Protective Effects of Resveratrol Against Perfluorooctanoic Acid-Induced Testicular and Epididymal Toxicity in Adult Rats Exposed During Their Prepubertal Period
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Procurement and Maintenance of Experimental Animals
2.3. Experimental Design
2.4. Fertility Studies
2.5. Collection of Blood and Necropsy
2.6. Spermiogram Analysis
2.7. Testicular Cholesterol Levels (TCLs)
2.8. Analysis of Indicators of Oxidative Stress
2.9. Activity of Caspase-3
2.10. Testicular Steroidogenic Marker Enzymes Assay
2.11. Estimation of Proteins
2.12. Molecular Studies
2.13. RT-qPCR Studies to Validate Transcriptomic Data
2.14. Testicular Histology
2.15. Circulatory Levels of Reproductive Hormones
2.16. Testosterone
2.17. Serum FSH and LH
2.18. Statistical Analysis
3. Results
3.1. General Observations
3.2. Effect of Prepubertal Exposure to PFOA on Fertility in Adult Rats With or Without RES Supplementation
3.3. Effect of Prepubertal Exposure to PFOA on Body Weight and Tissue Somatic Indices in Adult Rats With or Without RES Supplementation
3.4. Effect of Prepubertal Exposure to PFOA on Qualitative and Quantitative Sperm Parameters, Testicular Steroidogenesis, and Serum Reproductive Hormones in Adult Rats With or Without RES Supplementation
3.5. Effect of Prepubertal Exposure to PFOA on the Histology of Testis in Adult Rats With or Without RES Supplementation
3.6. Effect of Prepubertal Exposure to PFOA on Pro- and Anti-Oxidant Status in the Testes and Epididymal Regions of Adult Rats With or Without RES Supplementation
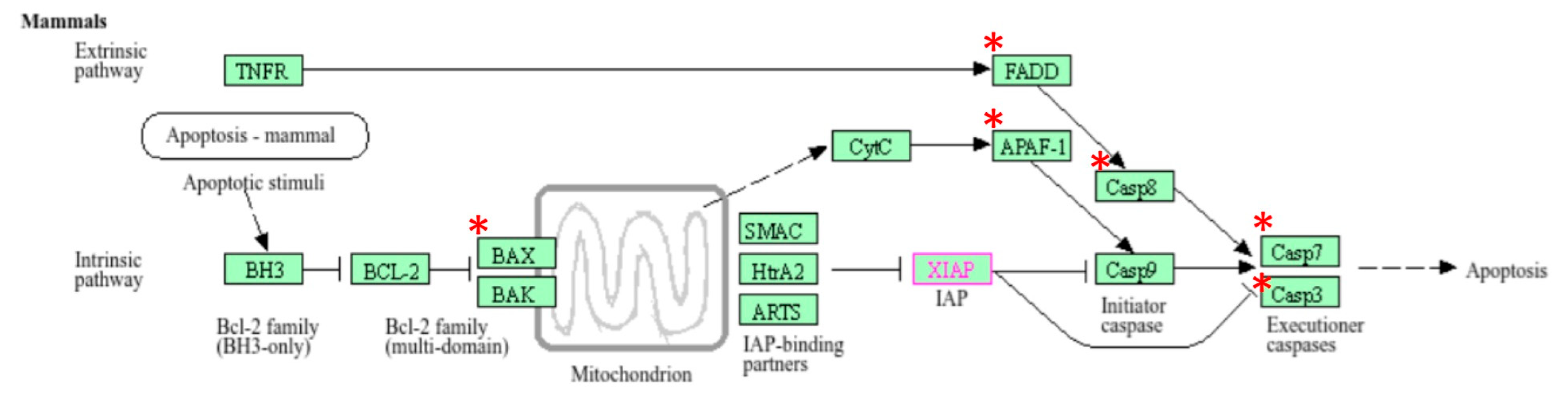
3.7. Effect of Prepubertal Exposure to Perfluorooctanoic Acid (PFOA) on Activity Levels of Caspase-3 in Rat Testes at Their Adulthood
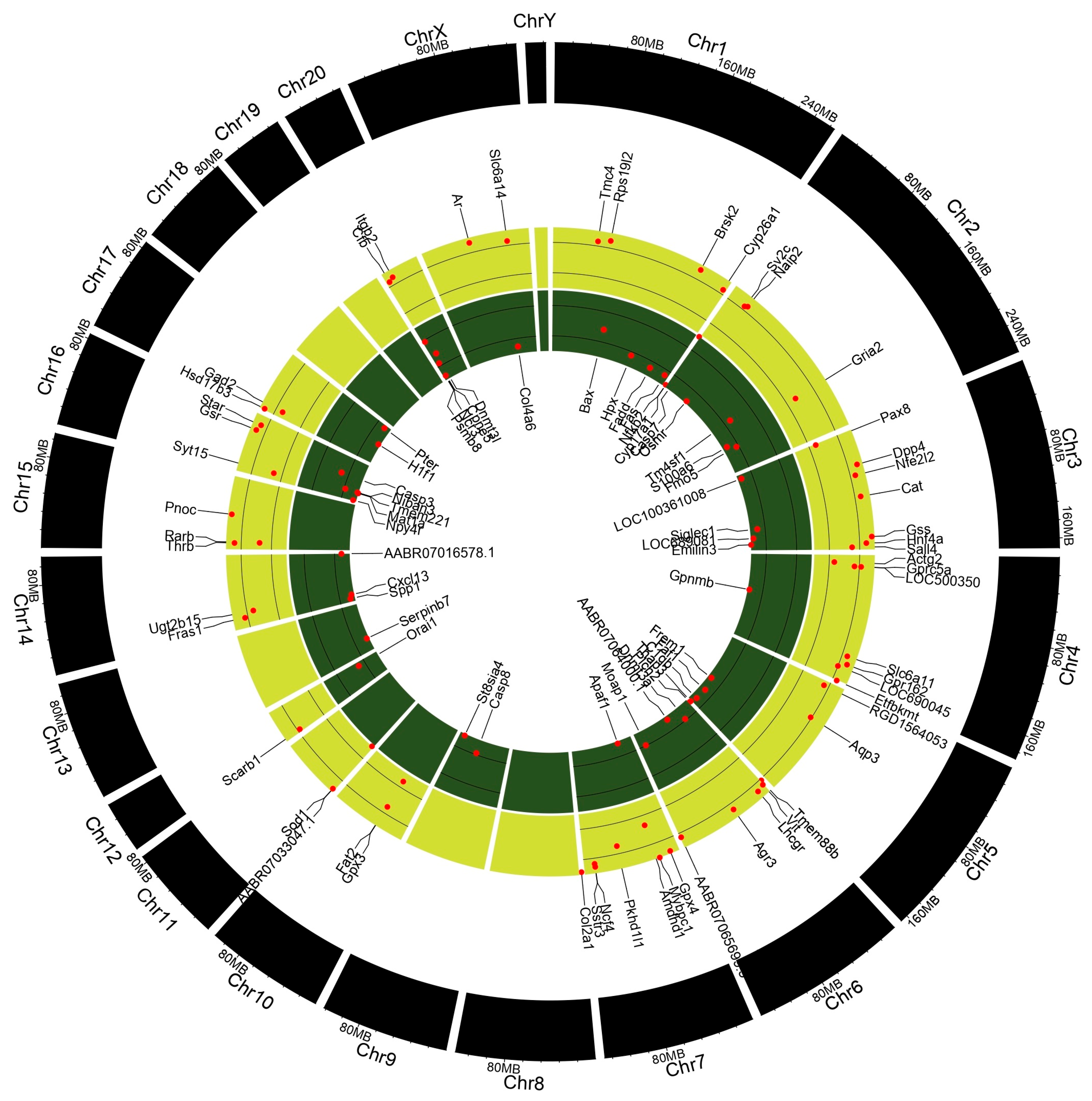
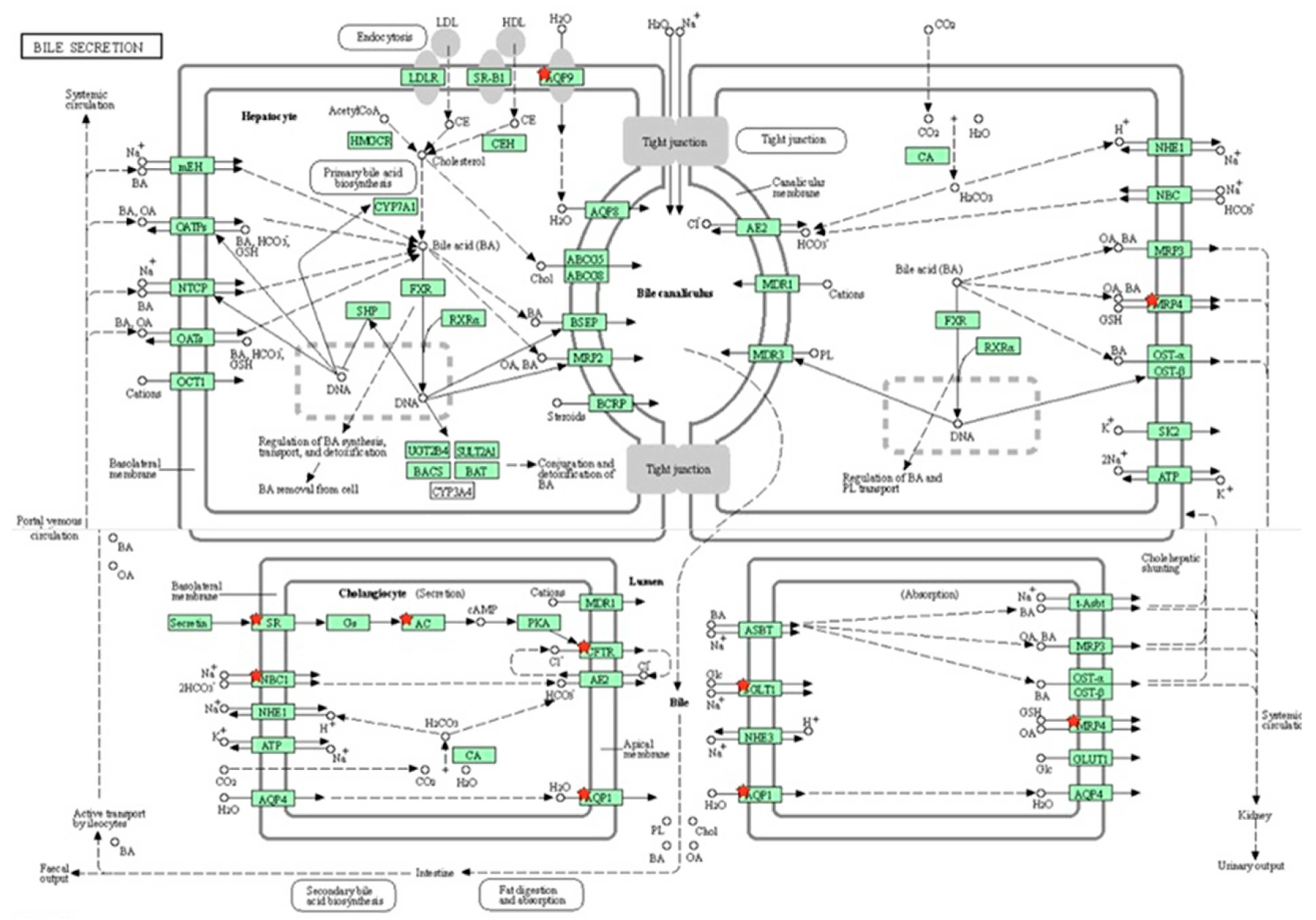
3.8. Gene Enrichment Analysis
3.9. Differential Gene Expression Analysis
3.10. Validation of RNA-Seq Data Using RT-qPCR
3.11. Gene Expression Studies
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selvaraju, V.; Baskaran, S.; Agarwal, A.; Henkel, R. Environmental contaminants and male infertility: Effects and mechanisms. Andrologia 2020, 53, e13646. [Google Scholar] [CrossRef]
- Buck, R.C.; Franklin, J.; Berger, U.; Conder, J.M.; Cousins, I.T.; Voogt, P.D.; Jensen, A.A.; Kannan, K.; Mabury, S.A.; van Leeuwen, S.P. Perfluoroalkyl and polyfluoroalkyl substances in the environment: Terminology, classification, and origins. Integr. Env. Assess. Manag. 2011, 7, 513–541. [Google Scholar] [CrossRef]
- Eggert, A.; Cisneros-Montalvo, S.; Anandan, S.; Musilli, S.; Stukenborg, J.-B.; Adamsson, A.; Nuemio, M.; Toppari, J. The effects of perfluorooctanoic acid (PFOA) on fetal and adult rat testis. Reprod. Toxicol. 2019, 90, 68–76. [Google Scholar] [CrossRef]
- Li, Y.; Fletcher, T.; Mucs, D.; Scott, K.; Lindh, C.H.; Tallving, P.; Jakobsson, K. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup. Environ. Med. 2018, 75, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Fraser, A.J.; Webster, T.F.; Watkins, D.J.; Nelson, J.W.; Stapleton, H.M.; Calafat, A.M.; Kato, K.; Shoeib, M.; Vieira, V.M.; McClean, M.D. Polyfluorinated compounds in serum linked to indoor air in office environments. Environ. Sci. Technol. 2012, 46, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Post, G.B.; Cohn, P.D.; Cooper, K.R. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: A critical review of recent literature. Environ. Res. 2013, 116, 93–117. [Google Scholar] [CrossRef]
- ATSDR (Agency for Toxic Substances and Disease Registry). Perfluoroalkyl and Polyfluor-Oalkyl Substances (PFAS) in the U.S. Population; ATSDR: Atlanta, GA, USA, 21 August 2017. [Google Scholar]
- Kaboré, H.A.; Vo Duy, S.; Munoz, G.; Méité, L.; Desrosiers, M.; Liu, J.; Sory, T.K.; Sauvé, S. Worldwide drinking water occurrence and levels of newly-identified perfluoroalkyl and polyfluoroalkyl substances. Sci. Total Environ. 2018, 616–617, 1089–1100. [Google Scholar] [CrossRef] [PubMed]
- Crone, B.C.; Speth, T.F.; Wahman, D.G.; Smith, S.J.; Abulikemu, G.; Kleiner, E.J.; Pressman, J.G. Occurrence of per- and polyfluoroalkyl substances (PFAS) in source water and their treatment in drinking water. Crit. Rev. Environ. Sci. Technol. 2019, 49, 2359–2396. [Google Scholar] [CrossRef] [PubMed]
- CDC. National Report on Human Exposure to Environmental Chemicals, Biomonitoring Data Tables for Environmental Chemicals; CDC: Atlanta, GA, USA, 2015. [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). Toxicological profile for perfluoroalkyls. In Draft for Public Comment; ATSDR: Atlanta, GA, USA, 2018. [Google Scholar]
- U.S. EPA. Drinking Water Health Advisory for Perfluorooctane Sulfonate (PFOS). In Office of Water Document 822-R-16-004; U.S. EPA: Washington, DC, USA, 2016. [Google Scholar]
- IARC Biennial Report 2016–2017. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Biennial-Reports/IARC-Biennial-Report-2016-2017 (accessed on 1 January 2020).
