The Effects of Fenobucarb on the Physiology, Behavior, and Growth of Silver Barb (Barbonymus gonionotus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Animals
2.2. Insecticide
2.3. Experimental Design
2.3.1. AChE Sensitivity Tests
2.3.2. Effects of Fenobucarb on Silver Barb
2.3.3. Recovery Test
2.3.4. Abnormal Physiological Expression Test
2.4. AChE Assay
2.5. Statistical Analysis
3. Result
3.1. AChE Sensitivity Test
3.2. Effect of Fenobucarb on Survival, Feed Intake, FCR, and Growth of Silver Barb
3.2.1. Survival Rate (SR)
3.2.2. Feed Intake (FI)
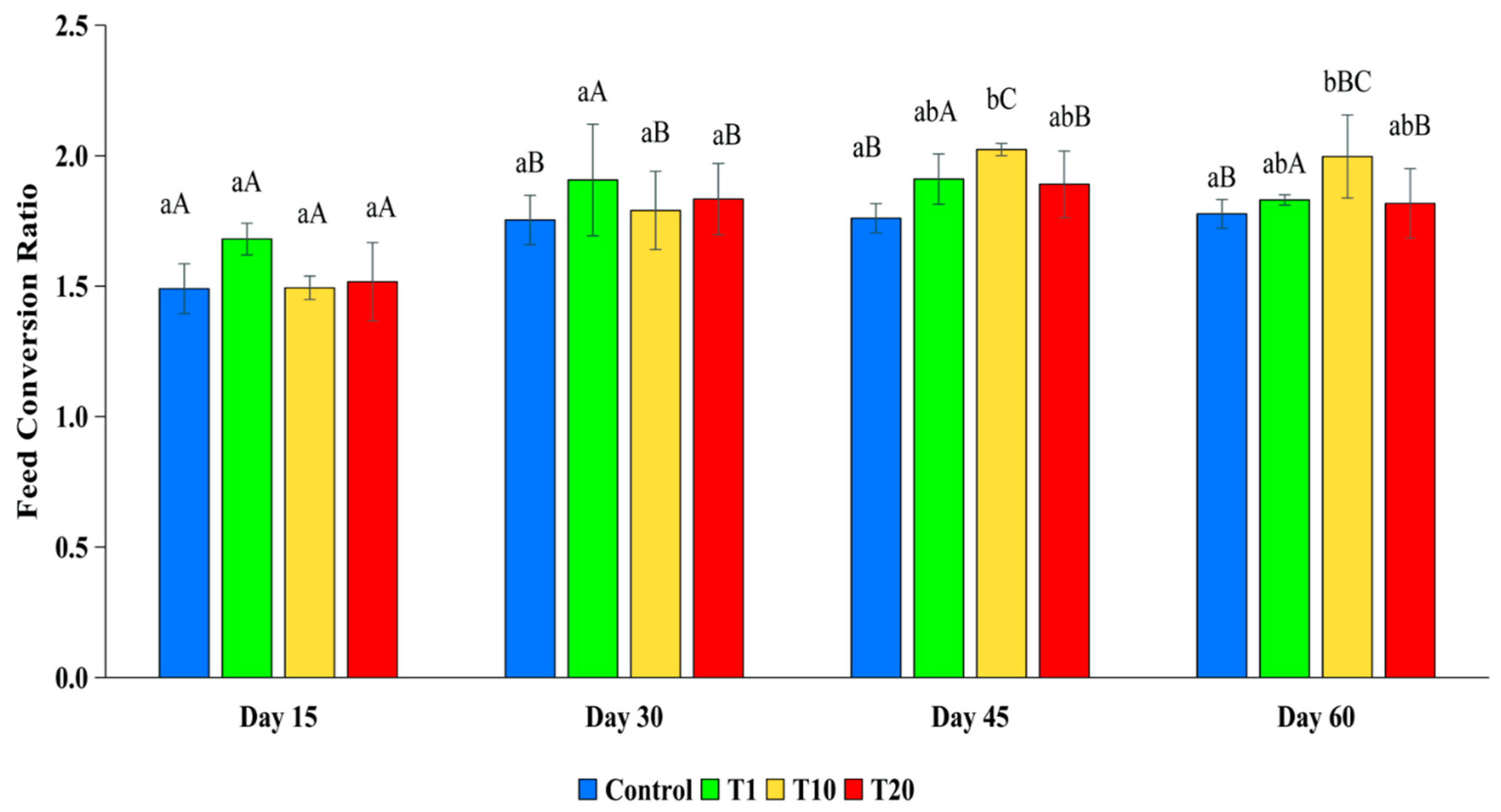
3.2.3. Food Conversion Ratio (FCR)
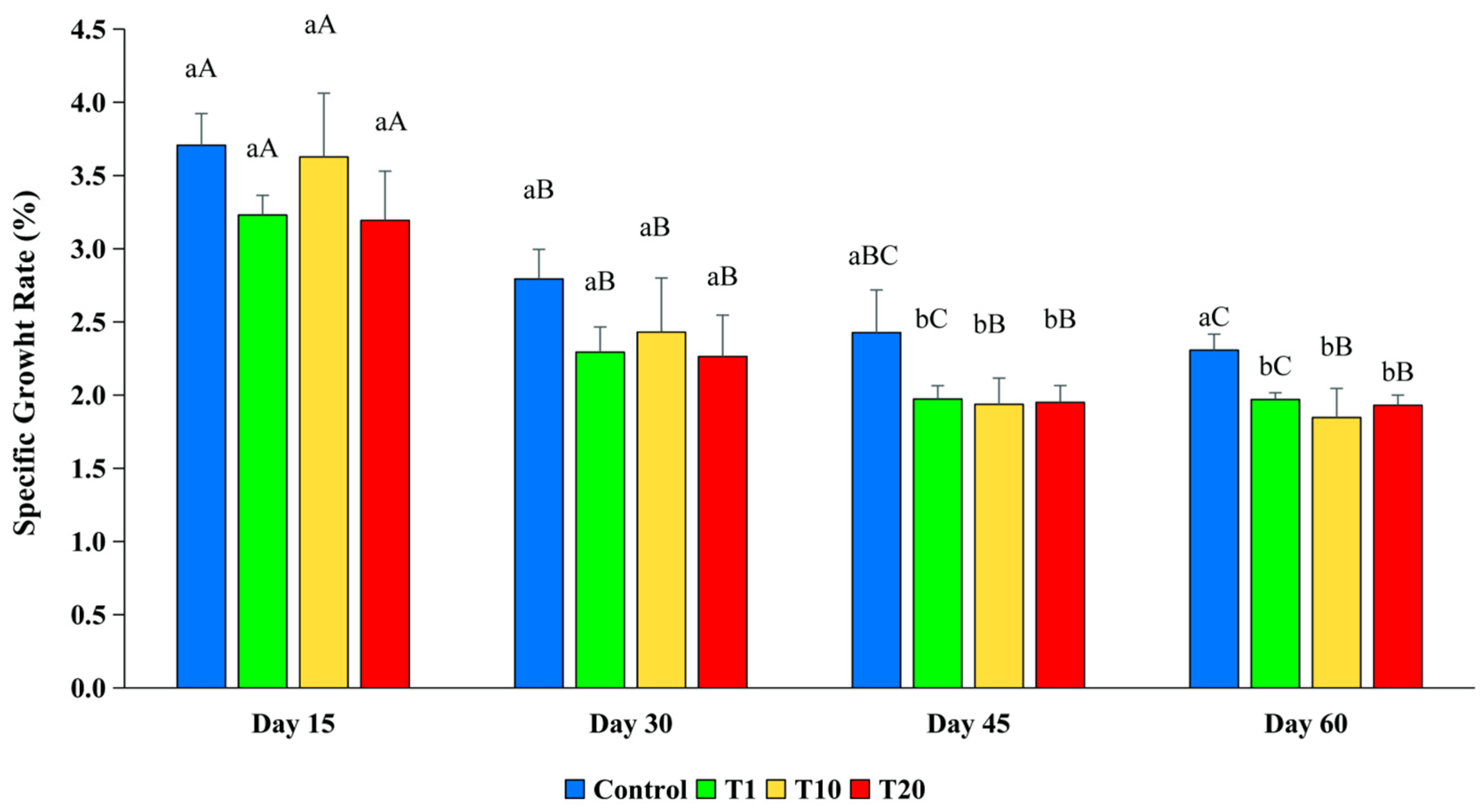
3.2.4. Specific Growth Rate (SGR)
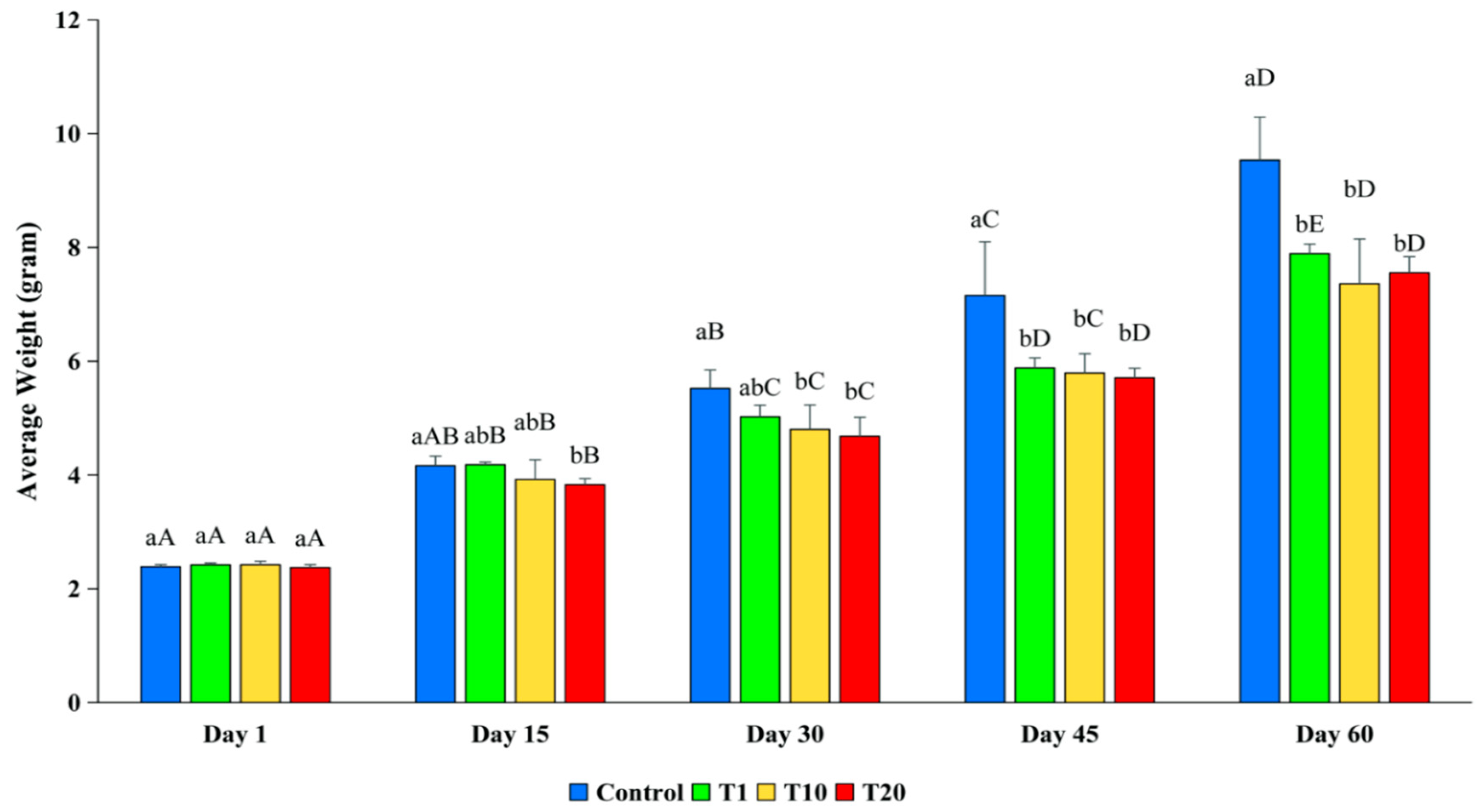
