal exposure to air pollution with clinically diagnosed attention deficit hyperactivity disorder: A systematic review. Front. Public Health 2024, 12, 1396251. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.; Leem, J.; Lee, S.; Kim, Y.; Yoo, M. Household survey for the victims due to humidifier disinfectants. Seoul. Spec. Investig. Comm. Humidifier Disinfect. Disaster April 2019, 16, 59–111. [Google Scholar]
- Kim, J.H.; Park, S.; Ha, E.K.; Yon, D.K.; Lee, S.W.; Koh, H.Y.; Han, M.Y. Association between humidifier disinfectant exposure during infancy and subsequent neuropsychiatric outcomes during childhood: A nation-wide cross-sectional study. BMC Pediatr. 2021, 21, 340. [Google Scholar] [CrossRef] [PubMed]
- Korea Environmental Industry & Technology Institute. Korea Environmental Industry & Technology Institute Humidifier Disinfectant Damage Support Site. Available online: https://healthrelief.or.kr/home/main.do (accessed on 20 August 2024).
- Park, D.-U.; Ryu, S.-H.; Lim, H.-K.; Kim, S.-K.; Choi, Y.-Y.; Ahn, J.-J.; Lee, E.; Hong, S.-B.; Do, K.-H.; Cho, J.-l.; et al. Types of household humidifier disinfectant and associated risk of lung injury (HDLI) in South Korea. Sci. Total Environ. 2017, 596–597, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment. Special Law for Relieving Damage from Humidifier Disinfectants; Ministry of Environment: Sejong, Republic of Korea, 2017. Available online: https://www.me.go.kr/home/web/board/read.do?menuId=286&boardId=807010&boardMasterId=1 (accessed on 21 August 2024).
- Shin, D.W.; Cho, B.; Guallar, E. Korean national health insurance database. JAMA Intern. Med. 2016, 176, 138. [Google Scholar] [CrossRef]
- Seo, J.C.; Jon, D.I.; Shim, S.H.; Sung, H.M.; Woo, Y.S.; Hong, J.; Park, S.Y.; Seo, J.S.; Bahk, W.M. Prevalence and Comorbidities of Attention Deficit Hyperactivity Disorder Among Adults and Children/Adolescents in Korea. Clin. Psychopharmacol. Neurosci. 2022, 20, 126–134. [Google Scholar] [CrossRef]
- Kim, S.; Kim, M.-S.; Kim, J.; Kim, J.S.; Hong, S.-B. Attention-deficit/hyperactivity disorder and subsequent diagnoses of major psychiatric disorders: A nationwide population-based study. Eur. Child Adolesc. Psychiatry 2024, 1–12. [Google Scholar] [CrossRef]
- Global Burden of Disease Collaborative Network. Global Burden of Disease Study 2019 (GBD 2019) Results; Institute for Health Metrics and Evaluation (IHME). Available online: http://ghdx.healthdata.org/gbd-results-tool (accessed on 30 August 2024).
- Kaas, T.H.; Vinding, R.K.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. Association between childhood asthma and attention deficit hyperactivity or autism spectrum disorders: A systematic review with meta-analysis. Clin. Exp. Allergy 2021, 51, 228–252. [Google Scholar] [CrossRef]
- Giannouli, V.; Markopoulou, A.; Kiosseoglou, G.; Kosmidis, M.H. Neuropsychological functioning in patients with interstitial lung disease. Appl. Neuropsychol. Adult 2022, 29, 1290–1295. [Google Scholar] [CrossRef]
- Korea Ministry of Government Legislation. Enforcement Decree of the Special Act on Remedy for Damage Caused by Humidifier Disinfectants. Available online: https://www.law.go.kr/%EB%B2%95%EB%A0%B9/%EA%B0%80%EC%8A%B5%EA%B8%B0%EC%82%B4%EA%B7%A0%EC%A0%9C%ED%94%BC%ED%95%B4%EA%B5%AC%EC%A0%9C%EB%A5%BC%EC%9C%84%ED%95%9C%ED%8A%B9%EB%B3%84%EB%B2%95%EC%8B%9C%ED%96%89%EB%A0%B9 (accessed on 3 September 2024).