- Ruan, Y.; Lalwani, D.; Kwok, K.Y.; Yamazaki, E.; Taniyasu, S.; Kumar, N.J.I.; Lam, P.K.S.; Yamashita, N. Assessing exposure to legacy and emerging per and polyfluoroalkyl substances via hair—the first nationwide survey in India. Chemosphere 2019, 229, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Land, M.; Wit, A.N.D.; Bignert, A.; Cousins, I.T.; Herzke, D.; Johansson, J.H.; Martin, J.W. What is the effect of phasing out long-chain per- and polyfluoroalkyl substances on the concentrations of perfluoroalkyl acids and their precursors in the environment? A systematic review. Environ. Evid. 2018, 7, 4. [Google Scholar] [CrossRef]
- Tarapore, P.; Ouyang, B. Perfluoroalkyl Chemicals and Male Reproductive Health: Do PFOA and PFOS Increase Risk for Male Infertility? Int. J. Environ. Res. Public Health 2021, 18, 3794. [Google Scholar] [CrossRef]
- Ducatman, A.; Tan, Y.; Nadeau, B.; Steenland, K. Perfluorooctanoic Acid (PFOA) Exposure and Abnormal Alanine Aminotransferase: Using Clinical Consensus Cutoffs Compared to Statistical Cutoffs for Abnormal Values. Toxics 2023, 11, 449. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yan, S.; Wang, P.; Chen, Q.; Liu, Y.; Cui, J.; Liang, Y.; Ren, S.; Gao, Y. Perfluorooctanoic acid (PFOA) exposure in relation to the kidneys: A review of current available literature. Front. Physiol. 2023, 14, 1103141. [Google Scholar] [CrossRef]
- Di Nisio, A.; Pannella, M.; Vogiatzis, S.; Sut, S.; Dall’Acqua, S.; Rocca, M.S.; Antonini, A.; Porzionato, A.; De Caro, R.; Bortolozzi, M.; et al. Impairment of human dopaminergic neurons at different developmental stages by perfluoro-octanoic acid (PFOA) and differential human brain areas accumulation of perfluoroalkyl chemicals. Environ. Int. 2022, 158, 106982. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Z.; Li, M.; Dong, H.; Li, J. Reproductive toxicity of PFOA, PFOS and their substitutes: A review based on epidemiological and toxicological evidence. Environ. Res. 2024, 250, 118485. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Awwad, O.; Rotondi, M.; Santini, F.; Imbriani, M.; Chiovato, L. Thyroid disruption by perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA). J. Endocrinol. Investig. 2017, 40, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Abudayyak, M.; Oztas, E.; Ozhan, G. Assessment of perfluorooctanoic acid toxicity in pancreatic cells. Toxicol. Vitr. 2021, 72, 105077. [Google Scholar] [CrossRef]
- Zhang, H.; Lu, Y.; Luo, B.; Yan, S.; Guo, X.; Dai, J. Proteomic analysis of mouse testis reveals perfluorooctanoic acid induced reproductive dysfunction via direct disturbance of testicular steroidogenic machinery. J. Proteome Res. 2014, 13, 3370–3385. [Google Scholar] [CrossRef]
- Liu, W.; Yang, B.; Wu, L.; Zou, W.; Pan, X.; Zou, T.; Liu, F.; Xia, L.; Wang, X.; Zhang, D. Involvement of NRF2 in Perfluorooctanoic Acid-Induced Testicular Damage in Male Mice. Biol. Reprod. 2015, 93, 41. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Li, D.; Wang, X.; Zhong, X. Effect of perfluorooctanoic acid exposure during pregnancy on the reproductive and development of male offspring mice. Andrology 2018, 50, 8. [Google Scholar]
- Lu, H.; Zhang, H.; Gao, J.; Li, Z.; Bao, S.; Chen, X.; Wang, Y.; Ge, R. Effect of perflurooctanoic acid on stem leydig cell function in the rat. Environ. Pollut. 2019, 250, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Owumi, S.E.; Akomolafe, A.P.; Imosemi, I.O.; Odunola, O.A.; Oyelere, A.K. N-acetyl cysteine co-treatment abates perfluorooctanoic acid-induced reproductive toxicity in male rats. Andrologia 2021, 53, e14037. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Cui, L.; Chen, L.; Zhang, J.; Zhang, X.; Kang, Q.; Jin, F.; Ye, Y. Parental plasma concentrations of perfluoroalkyl substances and in vitro fertilization outcomes. Environ. Pollut. 2021, 269, 116159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Mustieles, V.; Wang, Y.X.; Sun, Y.; Agudelo, J.; Bibi, Z.; Torres, N.; Oulhote, Y.; Slitt, A.; Messerlian, C. Folate concentrations and serum perfluoroalkyl and polyfluoroalkyl substance concentratios in adolescents and adult in the USA (National health and nutrition examination study 2003–2016): An observational study. Lancet 2023, 7, E449–E458. [Google Scholar]
- Couto-Santos, F.; Souza, A.C.F.; Bastos, D.S.S.; Ervilha, L.O.G.; Dias, F.C.R.; Araujo, L.D.S.; Guimaraes, S.E.F.; Oliveira, L.L.D.; Machado-Neves, M. Prepubertal exposure to arsenic alters male reproductive parameters in pubertal and adult rats. Toxicol. Appl. Pharmacol. 2020, 409, 115304. [Google Scholar] [CrossRef] [PubMed]
- Tung, B.T.; Rodriguez-Bies, E.; Ballesteros-Simarro, M.; Motilva, V.; Navas, P.; Lopez-Lluch, G. Modulation of endogenous antioxidant activity by resveratrol and excercise in mouse liver is age dependent. J. Gerontol. 2014, 69, 398–409. [Google Scholar] [CrossRef] [PubMed]
- Pallauf, K.; Rimbach, G.; Rupp, P.M.; Chin, D.; Wolf, I.M. Resveratrol and lifespan in model organisms. Curr. Med. Chem. 2016, 23, 4639–4680. [Google Scholar] [CrossRef]