3.2.5. Growth Rate
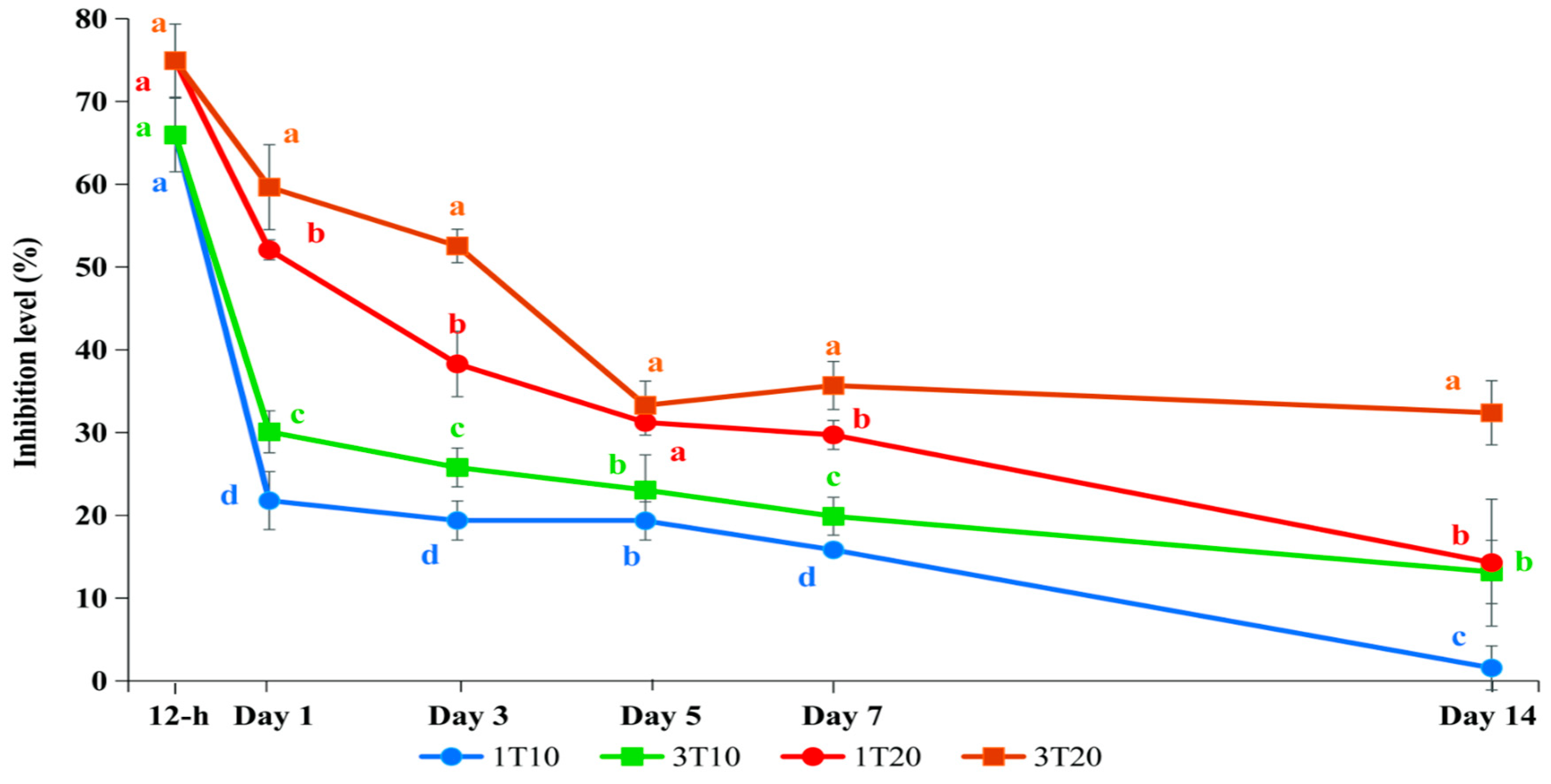
3.3. AChE Inhibition Levels That Cause Abnormal Physiological Behavior and Mortality of Silver Barb After Exposure to Fenobucarb
- (1)
- Abnormal swimming, where the fish became hyperactive, with quick, scattered swimming patterns (sometimes backward or spinning), often to the surface or into the bottom. Their gills moved fast, and convulsions occurred in some of the fish. These symptoms appeared 25 min after exposure in the treatment with 15.0 ppm of F, while they appeared after 30–55 min in the treatments with 13.5 and 12.2 ppm of F, respectively. At the onset of this symptom, the AChE activity in the fish was inhibited by 83.6%, compared to the control (p < 0.05) (Table 3).