- Ha, M.; Park, T.; Lee, J.H.; Kim, Y.; Lim, J.; Baek, Y.W.; Yu, S.; Chung, H.M.; Chung, K.H.; Cheong, H.K.; et al. Evidence integration on health damage for humidifier disinfectant exposure and legal presumption of causation. Epidemiol. Health 2023, 45, e2023095. [Google Scholar] [CrossRef]
- Sharma, A.; Couture, J. A review of the pathophysiology, etiology, and treatment of attention-deficit hyperactivity disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef]
- Cho, K.-H.; Kim, J.-R. Comparison study of dermal cell toxicity and zebrafish brain toxicity by humidifier sterilizer chemicals (PHMG, PGH, CMIT/MIT). Korean J. Environ. Biol. 2020, 38, 271–277. [Google Scholar] [CrossRef]
- Kim, H.; Ji, K. Exposure to humidifier disinfectants induces developmental effects and disrupts thyroid endocrine systems in zebrafish larvae. Ecotoxicol. Environ. Saf. 2019, 184, 109663. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.-N.; Park, S.; Lee, S.; Chun, H.-S.; Shin, W.-H.; Kim, W.-K. In vitro neurotoxicity evaluation of biocidal disinfectants in a human neuron–astrocyte co-culture model. Toxicol. Vitr. 2022, 84, 105449. [Google Scholar] [CrossRef] [PubMed]
- Rodier, P.M. Developing brain as a target of toxicity. Environ. Health Perspect. 1995, 103, 73–76. [Google Scholar] [PubMed]
- Lanphear, B.P. The impact of toxins on the developing brain. Annu. Rev. Public Health 2015, 36, 211–230. [Google Scholar] [CrossRef] [PubMed]

| N (%) or Mean ± SD | ADHD | Control | p | Total |
|---|---|---|---|---|
| (N = 84) | (N = 1513) | (N = 1597) | ||
| Sex | <0.001 | |||
| M | 66 (78.6%) | 864 (57.1%) | 930 (58.2%) | |
| F | 18 (21.4%) | 649 (42.9%) | 667 (41.8%) | |
| Birth year | 2006.4 ± 2.8 | 2006.7 ± 2.7 | 0.397 | 2006.7 ± 2.7 |
| Urbanization | 0.258 | |||
| Other place | 77 (91.7%) | 1313 (86.8%) | 1390 (87.0%) | |
| Rural area | 7 (8.3%) | 200 (13.2%) | 207 (13.0%) | |
| Birth season | 0.069 | |||
| Winter (December~February) | 17 (20.2%) | 406 (26.8%) | 378 (23.7%) | |
| Spring (March~May) | 20 (23.8%) | 371 (24.5%) | 391 (24.5%) | |
| Summer (June~Auguest) | 14 (16.7%) | 375 (24.8%) | 389 (24.4%) | |
| Fall (September~November) | 33 (39.3%) | 361 (23.9%) | 439 (27.5%) | |
| Exposure start age at month | 2.8 ± 10.0 | 5.5 ± 15.7 | 0.023 | 5.3 ± 15.4 |
| Exposure end age at month | 38.6 ± 27.1 | 37.7 ± 28.1 | 0.773 | 37.7 ± 28.1 |
| Antenatal exposure duration | 1.6 ± 2.9 | 1.9 ± 3.8 | 0.382 | 1.8 ± 3.7 |
| Cumulative exposure duration (before diagnosis ADHD), month | 33.5 ± 23.8 | 32.2 ± 26.2 | 0.651 | 32.3 ± 26.1 |
| Cumulative exposure duration (before diagnosis ADHD), Tertile | 0.595 | |||
| -T1 (≥1, <16) | 26 (31.0%) | 528 (34.9%) | 554 (34.7%) | |
| -T2 (≥16, <39) | 27 (32.1%) | 504 (33.3%) | 531 (33.2%) | |
| -T3 (≥39, <145) | 31 (36.9%) | 481 (31.8%) | 512 (32.1%) | |
| Cumulative exposure hours (before diagnosis ADHD) | 14,391.9 ± 14,078.7 | 12,265.8 ± 12,521.6 | 0.133 | 12,377.6 ± 12,612.3 |
| Cumulative exposure hours (before diagnosis ADHD), Tertile | 0.231 | |||
| -T1 (≥48, <4704) | 21 (25.0%) | 515 (34.0%) | 536 (33.6%) | |
| -T2 (≥4704, <13,244) | 31 (36.9%) | 499 (33.0%) | 530 (33.2%) | |
| -T3 (≥13,244, <91,392) | 32 (38.1%) | 499 (33.0%) | 531 (33.2%) | |