- Gu, B.; Zhang, L.; Dingenen, R.V.; Vieno, M.; Grinsven, H.J.V.; Zhng, X.; Zhang, S.; Chen, Y.; Wang, S.; Ren, C.; et al. Abating ammonia is more cost-effective than nitrogen oxides for mitigating PM2.5 air pollution. Science 2021, 374, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Anti-oxidative action of resveratrol: Implications for human health. Arab. J. Chem. 2011, 4, 293–298. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-Edged sword in health benefits. Biomed. 2018, 6, 91. [Google Scholar] [CrossRef]
- Turedi, S.; Yulug, E.; Alver, A.; Kutlu, O.; Kahraman, C. Effects of resveratrol on doxorubicin induced testicular damage in rats. Exp. Toxicol. Pathol. 2014, 67, 229–235. [Google Scholar] [CrossRef]
- Prathap Reddy, K.; Madhu, P.; Sreenivasula Reddy, P. Protective effects of resveratrol against cisplatin-induced testicular and epididymal toxicity in rats. Food Chem. Toxicol. 2016, 91, 65–72. [Google Scholar] [CrossRef]
- Hamdy, A.A.A.; Basma, G.E. Cisplatin induced testicular damage through mitochondria mediated apoptosis, inflammation and oxidative stress in rats: Impact of resveratrol. Endocr. J. 2020, 67, 969–980. [Google Scholar]
- Jalili, C.; Salahshoor, M.R.; Jalili, F.; Kakabaraei, S.; Akrami, A.; Sohrabi, M.; Ahookhash, M.; Ghanbari, A. Therapeutic effect of resveratrol on morphine-induced damage in male reproductive system of mice by reducing nitric oxide serum levels. Int. J. Morphol. 2017, 35, 1342–1347. [Google Scholar] [CrossRef]
- Banerjee, B.; Chakraborty, S.; Ghosh, D.; Raha, S.; Sen, P.C.; Jana, K. Benzo(a)pyrene Induced P53 mediated male germ cell apoptosis: Synergistic protective effects of curcumin and resveratrol. Front. Pharmacol. 2016, 7, 245. [Google Scholar] [CrossRef]
- Banerjee, B.; Chakraborty, S.; Chakraborty, P.; Ghosh, D.; Jana, K. Protective effect of resveratrol on benzo(a)pyrene induced dysfunctions steroidogenesis and steroidogenic acute regulatory gene expression in leydig cells. Front. Endocrinol. 2019, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Bahmanzadeh, M.; Goodarzi, M.T.; Farimani, A.R.; Fathi, N.; Alizadeh, Z. Resveratrol supplementation improves DNA integrity and sperm parameters in streptozotocin-nicotinamide-induced type 2 diabetic rats. Andrologia 2019, 51, e13313. [Google Scholar] [CrossRef] [PubMed]
- Erthal, R.P.; Siervo, G.E.M.L.; Silveira, L.T.R.; Scarano, W.R.; Fernandes, G.S.A. Can resveratrol attenuate testicular damage in neonatal and adult rats exposed to 2,3,7, 8-tetrachlorodibenzo-p-dioxin during gestation? Reprod. Fertile Dev. 2018, 30, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Jalili, C.; Roshankhah, S.; Salahshoor, M.R.; Mohammadi, M.M. Resveratrol attenuates Malathion induced damage in some reproductive parameters by decreasing oxidative stress and lipid peroxidation in male rats. J. Fam. Reprod. Health 2019, 13, 70–79. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Liu, C.; Wu, J.; Wang, Y.; Yuan, L.; Du, X.; Wang, R.; Marwa, P.W.; Zhuang, D.; et al. Resveratrol Ameliorates Microcystin-LR-Induced Testis Germ Cell Apoptosis in Rats via SIRT1 Signaling Pathway Activation. Toxins 2018, 10, 235. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.M.; Alabiad, M.A.; El Shaer, D.F. Resveratrol ameliorates the seminiferous tubules damages induced by finasteride in adult male rats. Microsc. Microanal. 2020, 26, 1176–1186. [Google Scholar] [CrossRef] [PubMed]
- Said, N.I.; Abd-Elrazek, A.M.; El-Dash, H.A. The protective role of resveratrol against sulfoxaflor-induced toxicity in testis of adult male rats. Environ. Toxicol. 2021, 36, 2105–2115. [Google Scholar] [CrossRef]
- Nakamura, N.; Sloper, D.T.; Del Valle, P.L. Gene expression profiling of cultured mouse testis fragments treated with ethinylestradiol. J. Toxicol. Sci. 2019, 44, 667–679. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Huang, Q.; Wang, H.; Martin, F.L.; Liu, L.; Zhang, J.; Shen, H. Biphasic effects of perfluorooctanoic acid on steroidogenesis in mouse Leydig tumour cells. Reprod. Toxicol. 2019, 83, 54–62. [Google Scholar] [CrossRef]
- Louvandini, H.; Correa, P.S.; Amorin, R.; Liu, L.; Leda, E.H.; Jimenez, C.R.; Tsai, S.M.; McManus, C.M.; Penagaricano, F. Gestational and lactational exposure to gossypol alters the testis transcriptome. BMC Genom. 2020, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, K.; Zhang, D.; Zhao, Z.; Huang, J.; Zhou, L.; Feng, M.; Shi, J.; Wei, H.; Li, L.; et al. GPx6 is involved in the invitro induced capacitation and acrosome reaction in porcine sperm. Theriogenology 2020, 156, 107–115. [Google Scholar] [CrossRef]
- Prathima, P.; Venkaiah, K.; Pavani, R.; Shrikanya Rao, K.V.L.; Gopi Krishna, P.; Kishori, B.; Dirisala, V.R.; Adi Pradeepkiran, J.; Sainath, S.B. Transcriptomic profiling identified altered expression of genes associated with testicular functions in adult F1 rats exposed to carbimazole during fetal period. Proteomics 2023, 274, 104811. [Google Scholar] [CrossRef]
- Committee for the Purpose of control and supervision on experiments on animals, CPCSEA guidelines for laboratory animal facility. Indian J. Pharmacol. 2003, 35, 257–274.