- (2)
- Belly flipping, where the fish lay still on the bottom of the tank, and the gills and fins moved only slowly. A few fish swam slowly towards the surface but then sank back to the bottom. These symptoms appeared in the treatments with 15.0 ppm and 13.5 ppm of F after 40 min and 55 min, respectively, while these symptoms appeared in the treatment with 12.2 ppm 190 min after exposure. When this symptom appeared, the AChE activity of the fish was inhibited by 84.5%, compared to the control treatment (p < 0.05) (Table 3).
- (3)
- Death, where the fish’s gills were closed, and the fish no longer moved or responded to knocking on the wall of the tank. The first dead fish was found three hours after exposure in the treatment with 15 ppm of F, while the first dead fish in the treatments with 12.2 and 13.5 ppm were found 17 h 30 min and 25 h 50 min after exposure, respectively. When the fish started to die, the AChE activity in the fish was inhibited by 89.5% (Table 4).
3.4. AChE Recovery Test
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huy, H.T. The economic transition and migration of Vietnam and the Mekong Delta region. CAS Discuss. Pap. 2011, 1–22. [Google Scholar]
- Tong, Y.D. Rice Intensive Cropping and Balanced Cropping in the Mekong Delta, Vietnam—Economic and Ecological Considerations. Ecol. Econ. 2017, 132, 205–212. [Google Scholar] [CrossRef]
- Sebesvari, Z.; Le, H.; Van Toan, P.; Arnold, U.; Renaud, F. Agriculture and Water Quality in the Vietnamese Mekong Delta. In The Mekong Delta System; Renaud, F.G., Kuenzer, C., Eds.; Springer Environmental Science and Engineering; Springer: Dordrecht, The Netherlands, 2012; pp. 331–361. [Google Scholar]
- Renaud, F.G.; Kuenzer, C. The Mekong Delta System: Interdisciplinary Analyses of a River Delta; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Berg, H.; Lan, T.H.P.; Tam, N.T.; Trang, D.H.; Van, P.H.T.; Duc, H.N.; Da, C.T. An ecological economic comparison between integrated rice-fish farming and rice monocultures with low and high dikes in the Mekong Delta, Vietnam. Ambio 2023, 52, 1462–1474. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, S.; Meisner, C.; Wheeler, D.; Xuyen, K.; Thi Lam, N. Pesticide poisoning of farm workers-implications of blood test results from Vietnam. Int. J. Hyg. Environ. Health 2007, 210, 121–132. [Google Scholar] [CrossRef] [PubMed]
- MARD. Ministry of Agriculture and Rural Development. Circular No. 10/2020/TT-BNNPTNT. 2020. Available online: https://thuvienphapluat.vn/van-ban/Tai-nguyen-Moi-truong/Thong-tu-10-2020-TT-BNNPTNT-danh-muc-thuoc-bao-ve-thuc-vat-duoc-phep-su-dung-tai-Viet-Nam-452219.aspx (accessed on 12 March 2023).
- MARD. Ministry of Agriculture and Rural Development. Report on the Current Situation and Solutions to Manage the Use of Fertilizers and Pesticide, Plant Protection, Growing Areas, and Agricultural Product Packaging Facilities in the Mekong Delta Provinces. 2021. Available online: https://faolex.fao.org/docs/pdf/vie209384.pdf (accessed on 23 July 2023).
- WHO. The WHO Recommended Classification of Pesticides by Hazard and Guidelines to Classification, 2019th ed.; WHO, Ed.; WHO: Geneva, Switzerland, 2019; p. 92.
- Silberman, J.; Taylor, A. Carbamate Toxicity. [Updated 2023 May 1]. In StatPearls [Internet]; StatPearls: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482183/ (accessed on 25 November 2024).