| Usage of PHMG/PGH | 0.471 | |||
| N | 12 (14.3%) | 168 (11.1%) | 180 (11.3%) | |
| Y | 72 (85.7%) | 1345 (88.9%) | 1417 (88.7%) | |
| Distance to humidifier | 0.296 | |||
| under 1 m | 60 (71.4%) | 987 (65.2%) | 1047 (65.6%) | |
| over 1 m | 24 (28.6%) | 526 (34.8%) | 550 (34.4%) | |
| Location of humidifier | 0.736 | |||
| Close to nose and mouth | 57 (67.9%) | 990 (65.4%) | 1047 (65.6%) | |
| Other locations | 27 (32.1%) | 523 (34.6%) | 550 (34.4%) | |
| Interstitial lung disease | 0.104 | |||
| N | 75 (89.3%) | 1426 (94.2%) | 1501 (94.0%) | |
| Y | 9 (10.7%) | 87 (5.8%) | 96 (6.0%) | |
| Asthma | 0.471 | |||
| N | 17 (20.2%) | 251 (16.6%) | 268 (16.8%) | |
| Y | 67 (79.8%) | 1262 (83.4%) | 1329 (83.2%) | |
| Preterm birth | 0.018 | |||
| N | 76 (90.5%) | 1457 (96.3%) | 1533 (96.0%) | |
| Y | 8 (9.5%) | 56 (3.7%) | 64 (4.0%) |
| Incidence (/1000 PY) | Male | Female | Total | ||
|---|---|---|---|---|---|
| Incidents | Incidence (95%CI) | Incidents | Incidence (95%CI) | Incidence (95%CI) | |
| 0–5 years-old | 7 | 1.23 (0.50–2.22) | 3 | 0.74 (0.15–1.62) | 1.03 (0.49–1.72) |
| 6–10 years-old | 61 | 13.33 (10.20–16.85) | 15 | 4.52 (2.53–6.99) | 9.63 (7.58–11.89) |
| Total | 68 | 6.63 (5.15–8.28) | 18 | 2.44 (1.45–3.66) | 4.88 (3.90–5.96) |
| N (%) or Mean ± SD | ADHD | Matched Control | p | Matched Total |
|---|---|---|---|---|
| (N = 84) | (N = 420) | (N = 504) | ||
| Sex | 1 | |||
| M | 66 (78.6%) | 330 (78.6%) | 396 (78.6%) | |
| F | 18 (21.4%) | 90 (21.4%) | 108 (21.4%) | |
| Birth year | 2006.4 ± 2.8 | 2006.4 ± 2.8 | 1 | 2006.4 ± 2.8 |
| Urbanization | 0.417 | |||
| Other place | 77 (91.7%) | 369 (87.9%) | 446 (88.5%) | |
| Rural area | 7 (8.3%) | 51 (12.1%) | 58 (11.5%) | |
| Birth season | 0.127 | |||
| Winter (December~February) | 17 (20.2%) | 109 (26.0%) | 126 (25.0%) | |
| Spring (March~May) | 20 (23.8%) | 98 (23.3%) | 118 (23.4%) | |
| Summer (June~Auguest) | 14 (16.7%) | 98 (23.3%) | 112 (22.2%) | |
| Fall (September~November) | 33 (39.3%) | 115 (27.4%) | 148 (29.4%) | |
| Exposure start age at month | 2.8 ± 10.0 | 7.7 ± 17.3 | <0.001 | 6.9 ± 16.4 |
| Exposure end age at month | 38.6 ± 27.1 | 38.6 ± 28.7 | 0.994 | 38.6 ± 28.4 |
| Antenatal exposure duration | 1.6 ± 2.9 | 1.6 ± 3.3 | 0.955 | 1.6 ± 3.2 |
| Cumulative exposure duration (before diagnosis ADHD), month | 33.5 ± 23.8 | 30.8 ± 25.8 | 0.374 | 31.3 ± 25.4 |
| Cumulative exposure duration (before diagnosis ADHD), Tertile | 0.366 | |||
| -T1 (≥1, <16) | 26 (31.0%) | 156 (37.1%) | 182 (36.1%) | |
| -T2 (≥16, <39) | 27 (32.1%) | 140 (33.3%) | 167 (33.1%) | |
| -T3 (≥39, <145) | 31 (36.9%) | 124 (29.5%) | 155 (30.8%) | |
| Cumulative exposure hours (before diagnosis ADHD) | 14,391.9 ± 14,078.7 | 11,189.1 ± 11,149.4 | 0.052 | 11,722.9 ± 11,733.9 |
| Cumulative exposure hours (before diagnosis ADHD), Tertile | 0.147 | |||
| -T1 (≥48, <4704) | 21 (25.0%) | 151 (36.0%) | 172 (34.1%) | |
| -T2 (≥4704, <13,244) | 31 (36.9%) | 138 (32.9%) | 169 (33.5%) | |
| -T3 (≥13,244, <91,392) | 32 (38.1%) | 131 (31.2%) | 163 (32.3%) | |
| Usage of PHMG/PGH | 0.371 | |||
| N | 12 (14.3%) | 43 (10.2%) | 55 (10.9%) | |
| Y | 72 (85.7%) | 377 (89.8%) | 449 (89.1%) | |
| Distance to humidifier | 0.099 | |||