- Chen, H.; Wang, Y.; Ge, R.; Zirkin, B.R. Leydig cell stem cells: Identification, proliferation and differentiation. Mol. Cell Endocrinol. 2017, 445, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Picut, C.A.; Ziejewski, M.K.; Stanislaus, D. Comparative aspects of pre and postnatal development of the male reproductive system. Birth Defects Res. 2018, 110, 190–227. [Google Scholar] [CrossRef] [PubMed]
- Agmo, A. Male rat sexual behaviour. Brain Res. Brain Res. Protoc. 1997, 2, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Robb, G.W.; Amann, R.P.; Killian, G.J. Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J. Reprod. Fertil. 1978, 54, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Blazak, W.F.; Treinen, K.A.; Juniewicz, P.E. Application of testicular sperm head counts in the assessment of male reproductive toxicity. In Methods in Toxicology, Volume 3, Male Reproductive Toxicology; Academic Press: Cambridge, MA, USA, 1993; pp. 86–94. [Google Scholar]
- Belsey, M.A.; Moshissi, K.S.; Eliasson, R.; Paulsen, C.A.; Callegos, A.J.; Prasad, M.R.N. Laboratory Manual for the Examination of Human Semen and Semen Cervical Mucus Interaction; Press Concern: Singapore, 1980; pp. 1–43. [Google Scholar]
- Talbot, P.; Chacon, R.S. A triple-stain technique for evaluating normal acrosome reactions of human sperm. J. Exp. Zool. 1981, 215, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Jeyendran, R.S.; Van Der Ven, H.H.; Zaneveld, L.J.D. The hypo—Osmotic swelling test, An update. Archiv. Androl. 1992, 29, 105–116. [Google Scholar] [CrossRef]
- Linder, R.E.; Strader, L.F.; Slott, V.L.; Suarez, J.K. Endpoints of spermatotoxicity in the rat after short duration exposures to fourteen reproductive toxicants. Reprod. Toxicol. 1992, 6, 491–505. [Google Scholar] [CrossRef]
- Miranda-Spooner, M.; Paccola, C.C.; Neves, F.M.; de Oliva, S.U.; Miraglia, S.M. Late reproductive analysis in rat male offspring exposed to nicotine during pregnancy and lactation. Andrology 2016, 4, 218–231. [Google Scholar] [CrossRef]
- Varshini, J.; Srinag, B.S.; Kalthur, G.; Krishnamurthy, H.; Kumar, P.; Rao, S.B.; Adiga, S.K. Poor sperm quality and advancing age are associated with increased sperm DNA damage in infertile men. Andrologia 2012, 44, 642–649. [Google Scholar] [CrossRef]
- Zlatkis, A.; Zak, B.; Boyle, A.J.; Mic, D. A new method for the direct determination of serum cholesterol. J. Lab. Clin. Med. 1953, 41, 486–492. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissue by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Pick, E.; Keisari, Y. Superoxide anion and H202 production by chemically elicited peritoneal macrophages induction by multiple nonphagocytic stimuli. Cell Immunol. 1981, 59, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Podczasy, J.J.; Wei, R. Reduction of iodinitrotetrazoliwn violet by superoxide radicals. Biochem. Biophys. Res. Commun. 1988, 150, 1294–1301. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidise. Methods Enzymol. 1955, 2, 764–775. [Google Scholar]
- Flohe, L.; Gunzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–121. [Google Scholar] [PubMed]
- Carlberg, I.; Mannervik, B. Purification and characterization of the flavoenzyme glutathione reductase from rat liver. J. Biol. Chem. 1975, 250, 5475–5480. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Beutler, E. The preparation of red cells for assay. In Red Cell Metabolism: A manual of Biochemical Methods; Beutler, E., Ed.; Grune and Straton Company: New York, NY, USA, 1975; pp. 8–18. [Google Scholar]
- Cid, C.; Alvarez-Cermeno, J.C.; Regidor, I.; Plaza, J.; Salinas, M.; Alcazar, A. Caspase inhibitors protect against neuronal apoptosis induced by cerebrospinal fluid from multiple sclerosis patients. J. Neuroimmunol. 2003, 136, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U. (Ed.) Methods of Enzymatic Analysis; Academic Press: New York, NY, USA, 1974; pp. 447–489. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. (Eds.) Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Huang, H.; Rong, H.; Li, X.; Tong, S.; Zhu, Z.; Niu, L.; Teng, M. The crystal structure and identification of NQM1/YGRo43C, a transaldolase from saccharomyces cerevisiae. Proteins 2008, 73, 1076–1081. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2019, 47, D419–D426. [Google Scholar] [CrossRef]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. g: Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]
- Kurowicka, B.; Chrusciel, M.; Zmijewska, A.; Doroszko, M.; Kotwica, G.; Rahman, N.A. Distinct testicular steroidogenic response mechanisms between neonatal and adult heat-acclimated male rats. Cell Physiol. Biochem. 2015, 35, 1729–1743. [Google Scholar] [CrossRef]
- Lee, S.O.; Ma, Z.; Yeh, C.-R.; Luo, J.; Lin, T.-H.; Lai, K.-P.; Yamashita, S.; Liang, L.; Tian, J.; Li, L.; et al. New therapy targeting differential androgen receptor signalling in prostate cancer stem/progenitor vs. Non-stem/progenitor cells. J. Mol. Cell Boil. 2013, 5, 14–26. [Google Scholar] [CrossRef]
- Ning, J.-Z.; Rao, T.; Cheng, F.; Yu, W.-M.; Ruan, Y.; Yuan, R.; Zhu, S.-M.; Du, Y.; Xiao, C.-C. Effect of varicocelectomy treatment on spermatogenesis and apoptosis via the induction of heat shock protein 70 in varicocele-induced rats. Mol. Med. Rep. 2017, 16, 1791–3004. [Google Scholar] [CrossRef] [PubMed]
- Elmallah, M.I.Y.; Elkhadragy, M.F.; Al-Olayan, E.M.; Abdel Moneim, A.E. Protective effect of fragaria ananassa crude extract on cadmium-induced lipid peroxidation, antioxidant enzymes suppression, and apoptosis in rat testes. Int. J. Mol. Sci. 2017, 18, 957. [Google Scholar] [CrossRef]
- El-Maddawy, Z.K.; El Naby, W.S.H. Effects of ivermectin and its combination with alpha lipoic acid on expression of IGFBP-3 and HSPA1 genes and male rat fertility. Andrologia 2017, 50, e12891. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Bancraft, J.D.; Stevens, A. Theory and Practice of Histological Techniques, 2nd ed.; Chruchill Livingstone: New York, NY, USA, 1982. [Google Scholar]
- Wang, Z.; Zhang, T.; Wu, J.; Wei, X.; Xu, A.; Wang, S.; Wang, Z. Male reproductive toxicity of perfluorooctanoate (PFOA): Rodent studies. Chemosphere 2021, 270, 128608. [Google Scholar] [CrossRef] [PubMed]