- Silman, I.; Sussman, J.L. Acetylcholinesterase: ‘classical’ and ‘non-classical’ functions and pharmacology. Curr. Opin. Pharmacol. 2005, 5, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Peakall, D.B. Animal Biomarkers As Pollution Indicators; Chapman & Hall: London, UK, 1992. [Google Scholar]
- Assis, C.R.D.; Bezerra, R.S.; Luiz, B.; Carvalho, J. Fish Cholinesterases as Biomarkers of Organophosphorus and Carbamate Pesticides. In Pesticides in the Modern World—Pests Control and Pesticides Exposure and Toxicity Assessment; Stoytcheva, M., Ed.; InTech: Toulon, France, 2011; p. 626. [Google Scholar]
- Tomlin, C. The Pesticide Manual: Incorporating the Agrochemicals Handbook: A World Compendium; The Royal Society of Chemisty: London, UK, 1995. [Google Scholar]
- Van Pham, T.; Mai Phuoc, V.; Van Nguyen, D.; Koyama, J. Treatment efficiency of a combination of alternative technologies in removing pollutants from pesticide containing wastewater. Environ. Eng. Res. 2021, 26, 200263. [Google Scholar] [CrossRef]
- Toan, P.V.; Sebesvari, Z.; Blasing, M.; Rosendahl, I.; Renaud, F.G. Pesticide management and their residues in sediments and surface and drinking water in the Mekong Delta, Vietnam. Sci. Total Environ. 2013, 452, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Chau, N.D.G.; Sebesvari, Z.; Amelung, W.; Renaud, F.G. Pesticide pollution of multiple drinking water sources in the Mekong Delta, Vietnam: Evidence from two provinces. Environ. Sci. Pollut. Res. 2015, 22, 9042–9058. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.T.; Berg, H.; Van Cong, N. Evaluation of the joint toxicity of chlorpyrifos ethyl and fenobucarb on climbing perch (Anabas testudineus) from Rice Fields in the Mekong Delta, Vietnam. Environ. Sci. Pollut. Res. Int. 2018, 25, 13226–13234. [Google Scholar] [CrossRef] [PubMed]
- Rainboth, W.J. Fishes of the Cambodian Mekong; Food and Agriculture Organization of the United Nations: Rome, Italy, 1996; p. 265. [Google Scholar]
- Anh, V.T.; Chiem, N.N.; Dung, D.T.; Sultana, P. Vietnam PRA Report. In Understanding Livelihoods Dependent on Inland Fisheries in Bangladesh and South East Asia—Synthesis Report for FMSP Project R8118; WorldFish Center: Penang, Malaysia, 2003. [Google Scholar]
- Edwards, P.; Little, D.C.; Yakupitiyage, A. A comparison of traditional and modified inland artisanal aquaculture systems. Aquac. Res. 1997, 28, 777–788. [Google Scholar] [CrossRef]
- Klemick, H.; Lichtenberg, E. Pesticide use and fish harvests in Vietnamese rice agroecosystems. Am. J. Agric. Econ. 2008, 90, 1–14. [Google Scholar] [CrossRef]
- Yen, N.T.H.; Phuc, T.N.T.; Cong, N.V. Acute toxicity and effects of Fenobucarb at lethal concentrations on respiration and cholinesterase activity of silver barb. Nat. Resour. Environ. E-Mag. 2022, 1, 36–38. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Feather-Stone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Bentsen, H.B.; Gjedrem, T.; Hao, N.V. Breeding Plan for Silver Barb (Puntius gonionotus) in Vietnam: Individual (Mass) Selection to Improve Growth Rate; The WorldFish Center: Penang, Malaysia, 1996; p. 12. [Google Scholar]
- Lan, T.T. Effect of Bassa 50EC on Some Physiological Parameters of Climbing perch (Anabas testudineus); Cantho University Press: Cantho City, Vietnam, 2004. [Google Scholar]