| under 1 m | 60 (71.4%) | 257 (61.2%) | 317 (62.9%) | |
| over 1 m | 24 (28.6%) | 163 (38.8%) | 187 (37.1%) | |
| Location of humidifier | 0.617 | |||
| Close to nose and mouth | 57 (67.9%) | 270 (64.3%) | 327 (64.9%) | |
| Other locations | 27 (32.1%) | 150 (35.7%) | 177 (35.1%) | |
| Interstitial lung disease | 0.517 | |||
| N | 75 (89.3%) | 387 (92.1%) | 462 (91.7%) | |
| Y | 9 (10.7%) | 33 (7.9%) | 42 (8.3%) | |
| Asthma | 0.601 | |||
| N | 17 (20.2%) | 72 (17.1%) | 89 (17.7%) | |
| Y | 67 (79.8%) | 348 (82.9%) | 49.7 ± 36.0 | |
| Preterm birth | 0.246 | |||
| N | 76 (90.5%) | 397 (94.5%) | 473 (93.8%) | |
| Y | 8 (9.5%) | 23 (5.5%) | 31 (6.2%) |
| Variables | Model1 HR (95%CI) | Model2 HR (95%CI) |
|---|---|---|
| From the end of exposure | ||
| Usage of PHMG/PGH, | ||
| Non PHMG/PGH | 1.00 (reference) | 1.00 (reference) |
| PHMG/PGH user | 0.67 (0.36–1.24) | 0.70 (0.37–1.31) |
| Distance to humidifier | ||
| Under 1 m | 1.00 (reference) | 1.00 (reference) |
| Over 1 m | 0.63 (0.38–1.04) | 0.64 (0.39–1.06) |
| Location of humidifier | ||
| close to nose and mouth | 1.00 (reference) | 1.00 (reference) |
| Others | 0.89 (0.55–1.44) | 0.94 (0.58–1.52) |
| Cumulative exposure duration (before diagnosis ADHD), Tertile | ||
| -T1 (≥1, <16) | 1.00 (reference) | 1.00 (reference) |
| -T2 (≥16, <39) | 1.12 (0.65–1.94) | 1.43 (0.77–2.64) |
| -T3 (≥39, <145) | 1.48 (0.85–2.59) | 2.86 (1.167–7.01) |
| Cumulative exposure hours (before diagnosis ADHD), Tertile | ||
| -T1 (≥48, <4704) | 1.00 (reference) | 1.00 (reference) |
| -T2 (≥4704, <13,244) | 1.56 (0.88–2.76) | 1.82 (1.01–3.27) |
| -T3 (≥13,244, <91,392) | 1.73 (0.97–3.10) | 2.15 (1.06–4.33) |
| Annual exposure duration | ||
| Prenatal period | 1.00 (0.93–1.07) | 1.00 (0.93–1.07) |
| 1st year | 1.06 (1.01–1.11) | 1.06 (1.01–1.11) |
| 2nd year | 1.06 (1.02–1.10) | 1.06 (1.02–1.11) |
| 3rd year | 1.05 (1.01–1.09) | 1.05 (1.01–1.09) |
| 4th year | 1.03 (0.99–1.07) | 1.03 (0.99–1.08) |
| 5th year | 1.00 (0.96–1.05) | 1.01 (0.96–1.06) |
| 6th year | 0.98 (0.92–1.04) | 0.99 (0.91–1.06) |
| 7th year | 0.92 (0.82–1.03) | 0.92 (0.80–1.06) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, H.; Lee, H.; Ahn, Y.-S. Prenatal and Childhood Exposure to Humidifier Disinfectants and Attention-Deficit/Hyperactivity Disorder (ADHD): Insights from a Retrospective Cohort Design. Toxics 2025, 13, 78. https://doi.org/10.3390/toxics13020078
Choi H, Lee H, Ahn Y-S. Prenatal and Childhood Exposure to Humidifier Disinfectants and Attention-Deficit/Hyperactivity Disorder (ADHD): Insights from a Retrospective Cohort Design. Toxics. 2025; 13(2):78. https://doi.org/10.3390/toxics13020078
Chicago/Turabian StyleChoi, Hyowon, Hunju Lee, and Yeon-Soon Ahn. 2025. "Prenatal and Childhood Exposure to Humidifier Disinfectants and Attention-Deficit/Hyperactivity Disorder (ADHD): Insights from a Retrospective Cohort Design" Toxics 13, no. 2: 78. https://doi.org/10.3390/toxics13020078
APA StyleChoi, H., Lee, H., & Ahn, Y.-S. (2025). Prenatal and Childhood Exposure to Humidifier Disinfectants and Attention-Deficit/Hyperactivity Disorder (ADHD): Insights from a Retrospective Cohort Design. Toxics, 13(2), 78. https://doi.org/10.3390/toxics13020078