- Calvert, L.; Green, M.P.; De Luliis, G.N.; Dun, M.D.; Turner, B.D.; Clarke, B.O.; Eamens, A.L.; Roman, S.D.; Nixon, B. Assessment of the Emerging Threat Posed by Perfluoroalkyl and Polyfluoroalkyl Substances to Male Reproduction in Humans. Front. Endocrinol. 2022, 12, 799043. [Google Scholar] [CrossRef]
- Hussain, T.; Kandeel, M.; Metwally, E.; Murtaza, G.; Kalhoro, D.H.; Yin, Y.; Tan, B.; Chughtai, M.I.; Yaseen, A.; Afzal, A.; et al. Unraveling the harmful effect of oxidative stress on male fertility: A mechanistic insight. Front. Endocrinol. 2023, 14, 1070692. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.P.; Scholten, J.T.; Mzyk, A.; Reyes-San-Martin, C.; Llumbet, A.E.; Hamoh, T.; Arts, E.G.; Schirhagl, R.; Cantineau, A.E. Male subfertility and oxidative stress. Redox Biol. 2021, 46, 102071. [Google Scholar] [CrossRef]
- Dean, R.T.; Fu, S.; Stocker, R.; Davies, M.J. Biochemistry and pathology of radical mediated protein oxidation. Biochem. J. 1997, 324, 1–18. [Google Scholar] [CrossRef]
- Kolasa-Wołosiuk, A.; Tarnowski, M.; Baranowska-Bosiacka, I.; Chlubek, D.; Wiszniewska, B. Antioxidant enzyme expression of mRNA and protein in the epididymis of finasteride-treated male rat offspring during postnatal development. AMS 2019, 15, 797–810. [Google Scholar] [CrossRef]
- Tremellen, K. Oxidative stress and male infertility–a clinical perspective. Hum. Reprod. Update 2008, 14, 243–258. [Google Scholar] [CrossRef]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the role of glutathione on oxidative stress and infertility. JBRA Assist. Reprod. 2018, 22, 61–66. [Google Scholar] [CrossRef]
- Singh, S.; Singh, S.K. Prepubertal exposure to perfluorooctanoic acid interferes with spermatogenesis and steroidogenesis in male mice. Ecotoxicol. Environ. Saf. 2019, 170, 590–599. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Ren, X.; Zhang, X.; Griffin, N.; Liu, H.; Wang, L. Rutin ameliorates perfluorooctanoic acid-induced testicular injury in mice by reducing oxidative stress and improving lipid metabolism. Drug Chem. Toxicol. 2022, 46, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, F.; Zhou, Y.; Cheng, J.; Tang, Y.; Wang, L. Rutin activates AMPKα to ameliorate liver damage caused by perfluorooctanoic acid in mice. Wei Sheng Yan Jiu 2021, 50, 609–614. [Google Scholar] [PubMed]
- Yang, B.; Zou, W.; Hu, Z.; Liu, F.; Zhou, L.; Yang, S.; Kuang, H.; Wu, L.; Wei, J.; Wang, J.; et al. Involvement of Oxidative Stress and Inflammation in Liver Injury Caused by Perfluorooctanoic Acid Exposure in Mice. BioMed Res. Int. 2014, 2014, 409837. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Liu, W.; Yang, B.; Wu, L.; Yang, J.; Zou, T.; Liu, F.; Xia, L.; Zhang, D. Quercetin protects against perfluorooctanoic acid-induced liver injury by attenuating oxidative stress and inflammatory response in mice. Int. Immunopharmacol. 2015, 28, 129–135. [Google Scholar] [CrossRef]
- Naderi, M.; Seyedabadi, M.; Amiri, F.T.; Akbari, S.; Akbari, S.; Shaki, F. Rutin mitigates perfluorooctanoic acid-induced liver injury via modulation of oxidative stress, apoptosis, and inflammation. Iran. J. Basic Med. Sci. 2023, 26, 1291–1297. [Google Scholar]
- Kamendulis, L.M.; Hocevar, J.M.; Stephens, M.; Sandusky, G.E.; Hocevar, B.A. Exposure to perfluorooctanoic acid leads to promotion of pancreatic cancer. Carcinogenesis 2022, 43, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Endirlik, B.U.; Eken, A.; Ozturk, F.; Gurbay, A. Assessing perfluorooctanoic acid toxicity in lung, heart, and testis tissues of mice: Evaluation of protective effects of taurine and coenzyme Q10. J. Res. Pharm. 2022, 27, 2630–6344. [Google Scholar]
- Ya-ping, Z.; Qian-hua, Z.; Hui-hui, W.; Bao-lu, L.; Shu-hua, Z.; Peng-gao, L.I. Effect of oral perfluorooctanoic acid exposure on serum total antioxidant capacity in SD rats. J. Food Microbiol. Saf. Hyg. 2013, 25, 509–511. [Google Scholar]
- Shaha, C.; Tripathi, R.; Mishra, D.P. Male germ cell apoptosis: Regulation and biology. Philos. Trans. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1501–1515. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Baker, M.A. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. J. Dev. Biol. 2013, 57, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Juarez-Rojas, A.L.; Garcia-Lorenzana, M.; Aragon-martinez, A.; Gomez-quiroz, L.E.; Retana-Marquez, M.D.S. Intrinsic and extrinsic apoptotic pathways are involved in rat testis by cold water immersion-induced acute and chronic stress. Syst. Biol. Reprod. Med. 2015, 61, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.H.; Lu, C.C.; Xu, C.; Chen, G.; Lin Qiu, L.; Jiang, J.K.; Ben, S.; Wang, Y.B.; Gu, A.H.; Wang, X.R. Perfluorooctanoate sulfonate-induced testicular toxicity and differential testicular expression of estrogen receptor in male mice. Environ. Toxicol. Pharmacol. 2016, 45, 150–157. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, Z.; Fang, X.; Xu, M.; Dai, J. Perfluorononanoic acid induces apoptosis involving the Fas death receptor signaling pathway in rat testis. Toxicol. Lett. 2009, 90, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.Y.; Xu, X.L.; Shen, X.Y.; Ruan, Q.; Hu, W.L. Analysis of apoptosis induced by perfluorooctane sulfonates (PFOS) in mouse leydig cells in vitro. Toxicol. Mech. Methods 2015, 25, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ge, S.; Lv, Z.; Wu, M.; Kuang, H.; Yang, B.; Yang, J.; Wu, L.; Zou, W.; Zhang, D. Attenuation of perfluorooctanoic acid-induced testicular oxidative stress and apoptosis by quercetin in mice. RSC. Adv. 2017, 7, 45045–45052. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, L.; Yue, J.Q.; Lv, Z.Q.; Xia, W.; Wan, Y.J.; Li, Y.Y.; Xu, S.Q. Prenatal PFOS exposure induces oxidative stress and apoptosis in the lung of rat off-spring. Repro. Toxicol. 2012, 33, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Suh, K.S.; Choi, E.M.; Kim, Y.J.; Hong, S.M.; Park, S.Y.; Rhee, S.Y.; Oh, S.; Kim, S.W.; Pak, Y.K.; Choe, W.; et al. Perfluorooctanoic acid induces oxidative damage and mitochondrial dysfunction in pancreatic β-cells. Mol. Med. Rep. 2017, 15, 3871–3878. [Google Scholar] [CrossRef]