- Anh, T.T.; Ha, N.T.K.; Trung, N.Q.; Huong, D.T.T.; Phuong, N.T. Effects of quinalphos on acetylcholinesterase and growth of Barbodes gonionotus. CTU J. Sci. 2012, 22a, 269–278. [Google Scholar]
- Aprea, C.; Colosio, C.; Mammone, T.; Minoia, C.; Maroni, M. Biological monitoring of pesticide exposure: A review of analytical methods. J. Chromatogr. B 2002, 769, 191–219. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.T.; Berg, H.; Laureus, J.; Cong, N.V.; Tedengren, M. Effects of Sequential Applications of Bassa 50EC (Fenobucarb) and Vitashield 40EC (Chlorpyrifos ethyl) on Acetylcholinesterase Activity in Climbing Perch (Anabas testudineus) Cultured in Rice Fields in the Mekong Delta, Vietnam. Bull. Environ. Contam. Toxicol. 2016, 97, 98–104. [Google Scholar] [CrossRef]
- Ito, H.C.; Shiraishi, H.; Nakagawa, M.; Takamura, N. Combined impact of pesticides and other environmental stressors on animal diversity in irrigation ponds. PLoS ONE 2020, 15, e0229052. [Google Scholar] [CrossRef] [PubMed]
- Tam, N.T.; Berg, H.; Van Cong, N. The combined effect of Bassa 50EC and Vitashield 40EC on the brain acetylcholinesterase activity in climbing perch (Anabas testudineus). Environ. Sci. Pollut. Res. Int. 2018, 25, 17207–17215. [Google Scholar] [CrossRef]
- Gill, T.S.; Pande, J.; Tewari, H. Sublethal effects of an organophosphorus insecticide on certain metabolite levels in a freshwater fish, Puntius conchonius hamilton. Peptic. Biochem. Physiol. 1990, 36, 290–299. [Google Scholar] [CrossRef]
- Ram, P.Y.; Digvijay, S.; Singh, S.K.; Ajay, S. Metabolic changes in freshwater fish Channa punctatus due to Stem-bark extract of Croton liglium. J. Biol. Sci. 2003, 6, 1223–1228. [Google Scholar]
- Stenersen, J. Chemical Pesticides. In Mode of Action and Toxicity; CRC Press: Boca Raton, FL, USA; London, UK, 2004. [Google Scholar]
- Moralev, S.N.; Rozengart, E.V. Comparative enzymology of Cholinesterase; International Universtity Line Inc.: La Jolla, CA, USA, 2007. [Google Scholar]
- Thanh, T.V. Toxicity of the Mixture of Vibasu 50ND (Diazinon) and Bassan (Fenobucarb) on Climbing perch (Anabas testudineus) Fingerling. Bachelor’s Thesis, Can Tho University, Can Tho city, Vietnam, 2010. [Google Scholar]
- Hue, D.T. Research on the Toxicity of the Mixture of Pesticides Containing 3% Chlopyrifos and 2% Fenobucarb on the Physiology and Biochemistry of Snakehead Fish (Channa striata). Master’s Thesis, Can Tho University, Can Tho, Vietnam, 2011. [Google Scholar]
- Guo, Z.; Cui, J.; Li, M.; Liu, H.; Zhang, M.; Meng, F.; Shi, G.; Wang, R.; He, X.; Zhao, Y. Effect of feeding frequency on growth performance, antioxidant status, immune response and resistance to hypoxia stress challenge on juvenile dolly varden char Salvelinus malma. Aquaculture 2018, 486, 197–201. [Google Scholar] [CrossRef]
- Pan, G.; Dutta, H.M. The inhibition of brain acetylcholinesterase activity of juvenile largemouth bass Micropterus salmoides by sublethal concentrations of diazinon. Environ. Res. 1998, 79, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Richendrfer, H.; Pelkowski, S.D.; Colwill, R.M.; Creton, R. Developmental sub-chronic exposure to chlorpyrifos reduces anxiety-related behavior in zebrafish larvaen. Neurotoxicol. Teratol. 2012, 34, 458–465. [Google Scholar] [CrossRef]