- Xu, M.; Liu, G.; Li, M.; Huo, M.; Zong, W.; Liu, R. Probing the cell apoptosis pathway induced by perfluorooctanoic acid and perfluorooctane sulfonate at the subcellular and molecular levels. J. Agric. Food Chem. 2020, 68, 633–641. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, H.; Lin, C.; Mao, Y.; Rao, J.; Lou, Y.; Yang, X.; Xu, X.R.; Jin, F. Perfluorooctanoic acid (PFOA) inhibits the gap junction intercellular communication and induces apoptosis in human ovarian granulosa cells. Reprod. Toxicol. 2020, 98, 125–133. [Google Scholar] [CrossRef]
- Jiang, W.; Deng, Y.; Song, Z.; Xie, Y.; Gong, L.; Chen, Y.; Kuang, H. Gestational perfluorooctanoic acid exposure inhibits placental development by dysregulation of labyrinth vessels and uNK cells and Apoptosis in mice. Front. Physiol. 2020, 11, 51. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, Y.; Zhang, L.; Wang, X. Effects of maternal exposure to PFOA on testes of male offspring mice. Chemosphere 2021, 272, 129585. [Google Scholar] [CrossRef]
- Sun, Z.; Wen, Y.; Wang, B.; Deng, S.; Zhang, F.; Fu, Z.; Yuan, Y.; Zhang, D. Toxic effects of per- and polyfluoroalkyl substances on sperm: Epidemiological and experimental evidence. Front. Endocrinol. 2023, 14, 1114463. [Google Scholar] [CrossRef] [PubMed]
- Gubina, N.; Leboeuf, D.; Piatkov, K.; Pyatkov, M. Novel apoptotic mediators identified by conservation of vertebrate caspase targets. Biomolecules 2020, 10, 612. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.J.; Wang, S.L.; Chen, P.C.; Guo, Y.L. Prenatal perfluorooctanoic acid exposure and glutathione s-transferase T1/M1 genotypes and their association with atopic dermatitis at 2 years of age. PLoS ONE 2019, 14, e0210708. [Google Scholar] [CrossRef]
- Tang, J.; Jia, X.; Gao, N.; Wu, Y.; Liu, Z.; Lu, X.; Du, Q.; He, J.; Li, N.; Chen, B.; et al. Role of the Nrf2-ARE pathway in perfluorooctanoic acid (PFOA)-induced hepatotoxicity in Rana nigromaculata. Environ. Pollut. 2018, 238, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Brigelius Folhe, R.; Kipp, A. Glutathione peroxidases in different stages of carcinogesis. Biochim. Biophys. Acta. 2009, 1790, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Remmen, H.V.; Frohlich, V.; Lechleiter, J.; Richarson, A.; Ran, Q. Gpx4 protects mitochondrial ATP generation against oxidative damage. Biochem. Biophys. Res. Commun. 2007, 356, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J.; Kundu, J.K.; Na, H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar] [CrossRef]
- Gregory, M.; Cyr, D.G. The blood-epididymis barrier and inflammation. Spermatogenesis 2014, 4, e979619. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, D.K.; Chen, L.M. The Physiology of Bicarbonate Transporters in Mammalian Reproduction. Biol. Reprod. 2012, 86, 99. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.Y.; Wang, D.K.; Wang, L.; Chen, L.M. Cloning and identification of two novel NBCe1 splice varients from mouse reproductive tract tissues: A comparative study of NCBT genes. Genomics 2011, 98, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Soler, N.; Pietrement, C.; Breton, S. Role of acid/base transporters in the male reproductive tract and potential consequences of their malfunction. Physiology 2005, 20, 417–428. [Google Scholar] [CrossRef]
- Lim, J.J.; Suh, Y.; Faustman, E.M.; Cui, J.Y. PFCAs with increasing carbon chain lengths up-regulate amino acid transporters and modulate compensatory response of xenobiotic transporters in HepaRG cells. Drug Metab Dispos. 2021, 50, 1396–1413. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Ma, X.; Griffin, N.; Liu, H. Perfluorooctanoic acid (PFOA) exposure induces renal filtration and reabsorption disorders via down-regulation of aquaporins. Toxicol. Lett. 2024, 392, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Shati, A.A. Resveratrol improves sperm parameter and testicular apoptosis in cisplatin-treated rats: Effects on ERK1/2, JNK, and Akt pathways. Syst. Biol. Reprod. Med. 2019, 65, 236–249. [Google Scholar] [CrossRef]
- Mustafa, S.; Wei, Q.; Ennab, W.; Lv, Z.; Nazar, K.; Siyal, F.A.; Rodeni, S.; Kavita, N.M.X.; Shi, F. Resveratrol Ameliorates Testicular Histopathology of Mice Exposed to Restraint Stress. Animals 2019, 9, 743. [Google Scholar] [CrossRef]
- Mustafa, S.; Ennab, W.; Nazar, K.; Wei, Q.; Lv, Z.; Shi, Z.; Shi, F. Positive Roles of Resveratrol in Early Development of Testicular Germ Cells against Maternal Restraint Stress in Mice. Animals 2020, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Chinwe, G.S.; Azuka, O.I.; Adaeze, N.C. Resveratrol supplementation rescues pool of growing follicles and ovarian stroma from Cisplatin-induced toxicity on the ovary in Sprague-Dawley rats: An experimental study. Int. J. Reprod. Biomed. 2018, 16, 19–30. [Google Scholar] [PubMed]
- Guo, Y.; Wang, A.; Liu, X. Effects of resveratrol on reducing spermatogenic dysfunction caused by high-intensity exercise. Reprod. Biol. Endocrinol. 2019, 17, 42. [Google Scholar] [CrossRef]
- Constantinescu, T.; Mihis, A.G. Resveratrol as a privileged molecule with antioxidant activity. Food Chem. Adv. 2023, 3, 100539. [Google Scholar] [CrossRef]
- Akbel, E.; Arslan-Acaroz, D.; Demirel, H.H.; Kucukkurt, I.; Ince, S. The subchronic exposure to malathion, an organophosphate pesticide, causes lipid peroxidation, oxidative stress, and tissue damage in rats; the protective role of resveratrol. Toxicol Res. 2018, 7, 503–512. [Google Scholar] [CrossRef]
- Wan, H.T.; Lai Kp Wong, C.K.C. Comparative analysis of PFOS and PFOA toxicity on sertoli cells. Environ. Sci. Technol. 2020, 54, 3465–3475. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Steroid hormone biosynthesis and actions in the materno-feno-placental. Unit. Clin. Perinatol. 1998, 25, 799–817. [Google Scholar] [CrossRef]
- Smith, L.B.; Walker, W.H. The Regulation of Spermatogenesis by Androgens. Semin. Cell Dev. Biol. 2014, 2, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Alharthy, S.A.; Hardej, D. The role of transcription factor Nrf2 in the toxicity of perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) in C57BL/6 mouse astrocytes. Environ. Toxicol. Pharmacol. 2021, 86, 103652. [Google Scholar] [CrossRef]
- Krajka-Kuzniak, V.; Paluszczak, J.; Baer-Dubowska, W. The Nrf2-ARE signaling pathway: An update on its regulation and possible role in cancer prevention and treatment. Pharmacol. Rep. 2017, 69, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef] [PubMed]
- Elfgen, V.; Mietens, A.; Mewe, M.; Hau, T.; Middendorff, R. Contractility of the epididymal duct: Function, regulation and potential drug effects. Reproduction 2018, 156, R125–R141. [Google Scholar] [CrossRef]
- Adedara, I.A.; Souza, T.P.; Canzian, J.; Olabiyi, A.A.; Borba, J.V.; Biasuz, E.; Sabadin, G.R.; Gonçalves, F.L.; Costa, F.V.; Schetinger, M.R.C.; et al. Induction of aggression and anxiety-like responses by perfluorooctanoic acid is accompanied by modulation of cholinergic- and purinergic signaling-related parameters in adult zebrafish. Ecotoxicol. Environ. Saf. 2022, 239, 113635. [Google Scholar] [CrossRef] [PubMed]
- Gacar, N.; Mutlu, O.; Utkan, T.; KomsuogluCelikyurt, I.; Gocmez, S.S.; Ulak, G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and Morris water maze tests in rats. Pharmacol. Biochem. Behav. 2011, 99, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Ho, C.H.; Ghai, G.; Chen, K.Y. Resveratrol analog, 3,4,5,4′-tetrahydroxystilbene, differentially induces pro-apoptotic p53/Bax gene expression and inhibits the growth of transformed cells but not their normal counterparts. Carcinogenesis 2001, 22, 321–328. [Google Scholar] [CrossRef]
- Tinhofer, I.; Bernhard, D.; Senfter, M.; Anether, G.; Loeffler, M.; Kroemer, G. Resveratrol, a tumor-suppressive compound from grapes, induces apoptosis via a novel mitochondrial pathway controlled by Bcl-2. FASEB J. 2001, 15, 1613–1615. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Choi, Y.J.; Suh, S.I.; Baek, W.K.; Suh, M.H.; Jin, I.N. Bcl-2 overexpression attenuates resveratrol-induced apoptosis in U937 cells by inhibition of caspase-3 activity. Carcinogenesis 2001, 22, 1633–1639. [Google Scholar] [CrossRef] [PubMed]
- Aly, H.A.A. Mitochondria-Mediated Apoptosis Induced Testicular Dysfunction in Diabetic Rats: Ameliorative Effect of Resveratrol. Endocrinology 2021, 162, bqab018. [Google Scholar] [CrossRef]
- Xu, M.; Wan, J.; Niu, Q.; Liu, R. PFOA and PFOS interact with superoxide dismutase and induce cytotoxicity in mouse primary hepatocytes: A combined cellular and molecular methods. Environ. Res. 2019, 175, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Rajak, P.; Ganguly, A. The ligand-docking approach explores the binding affinity of PFOS and PFOA for major endogenous antioxidants: A potential mechanism to fuel oxidative stress. Sustain. Chem. Environ. 2023, 4, 100047. [Google Scholar] [CrossRef]
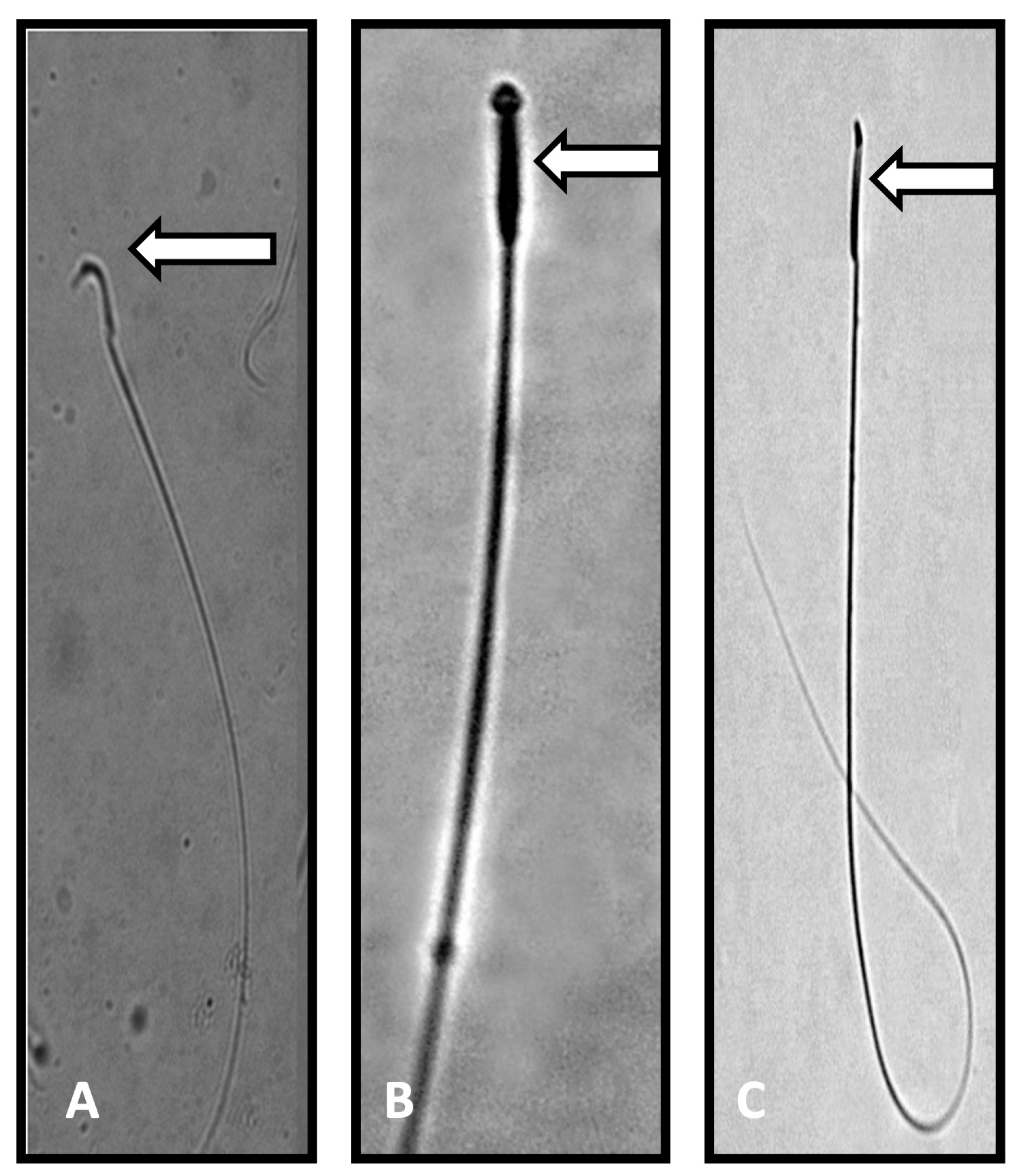
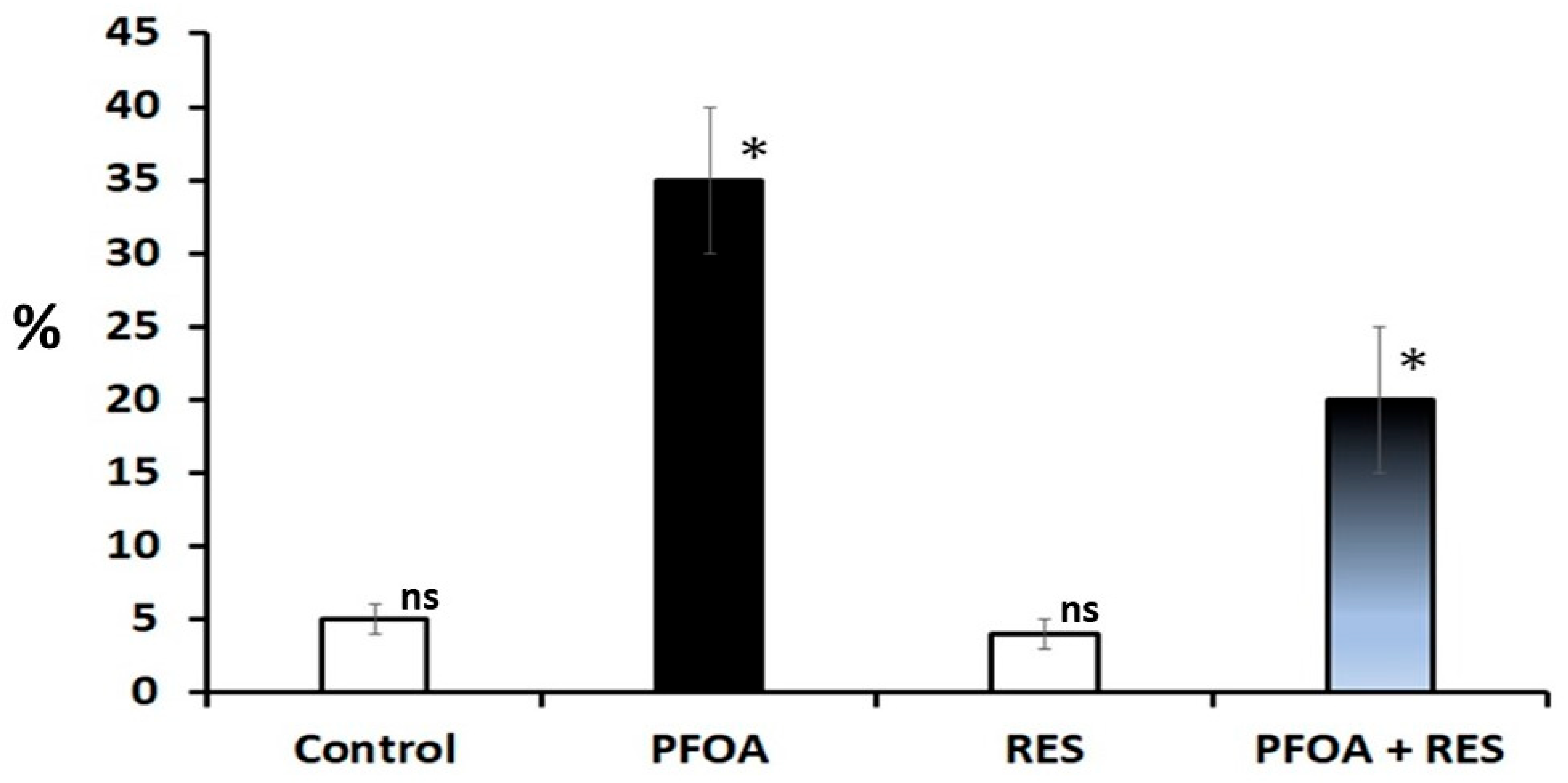
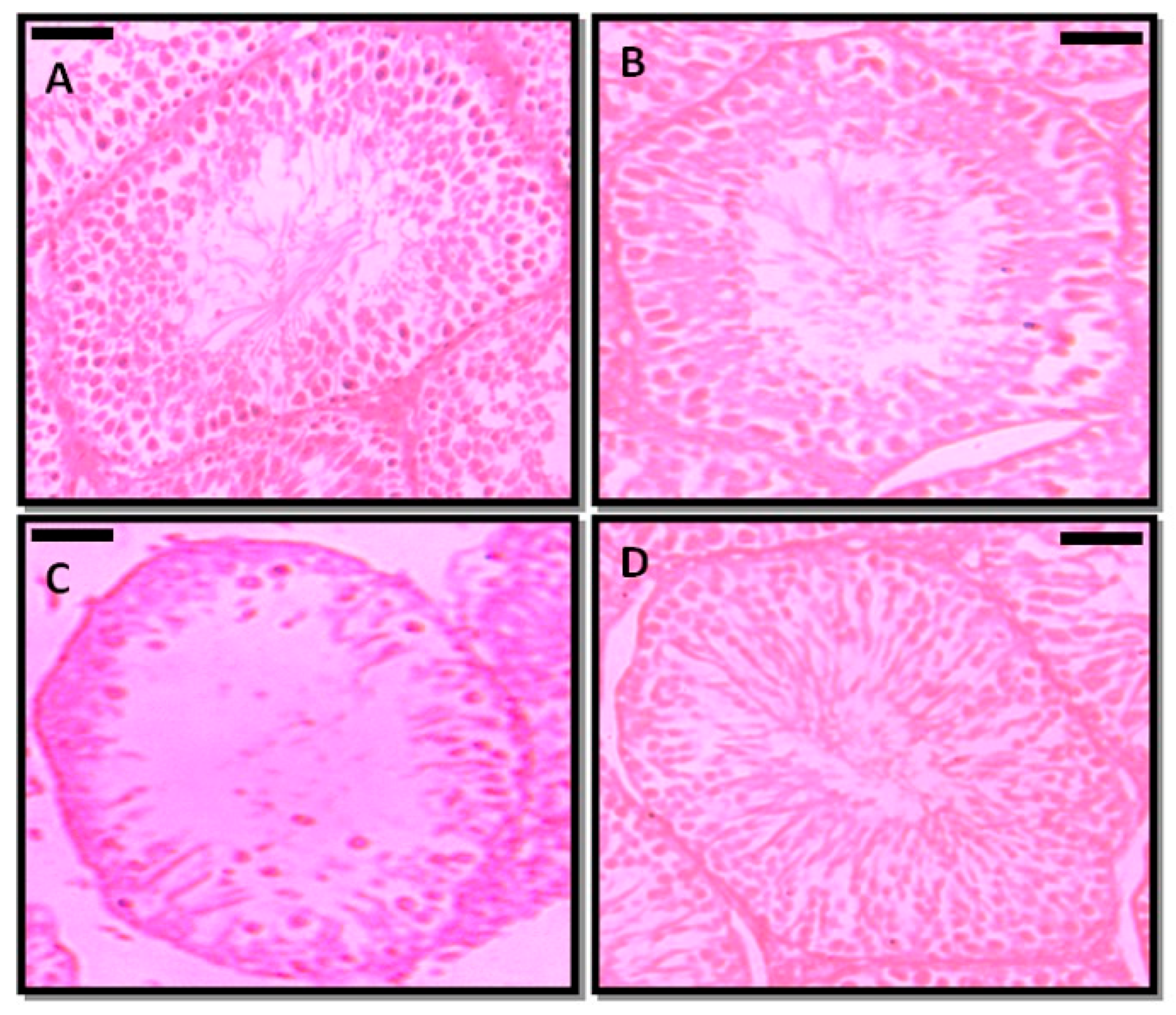

| Parameter | Control | PFOA Exposed | ||
|---|---|---|---|---|
| Untreated | RES | Untreated | RES | |
| Number of litters | 10 | 10 | 10 | 10 |
| No. of mating trails (days) | 1.48 a ± 0.27 | 1.52 a ± 0.31 (2.702) | 1.62 a ± 0.42 (9.459) | 1.49 a ± 0.39 (−1.97); (−8.02) |
| Mating Index (%) | 100 | 100 | 100 | 100 |
| Fertility Index (%) | 100 | 100 | 100 | 100 |
| No. of corpora lutea/rat | 13.65 a ± 1.43 | 13.42 a ± 1.31 (−1.68) | 13.58 a ± 1.48 (−0.51) | 13.13 a ± 1.92 (−3.31); (−2.160) |
| No. of implantations/rat | 12.78 a ± 1.02 | 12.89 a ± 1.21 (0.86) | 7.68 b ± 0.42 (−39.906) | 10.38 c ± 0.31 (−19.47); (35.156) |
| Pre implantation loss (%) | 6.37 | 4.11 | 43.44 | 19.42 |
| No. of live foetuses/rat | 12.02 a ± 0.39 | 11.91 b ± 0.28 (−0.92) | 5.07 c ± 0.18 (−57.820) | 10.21 d ± 0.28 (−14.27); (101.38) |
| Post implantation loss (%) | 5.94 | 7.60 | 33.984 | 1.637 |
| Body weight of pups (g) on PND 1 | 4.81 a ± 0.53 | 4.92 a ± 0.48 (2.286) | 4.84 a ± 0.51 (0.623) | 4.89 a ± 0.49 (−0.609); (1.03) |
| Body weight of pups (g) on PND 21 | 30.28 a ± 1.71 | 29.48 a ± 1.68 (−2.642) | 30.09 a ± 1.59 (−0.627) | 30.07 a ± 1.74 (2.001); (−0.066) |
| Parameters | Controls | PFOA Exposed | ||
|---|---|---|---|---|
| untreated | RES | Untreated | RES | |
| Daily sperm count (millions/g epididymis) | 26.29 a ± 1.43 | 25.76 a ± 1.62 (−2.015) | 12.68 b ± 2.18 (−51.768) | 17.28 c ± 1.09 (−32.919); (36.277) |
| Sperm analysis (epididymis) | ||||
| Sperm count (millions/mL) | 74.68 a ± 4.32 | 73.91 a ± 5.21 (−1.031) | 40.31 b ± 4.38 (−46.023) | 59.73 c ± 3.22 (−19.185); (48.176) |
| Sperm viability (%) | 73.9 a ± 3.2 | 72.98 a ± 4.33 (−1.244) | 39.28 b ± 4.09 (−46.847) | 60.12 c ± 4.82 (−17.621); (53.054) |
| Sperm motility (%) | 70.18 a ± 4.29 | 69.33 a ± 3.47 (−1.211) | 37.28 b ± 3.19 (−46.879) | 59.22 c ± 3.43 (−14.582); (58.851) |
| HOS tail coiled sperm (%) | 69.99 a ± 3.33 | 68.72 a ± 2.91 (−1.814) | 35.41 b ± 2.09 (−49.407) | 55.83 c ± 2.19 (−18.757); (57.667) |
| Abnormalities of sperm (%) | 2.28 a ± 0.72 | 2.92 a ± 0.16 (28.070) | 22.94 b ± 2.39 (906.140) | 14.97 c ± 3.23 (412.67); (−34.742) |
| Pin shaped (%) | 1 | 1 | 12 | 8 |
| Rod shaped (%) | 1 | 1 | 10 | 6 |
| Sperm transit time (days) | 6.91 a ± 1.02 | 7.01 a ± 1.22 (1.44) | 3.84 b ± 0.83 (−44.42) | 5.93 c ± 0.67 (−14.18); (35.24) |
| Controls | PFOA Exposed | |||
|---|---|---|---|---|
| Parameters | Untreated | RES | Untreated | RES |
| Total cholesterol (mg/g) | 6.01 a ± 0.41 | 5.92 a ± 0.31 (−1.497) | 10.08 b ± 0.16 (67.720) | 7.32 c ± 0.16 (23.648); (−27.3809) |
| 3β-HSD | 16.89 a ± 2.38 | 16.46 a ± 2.24 (−2.545) | 8.04 b ± 1.87 (−52.397) | 13.55 c ± 1.23 (−17.679); (68.532) |
| 17βHSD | 11.21 a ± 0.94 | 11.33 a ± 0.83 (1.070) | 5.01 b ± 0.32 (−55.307) | 8.05 c ± 0.66 (−28.949); (60.678) |
| T (ng/mL) | 7.37 a ± 0.19 | 7.22 a ± 0.23 (−2.035) | 3.47 b ± 0.13 (−52.917) | 5.01 c ± 0.14 (−30.61); (44.380) |
| FSH (ng/mL) | 8.27 a ± 0.91 | 8.17 a ± 0.31 (−1.209) | 15.97 b ± 0.81 (93.107) | 11.08 c ± 0.56 (35.62); (−30.62) |
| LH (ng/mL) | 6.33 a ± 0.54 | 6.62 a ± 0.67 (4.581) | 16.44 b ± 1.71 (159.715) | 11.21 c ± 1.08 (69.34); (−31.813) |
| Parameters | Control | PFOA exposed | |||
|---|---|---|---|---|---|
| Untreated | RES | Untreated | PFOA + RES | ||
| LPx | Testis | 9.18 a ± 2.08 | 10.11 a ± 0.98 (10.130) | 27.14 b ± 2.39 (195.642) | 17.28 c ± 1.97 (70.92); (−36.33) |
| Caput | 11.83 a ± 1.82 | 10.27 a ± 1.38 (−13.18) | 22.91 b ± 1.31 (93.660) | 14.38 c ± 0.97 (40.02); (−37.23) | |
| corpus | 13.18 a ± 2.09 | 12.18 a ± 1.90 (−7.587) | 24.28 b ± 2.19 (84.218) | 16.18 c ± 0.93 (32.84); (−33.36) | |
| Cauda | 10.21 a ± 1.03 | 11.27 a ± 2.03 (10.381) | 24.38 b ± 2.34 (138.785) | 15.28 c ±0.98 (35.58); (−37.33) | |
| PC | Testis | 1.91 a ± 0.38 | 1.78 a ± 0.21 (−6.806) | 6.02 c ± 0.71 (215.18) | 3.62 d ± 0.29 (103.37); (−39.86) |