- Sancho, E.; Fernandez-Vega, C.; Sanchez, M.; Ferrando, M.D.; Andreu-Moliner, E. Alterations on AChE activity of the fish Anguilla anguilla as response to herbicide-contaminated water. Ecotoxicol. Environ. Saf. 2000, 46, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Fulton, M.H.; Key, P.B. Acetylcholinesterase inhibition in estuarine fish and invertebrates as an indicator of organophosphorus insecticide exposure and effects. Environ. Toxicol. Chem. 2001, 20, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.P. Stress proteins. Their detection and uses in biomonitoring. ASTM (Am. Soc. Test. Mater.) Spec. Tech. Publ. 1990, 1096, 338–347. [Google Scholar]
- Smith, R.R. Nutritional energetic. In Fish Nutrition, 2nd ed.; Halver, J.E., Ed.; Academic Press: San Diego, CA, USA, 1989. [Google Scholar]


| Control | T1 | T10 | T20 | |
|---|---|---|---|---|
| Temperature (°C) | 26.0 ± 0.09 a | 26.0 ± 0.09 a | 26.1 ± 0.09 a | 26.1 ± 0.09 a |
| pH | 6.93 ± 0.04 c | 7.15 ± 0.04 b | 7.34 ± 0.03 a | 7.42 ± 0.03 a |
| DO (mg/L) | 6.82 ± 0.04 a | 6.77 ± 0.04 a | 6.71 ± 0.05 a | 6.79 ± 0.05 a |
| N-NH4+ (mg/L) | 1.72 ± 0.17 a | 1.74 ± 0.19 a | 1.63 ± 0.16 a | 1.77 ± 0.18 a |
| N-NO2− (mg/L) | 1.72 ± 0.27 a | 1.94 ± 0.28 a | 2.51 ± 0.33 a | 2.05 ± 0.27 a |
| F (ppm) | ||
|---|---|---|
| Treatment | Nominal Concentration | Actual Concentration |
| Control | Tap water | <MDL = 0.0001 |
| T1 | 0.127 | 0.095 (0.095–0.102) |
| T10 | 1.27 | 1.25 (1.24–1.26) |
| T20 | 2.54 | 2.19 (2.17–2.20) |
| Treatment | Day 15 | Day 30 | Day 45 | Day 60 |
|---|---|---|---|---|
| Control | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| T1 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 | 100 ± 0.0 |
| T10 | 100 ± 0.0 | 98.9 ± 1.9 | 98.9 ± 1.9 | 98.9 ± 1.9 |
| T20 | 98.9 ± 1.9 | 98.9 ± 1.9 | 98.9 ± 1.9 | 98.9 ± 1.9 |
| AChE (µM/g/min) | AChE Inhibition Level (%) | |
|---|---|---|
| Normal (control) | 13.5 ± 0.65 a | 0 |
| Erratic swimming | 2.23 ± 0.09 b | 83.6 |
| Belly flipping | 2.10 ± 0.10 b | 84.5 |
| Death | 1.42 ± 0.09 b | 89.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.; Berg, H.; Nguyen, L.N.; Nguyen, Y.T.H.; Nguyen, C.V. The Effects of Fenobucarb on the Physiology, Behavior, and Growth of Silver Barb (Barbonymus gonionotus). Toxics 2025, 13, 12. https://doi.org/10.3390/toxics13010012
Nguyen TT, Berg H, Nguyen LN, Nguyen YTH, Nguyen CV. The Effects of Fenobucarb on the Physiology, Behavior, and Growth of Silver Barb (Barbonymus gonionotus). Toxics. 2025; 13(1):12. https://doi.org/10.3390/toxics13010012
Chicago/Turabian StyleNguyen, Tam Thanh, Håkan Berg, Loi Ngoc Nguyen, Yen Thi Hai Nguyen, and Cong Van Nguyen. 2025. "The Effects of Fenobucarb on the Physiology, Behavior, and Growth of Silver Barb (Barbonymus gonionotus)" Toxics 13, no. 1: 12. https://doi.org/10.3390/toxics13010012
APA StyleNguyen, T. T., Berg, H., Nguyen, L. N., Nguyen, Y. T. H., & Nguyen, C. V. (2025). The Effects of Fenobucarb on the Physiology, Behavior, and Growth of Silver Barb (Barbonymus gonionotus). Toxics, 13(1), 12. https://doi.org/10.3390/toxics13010012