| Caput | 1.38 a ± 0.623 | 1.53 a ± 0.531 (10.869) | 1.41 a ± 0.591 (2.173) | 1.43 a ± 0.487 (−6.54); (1.418) | |
| corpus | 1.76 a ± 0.528 | 1.64 a ± 0.612 (−6.818) | 1.69 a ± 0.612 (−3.977) | 1.68 a ± 0.712 (2.43); (−0.59) | |
| Cauda | 1.49 a ± 0.209 | 1.68 a ± 0.318 (12.751) | 3.12 b ± 0.291 (109.395) | 1.51 a ± 0.337 (−10.12); (−51.6) | |
| H2 O2 | Testis | 10.38 a ± 0.42 | 9.27 b ± 0.32 (−10.693) | 22.19 c ± 1.38 (113.776) | 15.91 d ± 1.09 (71.63); (−28.30) |
| Caput | 10.13 a ± 1.28 | 9.73 b ± 1.31 (−3.94) | 17.33 c ± 1.228 (71.076) | 13.48 d ± 0.97 (38.54); (−22.22) | |
| corpus | 10.13 a ± 1.28 | 9.73 b ± 1.31 (−3.94) | 17.33 c ± 1.228 (71.076) | 13.48 d ± 0.97 (38.54); (−22.22) | |
| Cauda | 10.92 a ± 1.08 | 11.32 a ± 1.09 (3.66) | 18.39 b ± 0.981 (68.406) | 14.08 c ± 1.07 (24.38); (−23.44) | |
| O2− | Testis | 4.98 a ± 0.32 | 4.72 a ± 0.19 (−5.22) | 10.98 c ± 0.14 (120.481) | 7.27 d ± 0.18 (54.025); (−33.78) |
| Caput | 5.17 a ± 0.431 | 4.89 a ± 0.581 (−5.415) | 7.82 b ± 0.281 (51.257) | 5.23 c ± 0.61 (6.95); (−33.12) | |
| corpus | 4.27 a ± 0.312 | 4.31 a ± 0.302 (0.936) | 8.31 b ± 0.273 (94.613) | 5.18 c ± 0.428 (20.18); (−37.66) | |
| Cauda | 4.39 a ± 0.414 | 4.41 a ± 0.281 (0.455) | 8.21 b ± 0.314 (87.015) | 5.21 c ± 0.513 (18.14); (−36.54) | |
| Parameters | Controls | Treated | |||
|---|---|---|---|---|---|
| Control | RES | PFOA | PFOA +RES | ||
| SOD | Testis | 0.62 a ± 0.15 | 0.58 a ± 0.11 (−6.45) | 0.23 c ± 0.12 (−62.903) | 0.38 d ± 0.18 (−34.48); (65.217) |
| Caput | 1.73 a ± 0.072 | 1.68 a ± 0.061 (−2.89) | 1.02 b ± 0.023 (−41.040) | 1.43 c ± 0.029 (−14.88); (40.196) | |
| corpus | 1.54 a ± 0.033 | 1.52 a ± 0.042 (−1.29) | 1.08 c ± 0.019 (−29.870) | 1.39 d ± 0.013 (−8.552); (28.703) | |
| Cauda | 1.68 a ± 0.041 | 1.71 a ± 0.038 (1.785) | 1.12 b ± 0.017 (−33.333) | 1.48 c ± 0.019 (−13.45); (32.142) | |
| CAT | Testis | 0.48 a ± 0.013 | 0.51 a ± 0.041 (6.25) | 0.22 c ± 0.014 (−54.166) | 0.39 d ± 0.009 (−23.52); (77.27) |
| Caput | 5.08 a ± 0.47 | 5.18 a ± 0.37 (1.968) | 3.07 b ± 0.21 (−39.566) | 4.97 a ± 0.38 (−4.05); (61.88) | |
| corpus | 5.59 a ± 0.38 | 6.02 a ± 0.44 (7.692) | 3.12 b ± 0.29 (−44.186) | 4.82 c ± 0.37 (−19.93); (54.48) | |
| Cauda | 5.73 a ± 0.39 | 6.12 a ± 0.51 (6.806) | 3.24 b ± 0.23 (−43.455) | 4.93 c ± 0.30 (−19.44); (52.160) | |
| GR | Testis | 2.83 a ± 0.014 | 2.81 a ± 0.013 (−0.71) | 0.98 c ± 0.021 (−65.371) | 1.48 d ± 0.015 (−47.33); (51.020) |
| Caput | 3.28 a ± 0.031 | 3.25 a ± 0.040 (−0.91) | 3.31 a ± 0.039 (0.914) | 3.27 a ± 0.049 (0.615); (−1.208) | |
| corpus | 2.14 a ± 0.012 | 2.19 a ± 0.022 (2.23) | 4.29 c ± 0.027 (100.467) | 2.72 d ± 0.039 (24.200); (−36.596) | |
| Cauda | 3.91 a ± 0.017 | 3.95 a ± 0.043 (1.02) | 2.44 c ± 0.031 (−37.595) | 3.73 d ± 0.024 (−5.569); (52.868) | |
| GPx | Testis | 2.07 a ± 0.014 | 2.04 a ± 0.012 (−1.44) | 0.87 c ± 0.011 (−57.971) | 1.29 d ± 0.009 (−36.76); (48.27) |
| Caput | 6.02 a ± 0.042 | 5.98 a ± 0.053 (−0.66) | 6.05 a ± 0.067 (0.49) | 5.95 a ± 0.081 (−0.501); (−1.65) | |
| corpus | 5.11 a ± 0.039 | 5.13 a ± 0.023 (0.391) | 8.03 c ± 0.033 (57.142) | 5.13 a ± 0.063 (0); (−36.11) | |
| Cauda | 4.74 a ± 0.051 | 4.79 a ± 0.024 (1.054) | 2.18 c ± 0.019 (−54.008) | 3.67 d ± 0.020 (−23.38); (68.34) | |
| GSH | Testis | 10.48 a ± 1.21 | 11.29 a ± 2.08 (7.729) | 4.38 b ± 0.82 (−58.206) | 7.19 c ± 1.07 (−36.32); (64.15) |
| Caput | 9.38 a ± 0.29 | 9.48 a ± 0.33 (1.066) | 10.02 b ± 0.42 (6.823) | 10.12 c ± 0.36 (6.75); (0.99) | |
| corpus | 10.23 a ± 0.41 | 10.71 a ± 0.39 (4.692) | 11.18 b ± 0.46 (9.286) | 10.37 a ± 0.32 (−3.17); (−7.24) | |
| Cauda | 9.32 a ± 0.23 | 9.30 a ± 0.29 (−0.22) | 5.27 c ± 0.17 (−43.454) | 7.98 d ± 0.20 (−14.19); (51.42) | |
| Controls | PFOA exposed | ||
|---|---|---|---|
| Untreated | RES | Untreated | RES |
| 190.42 a ± 20.74 | 192.78 a ± 30.44 (1.239) | 392.48 b ± 29.48 (106.112) | 272.28 c ± 22.53 (41.23); (−30.62) |
| Term | Count | % | p-Value | Fold Enrichment |
|---|---|---|---|---|
| Testis | ||||
| rno04215: Apoptosis—multiple species (up-regulated) | 6 | 14.28571 | 1.65 × 10−08 | 67.46324 |
| rno00480: Glutathione metabolism (down-regulated)−pididymis | 4 | 8.695652 | 0.001779 | 15.92882 |
| rno00480: Glutathione metabolism (up-regulated) | 3 | 1.923077 | 0.048064 | 6.371528 |
| rno04976: Bile secretion (down-regulated) | 9 | 2.122642 | 9.61 × 10−04 | 4.392287 |
| Fold Change * | Fold Change *# | |
|---|---|---|
| Gene Symbol | PFOA | PFOA + RES |
| Testis | ||
| hsd17β3 | −2.52 ± 0.04 | 1.23 ± 0.09 (−148.81) |
| StAR | −1.84 ± 0.07 | 1.45 ± 0.11 (−178.80) |
| AR | −2.04 ± 0.08 | 0.94 ± 0.14 (−146.07) |
| Casp3 | 2.67 ± 0.09 | 1.02 ± 0.24 (−61.79) |
| Nfe2l2 | −2.07 ± 0.03 | 1.55 ± 0.13 (−174.87) |
| lhcgr | −2.79 ± 0.07 | 1.21 ± 0.11 (−143.36) |
| Epididymis | ||
| AR | −1.97 ± 0.05 | 0.96 ± 0.13 (−148.73) |
| Alpha adrenoceptor 1 | −3.18 ± 0.06 | 0.87 ± 0.13 (−127.35) |
| Muscarinic acetylcholine receptor 3 | −2.64 ± 0.11 | 1.11 ± 0.15 (−142.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavani, R.; Venkaiah, K.; Prakasam, P.G.; Dirisala, V.R.; Krishna, P.G.; Kishori, B.; Sainath, S.B. Protective Effects of Resveratrol Against Perfluorooctanoic Acid-Induced Testicular and Epididymal Toxicity in Adult Rats Exposed During Their Prepubertal Period. Toxics 2025, 13, 111. https://doi.org/10.3390/toxics13020111
Pavani R, Venkaiah K, Prakasam PG, Dirisala VR, Krishna PG, Kishori B, Sainath SB. Protective Effects of Resveratrol Against Perfluorooctanoic Acid-Induced Testicular and Epididymal Toxicity in Adult Rats Exposed During Their Prepubertal Period. Toxics. 2025; 13(2):111. https://doi.org/10.3390/toxics13020111
Chicago/Turabian StylePavani, R., K. Venkaiah, P. Gnana Prakasam, Vijaya R. Dirisala, P. Gopi Krishna, B. Kishori, and S. B. Sainath. 2025. "Protective Effects of Resveratrol Against Perfluorooctanoic Acid-Induced Testicular and Epididymal Toxicity in Adult Rats Exposed During Their Prepubertal Period" Toxics 13, no. 2: 111. https://doi.org/10.3390/toxics13020111
APA StylePavani, R., Venkaiah, K., Prakasam, P. G., Dirisala, V. R., Krishna, P. G., Kishori, B., & Sainath, S. B. (2025). Protective Effects of Resveratrol Against Perfluorooctanoic Acid-Induced Testicular and Epididymal Toxicity in Adult Rats Exposed During Their Prepubertal Period. Toxics, 13(2), 111. https://doi.org/10.3390/toxics13020111







